Abstract
Background
The ability to evade apoptosis is one of the key properties of cancer. The apoptogenic effect of nickel nanowires (Ni NWs) on cancer cell lines has never been adequately addressed. Due to the unique physicochemical characteristics of Ni NWs, we envision the development of a novel anticancer therapeutics specifically for pancreatic cancer. Thus, we investigated whether Ni NWs induce ROS-mediated apoptosis in human pancreatic adenocarcinoma (Panc-1) cells.
Methods
In this study Ni NWs were fabricated using the electrodeposition method. Synthesized Ni NWs were physically characterized by energy dispersive X-ray analysis, UV-Vis spectroscopy of NanoDrop 2000 (UV-Vis), magnetization study, scanning electron microscopy, and transmission electron microscopy. Assessment of morphological apoptotic characteristics by phase contrast microscopy (PCM), Ni-NWs-induced apoptosis staining with ethidium bromide (EB) and acridine orange (AO) followed by fluorescence microscopy (FM) was performed. For molecular biological and biochemical characterization, Panc-1 cell culture and cytotoxic effect of Ni NWs were determined by using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Quantitative apoptosis was analyzed by flow cytometry staining with propidium iodide through cell cycle arrest and generation of ROS using 2′, 7′-dichlorofluorescein diacetate fluorescence intensity. In all experiments, Panc-1 cancer cells without any treatment were used as the negative controls.
Results
The intracellular uptake of Ni NWs through endocytosis by Panc-1 cells was observed by PCM. EB and AO staining of FM and MTT assay qualitatively and quantitatively confirmed the extent of apoptosis. Flow cytometric cell cycle arrest and ROS generation indicated Ni NWs as inducers of apoptotic cell death.
Conclusion
We investigated the role of Ni NWs as inducers of ROS-mediated apoptosis in Panc-1 cells. These results suggested that Ni NWs could be an effective apoptotic agent for Panc-1 cells and have good potential for further research into a clinical treatment selective for pancreatic cancer.
Introduction
Pancreatic cancer has the highest fatality rates of all cancers; the 5-year survival rate is less than 5% due to intrinsic resistance of radiotherapy, immunotherapy, and chemotherapy. Citation1 Current pancreatic cancer treatment involves surgery, radiation, and drugs alone or in combination, which temporarily relieves symptoms, increases survival rates, and occasionally cures.Citation1 Apoptosis is derived from Greek “apo” meaning away from and “ptosis” meaning to drop or to fall.Citation2 Apoptosis is characterized by specific morphological features and biochemical cascades.Citation3 Morphological changes during apoptosis include plasma membrane blebbing, initial cell shrinkage, chromatin condensation, and formation of apoptotic bodies.Citation4 However, the organelles remain intact and healthy looking at the very late stage of death through continuous metabolic activity for some considerable time.Citation2 Cellular reactive oxygen species (ROS) are essential to cell survival. Additionally, ROS have important functions in pro-apoptotic pathways.Citation4 ROS are generated through nickel exposure in certain cell types and thought to be a key step in nickel-induced apoptosis due to an apoptotic response. Therefore, cancer cells may escape potentially carcinogenic ability.Citation5,Citation6
An ideal anticancer drug is expected to kill or inactivate cancer cells without causing excessive damage to normal cells.Citation7 These criteria are achievable only by apoptosis induction in cancerous cells because the lifespan of both normal and cancerous cells are significantly affected by the rate of apoptosis.Citation8 Thus, modulating or inducing apoptosis might be helpful in cancer therapy or preventions.Citation7,Citation9 Advanced nanomaterial-based drugs may offer the possibility of new and intriguing opportunities to eliminate the toxicity in human cancer cells.
Nickel (Ni) is a naturally occurring transition metal and trace element which is an essential nutrient for human and higher animals.Citation10 A possible health benefit of nickel includes optimal growth, healthy skin and bone structure.Citation11 However, nickel is a potent human carcinogen in large quantities with low mutational activity. The most carcinogenic ni-compounds are those that do not induce apoptosis.Citation12 Ni- induced apoptosis was reported on Chinese hamster ovary (CHO) cells, T-cell hybridoma, Jurkat cells, and mouse epidermal JB-6 cells.Citation5
By definition, a nanowire (NW) is a nanostructure that has a diameter restricted to tens of nanometers (10−9 m) or less and an unconstrained length which shows an aspect ratio (length-to-width) of ≥1000 higher. Nanowires (NWs) have numerous interesting properties that are not exhibited in bulk or 3-D materials. This is true because in nanowires, electrons are laterally quantum confined and occupied energy levels are different from the continuum of energy levels or bands of bulk substrates. The NWs have advantages over the same nanoparticles (NPs) in terms of geometrical anisotrophy, increased surface to volume ratio, and dipolar magnetic properties linked to the NW shape.Citation10
Choi et alCitation12 described Ni NWs are not toxic to cells and Guo et alCitation13 reported that Ni NWs are not immediately toxic to cells and chronic exposure is needed for them to act as carcinogens.Citation10,Citation14 Ni NWs can easily be internalized by cancer cells due to Ni+2 ions which are also present in metalloproteins. Citation10 Due to this property, it is possible that Ni NWs can also inhibit the proliferation of certain cancer cells,Citation14 thus implying their great potential in cancer biomedical and chemotherapeutic applications. Apoptosis suppression is a hallmark of human cancer.Citation15 Currently apoptotic cell death inducers and regulators are considered to have significant potential for cancer therapy which is a desired goal of many cancer treatments.Citation15 Though some nickel (II)-induced apoptosis has been reported recently, Citation5,Citation8 the findings do not represent the effects of Ni NWs on the pancreatic adenocarcinoma cells. Since nickel has the ability to induce apoptosis through generation of reactive oxygen species (ROS),Citation5 Ni NWs could be used to fight against cancer through tumor cell death induction.
Internalization of Ni NWs with cell cytoplasm may lead to internal reorganization of lysozomes and metalloproteins.Citation16 To the best of our knowledge this study is the first to delineate that Ni NWs induce cell cycle arrest, increase percentage of sub-G1 fractions, and trigger ROS generation in Panc-1 cells. These are the key characteristics for accomplishing apoptosis. Citation17 In close agreement with previous studies,Citation10,Citation12,Citation14,Citation16 our in vitro studies showed that the treatment of pancreatic cancer Panc-1 cells with Ni NWs resulted in a concentration and time dependent induction of apoptosis. Therefore, Ni NWs induced apoptosis shows promising potential as an innovative approach to pancreatic cancer therapy.
Materials and methods
Materials
Whatman Anodisc alumina filters (diameter of 25 mm with polypropylene support rings and 200 nanometer pores) were purchased from Voight global distributions Inc (Lawrence, KS) and Gallium Indium (GaIn) Eutectic was obtained from ProChem Inc (Rockeford, IL). Ni Wire (99% pure) and copper sheets were purchased from VWR International (Westchester, PA). Watts nickel plating solution (Watts Ni Pure recipes include 300 g/L NiSO4 · 6H2O, 45 g/L each H3BO3 and NiCl2 · 6H2O), and 6 M NaOH were prepared in our laboratory. Panc-1 cells, Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), 0.25 trypsin/0.53 mM EDTA (trypsin-EDTA), and penicillin-streptomycin antibiotics (P/S) were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Phosphate buffered saline (PBS) was purchased from Amresco Inc (Salon, OH) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) cell proliferation assay kits were obtained from Biotium, Inc (CA). 2′, 7′-dichlorofluorescein diacetate (DCF-DA) was purchased from EMD chemicals. Acridine orange (AO), ethidium bro-mide (EB), concentrated nitric acid (HNO3), microscope glass slides, and electrical tape were from our laboratory. PI and all other biochemicals not otherwise mentioned were all bought from Sigma-Aldrich (St Louis, MO).
Methods
Fabrication of Ni NWs
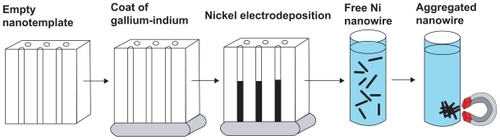
Ni NWs were synthesized by using the electrodeposition syringe method () with modifications.Citation18 A custom-made setting of 1.5 V (AA) battery holder and systems for the electrolysis of the nickel was used to prepare the Ni NWs. In brief, the synthesis of Ni NWs was accomplished by using an alumina (Al2O3) anodisc membrane as a template. One side of the alumina anodisc membrane was coated with the gallium-indium (GaIn) alloy which creates a conductive surface against a copper electrode. A pure Ni electrode was also added to the electrolytes. The synthesis of the Ni NWs takes place in a Ni plating solution. Following this, the alumina disc was removed and taped with its shiny side up onto a glass slide. Concentrated HNO3 was used to complete removal of all the GaIn from the alumina anodisc. The alumina anodisc was then washed with deionized water six times. Sodium hydroxide (6 M NaOH) was used to dissolve the alumina disc which had Ni NWs on its pore. The polystyrene support ring was then separated from the alumina filter on a beaker and discarded. Using a strong magnet, the Ni NWs were collected and the NaOH was poured off. The Ni NWs were washed at least 5 times to ensure the complete removal of the trace elements of NaOH. Finally, the Ni NWs were suspended in double distilled water (ddH20) in a 320 mL vial for analysis or extended storage. Structural and morphological characterization of synthesized Ni NWs was performed by using energy dispersive X-ray analysis (EDAX), UV-Vis spectroscopy, magnetization study, scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
Transmission electron microscopy
The sample of Ni NWs was collected and placed onto a formvar coated 200 mesh copper grid by evaporating a drop of ddH20 containing well dispersed Ni NWs and photographed with transmission electron microscope (JEOL JEM1200® EX-II) with AMT CCD imaging system at 120 kV.
Scanning electron microscopy
The synthesized Ni NWs were suspended in ddH20. A few drops of suspension were then put on a p-Si substrate using silver paint and allowed to dry overnight. A Philips XL 30® environmental scanning electron microscope (ESEM) was used to take the micrograph of the fabricated Ni NWs.
EDAX
Purity and elemental chemical analysis of prepared Ni NWs was performed by using EDAX attached with a Philips XL 30® ESEM.
Magnetization properties
Powerful magnets were used to characterize the magnetization properties of Ni NWs by suspending the Ni NWs in ddH2O and photographing them with a 5.1 megapixel Nikon digital camera.
UV-VIS spectroscopy
The UV-VIS spectrum of Ni NWs was monitored using a Fisher Scientific NanoDrop 2000 spectrophotometer while suspended in ddH2O with a baseline correction at 750 nm.
Concentrations of the fabricated Ni NWs working suspensions
Prepared Ni NWs were washed several times until a neutral pH (7.00) was obtained. These NWs were sonicated 10 minutes for well dispersion and resuspended in fresh DMEM cell culture medium to a final concentration of approximately 3 NWs for each cell (3:1). The concentration of the Ni NWs in aqueous suspension was measured by a haemacytometer (Hausser Scientific Bright Line) and observed with a 20× objective using a Zeiss inverted phase contrast 40 CFL microscope with Spot Basic camera software. In this study, the Ni NWs and cell ratio was kept very low (3) to guarantee a low cytotoxicity.Citation15
Cell culture and assessment of growth
The Panc-1 cells with a concentration ranging between 2 × 104 and 2 × 105 cells/cm2 were cultured in DMEM supplemented with 10% heat-inactivated FBS and 100 IU/mL P-S in a 75 cm2 flask. Cells were incubated at pH 7.4 at 37°C in a humidified chamber equilibrated with 95% air or 5% CO2. The medium was changed every 3 to 4 days and subculture was routinely performed at a 60% to 70% confluence using trypsin-EDTA to detach the cells. For all experiments, cells were seeded to provide experimental stages, 70% to 80% confluence in 6-, 24-, 96-wells plates grown for 24 hours. After 24 hours, cells were washed with pre-warmed 1 × PBS (pH 7.4) to remove all traces of serum. The cells were then grown in starvation medium (SMEM) (a normal MEM medium but with reduced 2% FBS) for an additional 24 hours before all experiments. Representative Panc-1 cultures were harvested and monitored for cell numbers by counting cell suspensions with hemacytometer.
Morphological analysis after internalization of Ni NWs and MTT metabolic activity study by phase contrast microscopy (PCM)
Intracellular uptake of Ni NWs through endocytosis and metabolic activity of Panc-1 cells was observed by Zeiss Axiovert 40 CFL® inverted phase contrast microscope in a configuration with transmitted light of 12 V, 35 W halogen lamp and 40 × /0.50 LD A-Plan.
Apoptosis assay by fluorescence microscopy
Ni NW-induced apoptosis in Panc-1 was investigated by staining the cells with a combination of fluorescent AO and EB dyes followed by BD Biosciences protocol with modifications. In short, cells were grown on an 18 mm × 18 mm cover slip in a 6-well plate up to 70% to 80% confluency (1 × 106 cells/well). Cells were treated with Ni NWs suspensions of various concentrations, which are mentioned separately in figure captions for the corresponding experiment. After the appropriate incubation time was completed, cells were washed with 1 × PBS and stained with 200 μL of dye mixture containing 15 mg/mL AO and 50 mg/mL of EB mixed in 1 × PBS for 2 to 3 minutes then were washed with PBS twice. The cover slips were then transferred to a sterile glass slide, turned upside down and observed and photographed immediately under fluorescence microscope using 3 to 5 randomly (Nikon Eclipse 90i® equipped with a digital camera DS-Fi1, NIS-Elements® version 3.1 software at a magnification of 40 times) selected fields of each sample. For negative control images, cells without any treatment stained with AO and EB were used. For apoptosis percentage determination, photographic images were used to determine the number of live cells that were fluorescence green and the number of apoptotic cells that were fluorescence red- orange. Approximately 200 to 300 cells per treatment were counted for apoptosis-related statistical analysis.
MTT assay
The viability of the Panc-1 cells was quantified by using MTT assay with slight modifications. In brief, Panc-1 cells were plated onto a flat-bottomed 96-well cell culture plate (Bioexpress) for 48 hours (until they had grown approximately 10,000 cells/well) using DMEM with 10% FBS and 1% P/S solution in the dark at 37°C incubator with 5% CO2. The Ni NWs treatment was done with concentrations of 0 μL (without Ni NWs as control), 10 μL, 50 μL, 100 μL, and 200 μL and allowed to grow for 24 hours. After the appropriate treatment time, the cell culture medium was discarded, and to remove the traces of uninternalized Ni NWs, each well was washed 3 times with 1 × PBS. After that, 10 μL MTT solution was added to each well of the 96-well plates containing Panc-1 cells, mixed by shaking gently on an orbital shaker for 5 minutes, and incubated for 4 hours at 37°C. Then the medium was removed and 200 μL of dimethyl sulfoxide was added into each well and pipetted up and down several times to dissolve the formazan created by the MTT. Absorbance was measured in a 96 well-microplate reader (BIOTEK μQuant®) at 570 nm with reference OD at 630 nm. Each treatment was replicated in triplicate and the mean value was used to calculate the data. For qualitative analysis, phase contrast images were taken randomly.
Cell cycle analysis using flow cytometry (FC)
To analyze cell cycle with FC of Ni NW-induced apoptotic Panc-1 cells, PI staining method was used with optimization and modification. Various concentrations of Ni NWs were used to induce apoptosis in Panc-1 cells using the methodology described in the apoptosis induction section above. A negative control was prepared by incubating cells without Ni NWs. After the incubation period of the appropriate treatment was completed, Panc-1 cells were harvested using TE and washed 3 times using pre-warmed 1 × PBS to remove the trace amount of the apoptosis inducing agent. The density of the cells was adjusted to ~1 × 106 cells/mL in PBS. To stain the Panc-1 cells on FC, for each assay of 1 mL cell suspension (in PBS), 0.5 μL of PI (1.5 μM) was added to the FC tubes. The stained cell suspension was then transferred and kept on ice to incubate for 30 minutes. Following this incubation period, the stained cells were quantified and data were produced by a Becton Dickinson (BD) FACS Calibur® flow cytometer. The Cell Quest Pro® software was then used to analyze the data. Approximately 10,000 events were quantified using a FACS Calibur® with an excitation wave-length of 536 nm and an emission wavelength of 617 nm.
Detection and quantification of intracellular ROS production
To determine the intracellular ROS generation, the Panc-1 cells were labeled with the fluorescent DCF-DA probe and measured with a BD FACSCalibur® flow cytometer. Panc-1 cells were seeded in 6-well plates up to 70%–80% confluence. The cell culture media was then replaced with SMEM 24 hours before appropriate Ni NW-treatment to induce apoptosis. After 24 hours of incubation, cells were incubated with 10 μM of DCF-DA at 37° C for 30 minutes. Following this, the cells were trypsinized, centrifuged, then the pellets were collected in FC tubes and resuspended in 1 mL of 1 × PBS and analyzed on BD FACSCalibur® with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. For data analyses the Cell Quest® Pro software was used.
Statistical analysis
All results were expressed as mean ± SE. The significance of difference was evaluated with one way analysis of variance (ANOVA). A probability level of P < 0.05 was considered statistically significant different from the control.
Results
Structural and morphological characterization of synthesized Ni NWs
In the EDAX spectrum () of the Ni NWs, the presence of nickel with a very tiny amount of oxygen and iron was found. Weight percentages by element are nickel 42.33, oxygen 4.58, iron 0.85, and silicon 52.24. This small portion of oxygen is due to the Ni NWs being exposed to air in the environment. The very minute level of iron resulted from pre-existing contamination in the ESEM and EDAX chamber. The silicon peak is justified by the silicon wafer chip, which was used to augment visibility of the Ni NWs in the SEM and EDAX by increasing contrast with a low background. Therefore, the EDAX data analysis revealed that the resultant Ni NWs were pure Ni metal.
Figure 2 Characterization of fabricated Ni NWs. (A) Energy dispersive X-ray analysis spectrum of Ni NWs for elemental analysis; (B) UV-Vis spectrum of Ni NWs suspended in ddH2O at neutral pH (7.00); (C) vertically aligned magnetic Ni NWs on the bottom of a beaker; (D) illustration of magnetic properties of Ni NWs; (E) Ni NWs suspended in aqueous media.
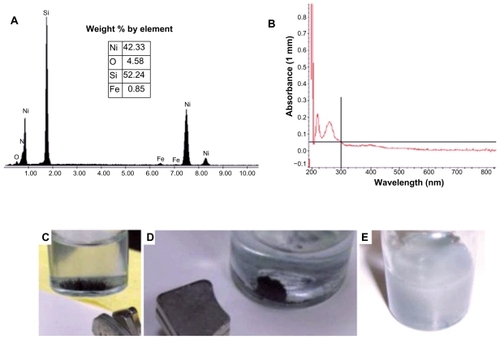
The UV-VIS spectrum () of the Ni NWs suspension was evaluated using the Fisher Scientific Nano-Drop 2000 spectrophotometer. The absorption spectrum displayed the optical properties and characteristic broadband at 264 nm with an absorbance of 0.18. The absorption spectrum has two peaks which may be attributed to the surface plasmon excitation.Citation19
illustrate the magnetic properties of Ni NWs in aqueous solution regulated with strong magnets. More specifically, in , Ni NWs are aggregated at the bottom of the vial in a vertical magnetic field and in , Ni NWs are collected on the sidewall of the vial illustrated by the black aggregate. depicts the Ni NWs in aqueous suspension which was used for apoptosis experiments in Panc-1.
Diameter and length of the fabricated Ni NWs
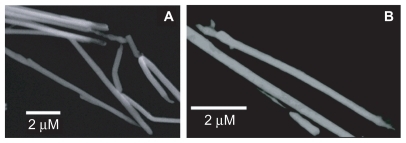
SEM and TEM were used to determine the length and diameter of Ni NWs. shows the representative SEM of the top view of Ni NWs and portrays a micrograph of a TEM view of the fabricated Ni NWs. The diameter of the fabricated Ni NWs ranged from 200 nm to 225 nm with the average of 215 nm. The average length of the Ni NWs was found to be 6.5 μm.
PCM for morphology and biocompatibility of internalized Ni NWs in Panc-1 cells
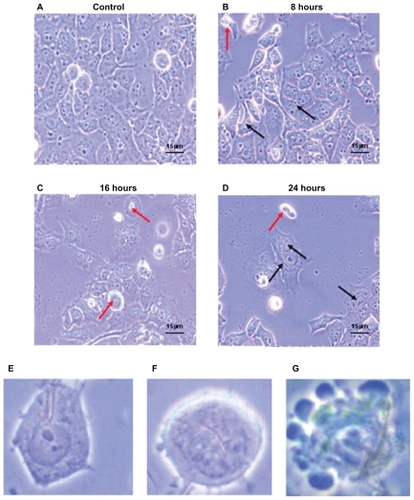
Compared with the control, as depicted by , there was increasing detachment of cells from the well surface in addition to internalization of Ni NWs with increasing exposure time. Apoptotic morphological changes are portrayed in showing cell shrinkage, round apoptotic bodies, and membrane blebbing in PCM (magnification 40×). The biocompatibility of the fabricated Ni NWs has been investigated in Panc-1 cell lines. It was determined that Ni NWs (diameter ~215 nm and length up to 7 μm) can easily be internalized by Panc-1 cells. The Ni NWs are indicated by arrows in the figures. Adherent cells with internalized Ni NWs are designated by black arrows and suspended cells with internalized Ni NWs are indicated by red arrows.
Figure 4 Morphological characteristics of Panc-1 cells which were treated with 200 μl of Ni NWs suspension for 0 (control), 8, 16, and 24 hours and visualized with phase contrast microscope (magnification 40×). Increasing detachment of cells from the well surface is seen with increasing exposure time. (A) Adherent cells without Ni NWs (control); (B) adherent (black arrows) and suspended (red arrows) cells with internalized Ni NWs after 8 hours; (C) adherent and a greater number of suspended cells with internalized Ni NWs after 16 hours; (D) Adherent and suspended cells with internalized Ni NWs after 24 hours. (E–G) represent more specific cellular morphological changes. (E) Uptake of Ni NWs by endocytosis to the cell cytoplasm; (F) internalization of Ni NWs to the cell nucleus followed by cell shrinkage, round shaped, and initiation of membrane blebbing; (G) apoptotic body formation through final membrane blebbing.
Fluorescent pictures of Panc-1 cells after Ni NW-induction of apoptosis
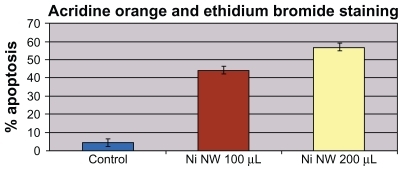
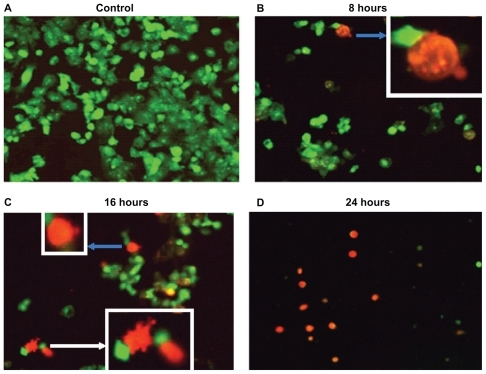
show fluorescence pictures with advancing exposure time to the Ni NWs suspension compared with the control. With increasing exposure time to Ni NWs, fewer Panc-1 cells survived. Cells were stained with AO and EB dyes. Viable Panc-1 cells displayed green nuclear fluorescence and apoptotic cells exhibited a red nucleus due to DNA binding capacity of EB. The bar graph in portrays a direct relationship between concentration of Ni NWs and extent of apoptosis in Panc-1 cells.
Figure 5 Panc-1 cells treated with Ni NWs which potentiates apoptosis. Cells were treated with 200 μL Ni NWs for 8, 16, and 24 hours and stained with ethidium bromide (EB) and acridine orange (AO) and were visualized and photographed immediately with fluorescence microscope. (A) Control; (B) 8 hours after treatment – blue arrow indicates initial membrane blebbing of the apoptosed cells; (C) 16 hours after treatment – the white arrow indicates late membrane blebbing; (D) 24 hours after treatment. Cells were stained with AO and EB where viable cells were green nuclear fluorescence and apoptotic cells exhibit a red nucleus.
MTT assay for cellular proliferation, viability, and metabolic activity study
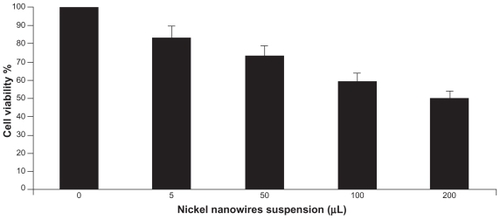
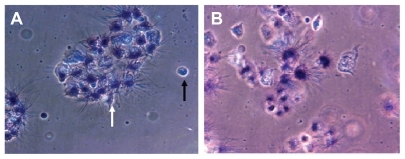
shows qualitative morphological study of PCM on Panc-1 cell viability and proliferation. It is evident that the morphology of Panc-1 cells treated with Ni NWs differs from the controls (ie, without treatment). As shown in , formazon grew on live or metabolically active cells as depicted by the deep purple cell nucleus. However, dead cells did not grow formazon and therefore the color was not changed. Black and white arrows in indicate dead and live cells respectively. For quantitative study of cell viability and proliferation, we used MTT assay and analyzed the results with a microplate reader. The bar graph in displays the dose and time-dependent effects of Ni NWs on the proliferation of Panc1 cells. In this figure, the percentages of viable cells with different concentrations of NI NWs were shown and compared with the control in which the cells are approximately 100% viable.
Figure 7 Qualitative morphological study of apoptosis by MTT assay. Phase contrast pictures of formazon in Ni NWs treated Panc-1 cells where (A) control, ie, untreated cells and (B) treated cells. Viable cells exhibited a deep purple cell nucleus with rod shaped formazon whereas apoptotic cells lacked formazon growth because of the lack of mitochondrial reductase and thus did not change in color. Dead cells are indicated by black arrows and live cells are identified by white arrows.
Apoptotic cell cycle analysis through FC
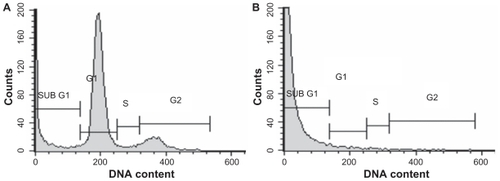
Cell cycle analysis of Ni NW-induced apoptosis in Panc-1 cells was shown in . The cells were incubated with Ni NWs () for 24 hours, stained with PI, and analyzed by FC (BDFACS Calibur®). These results were compared with the control. The increase of sub-G1 phase accumulation is the biomarker of apoptosis.Citation20 This is visualized in where the percentage of the sub-G1 peak is exclusively increased, which is an indicator of apoptosis. FC analysis showed that the Ni NW-induced Panc-1 cell proliferation was suppressed and cell cycle arrest occurred in G0/G1 phase. Thus, our study suggested that Ni NWs modulate cellular response through apoptosis effectors involved in cell cycle. In the cell cycle, 2 check points G1 and G2 play a critical role in the regulation of cells going from S and M phases such that apoptotic or dead cells stop DNA replication at G1 or G2.Citation17 In our study involving flow cytometric DNA content analysis, cell cycle was terminated in the sub-G1 phase. These data establish that the Panc-1 cell cycle was halted as a result of exposure to Ni NWs which clearly demonstrates apoptosis.
Figure 9 Cell cycle analysis after induction of apoptosis in Panc-1 cells by Ni NWs. The cells were incubated with Ni NWs for 24 hours, stained with PI and analyzed by Cell Quest Pro® on FC (BD-FACS Calibur®). A portrays untreated cells with all phases of the cell cycle, whereas B shows an exclusively increased sub-G1 peak with Ni NWs treatment.
Quantification of endogeneous ROS generation with DCF fluorescence of FC
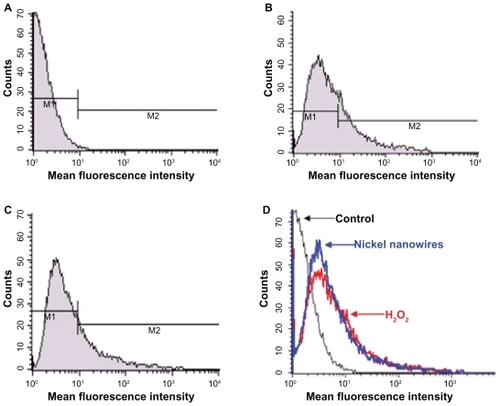
ROS has major functions in a series of biological responses and diverse signaling pathways including apoptosis.Citation21 ROS production by Ni NWs was measured by FC. shows Ni NWs-induced ROS generation of Panc-1 cells. Panc-1 cells were treated with different concentrations of Ni NWs over 24 hours. Cells without treatment were used as the control (), cells treated with H2O2 (0.3%) were used as the positive control (), and Ni NW-treated cells (). (representative overlay histograms of ) shows increases of mean fluorescence intensity (oxidant production) as right shifts on the x-axis.
Figure 10 Ni NWs-induced reactive oxygen species generation in Panc-1 cells were measured by flow cytometry. The fluorescence intensities of 10,000 cells were analyzed by BD-FACS Calibur®. Representative histograms show cell number on the y-axis and increases of fluorescence (oxidant production) as right shifts on the x-axis (mean fluorescence intensity). (A) Cells without treatment; (B) cells treated with 0.3% H2O2; (C) Ni NWs treated cells; (D) overlay histogram of A–C.
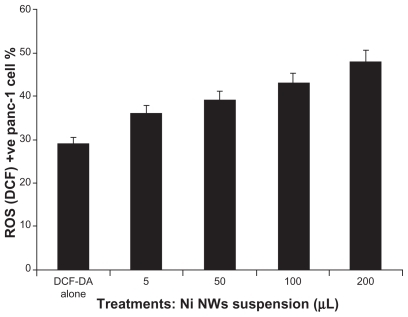
The bar graph in represents the concentration dependent ROS generation which is quantified using DCF-DA by FC, where the x-axis portrays the concentration of Ni NWs and the y-axis denotes the percentage of ROS (DCF) positive Panc-1 cells. We further examined the correlation between the rate of apoptosis and the ROS generation by Ni NW-treated cells. A significant positive correlation was observed with a correlation coefficient of 0.99373. These results clearly demonstrated that Ni NWs induced ROS generation and ultimately apoptotic cell death.
Figure 11 Quantification of reactive oxygen species (ROS) generation on Ni NW-induced Panc-1 cells stained with DCF-DA dye and measured by flow cytometry. This bar graph represents the concentration dependent ROS generation where the x-axis represents the concentration of Ni NWs and the y-axis denotes the percentage of ROS (DCF)-positive Panc-1 cells.
Discussion
Structural and morphological characterization of synthesized Ni NWs was determined by using EDAX, UV-Vis, magnetization study, SEM, and TEM. From these analyses we gained the compositions, diameter, length, and characteristics of the Ni NWs and came to the conclusion that Ni NWs prepared in our laboratory were pure Ni metal.
In this study, by using several standard apoptotic analysis strategies, we demonstrated that Ni NWs induced apoptosis through ROS generation. First, we microscopically observed morphological changes showing the evidence of Ni NWs induced apoptosis in Panc-1 cells through typical apoptosis features such as cell shrinkage, cell membrane blebbing, detachment from surface, nuclear condensation and apoptotic round-shape body formation. Secondly, we illustrated that Ni NWs enhanced cell death in Panc-1 cells as proven by an increased sub-G1 peak percentage of cell cycle arrest which mediates the increased apoptotic cell percentage in DNA FC. Third, since nanomaterials do not have the same properties as bulk materials,Citation13 in the global cytotoxicity perspective, cytotoxicity of nanomaterials in cancer cells should be distinguished from cell specific or cell receptor-specific toxicity.Citation21 To investigate the mechanism of Ni NWs-induced cytotoxicity, we examined the mitochondrial activity assay in Panc-1 cells both quantitatively and qualitatively. The viability result of MTT implies that the cytotoxic effect of Ni NWs is related to programmed cell death. In our study, it was found that the viability of the Panc-1 cancer cells decreased as the concentration of the Ni NWs and time of exposure increased.
ROS such as H2O2 have been considered as cytotoxic byproducts of cellular metabolism, and the overproduction of ROS in cells may enhance cell death.Citation22,Citation23 We next examined whether Ni NWs have the ability to generate endogeneous ROS in Panc-1 cells. For this reason, cells treated with Ni NWs were stained with ROS specific DCF-DA dye which is specific for H2O2. In , rightward shift of the peaks indicate that cellular ROS were strongly induced by Ni NWs through H2O2 accumulation, suggesting that Ni NWs function as an apoptotic agent in cancer cells. Furthermore, significant cellular ROS accumulation was detected in Ni NW-treated Panc-1 cells which exert anti-cancer effects through H2O2 generation.
Our results point to a positive correlation between the rate of apoptosis and the ROS generation induced by Ni NWs with a concentration dependent fashion. These findings are also confirmed by cellular and nuclear morphological changes, loss of viability in concentration-dependent manner (MTT assay), increase in PI uptake, and resultant increase in sub-G1 peak in DNA content analysis of FC. We have shown that without any specific surface functionalization, Ni NWs were able to induce apoptosis in certain cancer cell lines such as Panc-1.
Taken together, our results suggest the molecular mechanisms involved in Ni NWs induced apoptosis through ROS generation in Panc-1 cells. To the best of our knowledge this is the first report showing Ni NWs-induced ROS mediated apoptotic cell death in Panc-1 cancer cells.
Conclusion
In summary, the present study indicates that Ni NWs inhibit cell proliferation of Panc-1 cell lines and induce apoptosis by accumulating ROS. More specifically, we have shown that Ni NWs induce oxidative stress, resulting in ROS generation, which in response triggers apoptosis in Panc-1 cells. Overall our data suggest that Ni NWs have the properties of apoptotic agents through cell cycle arrest by diminishing fractions of cell cycle phases excluding the sub-G1 phase, increasing endogenous ROS generation and finally inducing apoptosis in Panc-1 cells. For these reasons, Ni NWs show good potential for the treatment of pancreatic cancer selectively and warrant further investigation.
Acknowledgments
The authors would like to thank Rozina Akter, PhD candidate, for critically reading the manuscript and Jill L Castleberry, MS candidate for English language copy editing. Md. Zakir Hossain thanks Dr William H Baltosser for his assistance and acknowledges the Graduate Institute of Technology, UALR, for a Graduate Assistantship.
Disclosure
The authors declare that there are no conflicts of competing interests in relation to this work.
References
- National Cancer Institute, US National Institutes of Health http://www.cancer.gov. and http://www.cancer.gov/cancertopics/types/pancreaticAccessed at April 18, 2011
- PottenCWilsonJApoptosis: the Life and Death of CellsCambridge University Press2004
- KanducDMittelmanASerpicoRSinigagliaECell death: apoptosis versus necrosis (review)Int J Oncol20022116517012063564
- HeldPNewickKUsing BioTek’s Synergy HT Reader to measure reactive oxygen species (ROS) generation in stimulated cellsTech Resources200813
- PanJChangQWangXReactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cellsChem Res Toxicol20102356857720112989
- LauSTLinZXLeungPSRole of reactive oxygen species in brucein D-mediated p38-mitogen-activated protein kinase and nuclear factor-κB signalling pathways in human pancreatic adenocarcinoma cellsBr J Cancer201010258359320068565
- AmitTKRoyMBhattacharyaRKNatural products as inducers of apoptosis: implication for cancer therapy and preventionCurr Sci2001801113871396
- ZhaoJBowmanLZhangXMetallic nickel nano- and fine particles induce JB6 cell apoptosis through a caspase-8/AIF mediated cytochrome c-independent pathwayJ Nanobiotechnology20097219379505
- SharmaMSharmaPDBansalMPSinghJLantadene A-induced apoptosis in human leukemia HL-60 cellsIndian J Pharmacol200739140144
- Available from: http://www.webelements.com/nickel/biology.htmlAccessed April 18, 2011
- CostaMMolecular biology of nickel carcinogenesisFresenius J Anal Chem1998361381385
- ChoiDParkJKimSHyperthermia with magnetic nanowires for inactivating living cellsJ Nanosci Nanotechnol200881518468051
- GuoDWuCLiJSynergistic effect of functionalized nickel nanoparticles and quercetin on inhibition of the SMMC-7721 cells proliferationNanoscale Res Lett200941395140220651919
- GaoNWangHYangEHAn experimental study on ferromagnetic nickel nanowires functionalized with antibodies for cell separationJ Nanotechnology20102110105107
- WardTHCummingsJDeanEMinireview biomarkers of apoptosisBr J Cancer20089984184619238626
- Prina-MelloADiaoZCoeyJMDInternalization of ferromagnetic nanowires by different living cellsJ Nanobiotechnology20064916953891
- ShiaoYHLeeSHKasprzakKSCell cycle arrest, apoptosis and p53 expression in nickel (II) acetate-treated Chinese hamster ovary cellsCarcinogenesis199819120312079683178
- BentleyAKFarhoudMEllisABLisenskyGCAnne-MarieCroneWCTemplate synthesis and magnetic manipulation of nickel nanowiresJ Chem Educ200582765768
- SapkalSBShelkeKFShingateBBShingareMSSynthesis, characterization and structural investigations of Ni nanoparticless: an ecofriendly and reusable catalyst for the synthesis of 3, 4-dihydropyrimidine-2(1H)-ones via Biginelli reactionBull Korean Chem Soc201031351
- KezhouCChongRZengliangYNickel-induced apoptosis and relevant signal transduction pathways in Caenorhabditis elegansToxicol Ind Health20102624925620237193
- PatraHKBanerjeeSChaudhuriULahiriPDasguptaAKCell selective response to gold nanoparticlesNanomedicine2007311111917572353
- WangQZhengXYangLReactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cellsJ Exp Clin Cancer Res20102915921143894
- EruslanovEKusmartsevSIdentification of ROS using oxidized DCFDA and flow-cytometryMethods Mol Biol2010594577220072909