Abstract
Nanomedicine is a medical application of biochemistry incorporated with materials chemistry at the scale of nanometer for the purpose of diagnosis, prevention, and treatment. New models and approaches are typically associated with nanomedicine for precise multifunctional diagnostic systems at molecular level. Hence, employing nanoparticles (NPs) has unveiled new opportunities for efficient therapies and remedy of difficult-to-cure diseases. Among all types of inorganic NPs, lanthanide-doped up-conversion nanoparticles (UCNPs) have shown excellent potential for biomedical applications, especially for multimodal bioimaging including fluorescence and electron microscopy. Association of these visualization techniques plus the capability for transporting biomaterials and drugs make them superior agents in the field of nanomedicine. Accordingly, in this review, we firstly presented a fundamental understanding of physical and optical properties of UCNPs and secondly, we illustrated some of the prominent associations with bioimaging, theranostics, cancer therapy, and optogenetics.
Introduction
Nanomedicine is an interdisciplinary, preclinical research field that is a combination of nanotechnology and biochemistry which facilitates biomedical sciences for diagnosis and treatment at molecular scale.Citation1 The majority of biological processes which occur at nanoscale, might provide new models and standards for improving the medical profession.Citation2 Accordingly, the use of nanoscale materials is unveiling a new world of highly-sensitive treatments for hard-to-cure diseases, especially for the purpose of precise drug delivery, prophylactics, biosensing, and imaging.Citation3 Due to the unique properties of materials of this size, such as high chemical/heat resistance, modifying and transporting capability, optoelectronic properties, quantum behavior, and proper sizes against dimension of a multitude of biomaterials such as proteins and nucleic acids, this field might be extraordinarily beneficial in medicine.Citation4 One of the exclusive and practical properties of materials at nanoscale is their size-dependency that eventuates various fundamental behaviors such as luminescence, conductivity, chemical reactivity and magnetic permeability from particles as a function of the size.Citation5,Citation6 Another distinctive property of materials at this scale is the high surface-area-to-volume ratio that provides a relatively large substrate for chemical or biomaterials’ attachment.Citation7 Researchers have been able to modify such surfaces on nanoparticles (NPs) to generate fine platforms that involve coating molecules with active peripheral sides to make well-tuned particles for loading drugs.Citation8 The sum of all these properties enhances the efficacy of medical applications and diminishes the side effects and toxicity of these materials.Citation9 However, employing inorganic NPs, either for treatment or diagnostics, is not absolutely compatible in the human body and there are several side effects with inherent toxicity of these undersized objects. Therefore, providing NPs with lower toxicity, such as lanthanide-doped up-conversion nanoparticles (UCNPs) might enhance the potential of utilizing this class of probes in medicine.
Usually, numerous difficulties arise with conventional medical approaches for delivering drugs and efficient targeting. In some cases, the drug molecules have low water solubility, or they can be well-absorbed by the target but are removed from the body before sufficient effect to the cells and organelles to provide beneficial treatment.Citation10 Also, drugs with a higher effectiveness can possess a higher toxic profile that may lead to adverse effects and most notably cause damage to normal and healthy sites. This is a well-known phenomenon of the poor specificity of treatment agents to be taken up by the disease-affected tissues.Citation11 Nanomedicines, by contrast, provides assurance of sufficient drug loading to the body, by having a prolonged circulation time in the blood and by delivering the drug specifically to the areas where the treatment is needed.Citation12 This helps to maintain the required dose of drug in the body, and importantly, prevents salient damage to the healthy tissues that would otherwise be caused by the therapeutic drug molecules.Citation13 In addition, these therapeutic reagents that are transported using nanomaterials, have shown an increased loading of the drugs to the organs in the body compared with chemicals in classical delivery matrices.Citation14 Nanomedicine has also been approved by the US Food and Drug Administration (FDA) for the aid of diverse nanotechnology diagnostic approaches and nanodrugs.Citation15,Citation16
NPs
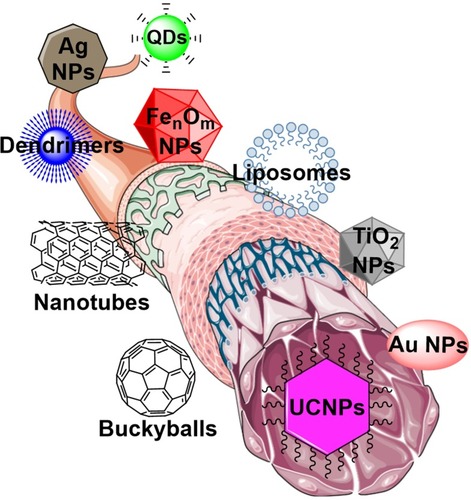
NPs, due to their small sizes, customized surface, multifunctional capabilities, and water solubility, are creative agents for transporting biomaterials and drugs to site-specific emplacements.Citation17 The expansion of NPs in medicine has also led to further classes of clinical studies such as early diagnosis of pathologies, prediction and prevention using smart nanodevices.Citation18 NPs positively manipulate the enhanced permeability and retention (EPR) effect to reach the pathological tissues or intended area in the body through their quantum property.Citation19 After targeting the tissue, cell penetration is the next challenging barrier for therapeutic effect of drugs which might be feasible with objects at nanoscale. Recent studies have demonstrated that the NPs less than 100 nm in size are able to pass through the cell membranes via endocytosis. Penetrability of cell membrane against NPs makes this important possible to reach the cell organelles such as the nucleus, lysosome, and mitochondrion.Citation20,Citation21 Since different types of nanomaterials can be manufactured, ranging from carbon nanotubes, metal oxides NPs, polymeric NPs, dendrimers, quantum dots (QDs), and UCNPs (), this might be a great advantage toward inventing more versatile diagnostic and treatment solutions for diseases such as different types of cancers, viral diseases, and neurodegenerative disorders.Citation22–Citation24
Figure 1 Most commonly used nanomaterials in nanomedicine manufactured from different substances.
Note: The figure was produced by smart servier medical art library in combination with ChemDrew.
Surface modification of NPs
A wide range of NPs exist with different properties and various precursors that demand mainstream attention. These materials might be organic such as semiconducting polymer dots, lipid drops, and nanosize carbon allotropes, or inorganic such as gold-, silver-, and lanthanide-doped NPs, rendering them particularly attractive as targeting and imaging reagents for biological specimens.Citation25 Nevertheless, NPs in the body act as a double-edged sword and despite their advantages, these small materials usually have inherent toxicity due to their sizes and potential to penetrate and accumulate in the main organs or bone marrow and generate new disorders.Citation26 This normally results in systemic toxicity and irreparable side effects; therefore, modification and functionalization of these particles with biocompatible molecules is being applied to convert them to low- or non-toxic objects with a safer result for diagnostics and therapeutics.Citation27
Modification of NPs for drug delivery requires interdisciplinary approaches and heeding the physical and chemical properties of pertinent materials in addition to subsequent responses in the biological environment. In order to have efficacious drug delivery, the NPs need to possess enough stability for modification and capacity for loading desirable drug molecules, plus protecting drug bioactivity and boosting biocompatibility.Citation28,Citation29
Surface modification requires stable and appropriate NPs that might load the drugs or biomaterials without alterations in physicochemical of therapeutic agents; moreover, these materials should be able to release the drug after delivery to the intended diseased sites. A variety of common drug loading methods have been used for modification of NPs, including encapsulation, direct adsorption of the drugs via hydrophobic interaction or Van-der-Waals bonding, and chemical reactions such as covalent bonding between the active groups on the surface of NPs and functional groups on the scaffold of drugs and biomaterials.Citation30
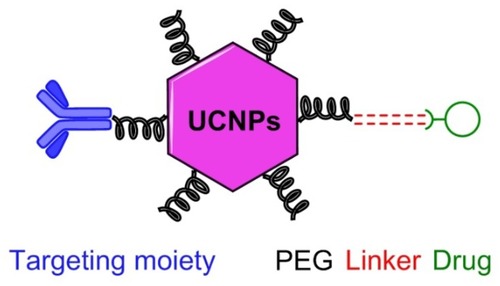
To develop noninvasive and impactful NPs, researchers, in the first step, attempt to coat them with biocompatible materials for therapeutic applications. Some of the most commonly used reagents are polyethylene glycol (PEG), succinic acid, thioglycolic acid, and silicon oxide that are loadable for biomaterials such as peptides, proteins, nucleic acids (e.g., DNA and RNA), and antibodies.Citation31–Citation33 This layout turns out a platform for biolabels followed by selective targeting, and also provides a basis for binding the drugs and dyes on peripheral side of metal NPs.Citation34 PEG is a typical non-immunogenic organic compound with a linear or branched polyether terminated with hydroxyl (–OH) or the other functional groups such as amine (–NH2), carboxyl (–COOH) or thiol (–SH). The general configuration of PEG incorporation with a drug delivery system is shown in together with a schematic illustration of UCNPs conjugated PEG-based prodrug with the targeting agent.Citation35
The PEG, in different sizes, has been used very routinely in various biological, chemical, and pharmaceutical applications due to its low toxicity and reproducibility of results.Citation36 This compound gives higher water solubility to the proteins, drugs, and even inorganic NPs and also diminishes aggregation of complex, besides providing high flexibility for labeling tags and cross-linkers without steric hindrance. PEGs have also been used to functionalize the surface of NPs in combination with hydrophobic polymer to assemble amphiphilic diblock co-polymers. This method can generate plasmonic vesicles or dimers to improve drug delivery and enhance optical signals.Citation37,Citation38
UCNPs
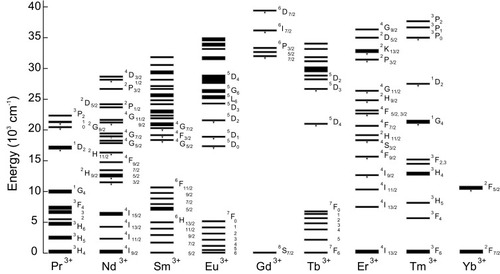
UCNPs are a class of luminescent nanomaterials that turn lower energy sources into higher energy luminescent emissions, which is a prodigious phenomenon in nonlinear optics.Citation39 These NPs, due to high fluorescence intensity under NIR irradiation and sufficient physical permanence, have been recently used for different demands, such as medical applications, new generations of printing, and even security of banknotes.Citation40–Citation49 The up-conversion phenomenon was first proposed by Bloembergen in 1959, who suggested the detection of NIR light by electron transferring and counting the sequential absorption between electron shells or energy states of single ions.Citation50 This concept was then pursued by Auzel, and in 1966 he could explain high efficient up-conversion by electron transferring between energy states of two lanthanides (Yb3+ and Er3+).Citation51 shows the general energy states diagram of the trivalent (tripositive) lanthanide ions doped in a low-symmetry crystal. The pointed lines in this figure represent practical up-conversion emissive excited layers that indicate the higher possibility of electron existence in these shells.Citation52,Citation53
Figure 3 General energy states diagram of the lanthanide ions doped in a low-symmetry crystal.Citation52,Citation53
Principle of lanthanides’ luminescence
Lanthanide ions in solids are either divalent or trivalent with 4fn, 5s2, 5p6 electron configuration with n=0–14. The electrons might be partly filled in 4f orbitals by different arrangements and it can give rise to specific optical and magnetic properties for each configuration.Citation54,Citation55 Since the electrons have a certain number of positions and orientations in available orbitals, the configuration possibility for electronic arrangement might take place within different levels of electron shells, and it can generate a wide range of energy values from NIR to ultraviolet (UV).Citation56 Principally, the most common valence states of the lanthanide ions in solids are the trivalent types, and doping the trivalent ions in different host lattices such as fluorides and oxides supplies stacks of absorption and emission wavelengths.Citation57 The independency of host lattices in this case and high yield energy transferring within the crystal lattice make the lanthanide ions suitable for spectral conversion and optical applications. The optical properties with these elements arise from luminescence phenomenon and electron transferring between allowed energy sates as well as common fluorophors.Citation58 As a matter of fact, the up-conversion process follows anti-Stokes emission since longer wavelengths convert to shorter wavelengths, and in this system essentially, two NIR photons incorporate to produce one visible photon.Citation59,Citation60
To date, a plethora of studies have investigated and proved the mechanisms of up-conversion luminescence (UCL).Citation61,Citation62 Principally, the long lifetime excited states of UCNPs affect the mechanism of the up-conversion energy transferring process and this has been developed different types of up-conversion mechanisms such as excited state absorption (ESA), up-conversion by energy transfer (ETU), two-photon absorption (TPA), cooperative sensitization up-conversion (CSU), cross-relaxation (CR), photon avalanche (PA), and energy migration-mediated up-conversion (EMU). Each of these mechanisms follows a specific pathway and they result in different efficiency in terms of luminescence output.Citation53,Citation63
UCNPs’ composition
UCNPs are composed of transparent inorganic crystals as host matrices that are co-doped with optical active trivalent lanthanide ions as the luminescent factors.Citation64 Luminescent lanthanide ions take place among the crystalline host matrices during the crystal lattice formation and the quantum yield (QY) of emissions (brightness) by these NPs is strongly dependent on the composition of substitutes within crystals.Citation65,Citation66
Host matrix
Host matrices play important roles in governing optical properties and chemical/heat stability of UCNPs. Different host matrices have been used for UCNPs and each of them has a specific coordination number, certain energy transfer distance from the luminescent core, and specific output of energy transfer.Citation67 Therefore, it is crucial to choose an appropriate host matrix as frame for these NPs. Host matrices require several properties to better assist with energy transferring in UCNPs, such as high crystal and chemical stability to reduce degradation, low photon cut-off energy to reduce non-radiative relaxation, low chemical symmetry to enhance transition possibility, and high transparency to enhance transmission of NIR photons into the crystal lattice.Citation68 In general, the host matrices need close lattice matching with dopant ions and low resonance energy of lattice vibrations within the crystals. As all trivalent lanthanides ions expose homogeneous ionic size and identical chemical properties, their inorganic compounds are consummate for host matrices of up-converting lanthanide dopant ions. Thus far, multifarious host materials such as LiYF4, NaYF4, NaGdF4, NaLuF4, BaYF5, KY3F10, BaGdF5, SrF2, and BaF2 have been developed and reviewed in various valuable articles.Citation56,Citation63,Citation69,Citation70 Different host lattices can provide different crystal structures and different morphologies of the nanocrystals, and each of these factors can be effective in luminescence efficiency. For instance, the KLu2F7 hexagonal-prism crystals, synthesized by controlling the ratio of F−/Ln3+, have shown optimal and higher UC emission intensity in comparison to the known β-NaREF4 (RE=Y and Gd).Citation71 Nevertheless, the NaREF4 family, due to the unique crystal composition and chemical stability among the series of host materials, has been utilized as the most popular host matrix for UCNPs’ fabrication.Citation72,Citation73 The cubic phase (α) and hexagonal phase (β) are two phases of this structure, and it has been frequently reported and proven that β-phase has higher luminescence efficiency than α-phase.Citation74
Sensitizers, activators, and energy mediators
An ideal sensitizer is able to improve the pumping efficiency of electrons to upper energy states of activators by having relatively high absorption cross-sections of NIR light and well-matched excited electron shells/energy states with the associated activator.Citation75 Yb3+ might be a superior candidate for donating and transferring energy into the entire lanthanide-doped nanocrystal due to its simple energy state configuration with large absorption cross-section (2F7/2–2F5/2). In the energy states diagram of the lanthanide ions illustrates that the electrons from the excited energy state of Yb3+ (2F5/2) can transfer to exited states of Er3+ and Tm3+ due to close electron states.Citation76 Gd3+, due to having a big energy gap (6P7/2–8S7/2), can be a supreme energy mediator to immigrate the energy through the layers nanocrystal unit.Citation77
Activators are responsible for generating and emitting the output fluorescence from the core of the UCNPs.Citation78 An ideal activator must possess numerous long-lived intermediate energy states. The ladder-like arrangement of these states might have an affirmative influence on the emission efficiency.Citation62 Excitation of electrons from the lowest state to the intermediate states of activators near the excited electron shells of sensitizers can elevate further transitions to the higher states. On the other hand, increasing the concentration of doped activators might quench the electron transmission of activators by non-radiative relaxation either within the same lanthanide or between two different elements with two pairs of electron shells with the same energy states.Citation78,Citation79 According to these characteristics, Er3+ and Tm3+ are ideal activators for Yb3+ doped UCNPs’ construction.Citation80,Citation81
UCNPs’ configuration
As outlined earlier, the function of host materials is very crucial in the luminescence process of lanthanide ions. The crystalline structure of host materials improves the f–f electronic transitions within individual lanthanide ions through the disturbing of 4f wave functions, and this affects the energy exchange interactions between dopant ions in several ways.Citation82,Citation83 The crystal structure and dopant concentration of sublattices might incorporate with the strength and direction of energy transferring through a particular interionic gap between dopant ions.Citation41 Well-defined concentration of lanthanide dopant ions is surrounded within the crystalline host matrices, besides, crystallization in the right spot might increase the efficiency of desirable energy transferring and photoluminescence.Citation84 In our previous work, we showed that optimized concentration of dopant ions might guarantee the highest efficiency of energy transferring and energy migration along the different layers of crystal lattice.Citation85 Tuning of the distances and junctions of lanthanide-doped ions within the crystal lattice might change the output of interior energy migration of NPs from the outer layer toward the inner layer.Citation86 This interior energy migration is also dependent on host sub-materials and their lattices’ structure and configurations.
The core-shell strategy may provide a controllable composition over dopant compartment that is substantially a self-sufficient system of either the host matrix or lanthanide dopants; therefore, it creates new opportunities to apply new dopant interactions and enhances the stability of NPs’ structure and photoluminescence lifetime.Citation87 Oftentimes, incompatible dopant ions which are separately located in different or even the same layers of nanocrystals can eliminate energy transmission or CRs within the crystal lattice and consequently quench the luminescence process.Citation88
Materials science has developed a wide verity of core-shell NP components that are a combination of different layers of dissimilar materials such as metals, polymers, and semiconductors. However, combination of most of these materials is not feasible in UCNP crystals due to chemical incompatibility and quenching the energy migration in quantum states.Citation89 In the core-shell UCNPs, typically a lanthanide fluoride-based lattice (e.g., YF4, LaF4 and NaYF4) employs as the host for the framework of these NPs. The rational tuning between fluorides and doping ions in UCNPs can influence the photoluminescence emission intensity. Furthermore, the host matrix needs to provide low photon cut-off energy and vibration of energy to situate a high concentration of lanthanide dopant ions and produce homogeneous doping.Citation88
Increasing the UCL efficiency and decreasing surface defects of up-conversion nanocrystals is crucial to improve their optical properties, and dense crystalline shell layers matched with the core of NPs lattices (epitaxial shells) can help to promote these factors ( left structure).Citation90 In addition, combination of discrete functional units on the top of the surface (non-epitaxial shells) is applicable to provide a platform for entrapping complex drugs and generating therapeutic opportunities for labeling, transporting, and light-activated therapy.Citation91 These core-shell coatings provide high photochemical stability and facilitate multi-shelled formations ( right structure).Citation92 A crucial point for generating core-shell structures is getting a maximized radiative spectral conversion and transmission; however, there are a few possibilities to make radiative channels. Non-epitaxial core-shell coating might be with organic substances (e.g., polymers and molecules as surfactant) and/or inorganic substances (e.g., SiO2 and TiO2).Citation93 To acquire a desired structure and to avoid non-radiative relaxations, the combination of epitaxial and non-epitaxial shell coatings might be utilized. Technically, the epitaxial core-shell NPs serve as the cores for non-epitaxial shell coatings.
Figure 4 (A) Schematic illustrations of the typical structures of epitaxial and non-epitaxial shells for core-shell tuned UCNPs. Reproduced with permission from Chen X, Peng D, Ju Q, Wang F. Photon upconversion in core-shell nanoparticles. Chem Soc Rev. 2015;44(6):1318–1330.Citation88 Copyright the Royal Society of Chemistry 2015. (B) Schematic illustration of synthesizing and coating process for multilayer NaYF4:Yb,Tm nanoparticles and TEM images from each step. Reproduced with permission from Su Q, Han S, Xie X, et al. The effect of surface coating on energy migration-mediated upconversion. J Am Chem Soc. 2012;134 (51):20849–20857.Citation96 Copyright American Chemistry Society 2012. (C) The left panel shows high-angle annular dark-field micrograph of a NaYF4:Yb,Er/NaGdF4 nanoparticle with the chemical maps from the presence of yttrium (Y) in the core and gadolinium (Gd) in the shell of nanocrystal. The right panel shows the electron energy loss spectroscopy of Y and Gd edges from the nanoparticle with 2.4 nm shell. Reproduced with permission from Zhang F, Che R, Li X, et al. Direct imaging the upconversion nanocrystal core/shell structure at the subnanometer level: shell thickness dependence in upconverting optical properties. Nano Lett. 2012;12(6):2852–2858.Citation98 Copyright American Chemistry Society 2012.

The other concern when manufacturing core-shell structures for UCNPs is to avoid luminescence quenching by the effect of hydroxyl groups on the lanthanide-doped elements such as Er3+ and Yb3+.Citation94,Citation95 Su et al found out that surface quenching can be prevented by growing an epitaxial inert NaYF4 shell around a core-shell gadolinium sublattice UCNP, as a result of boosting the excitation energy trapping by activator ().Citation96 This shell is able to make an optical active gap between sanitizers/activators and surface ligands and solvents to protect the luminescent output. The results from a study by Yi et al also indicated that NaYF4 shell could isolate the activator ions doped in the core from aqueous quencher and enhance the fluorescence efficiency of NaYF4:Yb,Er/Tm NPs.Citation97 The same results have been demonstrated by Zhang et al for NaYF4:Yb,Er/NaGdF4 core-shell NPs and the thickness of NaGdF4 showed direct dependency on the optical response from the nanocrystals and resistance to quenching effects by aqueous solvents ().Citation98
Even though using core-shell strategy could boost the luminescence efficiency of UCNPs, researchers have still been putting intemperate efforts into promoting the quantum yield (QY) of these NPs, especially for bioimaging proposes.Citation99 Developing the UCNPs with high QY might avoid the intense irradiation of NIR light to biological object that may overheat the tissues and/or cells. A recent report by Wisser et al represents an approach to remarkably enhance the up-conversion QY by conjugating a commercial fluorescence dye (ATTO 542) to the surface of UCNPs.Citation100 Efficient energy transferring happened between the organic dye and UCNP due to the equivalency in fluorescence energy band gap of the dye and the energy level of the activator (Er3+) from UCNP. The excited-state lifetime measurements indicated that the elevation of QY was related to the radiative rate gained by the conjugated dye. According to these results from the impact of emission sensitization, the dye-coated UCNPs showed better QY compared to the as-synthesized and ligand-stripped particles at different sizes.
Synthesis of UCNPs
According to the literature, three common methods, so-called thermal decomposition, hydrothermal method, and non-hydrolytic colloidal method, have been utilized by scientists to synthesize UCNPs.Citation101,Citation102 Several components are considered to get high luminescence efficiency and narrow size distributions of NPs with these methods, which are critical factors for high quality fabrication and various geometrical motifs.Citation103 Herein, we comprehensively discussed the two most common methods which have been utilized for synthesizing UCNPs with high UCL efficiency and homogeneous morphology of nanocrystals plus brief introductions on some other methods that are less utilized.
Thermal decomposition method
Practically, thermal decomposition is a bottom-up synthesizing method that has become the most common strategy for synthesizing UCNPs.Citation104 This technique can produce uniform size/shape/phase NPs from nanoscaled building blocks. In terms of reaction time, it could be executed in a relatively shorter time, whilst, the organometallic complex precursors dissolve in high temperature (280–325°C) organic solvents containing stabilizing surfactants. Oleylamine (OM), oleic acid (OA), and 1-octadecence (ODE) are typical surfactants with long chain primary alkylamine and polar end-capping groups.Citation105 The organic precursors commonly used are trifluoroacetate compounds. Much research has been dedicated to investigating the growth mechanisms of nanocrystals by optimizing different factors. Mai et al very comprehensively studied the growth mechanisms of different phases of up-conversion nanocrystals and their transition processed by conversional spectroscopy, transmission electron microscopy (TEM), and X-ray diffraction (XRD) techniques.Citation106
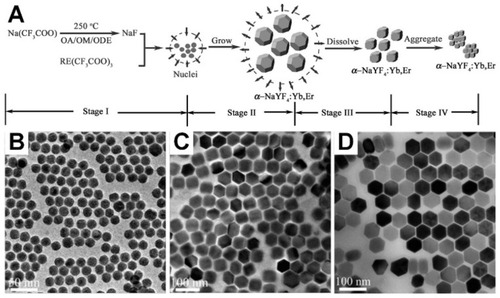
The synthesis reaction in thermal decomposition generally includes four steps: i) nucleation in a delayed time, ii) particle growth by monomer supply, iii) size shrinkage by dissolution, and iv) aggregation. demonstrates schematic synthesis workflow for α-NaYF4:Yb,Er UCNPs. The variety of reaction time, temperature, and concentration of reagents (organometallic compounds, surfactants, and solvents) provide various sizes, shapes, and also phases of NaYF4:Yb,Er UCNPs. shows different sizes of β-NaYF4-based NPs that have been synthesized under 330°C in OA/ODE for () 15 min, () 25 min, and () 45 min.Citation107 Even though thermal decomposition method renders a low size distribution and homogeneity in shape of NPs, this approach still suffers some drawbacks for high quality output, for instance, requiring relatively high temperature under the reflux reaction with oxygen-free synthesis flask with inert gas infusion.Citation108 Furthermore, most of the synthesized NPs are fixed by surfactants, which are usually in contrast to biological applications and it causes non-biocompatible covers around the NPs, thus surface modification would be necessary.Citation109
Figure 5 (A) Schematic procedure of synthesizing α-NaYF4:Yb,Er nanocrystals and growing stages consecutively through the thermal decomposition method. Reproduced with permission from Mai H-X, Zhang Y-W, Sun L-D, Yan C-H. Size- and phase-con-trolled synthesis of monodisperse NaYF4: Yb,ErNanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy. J Phys Chem C. 2007;111(37):13730–13739.Citation106 Copyright American Chemical Society 2007. Various sizes of NaYF4-based nanoparticles that have been synthesized under 330°C in OA/ODE (1/1) for (B) 15 min, (C) 25 min, and (D) 45 min. Reproduced with permission from Mai H-X, Zhang Y-W, Si R, et al. High-quality sodium rare-earth fluoride nanocrystals: controlled synthesis and optical properties. J Am Chem Soc. 2006;128(19):6426–6436.Citation107 Copyright American Chemical Society 2006.
Hydrothermal method
The hydrothermal method is a typical solution-based technique which has been used for synthesizing different types of nanocrystals.Citation110 The synthesis procedure can be relatively smoother than thermal decomposition in terms of temperature (130–240°C) and pressure. Nevertheless, the smoother synthesizing process suffers from a major drawback which is long reaction time depending on crystal growth and thermodynamic process.Citation111,Citation112 In this method, unlike the thermal decomposition that mainly occurs in organic solvents, water-based media can be applied, and this moderate system allows some biocompatible chelating ligands such as sodium citrate and ethylene diamine tetra acetate (EDTA) cover the nanocrystals instead of long chain non-biocompatible organic ligands.Citation113,Citation114 Moreover, several studies have reported hydrothermal synthesizing in high temperatures and pressure reaction conditions.Citation115–Citation117 It is likewise the thermal decomposition method that generates different morphologies and architectures by using and tuning different substances, solvents, and adjusting reaction time and temperature. Shen et al demonstrated that LaF3:Yb3+,RE3+ (RE=Er and Tm) NPs prepared via a hydrothermal co-precipitation method, followed by heat treatment at 180°C, 400°C, and 600°C separately. This investigation showed that the UCL intensity and particle size of these NPs were enhanced by increasing heat treatment temperatures.Citation117 An elegant study by Zhang et al introduced a general solution-based hydrothermal technique for generation of well-controlled morphologies nanoarray crystals of sodium lanthanide fluorides. Green and blue fluorescences have been yielded from nanoarrays of NaYF4 co-doped with Yb3+ as sensitizer and Er3+ and Tm3+, respectively, as the activators. These nanoarrays could propose superior potential as light sources for a new generation of solid-state lasers due to specific luminescence outputs and chemical flexibilities.Citation118
Lately, great efforts have been put into new approaches for synthesizing UCNPs to make the process faster, cheaper, and more efficient, not only for fundamental research, but also for high-tech applications.Citation41,Citation119–Citation121 Shao et al reported a fast and novel ion-exchange approach for synthesizing monodisperse β-NaYF4 micro/nanocrystals at 50°C. In this approach, the size of the crystals was adjusted according to the pH value. The results from this work indicated that by monotonously increasing the pH value the size of production reduced with no evident changes in morphology and monodispersity.Citation122 Lei et al also reported a super facile approach for synthesizing of hexagonal phase NaBiF4:Yb3+, Ln3+ (Ln=Er, Tm and Ho) UCNPs at room temperature. The replacement of bismuth (Bi) species instead of traditional host matrixes has made this method enormously economical. In the sense of energy efficiency for high volume fabrication of UCNPs, working in ambient and short time production is a substantial convenience which this approach might make it feasible.Citation123 The other approach for synthesizing UCNPs is polyol-mediated which is normally utilized for metal oxide particles such as Cu2O, SiO2, and TiO2 etc.Citation124,Citation125 This technique is a high boiling points method that can produce NPs with high dispersity in water. Glycol, diethylene glycol, and glycerol are three common polyols that can guarantee the stability of particles, control the particle growth, and prevent the agglomeration during and after the synthesis procedure.Citation126
UCNPs in medicine
UCNPs have recently attracted much attention in medicine due to numerous exclusivities that can ease diagnostic and therapeutic approaches. Higher detection sensitivity, broader signal dynamic ranges for biomarkers, and prediction of therapeutic responses are a few examples of positive contributions of these nanomaterials to medicine. UCNPs may be employed as multiplexed and sensitive nanobiomarkers with great ability to be excited in the tissue by infrared light instead of ultraviolet or visible lights to create photoluminescence emissions in a visible range.Citation127 Several advantages possessed by these NPs in biomedicine including capability for cell penetration due to their size (20–50 nm), high stability for surface modification, fine emission line widths (10–20 nm) compared with QDs (20–40 nm) and organic dyes (30–50 nm), and most importantly, low inherent toxicity.Citation128,Citation129 The combination of these properties makes UCNPs extremely practical in medicine, especially for cancer therapy.Citation130
Biocompatibility of UCNPs has been investigated with multiple functionalization and conditions.Citation131–Citation139 Here, we have summarized and listed recent efforts to study their toxicities in vitro (). As previously established, these NPs do not have significant toxicity; however, coating and different functionalities have been used to tune their interactions with cells. Our unpublished experimental encounter with UCNPs (size =35 nm, time =2 h, 8 h, and 24 h, concentration =10 µg/mL, 50 µg/mL, and 100 µg/mL, assay = resazurin) also showed their low toxicity profiles.
Table 1 Experimental cell viability results of UCNPs under different conditions
The unique photoactivity of UCNPs makes them a potential NIR adjuster probe that has attracted extensive attention in the past decade in the context of materials sciences. Forasmuch as the UCNPs have ability to be remotely utilized in regulated photodynamic inactivation and photo-triggered release systems, these materials exhibit superior potential for image-guided therapy and therapeutic studies.Citation140
Light, due to its easy handling and remote controlling capability, has been widely used as an external stimulus to affect photochemical reactions. Using UV and/or visible lights exhibit a variety of impediments based on the substantial high photon energy, such as material decomposition and low penetration into the tissue. In contrast, NIR light, with lower photon energy, renders less photo damage and higher penetration inside tissue.Citation141 The capability of UCNPs to turn NIR light to short-wavelength photons enables using low energy wavelength for photochemical reactions. Moreover, UCNPs, due to containing 4f electron orbital states of lanthanide ions, provide rich optoelectronic and magnetic properties.Citation142 Therefore, UCNPs have been explored for single-mode luminescence imaging as beam absorbent in several techniques such as magnetic resonance imaging (MRI), X-ray computed tomography (CT), positron emission tomography (PET), and single photon emission computed tomography (SPECT). In particular, UCL imaging has drawn remarkably increased attention in clinical studies of neurodegenerative diseases.Citation143,Citation144
There are a number of benefits and limitations of each bioimaging technique and the fact that any single imaging technique is not able to fulfill all of the requirements in a completed determination; accordingly, it would be appropriate to perform a series of modulation imaging to compensate for all the needs of visualization. Each imaging modality has unique characteristics and properties in terms of anatomical information. Different properties of these techniques such as sensitivity, depth of penetration, tissue discrimination, spatial resolution, and image properties (2D and 3D) can offer applicable monitoring of the biodistribution and location of UCNPs in animals’ bodies for therapy.Citation145,Citation146 There is increasing consideration about supplementing imaging techniques by combining different instrumental methodologies to compensate for the weakness of each technique individually. Hence, it is essential to employ a particular imaging contrast agent for each bioimaging strategy, utilizing a multifunctional contrast agent might be ideal to prevent injections of several agents into the body and reduce side effects by exterior reagents.Citation147,Citation148 Therefore, it might be practicable to create versatile UCNPs for bioimaging due to their unique physical and optical properties, such as containing high electron dense materials and positive signal enhancement which make them usable for electron microscopy, MRI, X-ray tomography and PET, and also multivalent targeting capability which converts them into appropriate cargos for drug delivery.Citation149,Citation150
Applications in optical imaging
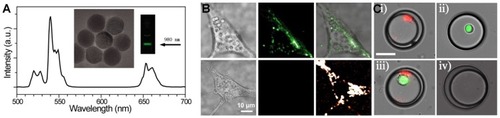
Optical imaging is a prominent and widely used tool in biological and clinical research due to its capability for visualizing the morphology and structure of cells (in vitro) and sub-cellular organelles (in vivo). The application of UCNPs can be extremely advantageous in cellular targeting, drug delivery to living objectives, fluorescence resonance energy transfer (FRET),Citation151 and for other purposes through surface conjugation of organic or biological molecules to generate various functional imaging probes. Zijlmans’s lab used up-converting phosphor particles conjugated with NeutrAvidin for detection of specific antigens in tissue sections or on cell membranes in 1999.Citation152 Recently, in similar work, Wu et al showed that UCNPs, with no on/off emission alteration, can be coated with amphiphilic biocompatible polymers. This study proved that the excitation of these materials with a modest-power continuous wave laser for optical microscopy investigation might provide strong UCL with low anti-Stokes autofluorescence, which is the ideal invention for single-molecule imaging ().Citation153 Recently, Vaithiyanathan et al designed a fluorescent microscopy-based microfluidic trapping array for simultaneously interrogate single-cell responses.Citation154 In this work, two different lanthanides (Eu3+ and Tb3+)-doped NaYF4 based NPs were employed to eliminate spectral overlap between tracking dyes and general fluorophores/biochemical stains. The fluorescent microscopy images from four different droplet subpopulations were: i) droplets with Eu3+-doped NPs, ii) droplets with green protein fluorescence (GFP)-expressing HeLa cell, iii) droplets with co-encapsulation of NPs and GFP-expressing cell, and iv) empty droplets (). Nevertheless, there is an unanswered question associated with the optical distance between the light source and the objective tissue or cell due to complexity of tissue scattering and absorption. Pominova et al have theoretically studied this topic on bioimaging by employing UCNPs.Citation155 In this study, optimal distance between two optical fibers, one at the position of laser source and the second at receiving point of biological tissue, was measured. This simulation by Monte Carlo modeling could calculate the intensity ratio of the UCNPs’ luminescence at different depths inside phantoms of biological tissues.
Figure 6 (A) Luminescence spectrum of the UCNPs excited at 980 nm and TEM image of the UCNPs. (B) Cell imaging using UCNPs (top left) brightfield image of a cell with internalized UCNPs, (top middle) fluorescence imaging of stained cell with UCNPs excited at 980 nm, and (top right) merged. (Bottom left) brightfield image of a cell without UCNPs, (bottom middle) fluorescence imaging of cell without UCNPs, and (bottom right) cellular autofluorescence excited at 532 nm. (A, B) Reproduced from Wu S, Han G, Milliron DJ, et al. Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals. Proc Natl Acad Sci. 2009;106(27):10917–10921.Citation153 (C) The fluorescent microscopy images from the trapped droplets by microfluidic array involving: iI) droplets with lanthanide-doped NPs, ii) droplets with GFP-expressing cell, iii) droplets with co-encapsulated NPs and GFP-expressing cell, and iv) empty droplets. Reprinted by permission from Springer Nature: Anal Bioanal Chem, Luminescent nanomaterials for droplet tracking in a microflui- dic trapping array, Vaithiyanathan M, R Bajgiran K, Darapaneni P, Safa N, Dorman JA, Melvin AT, Copyright 2019, doi:10.1007/s00216-018-1448-1.Citation154
Applications in cancer therapy
Employing light in medicine has a long history and it has been applied in many ancient cultures such as Greek, Egyptian, and Chinese for treatment of skin disorders, acne vulgaris, and eczema. A medical researcher in 1900, while conducting his study on the effect of an aromatic compound (acridine) on single-celled microorganisms, found that association of light with this fluorophore has a lethal effect on the cells.Citation156 This study discovered that the fluorescence output from the combination of acridine and light induced toxicity in the microorganisms. Later on, Tappeiner et al used this method in 1903 for the same purpose. They employed another type of fluorescent (eosin) using white light for skin tumor treatment and indicated it with photodynamic action phrase.Citation157 Since then, photodynamic therapy (PDT) has been investigated by researchers and medical specialties as an inoffensive approach in therapy, and fluorophores such as hematoporphyrin and coproporphyrin could improve the impact of PDT.Citation158–Citation160 Photosensitizers such as photofrin are also being used in PDT for different types of cancer therapy. In principle, in PDT, a source of light with certain wavelength and energy irradiates a photosensitizer and transfers the energy from ground state to excited state.Citation161 When energy is released from higher energy states, it may be transferred to the adjacent oxygen and produce oxygenated products (e.g., 1O2).Citation162 These reactive products with oxidative effects can progress inflammatory diseases and properly kill the cells.Citation163 In recent years, PDT has become an acceptable and prevalent technique as a cancer therapeutic method due to lower systemic toxicity for normal tissue and better selectivity for the tumor with fewer side effects in comparison with chemotherapy, radiation, and proton therapy.Citation164 Some NPs, such as QDs, gold nanomaterials, and polymers have been employed in PDT; however, most of these NPs turn short wavelengths into long wavelengths, which is called down-conversion luminescence, and this mechanism needs a higher energy density to activate photosensitizers.Citation165 Moreover, the higher energy lights can penetrate less into the tissue than lower energy lights and it brings difficulties to deep tissue imaging.Citation166 Therefore, a better solution for high PDT efficiency with fewer side effects is needed to employ this technique for further biomedical applications.Citation167 The use of UCNPs in PDT can cover a multitude of requirements as a result of deeper penetration of NIR light into the body, higher stability, and relatively easy surface modification. These materials in PDT usually require pre-coating with a shell which serves as a doping stage for photosensitizers, a probe platform for specific targeting, and UCNPs’ stabilization. The NaYF4:Yb,Er is one of the most commonly used UCNP types in PDT due to its high UCL efficiency.Citation168,Citation169
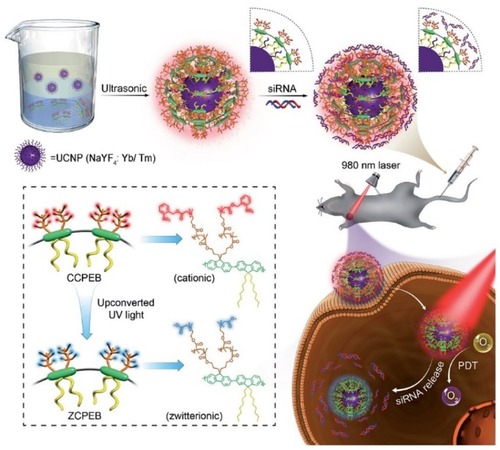
A multidisciplinary work by Zhao et al demonstrated the capability of UCNPs (NaYF4:Yb,Tm) for PDT and, simultaneously, it is a desirable platform for siRNA which may offer a major strategy in cancer therapy with superlative efficacy.Citation170 In this study, UCNPs were encapsulated with cationic conjugated polyelectrolyte brush (CCPEB). The richness of positive charges and photosensitizer behavior of this compound make it suitable for integrating the siRNA and the photosensitizer in a single molecule. The UCNPs coated with CCPEB showed efficacious PDT by producing 1O2, and turning the photoresponsive cationic side-chains of CCPEB into zwitterionic chains could accelerate the siRNA releasing up to 80% at pH 5.0 under 1 hr NIR irradiation. shows a schematic illustration of UCNPs@CCPEB/siRNA fabrication and photosensitization process under 980 nm laser irradiation. The combination of PDT and siRNA therapy on tumor-bearing mice showed notable therapeutic potential for cancer treatment. Liu et al also created a novel nanovehicle by coating the UCNPs with polydopamine (PDA). This complex is able to load indocyanine green (ICG), which can be triggered and heated by an 800–810 nm laser to overheat the tumor without damaging the surrounding tissue.Citation171
Figure 7 The top scheme indicates fabrication of UCNPs@CCPEB and loading the siRNA process. The bottom scheme indicates the procedure of releasing the siRNA and PDT simultaneously under NIR excitation and UV/visible emission from UCNPs. Reproduced with permission from Zhao H, Hu W, Ma H, et al. Photo-induced charge-variable conjugated polyelectrolyte brushes encapsulating upconversion nanoparticles for promoted siRNA release and collaborative photodynamic therapy under NIR light irradiation. Reproduced from Zhao H, Hu W, Ma H, et al. Photo-induced charge-variable conjugated polyelectrolyte brushes encapsulating upconversion nanoparticles for promoted siRNA release and collaborative photodynamic therapy under NIR light irradiation. Adv Funct Mater. 2017;27(44):1702592Citation170 Copyright John Wiley and Sons.
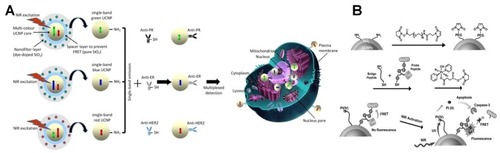
Zhou et al illustrated a multiplexed simultaneous in situ technique for diagnostication of different biomarkers in intact tumor specimens by using single-band UCNPs with different emissions that could provide a sensitive quantification of protein targeting. In this work, UCNPs were modified with a silicon dioxide (SiO2) layer containing organic dyes to create a selective nanofilter around the nanocrystals and remove unwanted emission bands ().Citation172 UCNPs covered with silicon dioxide layer in another study by Min et al, could provide a promising and innovatory platform for remote controlling of light activation of a specific antitumor platinum prodrug by using an apoptosis sensing peptide ().Citation173 Several studies have shown that nucleic acids-,Citation174 antibody-,Citation175 or peptide-conjugatedCitation176 UCNPs can be used to accomplish accurate molecular profiling and binding to protein targets in biodetections with high affinity and superior cell penetration.Citation177,Citation178
Figure 8 (A) Surface modified UCNPs with organic dyes doped within silicon to get single emission from NPs. Antibody conjugation of these modified nanoparticles creates influential probes for multiplexed in situ molecular mapping of tumor biomarkers. Reproduced with permission from Zhou L, Wang R, Yao C, et al. Single-band upconversion nanoprobes for multiplexed simultaneous in situ molecular mapping of cancer biomarkers. Nat Commun. 2015;6:6938.Citation172 Copyright Springer Nature 2015. (B) Schematic representation of prodrug activation using NIR and intracellular apoptosis by UCL. Reproduced with permission from Min Y, Li J, Liu F, Yeow EKL, Xing B. Near-infrared lightmediated photoactivation of a platinum antitumor prodrug and simultaneous cellular apoptosis imaging by upconversion-luminescent nanoparticles. Angew Chem Int Ed. 2014;53(4):1012–1016.Citation173 Copyright 2013, John Wiley and Sons.
The use of surface-modified UCNPs in animal experiments, chiefly in cancer research, for the purpose of drug delivery, bioimaging, and phototherapy has revealed reliable approaches for therapeutic innovations; however, there is an essential question about the statement and elimination of these particles from the body.Citation179–Citation182 Liu et al reported an inclusive study using PEG-modified NaGdF4:Yb,Er NPs for tumor targeting and in vivo imaging. The PEG was bearing maleimide on one side for attaching to a commercially available antitumor antibody and two phosphate groups on the other side for conjugating to the NP. One of the concerns in this study was pharmacokinetic interrogation on particle size-dependent biodistribution in mouse body and clearance pathways.Citation183 Three different sizes (5.1, 18.5, and 24.6 nm) of NPs were utilized for the output of post-injection examinations. The results from time-series in vivo experiments indicated major accumulation of these materials in liver and spleen, apart from the tumor. The clearance pathways investigation for NPs in this work showed the size dependency of elimination mechanism. Lower presence of smaller NPs in the liver and spleen at different time points post-injection, and also blood half-time calculation may prove that the smaller particles (5.1 nm) might have faster elimination from the renal pathway. The feces analysis showed that the biliary pathway could go forward with both big and small particle samples.
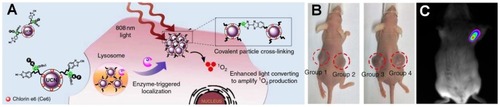
An innovative study by Ai et al has shown a microenvironment-sensitive system for covalent cross-linking of peptide-conjugated UCNPs and triggering the accumulation of nanocrystals into the tumor site ().Citation184 This accumulation could effectively enhance the emission density of UCNPs and intensify the production of reactive oxygen from the photosensitizers loaded on the nanocrystals for tumor treatment. In this work, targeted therapeutic evaluation was performed with UCNPs platforms to investigate PDT besides in vivo photoacoustic imaging. As shown in , two groups of tumor-bearing mice underwent treatment. The results showed that the implanted tumor that was injected with PDT agent (group 1 [G 1]) could significantly inhibit the tumor growth under NIR irradiation in comparison with control agent under NIR (G 2), PDT agent without NIR (G 3), and tumor treated with NIR light alone (G 4).
Figure 9 (A) Schematic illustration of microenvironment-sensitive strategy. Triggering the accumulation of UCNPs into the tumor. (B) Mice treated with PDT agent under NIR (G 1), control agent under NIR (G 2), PDT agent without NIR (G 3), and saline under NIR (G 4). Reproduced with permission from Ai X, Ho CJH, Aw J, et al. In vivo covalent cross-linking of photon-converted rare-earth nanostructures for tumour localization and theranostics. Nat Commun. 2016;7:10432.Citation184 (C) In vivo UCL imaging of a tumor-bearing mouse after intratumoral injection of UCNPs solution into the tumor site. Reproduced with permission from Dai Y, Xiao H, Liu J, et al. In vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans-platinum pro- drug-conjugated upconverison nanoparticles. J Am Chem Soc. 2013;135(50):18920–18929.Citation185 Copyright American Chemistry Society 2013.
Dai et al developed a type of nanotransducer with modification of core-shell Tm3+-doped UCNPs to trigger the platinum (iv) prodrug at the tumor site.Citation185 This photoactive platform, after entering the tumor cells via endocytosis, could convert the deeply penetrating NIR light into UV and eradicate the tumor by on-site release of prodrug. C shows the NIR to NIR UCL output observed from the tumor site after intratumoral injection of modified UCNPs in the left axilla. An accurate multifunctional nanotheranostic agent was designed by Dai et al for sensitive diagnosis and in vivo treatment of tumors.Citation186 In this work, a complex of PDA-coated NaYF4:Nd3+/NaLuF4 nanocomposites was synthesized by core-shell strategy and employed for dual-modal imaging, namely NIR-II optical imaging and X-ray CT imaging, to acquire in vivo photothermal therapy (PTT) which might be a low-invasive therapy. The X-ray CT imaging, using these well-designed nanocomposites, could elucidate the important physiological and anatomical details of the implant tumor through excellent spatial resolution and depth for in vivo imaging. Furthermore, these particular nanocomposites are considered theoretically superior to the traditional iodine-based commercial X-ray imaging agent (). The substantive amine and hydroxyl groups on the surface of the NP@PDA nanocomposites could maintain the stability and dispersity of these nanocomposites in polar solvents for up to 2 months at ambient temperature.
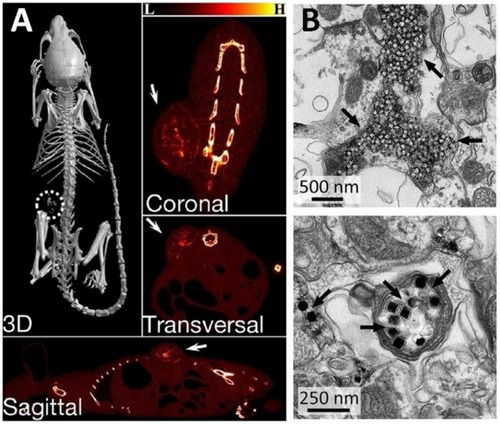
Figure 10 (A) X-ray CT images of the tumor-bearing mice after intratumoral injection of NP/PDA suspension. Reproduced with permission from Dai Y, Yang D, Yu D, et al. Mussel-inspired polydopamine-coated lanthanide nanoparticles for NIR-II/CT dual imaging and photothermal therapy. ACS Appl Mater Interfaces. 2017;9(32):26674– 26683.Citation186 Copyright American chemical society 2017. (B) Electron micrographs of UCNPs distributed in the neuronal tissue. Black arrows indicate clusters of UCNPs. Top image shows the distribution of most UCNPs in extracellular space, and the bottom image shows the uptake of UCNPs within an axon. From Chen S, Weitemier AZ, Zeng X, et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science. 2018;359(6376):679.Citation187 Reprinted with permission from AAAS https://science.sciencemag.org/content/359/6376/679.
Applications in optogenetics studies
One of the most interesting recent applications with UCNPs is optogenetics studies in small animals, this is only feasible due to the deep penetration of NIR light into the body. A ground-breaking study by Chen et al showed the usage of molecularly tailored UCNPs in the presence of transcranial NIR light for deep brain stimulation in genetically-tagged neurons.Citation187 These UCNPs were precisely tuned to a particular wavelength by selective lanthanide ion doping. By incorporating Tm3+ into Yb3+-doped host lattices, this produced blue emission matching the maximum absorption of channelrhodopsin-2 for activating neurons, while Yb3+, Er3+ couple was used to emit green light in a compatible way to activate halorhodopsin or archaerhodopsin for the purpose of inhibiting neuronal activity.
The concept of using UCNPs to mediate optogenetics is not new;Citation188–Citation192 however, this study was the first to show minimally invasive deep brain stimulation in a mammalian system. This important demonstration overcomes the challenging issue of visible light being a limiting factor in optogenetics as used for manipulating neuronal activity in deep tissue penetration and remote therapy. The researchers were able to successfully evoke brain oscillations, through activation of inhibitory neurons in a specific area deep in the brain, and physiologically eliminate seizures by inhibiting excitatory cells in another specific region deep within the brain. After injecting an adeno-associated virus encoding an enhanced yellow fluorescent protein into the ventral tegmentum area (VTA) deep within the brain, they activated Cre-dependent gene expression of channelrhodopsin-2 in dopaminergic neurons for neuronal activation purposes. Four weeks following injection, UCNPs were injected into the VTA region as well. Electron microscopy confirmed the presence of UCNPs primarily confined to the injection area with minimal dispersion, and that they were mainly distributed in extracellular spaces neighboring cell membranes and synaptic clefts, while a minority was shown as taken up by the neurons and clearly shown in their axons (). Real-time efficacy of the NIR-evoked excitation of these neurons was assessed by fast-scan cyclic voltammetry (FSCV) and confirmed dopamine release that was temporally confined to the transcranial NIR stimulation, with no dopamine release in control mice. With illumination at a distance of 0.5 mm, both the NIR and blue light triggered dopamine release in the ventral striatum area of the brain; and only NIR was able to elicit such a response in transcranial application. Besides neuronal activation, the researchers were also able to demonstrate the use of modified UCNPs for neuronal inhibition. It showed the ability of these modified UCNPs to inhibit activity of neurons in a different region of the brain, the hippocampus, which is primarily associated with memory formation and retention.
By demonstrating successful spectral tuning of UCNPs as compatible with the current toolbox of light-activated channels to the point of functionally activating and inhibiting neurons in various deep brain structures, these researchers have set a foundation for potential usage one day in human patients suffering from neurological conditions, such as Parkinson’s disease, in which cumbersome electrical equipment must be implanted both in the brain and chest of patients and tuned manually in the clinic at regular intervals. Lin et al used a UCNPs-based miniature device for neural inhibition in mouse brain. In this study, a sealed package of NaYF4:Yb,Er based UCNPs was placed precisely in animal brain for modulation of electrical activity of the neurons upon NIR (980 nm) irradiation.Citation144 This fiber-free technique allows the animal to move naturally and demonstrates the performance of deep brain inhibition with no attachments.
Conclusion
In this review, we summarized the principle of physical/chemical properties and photoactivity behavior of UCNPs, including up-conversion mechanisms, crystals compositions, and efficient methods for synthesization, in order to convey a better understanding of their potential for further theranostic use. Following that, the strongest emphasis in this article was the conceptual application of these materials in medicine for a variety of purposes: delivering, targeting, tumor therapy, and bioimaging in vitro and in vivo.
The discussions in this review article revealed that the unique functions of modified UCNPs in PDT by excitation of NIR light is a superior implement that might become a definite approach and inoffensive treatment for elimination of cancerous tissues. In addition to that, relatively high surface-area-to-volume ratio of these particles for loading the chemicals and biomaterials makes them an appropriate platform for drug and gene delivery for different diseases which need specific and selective targeting. Also, the high uptake of UCNPs by cells and tissues makes these functionalized fluorophore probes an indispensable tool for in vitro/in vivo imaging with high sensitivity and deep penetration of stimulators. In addition, reviewing general toxicity, the results from biodistribution in mouse body and excretion describe the reason for extending biomedical applications with these NPs.
Regarding the drawbacks of using UCNPs, it should be noted that due to unique optical properties and novelty of these materials in biochemistry and medicine, most of the commercial imaging equipment is not designed and compatible for direct application to UCNPs. Usually, the exciting light sources in confocal microscopes cover the range of UV and visible light, and the NIR sources of in vivo imaging machines are not focused and powerful enough to excite UCNPs; hence, scientists have to build their own homemade imaging instruments with suitable sources such as continuous wavelength lasers.
Overall, it is important to highlight the recently published reports, as they indicate how these novel NPs of different substances serve as appropriate alternatives to available mainstream techniques that otherwise pose arduous difficulties and limitations in detection and targeting, especially in hard-to-access areas of the body, such as deep brain structures. Harnessing the power of versatile UCNPs will be critical for developing modern and more tailored therapeutic approaches.
Abbreviations
UCNPs, up-conversion nanoparticles; FDA, US Food and Drug Administration; NPs, nanoparticles; EPR, enhanced permeability and retention; QDs, quantum dots; PEG, polyethylene glycol; NIR, infrared; UV, ultraviolet; UCL, up-conversion luminescence; ESA, state absorption; ETU, up-conversion by energy transfer; TPA, two-photon absorption; CSU, cooperative sensitization up-conversion; CR, cross-relaxation; PA, photon avalanche; EMU, energy migration-mediated up-conversion; QY, quantum yield; OM, oleylamine; OA, oleic acid; ODE, 1-octadecence; TEM, transmission electron microscopy; XRD, X-ray diffraction; EDTA, ethylene diamine tetra acetate; MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; FRET, fluorescence resonance energy transfer; GFP, green protein fluorescence; PDT, photodynamic therapy; CCPEB, cationic conjugated polyelectrolyte brush; PDA, polydopamine; ICG, indocyanine green; PTT, photothermal therapy; VTA, ventral tegmentum area; FSCV fast-scan cyclic voltammetry; Y, yttrium; Yb, ytterbium; Er, erbium; Tm, thulium; Gd, galadinium.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgment
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No 701647.
References
- Kim BYS, Rutka JT, Chan WCW. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. doi:10.1056/NEJMra091227321158659
- Chow EK-H, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5(216):216rv214–216rv214. doi:10.1126/scitranslmed.3005872
- Kairdolf BA, Qian X, Nie S. Bioconjugated nanoparticles for biosensing, in vivo imaging, and medical diagnostics. Anal Chem. 2017;89(2):1015–1031. doi:10.1021/acs.analchem.6b0487328043119
- Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer. 2007;120(12):2527–2537. doi:10.1002/ijc.2270917390371
- Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 2014;12:5. doi:10.1186/1477-3155-12-524491160
- Popović Z, Liu W, Chauhan VP, et al. A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed. 2010;49(46):8649–8652. doi:10.1002/anie.201003142
- Khajeh M, Laurent S, Dastafkan K. Nanoadsorbents: classification, preparation, and applications (with emphasis on aqueous media). Chem Rev. 2013;113(10):7728–7768. doi:10.1021/cr400086v23869773
- Yamada T, Fukuhara K, Matsuoka K, et al. Nanoparticle chemisorption printing technique for conductive silver patterning with submicron resolution. Nat Commun. 2016;7:11402. doi:10.1038/ncomms1140227091238
- De Jong WH, Borm PJA. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3(2):133–149.18686775
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed. 2014;53(46):12320–12364. doi:10.1002/anie.201403036
- De Crozals G, Bonnet R, Farre C, Chaix C. Nanoparticles with multiple properties for biomedical applications: a strategic guide. Nano Today. 2016;11(4):435–463. doi:10.1016/j.nantod.2016.07.002
- Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085–4109. doi:10.2147/IJN.S13278028615938
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Controlled Release. 2015;200:138–157. doi:10.1016/j.jconrel.2014.12.030
- Prencipe G, Tabakman SM, Welsher K, et al. PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation. J Am Chem Soc. 2009;131(13):4783–4787. doi:10.1021/ja809086q19173646
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi:10.1007/s11095-016-1958-527299311
- Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. PT. 2017;42(12):742–755.
- Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi:10.1016/j.yexmp.2008.12.00419186176
- Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23(12):H18–H40. doi:10.1002/adma.20110014021433100
- Rwei AY, Wang W, Kohane DS. Photoresponsive nanoparticles for drug delivery. Nano Today. 2015;10(4):451–467. doi:10.1016/j.nantod.2015.06.00426644797
- Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci. 2013;8(1):1–10. doi:10.1016/j.ajps.2013.07.001
- Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Controlled Release. 2014;190:485–499. doi:10.1016/j.jconrel.2014.06.038
- Min Y, Roche KC, Tian S, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nano. 2017 advance online publication. doi:10.1038/nnano.2017.113
- Singh L, Kruger HG, Maguire GEM, Govender T, Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis. 2017;4(4):105–131. doi:10.1177/204993611771359328748089
- Gendelman HE, Anantharam V, Bronich T, et al. Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomed. Nanotechnol Biol Med. 2015;11(3):751–767. doi:10.1016/j.nano.2014.12.014
- Park S-M, Aalipour A, Vermesh O, Yu JH, Gambhir SS. Towards clinically translatable in vivo nanodiagnostics. Nat Rev Mater. 2017;2:17014. doi:10.1038/natrevmats.2017.1429876137
- Jennifer M, Maciej W. Nanoparticle technology as a double-edged sword: cytotoxic, genotoxic and epigenetic effects on living cells. J Biomater Nanobiotechnol. 2013;04(01):11. doi:10.4236/jbnb.2013.41008
- Choueiri RM, Galati E, Thérien-Aubin H, et al. Surface patterning of nanoparticles with polymer patches. Nature. 2016;538(7623):79–83. doi:10.1038/nature1908927556943
- Zhang L, Li Y, Yu JC. Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J Mater Chem B. 2014;2(5):452–470. doi:10.1039/C3TB21196G
- Shen J, Zhao L, Han G. Lanthanide-doped upconverting luminescent nanoparticle platforms for optical imaging-guided drug delivery and therapy. Adv Drug Deliv Rev. 2013;65(5):744–755. doi:10.1016/j.addr.2012.05.00722626980
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi:10.1038/nrd261418758474
- Cao Y, Jin R, Mirkin CA. DNA-modified core−shell Ag/Au nanoparticles. J Am Chem Soc. 2001;123(32):7961–7962. doi:10.1021/ja011342n11493092
- Feng X, Yao J, Gao X, et al. Multi-targeting peptide-functionalized nanoparticles recognized vasculogenic mimicry, tumor neovasculature, and glioma cells for enhanced anti-glioma therapy. ACS Appl Mater Interfaces. 2015;7(50):27885–27899. doi:10.1021/acsami.5b0993426619329
- Lu J, Shi M, Shoichet MS. Click chemistry functionalized polymeric nanoparticles target corneal epithelial cells through RGD-cell surface receptors. Bioconjug Chem. 2009;20(1):87–94. doi:10.1021/bc800316719099361
- Sapsford KE, Algar WR, Berti L, et al. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113(3):1904–2074. doi:10.1021/cr300143v23432378
- Banerjee SS, Aher N, Patil R, Khandare J. Poly(ethylene glycol)-prodrug conjugates: concept, design, and applications. J Drug Deliv. 2012;2012:103973. doi:10.1155/2012/10397322645686
- Tao Z, Hong G, Shinji C, et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew Chem. 2013;125(49):13240–13244. doi:10.1002/ange.201307346
- Song J, Huang P, Chen X. Preparation of plasmonic vesicles from amphiphilic gold nanocrystals grafted with polymer brushes. Nat Protoc. 2016;11:2287. doi:10.1038/nprot.2016.13727763624
- Cheng L, Song J, Yin J, Duan H. Self-assembled plasmonic dimers of amphiphilic gold nanocrystals. J Phys Chem Lett. 2011;2(17):2258–2262. doi:10.1021/jz201011b
- Liu Y, Lu Y, Yang X, et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature. 2017;543:229. doi:10.1038/nature2136628225761
- Wang M, Zhu Y, Mao C. Synthesis of NIR-responsive NaYF4: Yb,ErUpconversion fluorescent nanoparticles using an optimized solvothermal method and their applications in enhanced development of latent fingerprints on various smooth substrates. Langmuir. 2015;31(25):7084–7090. doi:10.1021/acs.langmuir.5b0115126089129
- Zhou B, Shi B, Jin D, Liu X. Controlling upconversion nanocrystals for emerging applications. Nat Nanotechnol. 2015;10:924. doi:10.1038/nnano.2015.25126530022
- Zhu H, Chen X, Jin LM, Wang QJ, Wang F, Yu SF. Amplified spontaneous emission and lasing from lanthanide-doped up-conversion nanocrystals. ACS Nano. 2013;7(12):11420–11426. doi:10.1021/nn405387t24266853
- Yao W, Tian Q, Wu W. Tunable emissions of upconversion fluorescence for security applications. Adv Opt Mater. 2019;7(6):1801171. doi:10.1002/adom.v7.6
- Wang J, Wei T, Li X, et al. Near-infrared-light-mediated imaging of latent fingerprints based on molecular recognition. Angew Chem Int Ed. 2014;53(6):1616–1620. doi:10.1002/anie.201308843
- Zhang C, Zhou H-P, Liao L-Y, et al. Luminescence modulation of ordered upconversion nanopatterns by a photochromic diarylethene: rewritable optical storage with nondestructive readout. Adv Mater. 2010;22(5):633–637. doi:10.1002/adma.20090172220217763
- Liu H, Xu J, Wang H, et al. Tunable Resonator-Upconverted Emission (TRUE) color printing and applications in optical security. Adv Mater. 2019;31(15):1807900. doi:10.1002/adma.201802348
- Meruga JM, Baride A, Cross W, Kellar JJ, May PS. Red-green-blue printing using luminescence-upconversion inks. J Mater Chem C. 2014;2(12):2221–2227. doi:10.1039/c3tc32233e
- You M, Zhong J, Hong Y, Duan Z, Lin M, Xu F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale. 2015;7(10):4423–4431. doi:10.1039/c4nr06944g25613526
- You M, Lin M, Wang S, et al. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale. 2016;8(19):10096–10104. doi:10.1039/c6nr01353h27119377
- Bloembergen N. Solid state infrared quantum counters. Phys Rev Lett. 1959;2(3):84–85. doi:10.1103/PhysRevLett.2.84
- Auzel F. Compteur Quantique Par Transfert Denergie Entre Deux Ions De Terres Rares Dans Un Tungstate Mixte Et Dans Un Verre. CR Acad Sci Paris 1966;262:1016–1019.
- Dodson CM, Zia R. Magnetic dipole and electric quadrupole transitions in the trivalent lanthanide series: calculated emission rates and oscillator strengths. Phys Rev B. 2012;86(12):125102. doi:10.1103/PhysRevB.86.125102
- Dong H, Sun L-D, Yan C-H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem Soc Rev. 2015;44(6):1608–1634. doi:10.1039/c4cs00188e25242465
- Seth M, Dolg M, Fulde P, Schwerdtfeger P. Lanthanide and actinide contractions: relativistic and shell structure effects. J Am Chem Soc. 1995;117(24):6597–6598. doi:10.1021/ja00129a026
- Li X, Zhang F, Zhao D. Highly efficient lanthanide upconverting nanomaterials: progresses and challenges. Nano Today. 2013;8(6):643–676. doi:10.1016/j.nantod.2013.11.003
- Li X, Zhang F, Zhao D. Lab on upconversion nanoparticles: optical properties and applications engineering via designed nanostructure. Chem Soc Rev. 2015;44(6):1346–1378. doi:10.1039/c4cs00163j25052250
- Nakazawa E, Shionoya S. Energy transfer between trivalent rare‐earth ions in inorganic solids. J Chem Phys. 1967;47(9):3211–3219. doi:10.1063/1.1712377
- Balestrieri M, Colis S, Gallart M, et al. Photoluminescence properties of rare earth (Nd, Yb, Sm, Pr)-doped CeO2 pellets prepared by solid-state reaction. J Mater Chem C. 2015;3(27):7014–7021. doi:10.1039/C5TC00075K
- Downing E, Hesselink L, Ralston J, Macfarlane R. A three-color, solid-state, three-dimensional display. Science. 1996;273(5279):1185–1189. doi:10.1126/science.273.5279.1185
- Sivakumar S, van Veggel FCJM, Raudsepp M. Bright white light through up-conversion of a single NIR source from Sol−Gel-derived thin film made with Ln3+-Doped LaF3 nanoparticles. J Am Chem Soc. 2005;127(36):12464–12465. doi:10.1021/ja052583o16144374
- Sun L-D, Dong H, Zhang P-Z, Yan C-H. Upconversion of rare earth nanomaterials. Annu Rev Phys Chem. 2015;66(1):619–642. doi:10.1146/annurev-physchem-040214-12134425648487
- Wang F, Deng R, Wang J, et al. Tuning upconversion through energy migration in core–shell nanoparticles. Nat Mater. 2011;10:968. doi:10.1038/nmat308422019945
- Wang F, Liu X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev. 2009;38(4):976–989. doi:10.1039/b809132n19421576
- Ning K, Chao-Chao A, Ya-Ming Z, Zuo W, Lei R. Facile synthesis of upconversion nanoparticles with high purity using lanthanide oleate compounds. Nanotechnology. 2018;29(7):075601. doi:10.1088/1361-6528/aa96ee29083992
- Liu G. Advances in the theoretical understanding of photon upconversion in rare-earth activated nanophosphors. Chem Soc Rev. 2015;44(6):1635–1652. doi:10.1039/c4cs00187g25286989
- Suyver JF, Grimm J, van Veen MK, Biner D, Krämer KW, Güdel HU. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+. J Lumin. 2006;117(1):1–12. doi:10.1016/j.jlumin.2005.03.011
- Wang F, Deng R, Wang J, et al. Tuning upconversion through energy migration in core–shell nanoparticles. Nat Mater. 2011;10(12):968–973. doi:10.1038/nmat314922019945
- Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114(10):5161–5214. doi:10.1021/cr400425h24605868
- Gai S, Li C, Yang P, Lin J. Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem Rev. 2014;114(4):2343–2389. doi:10.1021/cr400159424344724
- Yang D, Dai Y, Liu J, et al. Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes. Biomaterials. 2014;35(6):2011–2023. doi:10.1016/j.biomaterials.2013.11.01824314558
- Xu D, Li A, Yao L, Lin H, Yang S, Zhang Y. Lanthanide-doped KLu2F7 nanoparticles with high upconversion luminescence performance: a comparative study by judd-ofelt analysis and energy transfer mechanistic investigation. Sci Rep. 2017;7:43189. doi:10.1038/srep4318928230083
- Liu D, Xu X, Du Y, et al. Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat Commun. 2016;7:10254. doi:10.1038/ncomms1025426743184
- Gargas DJ, Chan EM, Ostrowski AD, et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat Nanotechnol. 2014;9:300. doi:10.1038/nnano.2014.2924633523
- Wang F, Han Y, Lim CS, et al. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature. 2010;463(7284):1061–1065. doi:10.1038/nature0877720182508
- Gu M, Zhang Q, Lamon S. Nanomaterials for optical data storage. Nat Rev Mater. 2016;1:16070. doi:10.1038/natrevmats.2016.70
- Zhou B, Shi B, Jin D, Liu X. Controlling upconversion nanocrystals for emerging applications. Nat Nano. 2015;10(11):924–936. doi:10.1038/nnano.2015.251
- Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D. Advances in highly doped upconversion nanoparticles. Nat Commun. 2018;9(1):2415. doi:10.1038/s41467-018-04813-529925838
- Zhao J, Jin D, Schartner EP, et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat Nanotechnol. 2013;8:729. doi:10.1038/nnano.2013.17123995455
- Dexter DL, Schulman JH. Theory of concentration quenching in inorganic phosphors. J Chem Phys. 1954;22(6):1063–1070. doi:10.1063/1.1740265
- Tu L, Liu X, Wu F, Zhang H. Excitation energy migration dynamics in upconversion nanomaterials. Chem Soc Rev. 2015;44(6):1331–1345. doi:10.1039/c4cs00168k25223635
- Li M, Hao ZH, Peng XN, Li JB, Yu XF, Wang QQ. Controllable energy transfer in fluorescence upconversion of NdF3 and NaNdF4 nanocrystals. Opt Express. 2010;18(4):3364–3369. doi:10.1364/OE.18.00336420389345
- Taniguchi T, Murakami T, Funatsu A, Hatakeyama K, Koinuma M, Matsumoto Y. Reversibly tunable upconversion luminescence by host–guest chemistry. Inorg Chem. 2014;53(17):9151–9155. doi:10.1021/ic501129y25122035
- Deng R, Xie X, Vendrell M, Chang Y-T, Liu X. Intracellular glutathione detection using mno2-nanosheet-modified upconversion nanoparticles. J Am Chem Soc. 2011;133(50):20168–20171. doi:10.1021/ja210077422107163
- Ran W, Wang L, Tan L, Qu D, Shi J. Remote control effect of Li+, Na+, K+ Ions on the super energy transfer process in ZnMoO4: Eu3+,Bi3+ phosphors. Sci Rep. 2016;6:27657. doi:10.1038/srep2765727278286
- Zhong Y, Rostami I, Wang Z, Dai H, Hu Z. Energy migration engineering of bright rare‐earth upconversion nanoparticles for excitation by light‐emitting diodes. Adv Mater. 2015;27(41):6418–6422. doi:10.1002/adma.20150227226393770
- Zhong Y, Tian G, Gu Z, et al. Elimination of photon quenching by a transition layer to fabricate a quenching‐shield sandwich structure for 800 nm excited upconversion luminescence of Nd3+‐sensitized nanoparticles. Adv Mater. 2014;26(18):2831–2837. doi:10.1002/adma.20130490324338994
- Wang F, Deng R, Liu X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat Protoc. 2014;9:1634. doi:10.1038/nprot.2014.11124922272
- Chen X, Peng D, Ju Q, Wang F. Photon upconversion in core-shell nanoparticles. Chem Soc Rev. 2015;44(6):1318–1330. doi:10.1039/c4cs00151f25058157
- Wang F, Deng R, Liu X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat Protoc. 2014;9(7):1634–1644. doi:10.1038/nprot.2014.11124922272
- Li X, Shen D, Yang J, et al. Successive layer-by-layer strategy for multi-shell epitaxial growth: shell thickness and doping position dependence in upconverting optical properties. Chem Mater. 2013;25(1):106–112. doi:10.1021/cm3033498
- Chen G, Roy I, Yang C, et al. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. doi:10.1021/acs.chemrev.5b0014826799741
- Huang P, Tu D, Zheng W, Zhou S, Chen Z, Chen X. Inorganic lanthanide nanoprobes for background-free luminescent bioassays. Sci China Mater. 2015;58(2):156–177. doi:10.1007/s40843-015-0019-4
- Mir SH, Nagahara LA, Thundat T, Mokarian-Tabari P, Furukawa H, Khosla A. Review—organic-inorganic hybrid functional materials: an integrated platform for applied technologies. J Electrochem Soc. 2018;165(8):B3137–B3156. doi:10.1149/2.0191808jes
- Kuhn S, Tiegel M, Herrmann A, et al. Effect of hydroxyl concentration on Yb3+ luminescence properties in a peraluminous lithium-alumino-silicate glass. Opt Mater Express. 2015;5(2):430–440. doi:10.1364/OME.5.000430
- Yan Y, Faber AJ, de Waal H. Luminescence quenching by OH groups in highly Er-doped phosphate glasses. J Non Cryst Solids. 1995;181(3):283–290. doi:10.1016/S0022-3093(94)00528-1
- Su Q, Han S, Xie X, et al. The effect of surface coating on energy migration-mediated upconversion. J Am Chem Soc. 2012;134(51):20849–20857. doi:10.1021/ja311104823210614
- Yi G-S, Chow G-M. Water-soluble NaYF4: Yb,Er(Tm)/NaYF4/polymercore/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem Mater. 2007;19(3):341–343. doi:10.1021/cm062447y
- Zhang F, Che R, Li X, et al. Direct imaging the upconversion nanocrystal core/shell structure at the subnanometer level: shell thickness dependence in upconverting optical properties. Nano Lett. 2012;12(6):2852–2858. doi:10.1021/nl300421n22545710
- Zhan Q, He S, Qian J, Cheng H, Cai F. Optimization of optical excitation of upconversion nanoparticles for rapid microscopy and deeper tissue imaging with higher quantum yield. Theranostics. 2013;3(5):306–316. doi:10.7150/thno.600723650478
- Wisser MD, Fischer S, Siefe C, Alivisatos AP, Salleo A, Dionne JA. Improving quantum yield of upconverting nanoparticles in aqueous media via emission sensitization. Nano Lett. 2018;18(4):2689–2695. doi:10.1021/acs.nanolett.8b0063429589449
- Rafik N, Qing Y, CJ A. The fluoride host: nucleation, growth, and upconversion of lanthanide-doped nanoparticles. Adv Opt Mater. 2015;3(4):482–509. doi:10.1002/adom.201400628
- Mi C, Tian Z, Cao C, Wang Z, Mao C, Xu S. Novel microwave-assisted solvothermal synthesis of NaYF4: Yb,ErUpconversion nanoparticles and their application in cancer cell imaging. Langmuir. 2011;27(23):14632–14637. doi:10.1021/la204015m22029665
- Murali G, Lee BH, Mishra RK, et al. Synthesis, luminescence properties, and growth mechanisms of YF3: Yb3+/Er3+nanoplates. J Mater Chem C. 2015;3(39):10107–10113. doi:10.1039/C5TC02034D
- Liu X, Zhang X, Tian G, et al. A simple and efficient synthetic route for preparation of NaYF4 upconversion nanoparticles by thermo-decomposition of rare-earth oleates. CrystEngComm. 2014;16(25):5650–5661. doi:10.1021/acs.chemrev.5b00148
- Zhong Y, Ma Z, Zhu S, et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat Commun. 2017;8(1):737. doi:10.1038/s41467-017-00917-628963467
- Mai H-X, Zhang Y-W, Sun L-D, Yan C-H. Size- and phase-controlled synthesis of monodisperse NaYF4: Yb,ErNanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy. J Phys Chem C. 2007;111(37):13730–13739. doi:10.1021/jp073919e
- Mai H-X, Zhang Y-W, Si R, et al. High-quality sodium rare-earth fluoride nanocrystals: controlled synthesis and optical properties. J Am Chem Soc. 2006;128(19):6426–6436. doi:10.1021/ja060212h16683808
- Chen J, Zhao JX. Upconversion nanomaterials: synthesis, mechanism, and applications in sensing. Sensors. 2012;12(3):2414. doi:10.3390/s12030241422736958
- Chen G, Shen J, Ohulchanskyy TY, et al. (α-NaYbF4:Tm3+)/CaF2Core/shell nanoparticles with efficient near-infrared to near-infrared upconversion for high-contrast deep tissue bioimaging. ACS Nano. 2012;6(9):8280–8287. doi:10.1021/nn302972r22928629
- Dunne PW, Munn AS, Starkey CL, Huddle TA, Lester EH. Continuous-flow hydrothermal synthesis for the production of inorganic nanomaterials. Philos Trans R Soc Lond A. 2015;373(2057):20150015. doi:10.1098/rsta.2015.0015
- Ma Y, Chen M, Li M. Hydrothermal synthesis of hydrophilic NaYF4: Yb,ernanoparticles with bright upconversion luminescence as biological label. Mater Lett. 2015;139:22–25. doi:10.1016/j.matlet.2014.10.042
- Li C, Quan Z, Yang J, Yang P, Lin J. Highly uniform and monodisperse β-NaYF4: Ln3+(Ln = Eu, Tb, Yb/ Er,and Yb/Tm) hexagonal microprism crystals: hydrothermal synthesis and luminescent properties. Inorg Chem. 2007;46(16):6329–6337. doi:10.1021/ic070335i17602610
- Zeng JH, Su J, Li ZH, Yan RX, Li YD. Synthesis and upconversion luminescence of hexagonal-phase NaYF4: Yb,Er3+ phosphors of controlled size and morphology. Adv Mater. 2005;17(17):2119–2123. doi:10.1002/adma.200402046
- Zhou R, Li X. Effect of EDTA on the formation and upconversion of NaYF4:Yb3+/Er3+. Opt Mater Express. 2016;6(4):1313–1320. doi:10.1364/OME.6.001313
- Wang Y, Cai R, Liu Z. Controlled synthesis of NaYF4: Yb, Er nanocrystals with upconversion fluorescence via a facile hydrothermal procedure in aqueous solution. CrystEngComm. 2011;13(6):1772–1774. doi:10.1039/c0ce00708k
- Menyuk N, Dwight K, Pierce JW. NaYF4: Yb,Er—an efficient upconversion phosphor. Appl Phys Lett. 1972;21(4):159–161. doi:10.1063/1.1654325
- Shen H, Wang F, Fan X, Wang M. Synthesis of LaF3: Yb3+,Ln3+ nanoparticles with improved upconversion luminescence. J Exp Nanosci. 2007;2(4):303–311. doi:10.1080/17458080701724943
- Zhang F, Wan Y, Yu T, et al. Uniform nanostructured arrays of sodium rare‐earth fluorides for highly efficient multicolor upconversion luminescence. Angew Chem. 2007;119(42):8122–8125. doi:10.1002/ange.200702519
- Qiu P, Zhou N, Chen H, Zhang C, Gao G, Cui D. Recent advances in lanthanide-doped upconversion nanomaterials: synthesis, nanostructures and surface modification. Nanoscale. 2013;5(23):11512–11525. doi:10.1039/c3nr03642a24121736
- Ye X, Collins JE, Kang Y, et al. Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly. Proc Natl Acad Sci. 2010;107(52):22430–22435. doi:10.1073/pnas.100895810721148771
- Dong C, van Veggel FCJM. Cation exchange in lanthanide fluoride nanoparticles. ACS Nano. 2009;3(1):123–130. doi:10.1021/nn800474719206258
- Shao B, Zhao Q, Jia Y, et al. A novel synthetic route towards monodisperse β-NaYF4: Ln3+micro/nanocrystals from layered rare-earth hydroxides at ultra low temperature. Chem Commun. 2014;50(84):12706–12709. doi:10.1039/c4cc05191b
- Lei P, An R, Yao S, et al. Ultrafast synthesis of novel hexagonal phase NaBiF4 upconversion nanoparticles at room temperature. Adv Mater. 2017;29:22. doi:10.1002/adma.201700681
- Feldmann C, Jungk H-O. Preparation of sub-micrometer LnPO4 particles (Ln = La, Ce). J Mater Sci. 2002;37(15):3251–3254. doi:10.1023/A:1016131016637
- Feldmann C, Jungk H-O. Polyol-mediated preparation of nanoscale oxide particles. Angew Chem Int Ed. 2001;40(2):359–362. doi:10.1002/(ISSN)1521-3773
- Chen J, Herricks T, Xia Y. Polyol synthesis of platinum nanostructures: control of morphology through the manipulation of reduction kinetics. Angew Chem Int Ed. 2005;44(17):2589–2592. doi:10.1002/anie.200462668
- Dong H, Du S-R, Zheng X-Y, et al. Lanthanide nanoparticles: from design toward bioimaging and therapy. Chem Rev. 2015;115(19):10725–10815. doi:10.1021/acs.chemrev.5b0009126151155
- Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA. Upconverting nanoparticles: assessing the toxicity. Chem Soc Rev. 2015;44(6):1561–1584. doi:10.1039/c4cs00177j25176037
- Xiong L, Yang T, Yang Y, Xu C, Li F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials. 2010;31(27):7078–7085. doi:10.1016/j.biomaterials.2010.05.06520619791
- Wang M, Abbineni G, Clevenger A, Mao C, Xu S. Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed. Nanotechnol Biol Med. 2011;7(6):710–729. doi:10.1016/j.nano.2011.02.013
- He S, Krippes K, Ritz S, et al. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem Commun. 2015;51(2):431–434. doi:10.1039/c4cc07489k
- Li N, Wen X, Liu J, Wang B, Zhan Q, He S. Yb3+-enhanced UCNP@SiO2 nanocomposites for consecutive imaging, photothermal-controlled drug delivery and cancer therapy. Opt Mater Express. 2016;6(4):1161–1171. doi:10.1364/OME.6.001161
- Das GK, Stark DT, Kennedy IM. Potential toxicity of up-converting nanoparticles encapsulated with a bilayer formed by ligand attraction. Langmuir. 2014;30(27):8167–8176. doi:10.1021/la501595f24971524
- Wong PT, Chen D, Tang S, et al. Modular integration of upconverting nanocrystal–dendrimer composites for folate receptor-specific NIR imaging and light-triggered drug release. Small. 2015;11(45):6078–6090. doi:10.1002/smll.20150157526476917
- Wysokińska E, Cichos J, Kowalczyk A, et al. Toxicity mechanism of low doses of NaGdF4: Yb(3+),Er(3+)upconverting nanoparticles in activated macrophage cell lines. Biomolecules. 2019;9(1):14. doi:10.3390/biom9060228
- Guller AE, Generalova AN, Petersen EV, et al. Cytotoxicity and non-specific cellular uptake of bare and surface-modified upconversion nanoparticles in human skin cells. Nano Res. 2015;8(5):1546–1562. doi:10.1007/s12274-014-0641-6
- Wysokińska E, Cichos J, Zioło E, et al. Cytotoxic interactions of bare and coated NaGdF4: Yb3+:Er3+nanoparticles with macrophage and fibroblast cells. Toxicol in Vitro. 2016;32:16–25. doi:10.1016/j.tiv.2015.11.02126639924
- Ramasamy P, Chandra P, Rhee SW, Kim J. Enhanced upconversion luminescence in NaGdF4: Yb,Ernanocrystals by Fe3+ doping and their application in bioimaging. Nanoscale. 2013;5(18):8711–8717. doi:10.1039/c3nr01608k23900204
- Pem B, González-Mancebo D, Moros M, et al. Biocompatibility assessment of up-and down-converting nanoparticles: implications of interferences with in vitro assays. Method Appl Fluoresc. 2018;7(1):014001. doi:10.1088/2050-6120/aae9c8
- González-Béjar M, Francés-Soriano L, Pérez-Prieto J. Upconversion nanoparticles for bioimaging and regenerative medicine. Front Bioeng Biotechnol. 2016;4:47. doi:10.3389/fbioe.2016.0004727379231
- Wang F, Banerjee D, Liu Y, Chen X, Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst. 2010;135(8):1839–1854. doi:10.1039/c0an00144a20485777
- Chatterjee DK, Rufaihah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008;29(7):937–943. doi:10.1016/j.biomaterials.2007.10.05118061257
- Park YI, Lee KT, Suh YD, Hyeon T. Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem Soc Rev. 2015;44(6):1302–1317. doi:10.1039/c4cs00173g25042637
- Lin X, Chen X, Zhang W, et al. Core–shell–shell upconversion nanoparticles with enhanced emission for wireless optogenetic inhibition. Nano Lett. 2018;18(2):948–956. doi:10.1021/acs.nanolett.7b0433929278506
- Tsang M-K, Bai G, Hao J. Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chem Soc Rev. 2015;44(6):1585–1607. doi:10.1039/c4cs00171k25200182
- Xu CT, Zhan Q, Liu H, et al. Upconverting nanoparticles for pre‐clinical diffuse optical imaging, microscopy and sensing: current trends and future challenges. Laser Photon Rev. 2013;7(5):663–697. doi:10.1002/lpor.201200052
- Li X, Yi Z, Xue Z, Zeng S, Liu H. Multifunctional BaYbF5: Gd/Er upconversion nanoparticles for in vivo tri-modal upconversion optical, X-ray computed tomography and magnetic resonance imaging. Mater Sci Eng. 2017;75:510–516. doi:10.1016/j.msec.2017.02.085
- Guan M, Dong H, Ge J, et al. Multifunctional upconversion–nanoparticles–trismethylpyridylporphyrin–fullerene nanocomposite: a near-infrared light-triggered theranostic platform for imaging-guided photodynamic therapy. NPG Asia Mater. 2015;7:e205. doi:10.1038/am.2015.82
- Zhou J, Liu Z, Li F. Upconversion nanophosphors for small-animal imaging. Chem Soc Rev. 2012;41(3):1323–1349. doi:10.1039/c1cs15187h22008740
- Liu Q, Sun Y, Li C, et al. 18F-labeled magnetic-upconversion nanophosphors via rare-earth cation-assisted ligand assembly. ACS Nano. 2011;5(4):3146–3157. doi:10.1021/nn200298y21384900
- Zou W, Visser C, Maduro JA, Pshenichnikov MS, Hummelen JC. Broadband dye-sensitized upconversion of near-infrared light. Nat Photonics. 2012;6:560. doi:10.1038/nphoton.2012.158
- Zijlmans HJMAA, Bonnet J, Burton J, et al. Detection of cell and tissue surface antigens using up-converting phosphors: a new reporter technology. Anal Biochem. 1999;267(1):30–36. doi:10.1006/abio.1998.29659918652
- Wu S, Han G, Milliron DJ, et al. Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals. Proc Natl Acad Sci. 2009;106(27):10917–10921. doi:10.1073/pnas.090479210619541601
- Vaithiyanathan M, R Bajgiran K, Darapaneni P, Safa N, Dorman JA, Melvin AT. Luminescent nanomaterials for droplet tracking in a microfluidic trapping array. Anal Bioanal Chem. 2019;411(1):157–170. doi:10.1007/s00216-018-1448-130483856
- Pominova DV, Ryabova AV, Romanishkin ID, Makarov VI, Grachev PV. Bioimaging with controlled depth using upconversion nanoparticles. Paper presented at: SPIE Photonics Europe2018.
- Raab O. Uber die wirkung Fluorescirender Stoffe auf Infusorien. Z Biol. 1900;39:524–546.
- Von Tappenier H. Therapeutische versuche mit fluoreszierenden stoffen. Muench Med Wochenschr. 1903;47:2042–2044.
- Figge FH, Weiland GS, Manganiello LO. Cancer detection and therapy; affinity of neoplastic, embryonic, and traumatized tissues for porphyrins and metalloporphyrins. Proc Soc Exp Biol Med Soc Exp Bio Med. 1948;68(3):640. doi:10.3181/00379727-68-16580
- Schwartz S, Absolon K, Vermund H. Some relationships of porphyrins, X-rays and tumors. Univ Minn Med Bull. 1955;27:7.
- Kelly J, Snell M. Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol. 1976;115(2):150–151. doi:10.1016/s0022-5347(17)59108-91249866
- Idris NM, Jayakumar MKG, Bansal A, Zhang Y. Upconversion nanoparticles as versatile light nanotransducers for photoactivation applications. Chem Soc Rev. 2015;44(6):1449–1478. doi:10.1039/c4cs00158c24969662
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1(1):1–21. doi:10.1039/b108586g12659143
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2013;20(7):1126–1167. doi:10.1089/ars.2012.514923991888
- Stummer W, Hassan A, Kempski O, Goetz C. Photodynamic therapy within edematous brain tissue: considerations on sensitizer dose and time point of laser irradiation. J Photochem Photobiol B. 1996;36(2):179–181.9002256
- Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115(4):1990–2042. doi:10.1021/cr500419825602130
- Spyratou E, Makropoulou M, Efstathopoulos E, Georgakilas A, Sihver L. Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers. 2017;9(12):173. doi:10.3390/cancers9120173
- Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat Med. 2012;18(10):1580–1585. doi:10.1038/nm.293322983397
- Wang C, Cheng L, Liu Y, et al. Imaging‐guided pH‐sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near‐infrared light. Adv Funct Mater. 2013;23(24):3077–3086. doi:10.1002/adfm.201202992
- Chen Z, Chen H, Hu H, et al. Versatile synthesis strategy for carboxylic acid−functionalized upconverting nanophosphors as biological labels. J Am Chem Soc. 2008;130(10):3023–3029. doi:10.1021/ja076151k18278910
- Zhao H, Hu W, Ma H, et al. Photo-induced charge-variable conjugated polyelectrolyte brushes encapsulating upconversion nanoparticles for promoted siRNA release and collaborative photodynamic therapy under NIR light irradiation. Adv Funct Mater. 2017;27(44):1702592. doi:10.1002/adfm.201702592
- Liu B, Li C, Xing B, Yang P, Lin J. Multifunctional UCNPs@PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy. J Mater Chem B. 2016;4(28):4884–4894. doi:10.1039/C6TB00799F
- Zhou L, Wang R, Yao C, et al. Single-band upconversion nanoprobes for multiplexed simultaneous in situ molecular mapping of cancer biomarkers. Nat Commun. 2015;6:6938. doi:10.1038/ncomms793825907226
- Min Y, Li J, Liu F, Yeow EKL, Xing B. Near‐infrared light‐mediated photoactivation of a platinum antitumor prodrug and simultaneous cellular apoptosis imaging by upconversion‐luminescent nanoparticles. Angew Chem Int Ed. 2014;53(4):1012–1016. doi:10.1002/anie.201308834
- Yang Y, Liu F, Liu X, Xing B. NIR light controlled photorelease of siRNA and its targeted intracellular delivery based on upconversion nanoparticles. Nanoscale. 2013;5(1):231–238. doi:10.1039/c2nr32835f23154830
- Wang M, Mi -C-C, Wang W-X, et al. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4: Yb,ErUpconversion nanoparticles. ACS Nano. 2009;3(6):1580–1586. doi:10.1021/nn900491j19476317
- Xiong L, Chen Z, Tian Q, Cao T, Xu C, Li F. High contrast upconversion luminescence targeted imaging in vivo using peptide-labeled nanophosphors. Anal Chem. 2009;81(21):8687–8694. doi:10.1021/ac901960d19817386
- Wang C, Cheng L, Liu Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials. 2011;32(4):1110–1120. doi:10.1016/j.biomaterials.2010.09.06920965564
- Cardoso MM, Peca IN, Roque ACA. Antibody-conjugated nanoparticles for therapeutic applications. Curr Med Chem. 2012;19(19):3103–3127.22612698
- Feng W, Zhu X, Li F. Recent advances in the optimization and functionalization of upconversion nanomaterials for in vivo bioapplications. NPG Asia Mater. 2013;5:e75. doi:10.1038/am.2013.63
- Tsoi KM, MacParland SA, Ma X-Z, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212. doi:10.1038/nmat471827525571
- Liu J, Yu M, Zhou C, Zheng J. Renal clearable inorganic nanoparticles: a new frontier of bionanotechnology. Mater Today. 2013;16(12):477–486. doi:10.1016/j.mattod.2013.11.003
- Soo Choi H, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165. doi:10.1038/nbt127617891134
- Liu C, Gao Z, Zeng J, et al. Magnetic/upconversion fluorescent NaGdF4: Yb,ErNanoparticle-based dual-modal molecular probes for imaging tiny tumors in vivo. ACS Nano. 2013;7(8):7227–7240. doi:10.1021/nn403089823879437
- Ai X, Ho CJH, Aw J, et al. In vivo covalent cross-linking of photon-converted rare-earth nanostructures for tumour localization and theranostics. Nat Commun. 2016;7:10432. doi:10.1038/ncomms1043226786559
- Dai Y, Xiao H, Liu J, et al. In vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans-platinum pro-drug-conjugated upconverison nanoparticles. J Am Chem Soc. 2013;135(50):18920–18929. doi:10.1021/ja410028q24279316
- Dai Y, Yang D, Yu D, et al. Mussel-inspired polydopamine-coated lanthanide nanoparticles for NIR-II/CT dual imaging and photothermal therapy. ACS Appl Mater Interfaces. 2017;9(32):26674–26683. doi:10.1021/acsami.7b0610928726368
- Chen S, Weitemier AZ, Zeng X, et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science. 2018;359(6376):679. doi:10.1126/science.aaq114429439241
- Hososhima S, Yuasa H, Ishizuka T, et al. Near-infrared (NIR) up-conversion optogenetics. Sci Rep. 2015;5:16533. doi:10.1038/srep1653326552717
- Shah S, Liu J-J, Pasquale N, et al. Hybrid upconversion nanomaterials for optogenetic neuronal control. Nanoscale. 2015;7(40):16571–16577. doi:10.1039/c5nr03411f26415758
- Wu X, Zhang Y, Takle K, et al. Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS Nano. 2016;10(1):1060–1066. doi:10.1021/acsnano.5b0638326736013
- Akshaya B, Haichun L, Gnanasammandhan JMK, Stefan AE, Yong Z. Quasi‐continuous wave near‐infrared excitation of upconversion nanoparticles for optogenetic manipulation of C. elegans. Small. 2016;12(13):1732–1743. doi:10.1002/smll.20150379226849846
- Xiangzhao A, Linna L, Yang Z, et al. Remote regulation of membrane channel activity by site‐specific localization of lanthanide‐doped upconversion nanocrystals. Angew Chem Int Ed. 2017;56(11):3031–3035. doi:10.1002/anie.201612142