Abstract
Bioactivity and osteoconductivity of titanium degrade over time after surface processing. This time-dependent degradation is substantial and defined as the biological aging of titanium. UV treatment has shown to reactivate the aged surfaces, a process known as photofunctionalization. This study determined whether there is a difference in the behavior of biological aging for titanium with micro-nano-hybrid topography and titanium with microtopography alone, following functionalization. Titanium disks were acid etched to create micropits on the surface. Micro-nano-hybrid surfaces were created by depositioning 300-nm diameter TiO2 nodules onto the micropits using a previously established self-assembly protocol. These disks were stored for 8 weeks in the dark to allow sufficient aging, then treated with UV light for 48 hours. Rat bone marrow–derived osteoblasts were cultured on fresh disks (immediately after UV treatment), 3-day-old disks (disks stored for 3 days after UV treatment), and 7-day- old disks. The rates of cell attachment, spread, proliferation, and levels of alkaline phosphatase activity, and calcium deposition were reduced by 30%–50% on micropit surfaces, depending on the age of the titanium. In contrast, 7-day-old hybrid surfaces maintained equivalent levels of bioactivity compared with the fresh surfaces. Both micropit and micro-nano-hybrid surfaces were superhydrophilic immediately after UV treatment. However, after 7 days, the micro-nano- hybrid surfaces became hydrorepellent, while the micropit surfaces remained hydrophilic. The sustained bioactivity levels of the micro-nano-hybrid surfaces were nullified by treating these surfaces with Cl−anions. A thin TiO2 coating on the micropit surface without the formation of nanonodules did not result in the prevention or alleviation of the time-dependent decrease in biological activity. In conclusion, the micro-nano-hybrid titanium surfaces may slow the rate of time-dependent degradation of titanium bioactivity after UV photofunctionalization compared with titanium surfaces with microtopography alone. This antibiological aging effect was largely regulated by its sustained electropositivity uniquely conferred in TiO2 nanonodules, and was independent of the degree of hydrophilicity. These results demonstrate the potential usefulness of these hybrid surfaces to effectively utilize the benefits of UV photofunctionalization and provide a model to explore the mechanisms underlying antibiological aging properties.
Introduction
Recent reports on time-dependent degradation of bioactivity and osteoconductivity of titanium surfaces have provided considerable scientific and therapeutic impacts to the field of metallic implants.Citation1,Citation2 For instance, at an early healing stage in an animal model, the strength of implant fixation and the percentage of bone coverage around implants for 4-week-old surfaces were reduced to less than 50% of those for new surfaces.Citation1 Along with these compromised in vivo biological capabilities; significant impairments in in vitro bioactivity- related parameters were also indicated. The rate and degree of protein adsorption, cell attachment, and cell proliferation decreased by 30%–80% on 4-week-old titanium surfaces compared with new surfaces.Citation1–Citation3 Progressive accumulation of hydrocarbons, and time-related disappearance of hydrophilic properties and electrostatic positivity on titanium surfaces are thought to be responsible for this biological aging.Citation1,Citation4,Citation5
Currently, there are no effective means to prevent the biological aging of titanium. Titanium implant products have been sold as a storable medical device with no expiration date or conditional disclaimers regarding time-related changes to their biological capabilities. Therefore, the above-mentioned findings unleashed a pivotal implication that the biological capabilities of commercial implants may become unpredictably and unavoidably diminished.Citation1,Citation4 However, on the other hand, discovery of the biological aging of titanium revealed that new titanium surfaces possess a remarkably high level of bioactivity compared with titanium surfaces that are ordinarily available. This means that using new surfaces or preserving them, if possible, may result in significant advancements that could improve the biological capabilities of titanium surfaces, providing new strategies for the development of better osseous implants. Notably in the history of biomaterials science, because of the above-mentioned unique physicochemical properties, ie, superhydrophilic, electropositive, and clean (low carbon) surfaces, and a remarkably high potential to attract proteins and cells, new titanium surfaces have been redefined as bioactive, which have revised the long-held understanding that titanium is a bioinert material.Citation1,Citation4 Thus, prevention of the biological aging of titanium would be tantamount to the preservation of titanium in its bioactive state and a novel way of developing improved implant surfaces.
Treatment of titanium surfaces with UV light has been demonstrated to substantially increase their bioactivity and osteoconductivity.Citation5–Citation7 UV treatment is thought to counteract biological aging of titanium. The levels of bioactivity and osteoconductivity of UV-treated titanium surfaces exceeds the levels of newly prepared titanium surfaces.Citation2,Citation3 UV treatment reverses the time-dependent physicochemical changes of titanium surfaces.Citation4,Citation5 UV treatment of aged titanium surfaces decomposes hydrocarbons by inducing photocatalytic activity and restores superhydrophilicity. Moreover, UV-treated titanium surfaces are electropositive, whereas aged titanium surfaces that have not received UV treatment are electronegative.Citation4 These biological and physicochemical effects of UV treatment are collectively defined as photofunctionalization of titanium.Citation4,Citation5,Citation7 It is expected to be a new approach of surface enhancement to circumvent conventional surface modification technologies due to its technical simplicity, since it does not require additional chemical or mechanical processing of the original titanium surfaces. Here, a crucial question is raised about whether UV-photofunctionalized titanium surfaces are subjected to the same phenomena of biological aging as untreated titanium surfaces. If so, the sustainability of UV-induced highly bioactive titanium surfaces is an urgent matter to address.
A controllable, nanonodular structuring of TiO2 has recently been reported.Citation8,Citation9 Nanonodules can self-assemble onto specifically conditioned microstructured titanium surfaces during the chemical deposition of TiO2. When nanonodular structuring is applied to acid-etch-created microroughened titanium surfaces, a unique micro-nano-hybrid structure is created by the formation of TiO2 nanonodules within microscale compartments. These micro-nano-hybrid titanium surfaces have demonstrated the ability to considerably increase bioactivity and osteoconductivity compared with that of microtopography surfaces.Citation8 The micro-nano-hybrid titanium surface increases protein adsorption, cell attachment, and proliferation as well as the in vivo biomechanical strength of the bone-titanium interface. This effect varies with the size of nanonodules and is the most apparent with 300-nm diameter nodules.Citation8 TiO2 micro-nano-hybrid surfaces exhibit 4- to 5-fold increases in surface roughness and 1.6-fold increases in surface area compared with microtopographic surfaces.Citation8 In addition, micro-nano-hybrid surfaces have a thicker layer of titanium oxide depending on the deposition time of TiO2. A recent study demonstrated that UV treatment increases the biological property of micro-nano-hybrid surfaces.Citation10 The next crucial question would be whether and how UV-photofunctionalized micro-nano-hybrid surfaces are subjected to the biological aging. For instance, one simple but difficult question is whether the larger surface area of the micro-nano-hybrid surface compared to microfeatured surfaces has positive or negative effects on the progression of biological aging.
This study tested the following two major hypotheses: (1) biological aging, ie, time-dependent decrease in the bioactivity of titanium, occurs on UV-photofunctionalized titanium surfaces; and (2) micro-nano-hybrid surfaces modulate the rate of biological aging of titanium. Acid- etched titanium surfaces were used as microtextured surfaces because they have been topographically and biologically characterized in the literature and are one of the most commonly used surfaces in dental and orthopedic implants. Acid-etched, microtextured surfaces with 300-nm nodule deposits were used as the micro-nano-hybrid titanium surfaces.
Materials and methods
Creation of TiO2 micro-nano-hybrid topography
Titanium disks (20 mm in diameter and 1 mm in thickness) of grade 2 commercially pure titanium were prepared by machining. To create a microtexture with peaks and valleys (micropit surface), titanium samples were acid-etched with 3% HF and 66% H2SO4. We employed the previously established method of controllable nanonodule self-assembly to create TiO2 nanonodular structures within the micropits (micro-nano-hybrid surface).Citation8,Citation9 The technical strategy is schematically described in . TiO2 was depositioned onto the acid-etched surface using a sputter deposition system (Denton Discovery 550, Moorestown, NJ) with a deposition rate of 18.5 Å/minute. Deposition time was 164 minutes to form 300-nm nodules according to the previously established formula. The surface morphology of these surfaces was examined by scanning electron microscopy (SEM) (XL30, Philips, Eindhoven, Netherlands) and a laser profile microscope (VK-8500, Keyence, Osaka, Japan) for average roughness (Ra), peak-to-valley roughness (Rz), interirregularities space (Sm), and surface area. The surface area was measured in a 5 μm × 5 μm horizontal plane: if the surface is completely flat, the surface area is 25 μm2. The measurement is based on the non-contact optical scanning and these numbers are calculated using the pre-installed algorithms. To identify the role of nanonodules in potential anti-aging effects after UV functionalization, a very thin TiO2 coating without the formation of nanonodules was also examined for its biological property. It was reported that TiO2 sputter deposition of thinner than 100 nm does not result in the formation of defined nanonodular structures.Citation8 Therefore, 50-nm-thin deposition (27 min deposition with the same deposition rate as above) was carried out onto the acid-etched micropit surfaces.
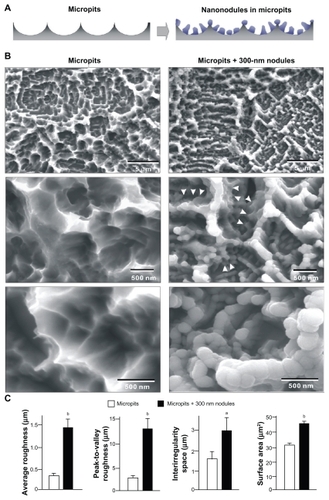
Figure 1 TiO2 nanonodules in a tailored size of 300 nm created within micropit architecture of titanium surfaces. (A) The strategic design of micro-nano-hybrid topography of TiO2 is described schematically. Addition of 300 nm nanonodules to the acid-etching-created micropit surface was planned. (B) Scanning electron micrography (SEM) before and after TiO2 sputter deposition onto the acid-etched (micropit) surface. Note that the nanonodular development of TiO2 at the flank (arrowheads) and top of existing peaks, while preserving the peak lines of the microscale compartments, manifesting the hybrid configuration of micro- and nano-architecture as a result. (C) Quantitative measurement of surface roughness and surface area of the micropit surface with and without 300 nm nanonodules (n = 9). The surface area was measured in a 5 μm × 5 μm horizontal plane.
Notes: aP < 0.05; bP < 0.01, indicating a statistically significant difference between the surfaces with micropits alone and with micropits and nanonodules.
UV photofunctionalization and hydrophilicity assessment
The prepared titanium disks were placed in a sealed container and stored in a dark room for 8 weeks to allow sufficient aging (sufficiently aged surface). After the storage, some titanium disks were treated with UV light for 48 hours under ambient conditions using a 15W bactericidal lamp (Toshiba, Tokyo, Japan); intensity; ca. 0.1 mW/cm2 (λ = 360 ± 20 nm) and 2 mW/cm2 (λ = 250 ± 20 nm). These UV-treated titanium disks were used immediately (fresh surface) for cell culture, or stored in the dark for 3 (3-day-old surface) or 7 days (7-day-old surface) before cell culture. Hydrophilicity of titanium surfaces was assessed by measuring the contact angle and spread area of a 10 μL ddH2O dropped onto the disks.
Osteoblast cell culture
As established previously,Citation5,Citation11 bone marrow cells isolated from the femurs of 8-week-old male Sprague-Dawley rats were placed into alpha-modified Eagle’s medium supplemented with 15% fetal bovine serum, 50 μg/mL ascorbic acid, 10 mM Na-β-glycerophosphate, 10−8 M dexamethasone, and antibiotic/antimycotic solution. Cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C. At 80% confluency, the cells were detached using 0.25% trypsin-1 mM EDTA-4 Na and seeded onto either sufficiently aged disks or differently aged UV-treated disks (fresh, 3-day-old, 7-day-old disks) with micropits or micro-nano- hybrid textures at a density of 4 × 104 cells/cm2. The culture medium was renewed every 3 days.
Cell attachment, density, and proliferation assays
Initial attachment of cells was evaluated by measuring the amount of cells attached to titanium disks after 3 hours (early stage attachment) and 24 hours (late-stage attachment) of incubation. Propagated cells were also quantified as cell density at day 3 of culture. These quantifications were performed using WST-1-based colorimetry (WST-1, Roche Applied Science, Mannheim, Germany). A culture well was incubated at 37°C for 4 hours with 100 μL tetrazolium salt (WST-1) reagent. The amount of formazan product was measured using an ELISA reader at 420 nm. The proliferative activity of cells was measured by BrdU incorporation during DNA synthesis. At day 3 of culture, 100 μL of 100 mM BrdU solution (Roche Applied Science) was added to the culture wells and incubated for 10 hours. After trypsinizing cells and denaturing DNA, cultures were incubated with anti-BrdU antibody conjugated with peroxidase for 90 minutes and reacted with tetramethylbenzidine for color development. Absorbance was measured using an ELISA reader at 370 nm (Synergy HT, BioTek Instruments, Winooski, VT).
Morphology and morphometry of cells
Spreading behavior and cytoskeletal arrangement of osteoblasts seeded onto titanium surfaces were examined using confocal laser scanning microscopy. At 6 hours after seeding, cells were fixed in 10% formalin and stained using fluorescent dye rhodamine phalloidin (actin filament, red color; Molecular Probes, OR). To observe the intracellular expression and localization of vinculin, a focal adhesion protein, cells were additionally stained with mouse antivinculin monoclonal antibody (Abcam, Cambridge, MA), followed by FITC-conjugated antimouse secondary antibody (Abcam). The area, perimeter, Feret’s diameter, and circularity of cells were quantified using an image analyzer (ImageJ, NIH, Bethesda, ML).
Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity of osteoblasts was examined at day 7 using a colorimetry-based assay. Cultures were rinsed with ddH2O followed by the addition of 250 μL p-Nitrophenylphosphate (LabAssay ATP, Wako Pure Chemicals, Richmond, VA), and then incubated at 37°C for 15 minutes. ALP activity was evaluated as the amount of nitrophenol released by the enzymatic reaction and measured using an ELISA reader at 405 nm.
Mineralization assay
The mineralization capability of cultured osteoblasts was examined by colorimetry-based quantification of calcium deposition at day 14. Cultures were washed with PBS and incubated overnight in 1 mL of 0.5 M HCl solution with gentle shaking. The solution was mixed with o-cresolphthalein complexone in an alkaline medium (calcium binding and buffer reagent, Sigma, St Louis, MO) to produce a red calcium-cresolphthalein complexone complex. Color intensity was measured by the absorbance at 575 nm using an ELISA reader.
Electrostatic treatment of titanium surfaces
To identify the role of surface electrostatic status of titanium surfaces in determining their bioactivity, osteoblast attachment was examined on fresh and 7-day-old UV-treated titanium surfaces with an additional ion treatment. The titanium disks were incubated for 24 hours at room temperature in 1 mL of 0.1 M NaCl. The disks were then washed thrice with ddH2O and left to completely dry at room temperature for 3 hours before seeding cells. Both micropit and micro-nano- hybrid surfaces were tested.
Statistical analyses
Three samples were used for all studies (n = 3) except for surface roughness assessment of titanium disks (n = 9) and cell morphometry (n = 10). One-way ANOVA was used to examine the effects of the different age of the titanium surfaces. If necessary, a post-hoc Bonferroni test was used as a multiple comparisons test; P < 0.05 was considered significant. Correlations between the 3 and 24 hour cell attachment and H2O contact angle were examined, and regression formulas were determined by least-squares mean approximation; P < 0.05 was considered significant.
Results
Topographical characterizations of micro-nano-hybrid TiO2 surfaces
Acid-etched titanium surfaces showed uniform microroughness features, so-called micropits, comprising peaks and valleys with intervals of 0.5–2.0 μm (low-magnification image, top of ). High-magnification images showed compartmental structures formed by sharp ridges (mid and bottom images in ). After TiO2 deposition of a theoretical thickness of 300 nm, the acid-etched surfaces showed a combination of micro and nanoscale topographical features; nanometer scale nodular structures were added to the micropit surfaces (). A low-magnification image of the depositioned surfaces showed a micron scale configuration formed by a preserved continuity of ridges (top image, ). High magnification images clearly showed the newly formed nanonodules within the micron scale compartments along the flank and top of the ridges (middle and bottom images, ). Nanonodules were evenly and densely formed with an average diameter of 295 ± 25 nm. Because of the depositioned nanonodules, the acid-etching-created peaks were rounded. Surface roughness analysis indicated that the average and the peak-to-valley roughness increased approximately 4- and 5-fold, respectively, on micropit + 300-nm nodule surfaces compared with surfaces with only micropits (). The interirregularity space increased twofold on the micropit-nanonodule surfaces, while the surface area increased by 60%.
Rapid reduction of hydrophilicity on micro-nano-hybrid surfaces
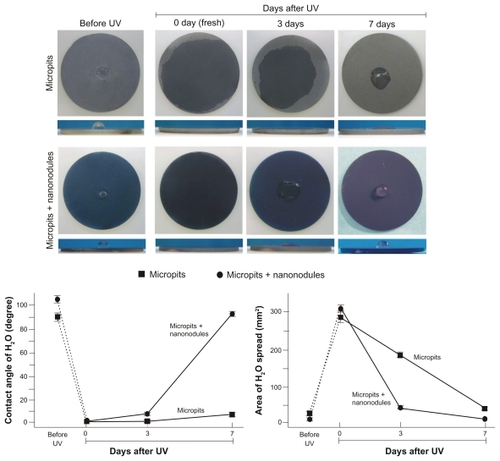
Time-related changes to UV-induced superhydrophilicity were examined on micropit and micropit-nanonodule surfaces. Acid-etching-created micropit surfaces and sputter-created micropit-nanonodule surfaces were stored for 8 weeks under dark, ambient conditions after processing, and then treated with UV light as described in the Materials and methods section. Both the micropit and micropitnanonodule surfaces, which were very hydrophobic (defined by a contact angle of >30°), became superhydrophilic (defined by a contact angle of <5°) with ddH2O contact angles becoming 0 ± 0° after UV treatment (images and line graphs, ). However, as the storage time after UV treatment increased, the hydrophilic nature of both surfaces attenuated. The rate of hydrophobic change was more rapid for micropit-nanonodule surfaces than for surfaces with micropits only. After 3 days of storage, micropit surfaces still maintained superhydrophilic properties, with a contact angle of <5°. Even after 7 days of storage, micropit surfaces were hydrophilic (defined by a contact angle between 5–30°) with a contact angle of <10°. In contrast, micropit-nanonodule surfaces changed from superhydrophilic to hydrophilic after 3 days of storage and became hydrophobic on day 7, demonstrating a contact angle of more than 90°. This rapid hydrophobic change is well illustrated in side- and top-view images of the ddH2O droplet (lower images, ). The area of ddH2O spread also reduced as hydrophilicity of both surfaces reduced (right line graph, ). However, again, the rate of the time-dependent reduction following UV treatment was more drastic on micropit-nanonodule surfaces.
Figure 2 Age-dependent changes in hydrophilicity of micropit and micro-nano-hybrid titanium surfaces after UV treatment. Fresh (immediately after UV treatment) and aged (3 and 7 days after UV treatment) UV-treated titanium surfaces as well as UV-untreated titanium surfaces are compared. Upper panels show the top and side views of titanium discs immediately after a 10 μL droplet of distilled H2O was placed. Lower panels show the calculated area of spread and the contact angle of H2O.
Note: Data are mean ± SD (n = 3).
Superior bioactivity of micro-nano-hybrid surface is further enhanced by UV
Before examining the time-related changes in the bioactivity of UV-treated titanium, we confirmed that UV treatment is effective in enhancing titanium surfaces with micro-nano-hybrid surfaces. Osteoblasts were cultured on titanium surfaces having only micropits and surfaces having micropits and nanonodules, either with or without UV treatment. The number of attached cells during 3-hour incubation (early stage attachment) was 2.5-fold higher on micro-nano-hybrid surfaces than on micropit surfaces (). UV treatment increased the number of attached cells on both surfaces in a similar proportion by more than twofold. The number of cells attached during 24-hour incubation (late-stage attachment) was also significantly increased by UV treatment on both surface types (). These results validated the necessity of further evaluation on possible time-related changes in UV-enhanced bioactivity of micro- nano-hybrid surfaces.
Figure 3 Age-dependent change of osteoblast attachment to UV-treated micropit and micro-nano-hybrid titanium surfaces. UV-treated titanium disks of different ages – fresh (immediately after UV treatment), 3-day-old (stored for 3 days in the dark after UV treatment) 0, and 7-day-old surfaces – were tested. Titanium disks before UV treatment are also compared. The number of rat bone marrow–derived osteoblasts attached to titanium surfaces after incubation for 3 hours ([A] early stage attachment) and 24 hours ([B] late-stage attachment), as evaluated by WST-1 colorimetry.
Notes: Data are mean ± SD (n = 3); aP < 0.05; bP < 0.01, indicating a statistically significant effect of age of titanium.
![Figure 3 Age-dependent change of osteoblast attachment to UV-treated micropit and micro-nano-hybrid titanium surfaces. UV-treated titanium disks of different ages – fresh (immediately after UV treatment), 3-day-old (stored for 3 days in the dark after UV treatment) 0, and 7-day-old surfaces – were tested. Titanium disks before UV treatment are also compared. The number of rat bone marrow–derived osteoblasts attached to titanium surfaces after incubation for 3 hours ([A] early stage attachment) and 24 hours ([B] late-stage attachment), as evaluated by WST-1 colorimetry.Notes: Data are mean ± SD (n = 3); aP < 0.05; bP < 0.01, indicating a statistically significant effect of age of titanium.](/cms/asset/4211525e-a1e2-4dcd-b223-0dfad072d987/dijn_a_22099_f0003_b.jpg)
UV-induced high osteoblast attraction on micro-nano-hybrid surfaces is sustainable over time
Osteoblasts were cultured on micropit and micropit-nanonodule surfaces that were stored for different storage times after UV treatment: 0-day-old (immediately after UV treatment), 3-day-old, and 7-day-old. After 3-hour incubation (early stage attachment), the number of cells attached to UV-treated micropit surfaces decreased as storage time increased (one-way ANOVA, P < 0.05; ). The number of cells attached to 7-day-old micropit surfaces was 30% less than the fresh (0-day-old surface) micropit surfaces. In contrast, the number of cells attached to UV-treated micropit-nanonodule surfaces was consistently high regardless of the storage time. The difference between micropit and micropit-nanonodule surfaces was 2.5-fold when they were freshly prepared, but this gap was widened to approximately 4-fold when the surfaces were 7 days old. The number of cells attached during 24-hour incubation (late-stage attachment) was also lower on older micropit surfaces (P < 0.01; ). The number of cells attached to 7-day-old micropit surfaces was half the number of cells attached to fresh counterparts. Such time-dependent reductions were not found on micro-nano-hybrid surfaces.
High osteoblast affinity for micro-nano-hybrid surfaces sustained after UV treatment
Confocal microscopic analysis was performed on osteoblasts 6 hours after seeding onto UV-treated micropit and micropit-nanonodule surfaces that had been stored for 0 or 7 days. Low-magnification images showed that there were more cells attached to UV-treated micro-nano-hybrid surfaces than UV-treated micropit surfaces (), confirming the results of the number of attached cells evaluated by WST1 assay (). Considerably fewer cells were found on 7-day-old UV-treated micropit surfaces, while such time-related reductions were not found on micropit-nanonodule surfaces (). These results, again re-affirm the results of WST1 cell attachment assay ().
Figure 4 Age-dependent change of osteoblast affinity of UV-treated titanium surfaces evaluated by the initial spread and cytoskeletal arrangement of osteoblasts. Osteoblasts were seeded onto UV-treated micropit and micro-nano-hybrid surfaces of different ages – fresh (immediately after UV treatment) and 7-day-old surfaces. Representative confocal microscopic images of cells stained with rhodamine phalloidin for actin filaments (red) and antivinculin (green) 6 hours after seeding are presented. Low (A) and high (B) magnification images, along with cytomorphometric evaluations (C), are presented.
Notes: Data are mean ± SD for panel C (n = 10); aP < 0.05; bP < 0.01, indicating a statistically significant effect of age of titanium.
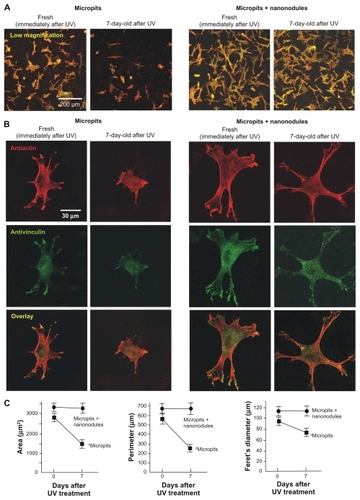
Representative high-magnification images of cells stained with rhodamine (antiactin) showed that immediately after UV treatment cells on both the micropit and micropit-nanonodule surfaces spread with their cell processes stretched and cytoskeletons formed (). In general, cells on fresh (0-day-old) micropit-nanonodule surfaces appeared larger than those on fresh (0-day-old) micropit surfaces. In these cells, the expression of vinculin, a focal adhesion protein, was detected extensively in the cytoplasm with, in particular, intensive expression at the tip of stretched cell processes. Seven days after UV treatment, cells on micropit surfaces were clearly smaller with less developed cell processes and cytoskeletons. In contrast, cells on 7-day-old UV-treated micropit-nanonodule surfaces appeared similar in size and configuration compared to those on corresponding fresh (0-day-old) surfaces. No noticeable differences were found between 0- and 7-day-old micropit-nanonodule surfaces in terms of the level of spread, cytoskeletal arrangement, and vinculin expression.
Cytomorphometry confirmed these observations (). Three parameters – cell area, perimeter, and Feret’s diameter – were significantly greater in cells cultured on fresh UV-treated micropit-nanonodule surfaces compared with cells cultured on fresh UV-treated micropit surfaces (P < 0.05). Cells cultured on micropit surfaces showed significant reductions of all these parameters when the surfaces were aged for 7 days (P < 0.05); similar reductions were not found in cells cultured on micro-nano-hybrid surfaces.
Osteoblast proliferation and function exclusively sustained on UV-treated micro-nano-hybrid surface
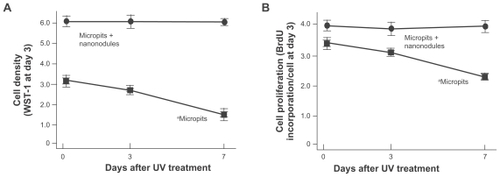
Cell density evaluated at day 3 of culture was significantly lower on 3-day-old and 7-day-old micropit surfaces than on fresh 0-day-old micropit surfaces (P < 0.01; ). Only half the number of cells were present on 7-day-old surfaces compared with the fresh counterparts. In contrast, cultures on micropit-nanonodule surfaces maintained the UV-induced high level of cell density over the storage times tested; ie, there was no reduction in cell density even after 7 days. The cell-based BrdU incorporation decreased in correlation with the storage time of micropit surfaces (P < 0.01; ), while no differences were found among cultures on micropit-nanonodule surfaces that had been stored for different times.
Figure 5 Osteoblast proliferation on differently aged UV-treated titanium surfaces. Osteoblasts were cultured on UV-treated micropit and micro-nano-hybrid titanium disks with different ages – fresh, 3-day-old, and 7-day-old surfaces. (A) Cell density at day 3 of culture. (B) Cell proliferative activity evaluated by BrdU incorporation per cell at day 3 of culture.
Notes: Data are mean ± SD (n = 3) for all panels; aP < 0.01, indicating a statistically significant effect of age of titanium.
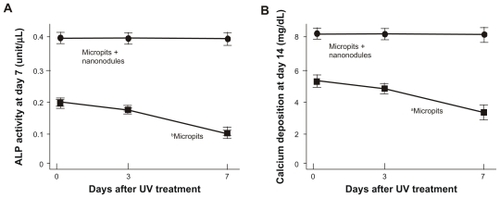
The ALP activity of cells cultured on micropit surfaces evaluated at day 7 of culturing was also found to be reduced in a storage time-dependent manner (P < 0.01; ); the ALP activity of cells on 7-day-old surfaces was reduced to half from the baseline level of the freshly prepared surfaces. Cultures on micropit-nanonodule surfaces maintained the baseline level regardless of the storage time. As a result, when comparing 7-day-old surfaces, there was a 3-fold difference in the ALP activity between micropit and micropit-nanonodule surfaces. A storage time-dependent decrease in the amount of calcium deposition was also observed on micropit surfaces (P < 0.05; ) but not on micropit-nanonodule surfaces.
Figure 6 Osteoblast functions on differently aged UV-treated titanium surfaces. Osteoblasts were cultured on UV-treated micropit and micro-nano-hybrid titanium disks with different ages – fresh, 3-day-old, and 7-day-old surfaces. (A) Alkaline phosphatase activity at day 7. (B) Calcium deposition at day 14 of culture.
Notes: Data are mean ± SD (n = 3) for all panels; aP < 0.05; bP < 0.01, indicating a statistically significant effect of age of titanium.
Ion treatment nullifies UV-induced high bioactivity
To explore the physicochemical properties responsible for decline in the bioactivity of UV-treated micropit surfaces and prolonged levels of high bioactivity of UV-treated micro-nano- hybrid surfaces, the relationship between the level of hydrophilicity and cell attraction capabilities were first examined. The contact angle of ddH2O and the number of attached cells during 3- (early stage attachment) and 24-hour (late-stage attachment) incubation, which varied with the amount of storage time after UV treatment, were plotted for each of the micropit and micro-nano-hybrid surfaces (). For micropit surfaces, although there was a noticeable trend linking a high number of attached cells with a low contact angle, there was no statistically significant correlation between these findings (correlation coefficients, P > 0.05). For micro-nano-hybrid surfaces, the number of attached cells was invariable and independent of the contact angle.
Figure 7 Experimental results addressing the mechanism underlying the sustained high bioactivity of UV-treated micro-nano-hybrid titanium surfaces. Osteoblast attachment after 3 hours (early stage attachment [A] and 24 hours late-stage attachment, [B] incubation plotted against the H2O contact angle on each of micropit and micro-nano-hybrid surfaces in various ages after UV treatment, showing no significant correlation between them). (C) Electrostatic effects on osteoblast attachment capability of titanium surfaces. UV-treated micropit and micro-nano-hybrid surfaces with different age (fresh and 7-day-old) were tested for cell attachment capability with and without an additional treatment of the surfaces with NaCl solution.
Notes: Data are mean ± SD (n = 3); aP < 0.01, indicating a statistically significant difference between NaCl-treated and untreated surfaces.
![Figure 7 Experimental results addressing the mechanism underlying the sustained high bioactivity of UV-treated micro-nano-hybrid titanium surfaces. Osteoblast attachment after 3 hours (early stage attachment [A] and 24 hours late-stage attachment, [B] incubation plotted against the H2O contact angle on each of micropit and micro-nano-hybrid surfaces in various ages after UV treatment, showing no significant correlation between them). (C) Electrostatic effects on osteoblast attachment capability of titanium surfaces. UV-treated micropit and micro-nano-hybrid surfaces with different age (fresh and 7-day-old) were tested for cell attachment capability with and without an additional treatment of the surfaces with NaCl solution.Notes: Data are mean ± SD (n = 3); aP < 0.01, indicating a statistically significant difference between NaCl-treated and untreated surfaces.](/cms/asset/3c2fc68c-6463-4204-81f5-de46af2f9b5e/dijn_a_22099_f0007_b.jpg)
Next, the effect of ion treatment on the bioactivity of fresh and 7-day-old UV-treated surfaces was examined. Cell attraction capability, measured by the number of attached cells during 24-hour incubation, of both the micropit and micropit-nanonodule surfaces was reduced to approximately half by treating the surfaces with NaCl immediately after UV treatment (). Likewise, 7-day-old micropit-nanonodule surfaces showed a substantial reduction in cell attachment after NaCl treatment. In contrast, cell attachment to 7-day-old micropit surfaces was not affected by NaCl treatment.
Thin TiO2 coating does not mitigate time-dependent decrease of cell attraction
To identify the mechanism behind the prevented time- dependent decrease of biological properties on UV-treated micro-nano-hybrid surfaces, micropit surfaces with 50-nm-thin TiO2 coating was examined. This surface showed a similar morphology to the original micropit surfaces (). The sharp ridges and compartmental structures were preserved without the formation of defined nanonodular structures. Early and late stage cell attachment significantly decreased as the 50-nm-thin TiO2 coated surfaces aged (). Approximately 30% reduction in late-stage cell attraction capability was observed on the 7-day-old surface compared with the fresh surface.
Figure 8 Age-dependent change of osteoblast attachment to UV-treated micropit surfaces with 50-nm-thin TiO2 coating. (A) SEM images of the 50-nm TiO2 coated micropit surfaces. The number of rat bone marrow–derived osteoblasts attached to titanium surfaces after incubation for 3 hours ([A] early stage attachment) and 24 hours ([B] late-stage attachment), as evaluated by WST-1 colorimetry.
Notes: Data are mean ± SD (n = 3); UV-treated titanium disks of different ages – fresh (immediately after UV treatment), 3-day-old (stored for 3 days in the dark after UV treatment), and 7-day-old surfaces – were tested; the data from micro-nano-hybrid surfaces (300 nm nodules in micropits) are also presented; aP < 0.05, indicating a statistically significant effect of age of titanium.
![Figure 8 Age-dependent change of osteoblast attachment to UV-treated micropit surfaces with 50-nm-thin TiO2 coating. (A) SEM images of the 50-nm TiO2 coated micropit surfaces. The number of rat bone marrow–derived osteoblasts attached to titanium surfaces after incubation for 3 hours ([A] early stage attachment) and 24 hours ([B] late-stage attachment), as evaluated by WST-1 colorimetry.Notes: Data are mean ± SD (n = 3); UV-treated titanium disks of different ages – fresh (immediately after UV treatment), 3-day-old (stored for 3 days in the dark after UV treatment), and 7-day-old surfaces – were tested; the data from micro-nano-hybrid surfaces (300 nm nodules in micropits) are also presented; aP < 0.05, indicating a statistically significant effect of age of titanium.](/cms/asset/4bb9e233-3bbf-43ae-b86c-3d661b39c4d8/dijn_a_22099_f0008_b.jpg)
Discussion
This study explored methods to overcome or at least slow the biological aging of titanium, and discovered an antibiological aging property of UV-treated micro-nano-hybrid TiO2 surfaces consisting of micropits and nanonodules. The bio-mimetic morphological features of these micro-nano-hybrid surfaces have been previously illustrated, and their proosteoblast and antifibroblast selective cell affinities have been demonstrated.Citation8 The present study has uncovered another feature of functionality inherent to this micro-nano-hybrid TiO2 surface.
We initiated this study by confirming that UV treatment can functionalize micro-nano-hybrid TiO2 surfaces as effectively as surfaces with micropits alone. Since the baseline bioactivity of micro-nano-hybrid surfaces before UV treatment was higher than surfaces with micropits alone, the resulting bioactivity after UV treatment was proportionally higher for micro-nano-hybrid surfaces. As anticipated, UV-induced bioactivity decreased in a time-dependent manner on micropit surfaces, as demonstrated by the reduction of all bioactivity parameters. Most importantly, while micropit titanium surfaces experienced time-dependent degradation, micro-nano-hybrid surfaces maintained high levels of UV treatment-induced bioactivity for the 7 days that we followed up in this study. Despite having larger surface areas, micro-nano- hybrid surfaces turned out to be more resistant to the biological aging of titanium.
The results of this study uncovered a significant issue concerning the assessment and control of the biological capabilities of titanium. Since this study revealed that the rates of biological aging were largely different for the surfaces examined – ranging from approximately 50% reduction for micropit surfaces to virtually 0% reduction for micro-nano-hybrid surfaces after 7 days – there should be a more significant impact on biological aging as the storage time increases. For instance, the number of cells attached to micropit surfaces during the 24-hour incubation period was half compared to that of the micro-nano-hybrid surfaces, when both surfaces were fresh. However, when compared to 7-day-old surfaces of the respective titanium samples, the difference increased by more than 3-fold. Likewise, the ALP activity of cells on 7-day-old micropit surfaces after 7 days of culturing was considerably lower – by as much as 70% – compared with 7-day-old micro-nano-hybrid surfaces, although the difference was 50% when these two surfaces were fresh right after UV treatment. These time-dependently growing differences happened because the micropit surfaces, which possess lower levels of bioactivity at the time the surfaces were prepared, showed more rapid rates of biological aging. If surfaces that had lower levels of bioactivity showed slower rates of biological aging, the consequence would be the opposite. In summary, biological aging could pronounce or diminish differences in the inherent biological capabilities of different titanium surfaces, depending on the speed of biological aging, which was shown to largely vary in this study. Previous studies have demonstrated that biological aging occurs on different surface topographies of machined, sandblasted, and chemically deposited titanium surfaces.Citation1–Citation3,Citation12 Importantly, the rate of aging varies significantly among different surface topographies. Along with these reports, the present study demonstrates the impact of titanium age-related heterogenetic degradation on the evaluation and control of the biological capabilities of titanium. Simultaneously, additional scientific interests and potential therapeutic values of micro-nano-hybrid surfaces have been uncovered.
We investigated the mechanisms underlying the prolonged enhancement of UV-induced high bioactivity levels on micro-nano-hybrid surfaces. Surprisingly, micro-nano-hybrid surfaces, which showed long lasting cell attraction and proliferation capabilities, lost hydrophilic properties more rapidly than micropit surfaces. After 7 days of storage, UV-treated micro-nano-hybrid surfaces were very hydrophobic with contact angles of >90° (this surfaces could be termed hydrorepellent, as defined by a contact angle of >90°). Despite the extremely poor wettability of this surface, and the dramatic change from superhydrophilic to hydrophobic immediately after UV treatment, 7-day-old micro-nano-hybrid surfaces sustained high levels of bioactivity comparable to the baseline level of fresh counterparts, providing a crucial suggestion that the biological activity of titanium is not necessarily dependent on the degree of hydrophilicity. In contrast, cell attraction tended to decrease in a time-dependent manner as the micropit surfaces became less hydrophilic; however, this relationship was not statistically significant. The role of surface hydrophilicity of biomaterials in determining their bioactivity is highly contentious. It is not a universally acknowledged principle that the hydrophilicity of the surface is proportional to the bioactivity of the material.Citation13–Citation16 A series of previous studies demonstrated that maintaining hydrophilic properties of titanium surfaces appears to have a positive effect on increasing attachment, spread, and proliferation of osteogenic cells; however, no evidence has been found that indicates the fact that hydrophilicity of a material is critical in determining its bioactivity or osteoconductivity.Citation1,Citation2,Citation5,Citation10 Specifically, the bioactivity of titanium surfaces did not correlate with the level of their hydrophilicity as it changes during biological aging and UV photofunctionalization.
We then explored new physicochemical properties related to post-UV degradation of biological capabilities. It was found that the electrostatic potential of UV-treated surfaces plays a key regulatory role in determining their bioactivity. Anion treatment with Cl− was employed to fresh and 7-day-old UV-treated surfaces, which has been previously demonstrated as a useful method to determine the role of electrostatic properties on the biological capabilities of a material.Citation1,Citation4 The enhanced bioactivity of fresh UV-treated micropit surfaces and UV-treated fresh and 7-day-old micro-nano-hybrid surfaces, as demonstrated by increased cell attachment, was diminished substantially by anion treatment. As previously reported,Citation4 it was thought that UV-treated surfaces, which acquire positive surface charge, would be neutralized by Cl− and would not be able to aggressively attract cells. The positively charged surfaces are assumed to be cell-attractive because cell membranes are negatively charged.Citation1,Citation4 Seven-day-old micropit surfaces that are not responsive to the anion treatment imply that these surfaces were no longer electropositive after 7 days of storage. Titanium surfaces (exactly speaking titanium dioxide surfaces) have been long considered electronegative. Titanium surfaces must therefore first be bridged by divalent cations such as Ca2+ to attract anionic proteins and the adsorbed proteins, then, attract osteogenic cells through their RGD sequence.Citation17–Citation19 This is the reason why titanium has been defined as a bioinert material. A recent study, however, revealed that new titanium surfaces immediately after processing are electropositive and lose the electropositivity over time while the hydrocarbon unavoidably accumulates on the surface.Citation1,Citation2 The surfaces become electronegative when they are sufficiently aged, which turned out to be within 4 weeks.Citation1 Although the storage for 7 days seemed to be too soon, the present result indicated that such time-dependent conversion of electrostatic status took place on UV-treated micropit surfaces. On such electronegative surfaces, the presence of monovalent cations and anions may not affect their surface bioactivity significantly. The monovalent cations would just fill the monovalent negativity of the titanium surfaces and neutralize it, while the monovalent anions are repellent to the negative titanium surfaces. These may explain why NaCl treatment of 7-day-old micropit surfaces did not modulate their cell attraction ability. In contrast, electropositivity was maintained on 7-day-old micro-nano-hybrid surfaces and abolished by anion treatment. Moreover, an almost equal reduction in the cell attraction capability by anion treatment was found in both fresh and 7-day-old UV-treated micro-nano-hybrid surfaces. Given that these two surfaces showed completely opposite hydrophilic properties (superhydrophilicity and hydrorepellency, respectively), it was thought that UV-acquired electropositivity, not the level of hydrophilicity, would be the primary reason for enhanced bioactivity and that the sustained high levels of bioactivity on 7-day-old micro-nano-hybrid surfaces would be due to long-lasting maintenance of electropositive properties.
Although the precise mechanisms that enabled long-lasting maintenance of electropositivity remain to be investigated, we hypothesized that a thicker layer of TiO2 on micro-nano-hybrid surfaces may be a possible factor. Bulk titanium rapidly forms a layer of TiO2 on its superficial surface when exposed to the atmosphere. Although no definitive data are available for the thickness of TiO2 that can form on either the micropit and micro-nano-hybrid surfaces used in this study, micro-nano-hybrid surfaces tested in the present study had an additional layer of TiO2 of up to 300 nm during sputter deposition. To test the hypothesis, we compared the micro-nano-hybrid surface with the micropit surface with 50-nm-thin TiO2 coating. As reported previously, TiO2 sputter coating of less than 100 nm did not create the typical nanonodules (). Therefore, the 50-nm-thin TiO2-coated micropit surface resembled the original micropit surface. Interestingly, there was no significant alleviation of biological aging on the 50-nm-thin TiO2-coated micropit surface (). This finding suggests that the presence of a TiO2 layer may not be critical but the nature of the TiO2 layer may have a role in the long-lasting electrostatic status. The nature of a TiO2 layer hypothetically includes the architecture (ie, in nanonodular form), thickness (eg, it should be 300-nm thick or more), and surface area (eg, the larger, the better). Exposing TiO2 to UV light results in the excitement of an electron from the valence band to the conduction band.Citation20,Citation21 This physicochemical change – the excited electron along with the positive hole created in the superficial layer of TiO2 – is part of the explanation for why UV-mediated photocatalysis occurs. UV treatment of TiO2 surfaces that are thicker or larger in area may affect the nature and sustainability of these electrostatic alterations. In fact, machined and acid-etched surfaces with different thickness of TiO2 show different light absorbance curves for wavelengths under 400 nm,Citation5 suggesting different sensitivities to UV light and probably different capacities and efficiencies of UV-induced photocatalysis. Lastly, as shown in the results, the surface areas of micro-nano-hybrid surfaces were approximately 60% greater than those of the micropit surfaces. Addressing these questions and hypotheses will be important guides for future research.
The roles of monocyte/macrophage in the process of bone–titanium integration or wound healing process around implants are proposed from the osteogenic and inflammatory reaction perspectives.Citation22–Citation25 Surface topography alteration affects the expression of osteoinductive growth factors and cytokines in macrophages, which may play an important role in the process of peri-implant osteogenesis.Citation22 The expression of proinflammatory cytokines possibly modulated by surface topography of titanium substrates may affect the process and maintenance of bone-implant integration.Citation23,Citation25 The surfaces tested in the present study show a various combination of topography, hydrophilicity, and hypothetically electrostatic status, which we believe will provide a unique set of in vitro experimental platforms to further explore the titanium-cell interaction. The interaction of these surfaces with macrophages and its effect on osteogenic cells would be of great interest.
Titanium implant products, regardless of use in either dental or orthopedic reconstruction, are available as storable devices, as previously mentioned. However, no considerations have been made regarding expiration or time-sensitive quality control. After the discovery of biological aging of titanium, effective measures to prevent, or at least minimize, this adverse phenomenon is desired. UV-mediated photofunctionalization has been demonstrated to restore reduced bioactivity levels to baseline levels of fresh titanium surfaces, and even enhance the bioactivity to even higher levels.Citation2 Photofunctionalized titanium surfaces are highly osteoconductive in vivo, enabling bone formation up to 100% in areas of bone-implant contact.Citation5 Another notable finding of UV-treated titanium surfaces is the remarkably reduced chance of soft tissue intervention between newly formed bone and titanium surfaces.Citation5 Since fibrous soft tissue formation, which has been considered unavoidable, competes against bone–titanium integration (osseointegration), the blocking process could be rephrased as zero resistance for osseointegration. Zero resistance is a term used to delineate super conductors in the field of engineering. Therefore, bone morphogenesis induced around UV-treated titanium surfaces could be defined as super osseointegration,Citation10,Citation26 not just because of the UV-induced, highly osteoconductive surfaces, but due to a near complete block of soft tissue formation around these surfaces. The present study found that microtopographic surfaces are not able to maintain UV-induced high levels of bioactivity, but micro-nano-hybrid surfaces are able to maintain UV-induced even higher levels of bioactivity, at least for the 7 days they were monitored. Although the commercial significance of 7 days should be determined from an industrial point of view, this study demonstrates that micro-nano-hybrid surfaces have promising therapeutic applications, and could lead to the development of effective delivery mechanisms and expanded opportunities of the use of UV photofunctionalization. Adding nanotopography to microtopography may be explored as a new approach to counteract the biological aging of titanium after UV photofunctionalization. Discovering an aging free, UV-treated titanium surface is the ultimate goal. In vivo testing of UV-treated micro-nano-hybrid surfaces and long-term follow-up studies of bioactivity will be the next step.
Conclusion
This study addressed the sustainability of UV-treated, highly bioactive titanium surfaces using micro-nano-hybrid structured titanium surfaces. Titanium disks with only micropits and with micropits and TiO2 300-nm nanonodules were prepared. After storing these disks for 8 weeks in the dark to allow sufficient aging, they were treated with UV light. Rat bone marrow–derived osteoblasts were cultured on fresh (immediately after UV treatment), 3-day-old (stored for 3 days after UV treatment), and 7-day-old disks. The number of cells attached to the titanium surfaces after 24 hours of culturing decreased with the age of the micropit surfaces (ie, 40% fewer cells were attached to 7-day-old surfaces compared with fresh surfaces); this phenomenon was not observed on micropit-nanonodule surfaces. The rates of cell spread, proliferation, ALP activity, and calcium deposition were 30%–50% lower on the aged micropit surfaces. The micropit-nanonodule surfaces maintained equivalent levels of bioactivity, regardless of age. Both micropit and micropit-nanonodule surfaces were superhydrophilic immediately after UV treatment. However, superhydrophilicity disappeared sooner on micropit-nanonodule surfaces. Sustained high levels of bioactivity of the micro-nano-hybrid surfaces were nullified by treating the surfaces with Cl− anions. A thin TiO2 coating on the micropit surface without the formation of nanonodules did not result in the prevention or alleviation of the titanium age-dependent decrease in biological activity. In conclusion, the micro-nano-hybrid titanium surface consisting of TiO2 nanonodules within micropits may prevent for a certain period of time or slow time-dependent degradation of titanium bioactivity after UV photofunctionalization, while the microtopographic surface was subjected to a rapid and substantial biological degradation. The antibiological aging nature is regulated by the unique surface electrostatic status of the UV-treated micro-nano-hybrid surfaces associated with the form and thickness of the TiO2 layer, and is independent from the degree of hydrophilicity. These results indicate the utility of micro-nano-hybrid surfaces to effectively convey UV-photofunctionalization technology and warrant further exploration to develop antibiological aging endosseous implant surfaces.
Acknowledgments
This work was supported by JAMSEA. The study was conducted in a facility constructed with support from the Research Facilities Improvement Program, grant nr C06RR014529, of the National Center for Research Resources, National Institute of Health.
Disclosure
The authors declare no conflicts of interest in relation to this paper.
References
- AttWHoriNTakeuchiMTime-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materialsBiomaterials2009305352536319595450
- AttWHoriNIwasaFYamadaMUenoTOgawaTThe effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloysBiomaterials2009304268427619473697
- SuzukiTHoriNAttWUltraviolet treatment overcomes time-related degrading bioactivity of titaniumTissue Eng Part A2009153679368819397472
- IwasaFHoriNUenoTMinamikawaHYamadaMOgawaTEnhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanismBiomaterials2010312717272720035996
- AitaHHoriNTakeuchiMThe effect of ultraviolet functionalization of titanium on integration with boneBiomaterials2009301015102519042016
- UenoTYamadaMSuzukiTEnhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing modelBiomaterials2010311546155719962757
- MiyauchiTYamadaMYamamotoAThe enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfacesBiomaterials2010313827383920153521
- KuboKTsukimuraNIwasaFCellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy modelBiomaterials2009305319532919589591
- OgawaTSaruwatariLTakeuchiKAitaHOhnoNTi nano-nodular structuring for bone integration and regenerationJ Dent Res20088775175618650547
- TsukimuraNYamadaMIwasaFSynergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titaniumBiomaterials2011324358436821421270
- SaruwatariLAitaHButzFOsteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructureJ Bone Miner Res2005202002201616234974
- HoriNAttWUenoTAge-dependent degradation of the protein adsorption capacity of titaniumJ Dent Res20098866366719641155
- HeBWanYBeiJWangSSynthesis and cell affinity of functionalized poly(L-lactide-co-beta-malic acid) with high molecular weightBiomaterials2004255239524715110475
- WangYWWuQChenGQReduced mouse fibroblast cell growth by increased hydrophilicity of microbial polyhydroxyalkanoates via hyaluronan coatingBiomaterials2003244621462912951005
- BerSTorun KoseGHasirciVBone tissue engineering on patterned collagen films: an in vitro studyBiomaterials2005261977198615576172
- JansenEJSladekREBaharHHydrophobicity as a design criterion for polymer scaffolds in bone tissue engineeringBiomaterials2005264423443115701371
- KlingerASteinbergDKohaviDSelaMNMechanism of adsorption of human albumin to titanium in vitroJ Biomed Mater Res1997363873929260109
- EllingsenJEA study on the mechanism of protein adsorption to TiO2Biomaterials1991125935961663394
- SteinbergDKlingerAKohaviDSelaMNAdsorption of human salivary proteins to titanium powder. I. Adsorption of human salivary albuminBiomaterials199516133913438573673
- WangRHashimotoKFujishimaALight-induced amphiphilic surfacesNature1997388431432
- ZubkovTStahlDThompsonTLPanayotovDDiwaldOYatesJTUltraviolet light-induced hydrophilicity effect on TiO2(110)(1x1). Dominant role of the photooxidation of adsorbed hydrocarbons causing wetting by water dropletsJ Phys Chem B2005109154541546216852960
- TakebeJItoSChampagneCMCooperLFIshibashiKAnodic oxidation and hydrothermal treatment of commercially pure titanium surfaces increases expression of bone morphogenetic protein-2 in the adherent macrophage cell line J774A.1J Biomed Mat Res Part A200780711718
- TanKSQianLRosadoRFloodPMCooperLFThe role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide productionBiomaterials2006275170517716808973
- RiceJMHuntJAGallagherJAHanarpPSutherlandDSGoldJQuantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitroBiomaterials2003244799481814530077
- TairaMNezuTSasakiMGene expression analyses of human macrophage phagocytizing sub-micro titanium particles by allergy DNA chip (Genopal)Biomed Mater Eng200919637019458447
- HoriNUenoTMinamikawaHElectrostatic control of protein adsorption on UV-photofunctionalized titaniumActa Biomater201064175418020466081