Abstract
The biodynamics of ultrasmall and small superparamagnetic iron oxide (USPIO and SPIO, respectively) particles that were injected intraperitoneally into 36 C57BL/6 mice were investigated chronologically. Their distribution was studied histologically at six time points by measuring iron-positive areas (μm2) in organ sections stained with Prussian blue. The uptake of the differently sized particles was also compared by cultured murine macrophages (J774.1). Iron-positive areas in the liver were significantly larger in the mice injected with USPIO than those injected with SPIO at the first three time points (P < 0.05). The amount of USPIO in the lung parenchyma around the airway was larger than that of SPIO at four time points (P < 0.05); distribution to the lymph nodes was not significantly different. The amount of iron was significantly larger in SPIO- than USPIO-treated cultured cells (P < 0.05). In conclusion, it is suggested that intra peritoneally injected USPIO particles could be used more quickly than SPIO to make Kupffer images of the liver and that both agents could help get lymph node images of similar quality.
Introduction
The uptake of superparamagnetic iron oxide (SPIO) particles by cells of the mononuclear phagocytic system (MPS) results in hepatic, splenic, bone marrow, and nodal iron accumulation.Citation1,Citation2 The selective accumulation of these particles by the MPS is exploited at organ-specific magnetic resonance imaging (MRI).Citation3–Citation10 The biodynamics of iron oxide nanoparticles vary depending on particle diameter. Unlike SPIO preparations comprised of large particles and magnetite microspheres (mean particle diameter, 72 nm and 1–5 μm, respectively), ultrasmall superparamagnetic iron oxide (USPIO) particles (mean diameter, 18 nm) are not immediately recognized by the hepatic and splenic MPS.Citation11,Citation12 The resultant prolongation of its intravascular half-life, together with its inherent T1 shortening properties, render USPIO a useful magnetic resonance angiography blood-pool agent.Citation13,Citation14 In contrast to large-particle superparamagnetic agents, the size of USPIO particles allows the extravasation of USPIO through capillary pores, the diameters of which range from 5 to 100 nm, and this capillary permeability facilitates the uptake of USPIO in MPS cells throughout the body.Citation15
Although information on the biodynamics of iron oxide nanoparticles of different diameters is important for various organ-specific MRI studies, to date this issue has not been addressed histologically. Therefore, a comparative histological study of the sequential biodynamics of USPIO and SPIO was performed.
Material and methods
Shiga University of Medical Science’s animal experimentation committee approved all experimental protocols and all experiments were conducted in accordance with the animal care guidelines of the university.
Iron oxide nanoparticles
Two kinds of iron oxide were studied: USPIO (Meito Sangyo Co, Ltd, Kiyosu, Aichi, Japan) and SPIO (Resovist®; I’rom Pharmaceutical Co, Ltd, Shinagawa-ku, Tokyo, Japan). The mean particle diameters and iron concentrations of USPIO and SPIO were 30 nm and 56 mg/mL and 57 nm and 28 mg/mL, respectively. Average diameter of the magnetic core was the same (5 nm) in both particle types. The iron oxide core of both particle types was coated with dextran; their magnetic susceptibility was almost the same (about 0.027 cgs).
In vivo study
USPIO or SPIO was injected intraperitoneally (500 μmol Fe/kg) into 8-week-old female C57BL/6 mice (Japan SLC, Inc, Tokyo, Japan), each weighing approximately 30 g. Groups of three mice each were treated with USPIO or SPIO and sacrificed at time points of 30 minutes and 1, 3, 12, 24, and 48 hours after-ward; two mice were the controls. Organs to be studied were fixed in 10% paraformaldehyde and examined histologically.
Histological examination
The mice were sacrificed at the indicated time points and their organs were removed for histological study. Sections of the right lobe of the liver were cut in the axial plane; splenic sections were also cut in the axial plane and included the hilum. Lung sections were cut in the coronal plane and included the hilum. Sections from the heart, great vessels, gastrointestinal tract, and kidney were cut in the coronal plane. Sections (4 μm in thickness) of paraffin-embedded organs were stained with Prussian blue to identify the accumulation of iron oxide. Macrophages were immunohistochemically stained with F4/80. Areas stained with Prussian blue and F4/80 were compared. For quantitative assessment, Prussian-blue-stained areas (μm2) in a single field of view (magnification ×200) of three different lesions for each histological section were measured using Image-Pro® Plus software (Media Cybernetics, Bethesda, MD) and average values were calculated.
In vitro study
To compare phagocytosis of USPIO and SPIO by cultured cells murine macrophage cell line J774.1 (RIKEN Cell Bank, Wako, Saitama, Japan) was used, which is widely used in research on macrophages.Citation16–Citation18 Cells (5 × 104) were seeded in 12-well multiple well culture plates (Costar®; Corning Inc, Corning, NY) and grown at 37°C for 24 hours in 1 mL of growth medium (RPMI-1640; Nacalai Tesque, Inc, Kyoto, Japan) supplemented with 10% (v/v) fetal bovine serum (FBS) (Invitrogen Ltd, Carlsbad, CA) and 1% (w/v) penicillin and streptomycin solution (Nacalai Tesque, Inc). After 24 hours, the medium was replaced, USPIO or SPIO was added (10 μg : 100 μL) and the plates were incubated for 1 hour for cell labeling. After this, the medium was again replaced and the wells then incubated for 24 hours. To identify intracellular iron oxide accumulation, the cells were stained with Prussian blue. The amount of iron contained in cell lysates by atomic absorption photometry (AA-6800; Shimadzu Co, Kyoto, Japan) was measured. Cell labeling was as described in a previous study.Citation19
Statistical analysis
Statistical analysis was carried out using Dr SPSS II for Windows (SPSS Japan Inc, Tokyo, Japan). Differences in the uptake of USPIO and SPIO in murine tissues and the uptake of iron by cultured cells were determined with the one-tailed Student’s t-test. A P-value < 0.05 was considered statistically significant.
Results
Quantitative histological analysis
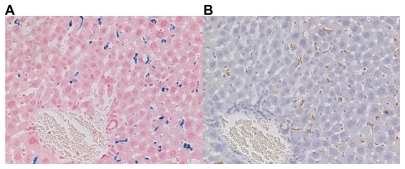
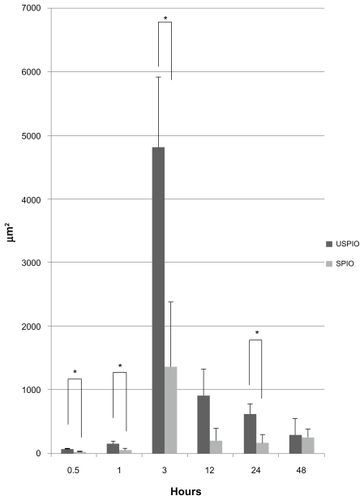
Quantitative analysis based on the measurement of Prussian-blue- stained areas revealed that iron-positive areas in the liver were larger in the mice injected with USPIO than those injected with SPIO at almost all time points except the last one (828 vs 0 μm2 at 30 minutes, 1299 vs 297 μm2 at 1 hour, 3647 vs 853 μm2 at 3 hours, 2256 vs 1497 μm2 at 12 hours, 1885 vs 1168 μm2 at 24 hours, and 1948 vs 2483 μm2 at 48 hours), as shown in and . The difference was significant at 30 minutes, 1, and 3 hours (P < 0.05). USPIO was quickly distributed throughout the liver; its distribution increased until 3 hours postinjection and decreased thereafter. On the other hand, the distribution of SPIO occurred more slowly and increased over a longer period of time. Unlike USPIO, SPIO was not detected in the liver at 30 minutes. Iron-positive areas at the hepatic sinusoid corresponding to the area harboring Kupffer cells coincided with macrophage-positive F4/80-stained areas, as shown in .
Figure 1 Histological study of mouse liver specimens stained with Prussian blue (magnification ×200). (A), (B), (C): 30 minutes, 1, and 3 hours, respectively, after intraperitoneal (IP) injection of ultrasmall superparamagnetic iron oxide. (D), (E), (F): 30 minutes, 1, and 3 hours, respectively, after IP injection of small superparamagnetic iron oxide.
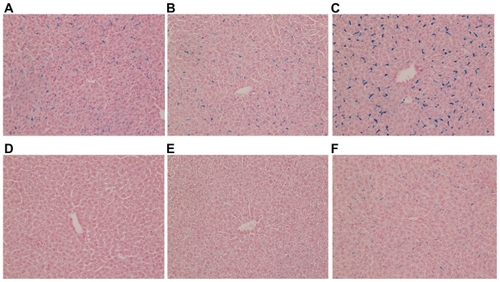
Figure 2 Comparison of Prussian-blue-stained areas (μm2) in the liver. At 30 minutes, 1, and 3 hours after intraperitoneal injection, iron-positive areas were significantly larger in mice treated with ultrasmall superparamagnetic iron oxide (USPIO) than with small superparamagnetic iron oxide (SPIO).
Note: *P < 0.05.
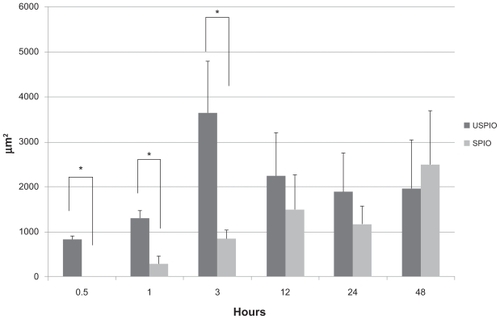
Figure 3 Histological study of the liver of mice sacrificed 48 hours after the intraperitoneal administration of ultrasmall superparamagnetic iron oxide. (A) Prussian-blue and (B) F4/80 stain (magnification ×200). Iron-positive areas were located at the hepatic sinusoid corresponding to the area harboring Kupffer cells and were consistent with areas positive for immunohistochemical staining.
In lung specimens, a few USPIO and SPIO particles were observed early; they were located in the parenchyma around the airway and their number was increased dramatically at 3 hours postinjection and decreased thereafter, as shown in . At all time points, iron positive areas in the lung parenchyma were larger in the mice injected with USPIO than those injected with SPIO (65 vs 29 at 30 minutes, 149 vs 52 at 1 hour, 4812 vs 1364 at 3 hours, 906 vs 201 at 12 hours, 616 vs 167 at 24 hours, and 288 vs 249 at 48 hours), as shown in . In addition, at 30 minutes, 1, 3, and 24 hours the iron-positive areas were significantly larger in mice injected with USPIO (P < 0.05).
Figure 4 Prussian-blue staining of lung parenchyma around the airway (magnification ×200). (A), (B), (C): 30 minutes, 3, and 48 hours, respectively, after intraperitoneal (IP) injection of ultrasmall superparamagnetic iron oxide. (D), (E), (F): 30 minutes, 3, and 48 hours, respectively, after IP injection of small superparamagnetic iron oxide.
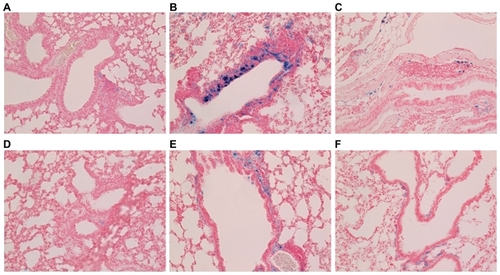
Figure 5 Comparison of Prussian-blue-stained areas (μm2) in the lung parenchyma. The iron-positive areas were significantly larger in mice intraperitoneally injected with ultrasmall superparamagnetic iron oxide (USPIO) than with small superparamagnetic iron oxide (SPIO) at 30 minutes, 1, 3, and 24 hours.
Note: *P < 0.05.
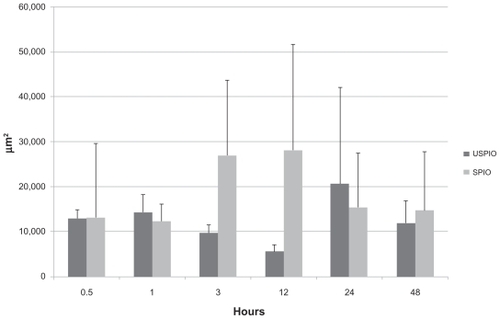
Both USPIO and SPIO were abundant at all time points in mediastinal lymph nodes; there was no difference between the two groups of mice (P > 0.05), as shown in and . As in liver specimens, iron-positive areas coincided with macrophages in lung and lymph node samples.
Figure 6 Prussian-blue staining of mediastinal lymph nodes (magnification ×100). (A), (B): 30 minutes and 1 hour after intraperitoneal (IP) injection of ultrasmall superparamagnetic iron oxide. (C), (D): 30 minutes and 1 hour after IP injection of small superparamagnetic iron oxide.
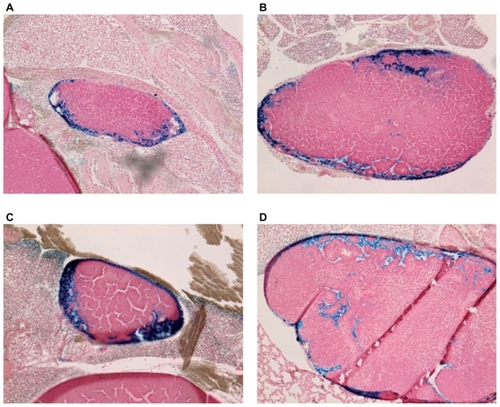
Figure 7 Comparison of distribution of ultrasmall superparamagnetic iron oxide (USPIO) and small superparamagnetic iron oxide (SPIO) in the lymph nodes.
Note: P > 0.05 at all time points.
There was no significant accumulation in the heart, great vessels, kidneys, or gastrointestinal tract. As the controls harbored abundant stores of iron, visualized as Prussian-blue- positive areas, macroscopically it was observed that there was almost no difference between them and USPIO- or SPIO-treated mice with respect to the spleen. Control mice manifested no significant iron deposits in organs other than the spleen.
In vitro study
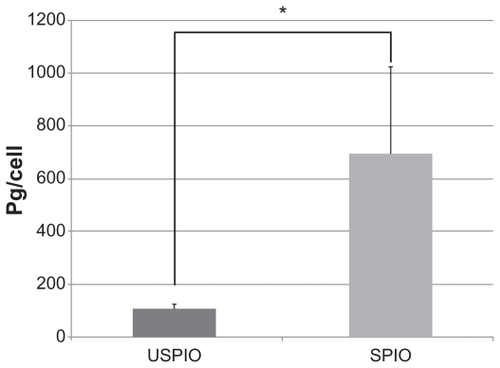
While almost all cultured J774.1 cells phagocytized USPIO and SPIO, the amount of intracellular iron measured by atomic absorption photometry was significantly higher in cells treated with SPIO than with USPIO (695 vs 108 pg/cell, P < 0.05), as shown in .
Discussion
To our knowledge, this is the first study comparing the biodynamics of intraperitoneally injected USPIO and SPIO. SPIO (Resovist) is the contrast agent used in MRI and generally its safety in the clinical setting has already been established. USPIO used in this study is different in particle size from that of SPIO, but very similar in composition. Ferumoxtran-10, similar to the USPIO used in this study, was well tolerated in phase I and II studies in adult humans.Citation20,Citation21 In addition, in a safety study conducted in adult rabbits, no inflammation or fibrosis occurred after intraperitoneal (IP) injection of NC100150, a USPIO similar to ferumoxtran-10.Citation22 IP iron-dextran supplementation is known to be effective and safe in correcting iron deficiency in rats and humans.Citation23,Citation24 In our study, IP injection was safe and never caused the death of any mouse.
USPIO was distributed to the liver as early as 30 minutes postinjection; it peaked at 3 hours and decreased thereafter. SPIO appeared later and its distribution throughout the liver increased over time. At 12 hours postinjection, the hepatic distribution of both agents was comparable. A greater amount of USPIO than SPIO tended to be distributed to the lung parenchyma around the airway, possibly because USPIO was presented more easily to the small lymphatic vessels around the airway. There was no significant difference in lymph node accumulation between the two groups of mice.
The early distribution of intraperitoneally injected USPIO to the liver and the absence of significant differences in the lymph node distribution of USPIO and SPIO are primarily attributable to differences in the absorption route. The trapping of intravenously administered USPIO by reticuloendothelial cells in the liver and spleen has been reported to be difficult because the particle diameter is small and the particles persist in the blood.Citation11,Citation12 Lukas et alCitation25 who studied the absorption route of intraperitoneally administered compounds, suggested that particles absorbed by the subperitoneal capillary system reached the liver via the hepatic portal system. The earlier hepatic distribution of USPIO suggests that it is delivered via this pathway. Intercellular spaces directly open to lymphatic vessels are present in the mesothelial layer of the peritoneum. The peritoneal cavity is the origin of two major lymphatic networks: the thoracic duct drains the retroperitoneal region and enters the left subclavian vein; the right lymphatic duct collects lymph from parasternal and intercostal-paravertebral lymph ducts and drains mainly through the diaphragm and, ultimately, into the right subclavian vein. In the former network, lymph passes through iliac, renal, splenic, posterior gastric, portal, superior mesenteric, and cisternal lymph nodes; in the latter network it passes through more distal (eg, parathymic) and left and right posterior mediastinal lymph nodes.Citation26,Citation27 The authors posited that there was not only USPIO present in abundant amounts in the lymphatic tracts due to these networks but also SPIO, which tended to be trapped in the liver with only slight distribution to the lymph nodes.
Cultured macrophages absorbed SPIO more readily than USPIO. Under conditions where the same number of macrophages respond to iron oxide compounds containing the same amount of iron, larger particles are recognized more easily. This is consistent with the hypothesis that upon intravenous (IV) infusion, reticuloendothelial cells in the liver and spleen trap SPIO more readily than USPIO.
This study had some limitations. First, the number of mice was too small for an accurate elucidation of the pharmacokinetics and prevented the exclusion of interindividual differences. Second, it could not be confirmed that the minute amounts of USPIO and SPIO used were injected accurately into the peritoneal cavity. In some instances, the agent might have been injected subcutaneously or into the bowel. Third, due to technical challenges, the pharmacokinetics of intravenously delivered USPIO or SPIO was not studied. However, considering the mechanisms by which USPIO reached the liver earlier than SPIO and the result that similar amounts of USPIO and SPIO were distributed throughout the lymph nodes, it is important to compare the differences of distribution between USPIO and SPIO via two different injection routes (IP and IV).
The findings suggest that the IP delivery of both USPIO and SPIO might be useful as a contrast agent for the study of lymph nodes in a clinical setting. The IP delivery of USPIO might also be used as a contrast agent for studying the liver. As IP delivery is not a popular injection route in clinical settings, drugs or contrast agents are currently intraperitoneally injected in limited cases of patients with cancer, peritonitis, or chronic renal failure requiring continuous ambulatory peritoneal dialysis (CAPD).Citation24,Citation28–Citation30 Although clinical application is limited, the authors suggest, for cases of patients with ascites of unknown origin, that intraperitoneally injected iron particles may be very useful for getting Kupffer images of the liver and lymph node images to help diagnose metastases in these areas. Also, in cases of iron-deficient CAPD patients, intraperitoneally injected iron dextran has potential for filling both roles, as a route of entry in iron therapy and as a contrast agent for the liver and lymph nodes. In addition, depending on the diameter of the administered particles, it may be possible to deliver agents other than USPIO and SPIO intraperitoneally for the study of various organs.
Conclusion
This study suggests that intraperitoneally injected USPIO could be used more quickly than SPIO can to make Kupffer images of the liver and that both agents could help get lymph node images of similar quality.
Disclosure
The authors report no conflicts of interest in this work.
References
- SainiSStarkDDHahnPFWittenbergJFerrite particles: a superparamagnetic MR contrast agent for the reticuloendothelial systemRadiology19871621 Pt 12112163786765
- WeisslederRStarkDDEngelstadBLSuperparamagnetic iron oxide: pharmacokinetics and toxicityAJR Am J Roentgenol198915211671732783272
- WeisslederRElizondoGWittenbergJUltrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imagingRadiology199017524944982326475
- WillOPurkayasthaSChanCDiagnostic precision of nanoparticle- enhanced MRI for lymph-node metastases: a meta- analysisLancet Oncol200671526016389184
- SigalRVoglTCasselmanJLymph node metastases from head and neck squamous cell carcinoma: MR imaging with ultrasmall superparamagnetic iron oxide particles (Sinerem MR): results of a phase-III multicenter clinical trialEur Radiol20021251104111311976854
- DaldrupHELinkTMBlasiusSMonitoring radiation-induced changes in bone marrow histopathology with ultra-small superparamagnetic iron oxide (USPIO)-enhanced MRIJ Magn Reson Imaging19999564365210331759
- SenéterreEWeisslederRJaramilloDBone marrow: ultrasmall superparamagnetic iron oxide for MR imagingRadiology199117925295332014305
- HarisinghaniMGSainiSWeisslederRSplenic imaging with ultrasmall superparamagnetic iron oxide ferumoxtran-10 (AMI-7227): preliminary observationsJ Comput Assist Tomogr200125577077611584239
- WeisslederRHahnPFStarkDDSuperparamagnetic iron oxide: enhanced detection of focal splenic tumors with MR imagingRadiology198816923994033174987
- StarkDDWeisslederRElizondoGSuperparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liverRadiology198816822973013393649
- WeisslederRElizondoGWittenbergJUltrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imagingRadiology199017524894932326474
- VassalloPMateiCHestonWDAMI-227-enhanced MR lymphography: usefulness for differentiating reactive from tumor-bearing lymph nodesRadiology11199419325015067972768
- AnzaiYPrinceMRChenevertTLMR angiography with an ultrasmall superparamagnetic iron oxide blood pool agentJ Magn Reson Imaging1997712092149039617
- LoubeyrePZhaoSCanetEUltrasmall superparamagnetic iron oxide particles (AMI 227) as a blood pool contrast agent for MR angiography: experimental study in rabbitsJ Magn Reson Imaging1997769589629400837
- RenkinEMMultiple pathways of capillary permeabilityCirc Res1977416735743923024
- BanerjeeSNarayananKMizutaniTMurine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cellsJ Virol200276125937594812021326
- KhouryMEscriouVCourtiesGEfficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexesArthritis Rheum20085882356236718668557
- TaniguchiSYanaseTKobayashiKDehydroepiandrosterone markedly inhibits the accumulation of cholesteryl ester in mouse macrophage J774-771 cellsAtherosclerosis199612611431548879442
- JoJAokiITabataYDesign of iron oxide nanoparticles with different sizes and surface charges for simple and efficient labeling of mesenchymal stem cellsJ Control Release2010142346547319932720
- SharmaRSainiSRosPRSafety profile of ultrasmall superparamagnetic iron oxide ferumoxtran-10: phase II clinical trial dataJ Magn Reson Imaging19999229129410077027
- McLachlanSJMorrisMRLucasMAPhase I clinical evaluation of a new iron oxide MR contrast agentJ Magn Reson Imaging1994433013078061425
- HilfikerPRDebatinJFTarloKAssessment of peritoneal tolerance of a new MR blood pool contrast agent in rabbitsInvest Radiol1999341172272710548385
- ReddyDKMooreHLLeeJHChronic peritoneal dialysis in iron-deficient rats with solutions containing iron dextranKidney Int200159276477311168960
- BastaniBGalleySIntraperitoneal iron-dextran as a potential route of iron therapy in CAPD patientsPerit Dial Int19961666466488981540
- LukasGBrindleSDGreengardPThe route of absorption of intraperitoneally administered compoundsJ Pharmacol Exp Ther197117835625645571904
- RusznyákLFöldiMSzaboGFiltration and absorption through serous membranesLymphatics and Lymph Circulation2nd edOxfordPergamon Press1967478497
- TilneyNLPatterns of lymphatic drainage in the adult laboratory ratJ Anat1971109Pt 33693835153800
- ZylberbergBDormontDAntoineJMFirst line immunochemotherapy with cisplatin-based protocol by intraperitoneal and intravenous routes in ovarian cancer: technique and results of 82 casesEur J Obstet Gynecol Reprod Biol199666157648735760
- RufPKlugeMJägerMPharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patientsBr J Clin Pharmacol201069661762520565453
- TamanoMHashimotoTKojimaKDiagnosis of hepatic hydrothorax using contrast-enhanced ultrasonography with intraperitoneal injection of SonazoidJ Gastroenterol Hepatol201025238338619817961