Abstract
Aims
Different kinds of vitamins can be used as promising candidates to mitigate the structural changes of proteins and associated cytotoxicity stimulated by NPs. Therefore, the structural changes of α-syn molecules and their associated cytotoxicity in the presence of SWCNTs either alone or co-incubated with vitamin K1 were studied by spectroscopic, bioinformatical, and cellular assays.
Methods
Intrinsic and ThT fluorescence, CD, and Congo red absorption spectroscopic approaches as well as TEM investigation, molecular docking, and molecular dynamics were used to explore the protective effect of vitamin K1 on the structural changes of α-syn induced by SWCNTs. The cytotoxicity of α-syn/SWCNTs co-incubated with vitamin K1 against SH-SY5Y cells was also carried out by MTT, LDH, and caspase-3 assays.
Results
Fluorescence spectroscopy showed that vitamin K1 has a significant effect in reducing SWCNT-induced fluorescence quenching and aggregation of α- syn. CD, Congo red adsorption, and TEM investigations determined that co-incubation of α- syn with vitamin K1 inhibited the propensity of α-syn into the structural changes and amorphous aggregation in the presence of SWCNT. Docking studies determined the occupation of preferred docked site of SWCNT by vitamin K1 on α- syn conformation. A molecular dynamics study also showed that vitamin K1 reduced the structural changes of α- syn induced by SWCNT. Cellular data exhibited that the cytotoxicity of α- syn co-incubated with vitamin K1 in the presence of SWCNTs is less than the outcomes obtained in the absence of the vitamin K1.
Conclusion
It may be concluded that vitamin K1 decreases the propensity of α- syn aggregation in the presence of SWCNTs and induction of cytotoxicity.
Introduction
Due to the unique and extraordinary mechanical, electronic, optical, geometric, and biological properties of CNTs,Citation1 they have been widely used in biological activities such as drug delivery, tissue engineering, cancer treatment, and gene therapy,Citation2 recently. In general, CNTs are one-dimensional molecules with one or more walls of graphene that in the form of SWCNTs with a diameter of 0.4 to 3.5 nm can cross the lipid membrane.Citation3 Despite considerable attention in both fields of pharmacologicalCitation4 and toxicologicalCitation5,Citation6 profiles, it is still vital to study the critical effects of CNTs on bimolecular structures and cell integrity. Several reports have shown that CNT causes chronic brain inflammation, multiple sclerosis, an increase of autism risk, a decrease of the IQ in children, neurodegenerative diseases, and microglia activation.Citation7–Citation10 Despite the mentioned reports, dynamic and atomic interactions between SWCNTs and biomolecules are still unknown.Citation10 Because it has been reported that SWCNTs alter the secondary structure of specific proteins in various formsCitation11,Citation12 and accelerate their aggregation based on the conditions of each assay.Citation13 The fibrillation of some proteins including Aβ, α-syn, and tau proteins is linked to a group of neurological diseases such as Alzheimer’s and Parkinson’s,Citation1,Citation14 which have been affected by CNTs.Citation13 Generally, CNTs specifically SWCNTs affect protein aggregation by stimulating nucleation and polymerization growth.Citation15 However, surface changes such as functionalization of SWCNTs can reduce their cytotoxicity.Citation16 In this regard, Liu et al.Citation16 revealed that the use of hydroxylating agents on SWCNTs could significantly reduce the amyloid and amorphous protein aggregation, which are very suitable for Alzheimer’s treatment.
α-Syn is a soluble protein with 140 amino acids, which are found mainly in the nervous system and red blood cells.Citation17 Despite the initial recognition of α-syn in the presynaptic nerve terminal, its natural role remains unknown.Citation18 The most common functions for α-syn are the regulation of soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor, production of dopamine and control of synaptic vesicle recycling.Citation19 The fact is that increase in α-syn aggregation, which can be due to gene amplification, nucleotide polymorphism, and ageing,Citation20 result in neuropathology such as Parkinson’s and Alzheimer’s diseases.Citation21 In this field, Alarcón-Arís et alCitation22 exhibited that reducing the level of α-syn and eliminating their aggregation increases the potential treatment of Parkinson’s disease. However, due to the effect of soluble α-syn on the function and development of neurons, it is not possible to ultimately decrease the level of these molecules.Citation23 Nevertheless, many compounds have been reported to reduce α-syn protein aggregation, including rifampicin,Citation24 dopamine analog compounds,Citation25 quercetin,Citation26 vitamin K,Citation27 gallic acid,Citation28 cathepsin D,Citation29 and curcumin compounds.Citation30
Different types of vitamins, specifically fat-soluble vitamin (A, E, D, and K), have shown to reduce the aggregation of the protein.Citation31 In this line, experiments have determined that vitamin K (1,4-naphthoquinones) has a wide range of activities on neurological disorders such as Hsp90 inhibition,Citation32 inhibition of monoamine oxidase activity,Citation33 and inhibition of protein aggregation.Citation31 In this regard, da Silva et alCitation27 reported an inhibitory effect of vitamin K against α-syn fibrillation. Hence, in this paper, we examined the effect of SWCNTs on structural changes and subsequent aggregation of α-syn either alone or co-incubated with vitamin K1. Also, the cytotoxicity of α-syn/SWCNT complex co-incubated with or without vitamin K1 against neuron-like cells (SH-SY5Y) was explored.
Materials And Methods
Materials
α-Syn, ThT, Congo red, vitamin K1, DMEM: F12, FBS, L-glutamine, penicillin, streptomycin, NGF, and MTT were purchased from Sigma (St. Louis, MO, USA). LDH Assay Kit (ab102526) and Annexin V-FITC Apoptosis Staining Kit (ab14085) were purchased from Abcam (Cambridge, CB4 0FL, UK). Caspase-3 activity kit (E13183) was obtained from Thermo Fisher Scientific, Massachusetts, USA.
Sample Preparation
A stock solution of α-syn was prepared in 20 mM phosphate buffer pH 7.4, and protein concentration with an extinction coefficient of 5120 M−1cm−1 was calculated by a UV-visible (vis) spectrophotometer (Varian, Carry 100 Bio, Australia) at 280 nm. SWCNT was dissolved in DMSO (0.5%, v/v) and sonicated for 20 min using a sonicator probe (Misonix- S3000, USA). Vitamin K1 was also dissolved in DMSO (0.5%, v/v). In the present study, α-syn (50 µM) either alone or with SWCNT (10 µg/mL) was co-incubated with a similar molar ratio of vitamin K1 for 24 hrs. In the future studies, the concentration-dependent inhibition spectra of vitamin K1 against α-syn structural changes induced by nanomaterial can be investigated by spectroscopic assays.
Intrinsic Fluorescence Assay
Intrinsic fluorescence assay was performed with a fluorescence spectrophotometer (Varian, Cary eclipse, Australia). Aliquots of α-syn samples either alone or with vitamin K1 incubated with the single dose of SWCNT for 24 hrs were then removed and diluted ten-fold with 20 mM phosphate buffer pH 7.4 at final concentrations of 5 µM. The excitation was fixed at 270 nm (slit width: 10 nm), and intensity was recorded from 290 to 370 nm (slit width: 10 nm). The resulting spectra were corrected against buffer blank, SWCNT solution, vitamin solution, and inner filter effects.
ThT Fluorescence Assay
Protein samples (5 µM) mixed with ThT to achieve the final ThT concentration of 10 μM. Samples were then incubated in the dark for 15 min. The excitation was fixed at 440 nm (slit width: 10 nm), and intensity was recorded from 450 to 550 nm (slit width: 10 nm). The resulting spectra were corrected against the blanks.
Congo Red Binding Assay
The protein and Congo red concentrations were mixed at 5 and 20 μM, respectively, and incubated in the dark for 30 mins. The absorbance spectra (400–650 nm) were then recorded with a UV-vis spectrophotometer (Varian, Carry 100 Bio, Australia).
Far-UV CD Assay
The CD signals of α-syn with or without SWCNT either alone or co-incubated with vitamin K1 were reordered using an AVIV 215 spectropolarimeter (Aviv, 215, Lakewood, NJ, USA). Signals as ellipticity changes [θ] were scanned in the range of 190–250 nm and their blanks (SWCNT and vitamin K1) were subtracted from the protein spectrum.
TEM
TEM images were taken on a Zeiss microscope (EM10C, 100 kV, Germany). The aggregation formation was determined by applying 10 μL of α-syn (50 μM) in the absence and presence of vitamin K1 with SWCNT on a 200-mesh copper grid. Girds were then dried at room temperature for 30 mins.
Simulation Methods
A (6, 6) armchair CNT model of lengths of about 7 nm was constructed and HEX 6.3 software was used to perform docking.Citation34 The structure of the human α-syn protein (PDB ID: 1XQ8) was downloaded from Brookhaven Protein Data Bank. The structure of vitamin K1 was obtained from Avogadro software (Libavogadro Library, Pittsburgh, PA, USA).Citation35 Water molecules and ions were replaced with hydrogen atoms. The protein was fixed to be rigid, and the effect of solvent molecules on docking was ignored. The molecular dynamics simulations were done using the Forcite code and the Dreiding force field.Citation36 The smaller CNT model and α-syn in the absence and presence of vitamin K1 were surrounded by 1000 water molecules, and the geometries were optimized. A time step of 1 fs and a total simulation time of 500 ps were used in the simulation.
Cell Culture
SH-SY5Y cells obtained from Royan institute (Tehran, Iran) were cultured in DMEM: F12 medium in a humidified atmosphere with 5% (v/v) CO2/air at 37°C containing 10% (v/v) FBS and 100 U/mL penicillin and streptomycin.
Cell Viability Assay
MTT assay was explored to assay the cell viability in the presence of different species of α-syn molecules. For cellular assays, the aliquots of α-syn either alone or co-incubated with vitamin K1 in the presence or absence of SWCNT for 24 hrs were then removed and added to the cell culture medium with a final concentration of 5µM in the 96-well plates. Briefly, cells were seeded at 10Citation4 cells/well with the differentiation medium, treated with an aliquot of α-syn/SWCNT complex with or without vitamin K1 for 24 hrs, followed by addition of MTT (concentration of 0.5 mg/mL), incubated for 4 hrs at 37°C, and followed by removal of supernatant and addition of DMSO (200 µL). Afterward, the absorbance was read at 570 nm using an ELISA reader (Expert 96, Asys Hitch, Ec Austria).
LDH Release Assay
LDH release assay was performed using the LDH Cytotoxicity Detection kit (ab102526, UK). After treatment, 50 μL of supernatant was added to the cell culture medium and mixed with 50 μL reaction mixture (30 mins) followed by the addition of 50 μL of stop solution. The optical density was read at 470 nm using an ELISA reader (Expert 96, Asys Hitch, Ec Austria).
Caspase-3 Activity Assay
Caspase-3 activity was carried out by employing the caspase-3 activity kit (E13183, Thermo Fisher Scientific). Briefly, cells after treatment were homogenized in reaction buffer followed by determination of protein concentration by Bradford assay. Then, 2mM caspase-3 substrate was added and incubated for 2 hrs at 37°C. The absorbance was finally read at 405 nm using an ELISA reader (Expert 96, Asys Hitch, Ec Austria).
Statistical Analysis
The statistical analysis was done by performing one-way ANOVA for three independent experiments. The significance of outcomes was explored as P≤ 0.05.
Results
CNT Characterization
SWCNT characterization was fully carried out in our previous study.Citation14 Briefly, it was shown that SWCNT shows microns long with uniform outer diameters of around 1–2 nm.Citation14 Also, the hydrodynamic radius of SWCNT was around 40–60 nm.Citation14
Intrinsic Fluorescence Study
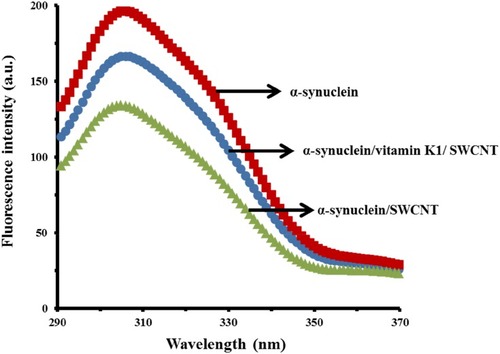
To explore the structural changes of α-syn upon interaction with SWCNT, tyrosine fluorescence emission spectrum was acquired for the α-syn in the presence of a single dose of SWCNT after 24 hrs incubation. It was detected that SWCNT could interact with α-syn monomers, eventually causing a quenching impact on the intrinsic fluorescence of α-syn. However, when α-syn co-incubated with vitamin K1 for 24 hrs, the quenching effect of SWCNT on the intrinsic fluorescence of protein was reduced. Although SWCNTs have induced some pronounces structural change in α-syn, these effects were decreased in the presence of vitamin K1 (). The higher quenching effect of SWCNT in the absence of vitamin K1 may indicate the better adsorption of α-syn on the surface of SWCNT. It could be suggested that vitamin K1 binds α-syn and mask the binding site of SWCNT on the protein surface, which may explain the inhibiting effect of vitamin K1 on α-syn structural changes induced by SWCNT.
ThT Study
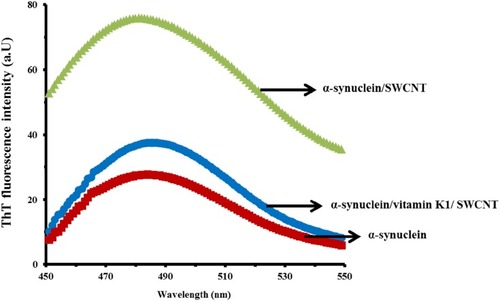
Aggregation of α-syn induced by SWCNT and the inhibitory effect of vitamin K1 can be explored by using ThT fluorescence study. The interaction of SWCNTs with α-syn for 24 hrs substantially significantly enhanced α-syn aggregation as determined by ThT fluorescence measurements (). However, ThT fluorescence intensity of the α-syn/SWCNT complex was reduced when protein samples were co-incubated with vitamin K1 for 24 hrs. These data indicate that the SWCNTs can enhance the aggregated formation of α-syn. This effect is dependent on the presence of the inhibitor as checked by the presence of vitamin K1 (). Indeed, ThT fluorescence intensity of α-syn was varied in a more significant manner by SWCNT relative to α-syn/vitamin K1 complex.
Congo Red Absorption Study
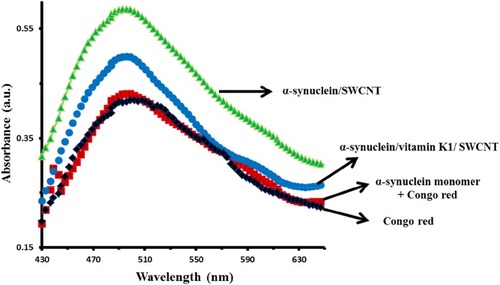
Congo red is known as an important dye, which is vastly employed to determine the presence of amyloid fibrils.Citation37 Indeed, binding to amyloid fibrils results in an increased absorbance and red-shift in the maximum optical density of Congo red probe. As shown in the Congo red absorption spectra of α-syn/SWCNT (), a significant enhancement in optical density is detected at 493 nm. However, this increase is not accompanied by a detectable red-shift from 493 nm to higher wavelength, determining the formation of non-ordered fibrillar species in the α-syn sample upon interaction with SWCNT. However, the Congo red absorbance (493 nm) decreased after co-incubation of α-syn with vitamin K1 in the presence of SWCNT, revealing the reduction in aggregation propensity of α-syn molecules. Therefore, ThT fluorescence and Congo red absorbance outcomes revealed that the formation of protein aggregates in the presence of SWCNTs exhibited nonfibrillar features and vitamin K1 reduces the formation of these aggregated species.
CD Study
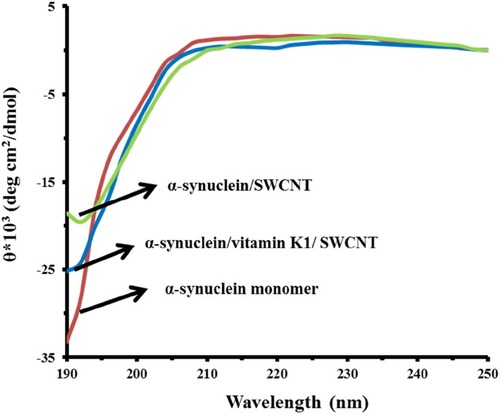
CD is considered a widely used spectroscopic technique to explore the secondary structural changes of biomolecules.Citation14,Citation37 The CD spectra of α-syn monomer demonstrated the typical band of predominantly random coil conformation. As shown in , the aggregation of α-syn while incubating with SWCNT at 25°C for 24 hrs caused a reduction in random coil structure. However, no considerable concomitant enhancement in the β-sheet fraction was observed, indicating the aggregation of protein into an amorphous morphology. Also, we observed a less reduction in single minima around 195 nm in α-syn samples co-incubated with K1 in the presence of SWCNT compared to the samples without vitamin K1. These data indicated that structural transition in α-syn by SWCNT was decreased upon co-incubation with vitamin K1.
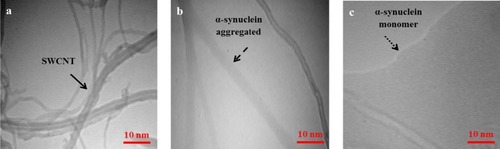
TEM Analysis
To further study the morphology of α-syn aggregation in the presence of SWCNT and the efficacy of vitamin K1 on α-syn aggregation inhibition, TEM study was done to detect the morphological characteristics of α-syn/SWCNT samples in the absence and presence of vitamin K1 after 24 hrs of incubation. As displayed in , SWCNT shows an outer diameter of around 1–2 nm with a worm-like shape. The micrograph of α-syn in the presence of SWCNT shows large quantities of branched nonfibrillar aggregates of ~5 nm in diameter and several µm in length, indicating amorphous aggregated species (). However, as compared to the α-syn/SWCNT sample, α-syn sample co-incubated with 50 µM vitamin K1 exhibits less aggregated species and are sparsely populated in the presence of SWCNT (). All in all, intrinsic and ThT fluorescence spectroscopy, Congo red binding spectroscopy, CD spectroscopy, and TEM data indicated that SWCNTs induced amorphous aggregation of α-syn, whereas co-incubation of α-syn with vitamin K1 leads to suppression of these nonfibrillar aggregates induced by SWCNT.
Molecular Docking Study
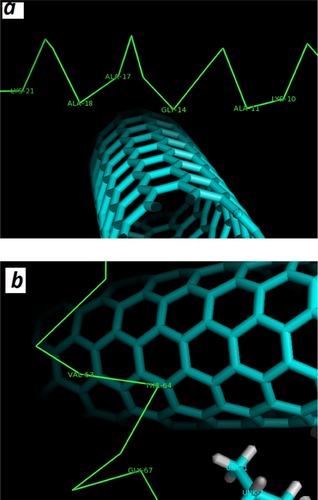
Molecular docking was run to determine the kind of interactions involved between vitamin K1 and α-syn that control the protein aggregation. The molecular docking was performed with a model of CNT and α-syn in the absence or presence of a stable conformer of vitamin K1. The resulting binding energy for CNT and α-syn was −286.32 E-value. To investigate the α-syn affinity to CNTs in the presence of vitamin K1, a successive molecular docking was performed with human α-syn as receptor and 30 molecules of vitamin K1 as ligands. Then, a molecular docking study was performed by the obtained complex and CNT model. The obtained binding energy was −220.63 E-value which reveals a lower affinity of CNT to interact with α-syn/SWCNT complex relative to free α-syn. The docked site was visualized by using CHIMERA (www.cgl.ucsf.edu/chimera) and PyMOL (http://pymol.sourceforge.net/) tools. The docked complexes for α-syn with SWCNT either alone or with K1 are shown in or , respectively. As can be seen in , in the absence of vitamin K1 the nearest residues are Lys-21, Ala-17, Ala-18, Gly-14, Ala-11, Lys-10, while in the presence of vitamin K1, the preferred sites of α-syn for CNT are occupied by vitamin K1 molecules () which can be the essential factor in decreasing the affinity of CNT to protein/vitamin complex. In this case, the CNT interacts with Val-63, Thr-64, Gly-67 (). Therefore, it may be concluded that vitamin K1 prevented aggregation of α-syn after interaction with SWCNT through non-covalent interaction.
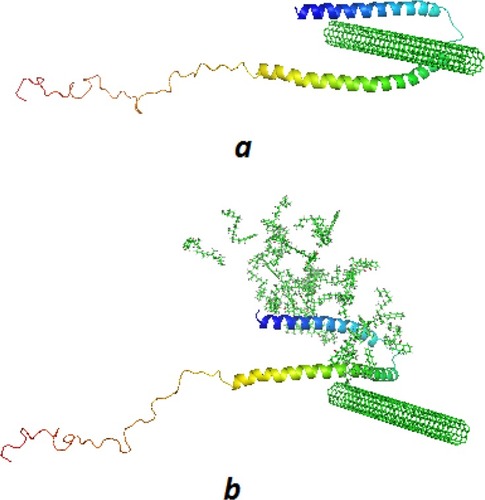
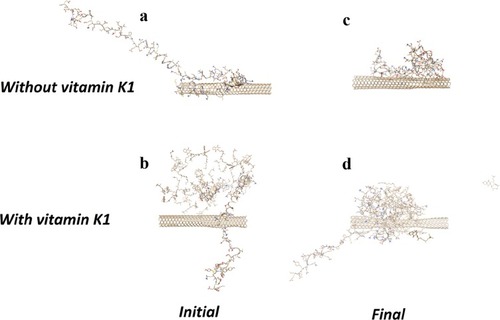
Molecular Dynamics Study
The conformation of α-syn either alone (A) or with vitamin K1 () in the beginning and after 500 ps evolution without () and with vitamin K1 () has been shown in . As can be observed, the α-syn upon interaction with CNT in the absence of vitamin K1 tends to attach the CNT wall and be crowded at the nanotube surface which leads to folding in the protein chain. However, the presence of vitamin K1 inhibited the SWCNT-induced structural changes of α-syn by interacting with residues involved in aggregation formation. The molecular dynamics data is in good agreement with experimental spectroscopy outcomes.
MTT Assay
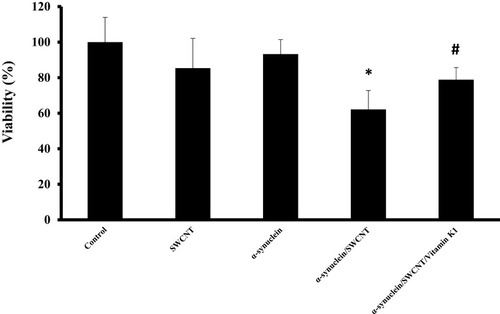
As indicated by MTT assay, SWCNT (1µg/mL) or α-syn (5µM) showed no cytotoxicity against SH-SY5Y cells after 24 hrs (). However, cell viability was markedly decreased to 62.1% ±10.69% (*P<0.05) after a 24-hrs exposure to 5µM α-syn/SWCNT complex. Afterward, when cells were treated with an aliquot of α-syn/SWCNT complex co-incubated with vitamin K1 for 24 hrs, cell toxicity was dramatically attenuated (#P<0.05) (). Indeed, treatment of cells with α-syn/vitamin K1/SWCNT complex for 24 hrs significantly elevated the cell viability to 78.88±6.80. Vitamin K1 did not possess remarkable SH-SY5Y cytotoxic capacities with a concentration of 5µM (data not shown).
LDH Assay
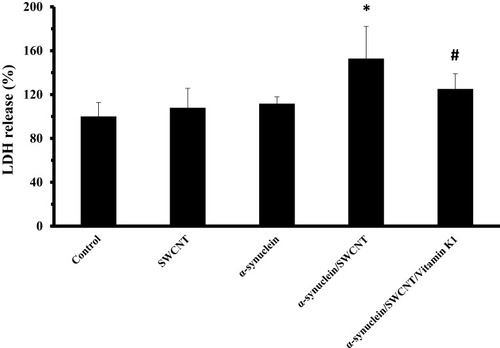
To further investigate the cytotoxic effect of α-syn/SWCNT complex with or without vitamin K1, the release of LDH, another indicator of cytotoxicity, was explored. As displayed in , a remarkable increase in LDH release (*P<0.05) was detected after 24 hrs exposures to 5 µM α-syn/SWCNT complex. However, treatment of the cells with an aliquot of α-syn/SWCNT complex co-incubated with vitamin K1 for 24 hrs attenuated this enhancement markedly (#P<0.05). Our data clearly showed that α-syn/SWCNT complex-induced membrane leakage in SH-SY5Y cells was attenuated in the presence of vitamin K1.
Figure 10 SH-SY5Y cell LDH release after being exposed to aliquots of α-syn (5 µM), SWCNT (1 µg mL−1), α-syn/SWCNT complex formed in the absence and presence of vitamin K1. *P ≤ 0.05 statistically different from the control group, #P ≤ 0.05 statistically different from the α-syn/SWCNT complex group.
Caspase-3 Assay
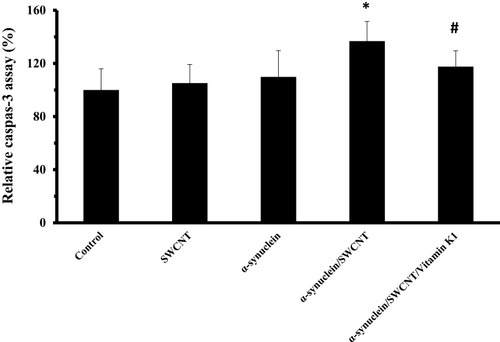
Caspase-3 as the key apoptotic executive protein can be activated by both extrinsic and intrinsic pathways. exhibits that treatment of SH-SY5Y cells with α-syn/SWCNT complex for 24 hrs significantly increased the activity of caspase-3 (*P<0.05), and this enhancement was remarkably reduced by an aliquot of α-syn/SWCNT complex co-incubated with vitamin K1 for 24 hrs (#P<0.05).
Figure 11 SH-SY5Y cell caspase-3 assay after being exposed to aliquots of α-syn (5 µM), SWCNT (1 µg mL−1), α-syn/SWCNT complex formed in the absence and presence of vitamin K1. *P ≤ 0.05 statistically different from the control group, #P ≤ 0.05 statistically different from the α-syn/SWCNT complex group.
Discussion
Although some reports have shown that oxidative stress or post-translational changes can play a vital role in oligomerization and α-syn aggregation,Citation38,Citation39 further investigations have demonstrated that α-syn aggregation causing neurological problems such as Alzheimer’s and Parkinson’s diseases are exacerbated by nanomaterial such as CNTs.Citation8 Therefore, understanding the role of CNTs in α-syn aggregation and introduction of some protective agents is crucial for the development of new therapies, which this study examines the protective effect of vitamin K1 against α-syn aggregation and their associated cytotoxicity in SH-SY5Y cell model induced by SWCNT. CNTs are basically insoluble in aqueous solutions and are not suitable for use in medical programs due to biological activity at their surfaces, such as increasing the aggregation of tau and α-syn proteins.Citation15 Similar to our results, Cavallo et al,Citation40 Avti et al,Citation41 and YuCitation42 reported that the use of SWCNT at concentrations of below 10 μg/mL does not create cytotoxicity. Also, in agreement with our results, Zeinabad et alCitation14 described that the use of SWCNTs did not affect LDH activity and cell membrane potential. Whereas, Pichardo et al,Citation43 Toyokuni et al,Citation44 and Syama et alCitation45 exhibited that the use of SWCNTs could induce cytotoxicity even at a concentration of 0.1 μg/mL. The difference in reports is due to the variation in the SWCNTs production method, the difference in surface factors, the type of target cells, length and diameter of the SWCNTs, the type of cytotoxicity assays, and even the media used in cellular studies. However, when CNTs interact with proteins, their effects on structural changes of proteins and associated cytotoxicity are not comprehensively understood. In this regard, the results of Ge et al,Citation46 Ebrahim-Habibi et al,Citation47 and Raghavendra et alCitation48 displayed that the interaction of proteins with SWCNTs would be dictated by functional groups on nanotube surface, which will be very effective in controlling the cytotoxicity of SWCNTs. On the other hand, the results of Du et alCitation49 demonstrated that SWCNTs caused the aggregation of lysozyme protein with a change in the protein structure. It was also found that lysozyme aggregation in multiwall CNTs was more severe than SWCNTs. However, changing surface factors from hydroxylates to carboxylates reduces the cytotoxicity of SWCNTs by reducing the accumulation of proteins. Also, SWCNTs result in more lysozyme aggregation (300-fold) compared to albumin protein corona based on multilayer accumulation at the CNT surface.Citation49 Analogously, Xie et alCitation50 demonstrated that in the presence of the hydroxylating agents on the SWCNT surface, they could significantly reduce the level of protein aggregation in the solution. It was determined that the reduction in protein aggregation is due to increased solubility of SWCNTs in the presence of the hydroxyl groups. Previously using molecular simulations, Li et alCitation51 and Jana et alCitation52 estimated that the hydrophobic interactions between SWCNTs and soluble proteins are considered as main sources in the initiation of protein aggregation. In line with the impact of carbon nanomaterial on the protein aggregation, it has been reported that graphene accelerates the α-syn aggregation and increases the cytotoxicity caused by the tendency of protein to aggregation.Citation53 The presence of some small molecules like vitamins also can play a potential role in the interaction of proteins and NPs. In this study, the protective effects of vitamin K1 against α-syn aggregation stimulated by SWCNTs were explored by fluorescence, CD, UV-vis, TEM, and bioinformatical investigations. It was shown that minor changes occur in the content of the α-syn structure co-incubated with vitamin K1 after interaction with SWCNTs, while the α-syn structure without vitamin K1 showed a significant structural change and subsequent aggregation after addition of SWCNT. Increasing absorption of α-syn on the SWCNT surface results in high levels of disorder in protein structure and function compared to the control group. More data are needed to evaluate the inhibitory effects of vitamin K1 against aggregation triggered by SWCNTs. Besides, the cytotoxicity of α-syn co-incubated with vitamin K1 in the presence of SWCNT on SH-SY5Y cells was also determined by MTT, LDH, and caspase-3 assays. Our results are consistent with reports by da Silva et al,Citation27 Xie et al,Citation50 Du et al,Citation49 and PangCitation54 which showed that by controlling carbon nanomaterial with auxiliary compounds, it is possible to inhibit the protein aggregation and associated cytotoxicity induced by CNTs.
Despite minimal information on the use of vitamins to control the toxicity of CNTs against protein aggregation, similar to our finding, da Silva et alCitation27 illustrated that vitamin K not only prevented the α-syn aggregation, but also reduced the population of formed oligomers. In addition, they reported that vitamin K, by attaching to the lysine residues of α-syn, causes the formation of amorphous aggregation from the oligomer species.Citation27 Likewise, it was determined that the use of vitamin E along with SWCNTs can prevent the reduction of intracellular protein content and significantly reduce the cytotoxicity of SWCNTs.Citation55,Citation56 It has been revealed that the connection between SWCNTs and proteins can increase the active sites for fibril formation and decrease the soluble protein content. The deposition and changing the intracellular protein activity by SWCNTs resulted in increased cell death or apoptosis and exacerbation of pulmonary inflammation in vivo. In another form, Alam et alCitation31 reported that the presence of vitamin K could prevent the aggregation of lysozyme and Aβ peptide which improves cellular viability in the SH-SY5Y cell line. In addition to vitamin E and K, vitamin A has also been reported as a potential inhibitory molecule against α-syn aggregation.Citation57 However, the biological properties of α-syn in the presence of vitamin A are not fully investigated and may be related to the high hydrophobicity of these molecules.
In general, it should be emphasized that intensive care should be considered to apply CNTs in medical applications,Citation58–Citation63 especially for the treatment of neurological diseases.
Conclusion
Herein, vitamin K1 was revealed to considerably prevent the formation of amorphous aggregation of α-syn in the presence of SWCNT and its relevant cytotoxicity. Our data suggested that vitamin K1 binds to α-syn monomer and mask the binding site of SWCNT. Furthermore, cytotoxicity experiments revealed that the amorphous aggregates formed by SWCNT in the absence of vitamin K1 are more toxic compared to non-aggregated species produced in the presence of vitamin K1. The data obtained may provide in-depth insight into probable mechanisms of aggregation inhibition by vitamins and an applicable suggestion about designing unique inhibitors.
Abbreviations
α-syn, α-synuclein; CNTs, carbon nanotubes; CD, circular dichroism; DMEM: F12, Dulbecco’s minimum essential medium and Ham’s F12 (1: 1); FBS, fetal bovine serum; LDH, lactate dehydrogenase; NP, nanoparticle; NGF, nerve growth factor; SWCNTs, single-walled carbon nanotubes; ThT, thioflavin T; TEM, transmission electron microscopy; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgement
This article was made possible by the grant NPRP10-120-170-211 from Qatar National Research Fund (a part of Qatar Foundation). All statements here are the sole responsibility of the authors.
References
- Butnaru D, Chapman J. The impact of self-replicating proteins on inflammation, autoimmunity and neurodegeneration—an untraveled path. Autoimmun Rev. 2019. doi:10.1016/j.autrev.2018.09.009
- Mohseni-Dargah M, Akbari-Birgani S, Madadi Z, Saghatchi F, Kaboudin B. Carbon nanotube-delivered iC9 suicide gene therapy for killing breast cancer cells in vitro. Nanomedicine 2019;14(8):1033–1047.
- Tagmatarchis N, Prato M. Functionalization of carbon nanotubes via 1, 3-dipolar cycloadditions. J Mater Chem. 2004;14(4):437–439. doi:10.1039/b314039c
- Ali-Boucetta H, Kostarelos K. Pharmacology of carbon nanotubes: toxicokinetics, excretion and tissue accumulation. Adv Drug Del Rev. 2013;65(15):2111–2119. doi:10.1016/j.addr.2013.10.004
- Zhao X, Lu D, Hao F, Liu R. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J Hazard Mater. 2015;292:98–107. doi:10.1016/j.jhazmat.2015.03.02325797928
- Zhao X, Hao F, Lu D, Liu W, Zhou Q, Jiang G. Influence of the surface functional group density on the carbon-nanotube-induced α-chymotrypsin structure and activity alterations. ACS Appl Mater Interfaces. 2015;7(33):18880–18890. doi:10.1021/acsami.5b0589526248557
- Bussy C, Al-Jamal KT, Boczkowski J, et al. Microglia determine brain region-specific neurotoxic responses to chemically functionalized carbon nanotubes. ACS Nano. 2015;9(8):7815–7830. doi:10.1021/acsnano.5b0235826043308
- Migliore L, Uboldi C, Di Bucchianico S, Coppedè F. Nanomaterials and neurodegeneration. Environ Mol Mutagen. 2015;56(2):149–170. doi:10.1002/em.2193125627719
- Costa PM, Bourgognon M, Wang JT, Al-Jamal KT. Functionalised carbon nanotubes: from intracellular uptake and cell-related toxicity to systemic brain delivery. J Control Release. 2016;241:200–219. doi:10.1016/j.jconrel.2016.09.03327693751
- Kafa H, Wang JT-W, Rubio N, et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood–brain barrier in vitro and in vivo. J Control Release. 2016;225:217–225. doi:10.1016/j.jconrel.2016.01.03126809004
- Luo J, Wärmländer SKTS, Yu C-H, Muhammad K, Gräslund A, Pieter Abrahams J. The Aβ peptide forms non-amyloid fibrils in the presence of carbon nanotubes. Nanoscale. 2014;6(12):6720–6726. doi:10.1039/c4nr00291a24820873
- Park M, Park J, Lee J, Ju S-Y. Scaling of binding affinities and cooperativities of surfactants on carbon nanotubes. Carbon. 2018;139:427–436. doi:10.1016/j.carbon.2018.07.003
- Allegri M, Perivoliotis DK, Bianchi MG, et al. Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep. 2016;3:230–243. doi:10.1016/j.toxrep.2016.01.01128959543
- Zeinabad HA, Zarrabian A, Saboury AA, Alizadeh AM, Falahati M. Interaction of single and multi wall carbon nanotubes with the biological systems: tau protein and PC12 cells as targets. Sci Rep. 2016;6:26508–26518. doi:10.1038/srep2650827216374
- Li C, Mezzenga R. The interplay between carbon nanomaterials and amyloid fibrils in bio-nanotechnology. Nanoscale. 2013;5(14):6207–6218. doi:10.1039/c3nr01644g23744243
- Liu F, Wang W, Sang J, Jia L, Lu F. Hydroxylated single-walled carbon nanotubes inhibit aβ42 fibrillogenesis, disaggregate mature fibrils, and protect against Aβ42-induced cytotoxicity. ACS Chem Neurosci. 2019;10(1):588–598. doi:10.1021/acschemneuro.8b0044130335950
- Marques O, Outeiro TF. Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis. 2012;3:350–360. doi:10.1038/cddis.2012.94
- Wales P, Pinho R, Lázaro DF, Outeiro TF. Limelight on alpha-synuclein: pathological and mechanistic implications in neurodegeneration. J Parkinsons Dis. 2013;3(4):415–459. doi:10.3233/JPD-13021624270242
- Valdinocci D, Radford RAW, Siow SM, Chung RS, Pountney DL. Potential modes of intercellular α-synuclein transmission. Int J Mol Sci. 2017;18(2):469–480. doi:10.3390/ijms18020469
- Book A, Guella I, Candido T, et al. A meta-analysis of α-Synuclein multiplication in familial parkinsonism. Front Neurol. 2018;9:1–10. doi:10.3389/fneur.2018.0000129403429
- Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol. 2017;298:225–235. doi:10.1016/j.expneurol.2017.10.00328987463
- Alarcón-Arís D, Recasens A, Galofré M, et al. Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for parkinson’s disease. Mol Ther. 2018;26(2):550–567. doi:10.1016/j.ymthe.2017.11.01529273501
- Pujols J, Peña-Díaz S, Lázaro DF, et al. Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc Natl Acad Sci. 2018;115(41):10481–10486. doi:10.1073/pnas.180419811530249646
- Li J, Zhu M, Rajamani S, Uversky VN, Fink AL, Inhibits R. α-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11(11):1513–1521. doi:10.1016/j.chembiol.2004.08.02515556002
- Li H-T, Lin D-H, Luo X-Y, et al. Inhibition of α-synuclein fibrillization by dopamine analogs via reaction with the amino groups of α-synuclein. Febs J. 2005;272(14):3661–3672. doi:10.1111/j.1742-4658.2005.04792.x16008565
- Zhu M, Han S, Fink AL. Oxidized quercetin inhibits α-synuclein fibrillization. Biochimi Et Biophys Acta. 2013;1830(4):2872–2881. doi:10.1016/j.bbagen.2012.12.027
- da Silva FL, Coelho Cerqueira E, de Freitas MS, Gonçalves DL, Costa LT, Follmer C. Vitamins K interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem Int. 2013;62(1):103–112. doi:10.1016/j.neuint.2012.10.00123064431
- Ardah MT, Paleologou KE, Lv G, et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front Aging Neurosci. 2014;6(197):1–10. doi:10.3389/fnagi.2014.0000124478697
- McGlinchey RP, Lee JC. Cysteine cathepsins are essential in lysosomal degradation of α-synuclein. Proc Natl Acad Sci. 2015;112(30):9322–9327. doi:10.1073/pnas.150093711226170293
- Jha NN, Ghosh D, Das S, et al. Effect of curcumin analogs onα-synuclein aggregation and cytotoxicity. Sci Rep. 2016;6:28511–28520. doi:10.1038/srep2851127338805
- Alam P, Chaturvedi SK, Siddiqi MK, et al. Vitamin k3 inhibits protein aggregation: implication in the treatment of amyloid diseases. Sci Rep. 2016;6:26759–26765. doi:10.1038/srep2675927230476
- Hadden MK, Hill SA, Davenport J, Matts RL, Blagg BS. Synthesis and evaluation of Hsp90 inhibitors that contain the 1, 4-naphthoquinone scaffold. Biorg Med Chem. 2009;17(2):634–640. doi:10.1016/j.bmc.2008.11.064
- Cerqueira EC, Netz PA, Diniz C, do Canto VP, Follmer C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1, 4-naphthoquinone: evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Biorg Med Chem. 2011;19(24):7416–7424. doi:10.1016/j.bmc.2011.10.049
- Ritchie DW, Venkatraman V. Ultra-fast FFT protein docking on graphics processors. Bioinformatics. 2010;26(19):2398–2405. doi:10.1093/bioinformatics/btq44420685958
- Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4(1):17–25. doi:10.1186/1758-2946-4-1722889332
- Mayo SL, Olafson BD, Goddard WA. Dreiding—A generic force-field for molecular simulations. J Phys Chem. 1990;94:8897–8909. doi:10.1021/j100389a010
- Mahdavimehr M, Meratan AA, Ghobeh M, Ghasemi A, Saboury AA, Nemat-Gorgani M. Inhibition of HEWL fibril formation by taxifolin: mechanism of action. PLoS One. 2017;12(11):0187841–0187845. doi:10.1371/journal.pone.0187841
- Breydo L, Wu JW, Uversky VN. α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis. 2012;1822(2):261–285. doi:10.1016/j.bbadis.2011.10.002
- Wan OW, Chung KK. The role of alpha-synuclein oligomerization and aggregation in cellular and animal models of Parkinson’s disease. PLoS One. 2012;7(6):38545–38554. doi:10.1371/journal.pone.0038545
- Cavallo D, Fanizza C, Ursini CL, et al. Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. J Appl Toxicol. 2012;32(6):454–464. doi:10.1002/jat.271122271384
- Avti PK, Caparelli ED, Sitharaman B. Cytotoxicity, cytocompatibility, cell-labeling efficiency, and in vitro cellular magnetic resonance imaging of gadolinium-catalyzed single-walled carbon nanotubes. J Biomed Mater Res A. 2013;101(12):3580–3591. doi:10.1002/jbm.a.3464323686792
- Yu IJ. Single-Wall Carbon Nanotubes (SWCNT) Induce Cytotoxicity and Genotoxicity Produced by Reactive Oxygen Species (ROS) Generation in Phytohemagglutinin (PHA)-stimulated male human peripheral blood lymphocytes AU - Kim, Jin Sik. J Toxicol Environ Health A. 2014;77(19):1141–1153. doi:10.1080/15287394.2014.91706225119736
- Pichardo S, Gutiérrez-Praena D, Puerto M, et al. Oxidative stress responses to carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicol In Vitro. 2012;26(5):672–677. doi:10.1016/j.tiv.2012.03.00722449549
- Toyokuni S, Jiang LI, Kitaura R, Shinohara H. Minimal inflammogenicity of pristine single-wall carbon nanotubes. Nagoya J Med Sci. 2015;77(1–2):195–202.25797984
- Syama S, Mohanan PV. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int J Biol Macromol. 2016;86:546–555. doi:10.1016/j.ijbiomac.2016.01.11626851208
- Ge C, Du J, Zhao L, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci USA. 2011;108(41):16968–16973. doi:10.1073/pnas.110527010821969544
- Ebrahim-Habibi MB, Ghobeh M, Mahyari FA, Rafii-Tabar H, Sasanpour P. An investigation into non-covalent functionalization of a single-walled carbon nanotube and a graphene sheet with protein G: A combined experimental and molecular dynamics study. Scientific Reports. 2019;9(1):1273.30718580
- Raghavendra AJ, Fritz K, Fu S, Brown JM, Podila R, Shannahan JH. Variations in biocorona formation related to defects in the structure of single walled carbon nanotubes and the hyperlipidemic disease state. Sci Rep. 2017;7(1):8382–8390. doi:10.1038/s41598-017-08896-w28814800
- Du P, Zhao J, Mashayekhi H, Xing B. Adsorption of bovine serum albumin and lysozyme on functionalized carbon nanotubes. J Phys Chem C. 2014;118(38):22249–22257. doi:10.1021/jp5044943
- Xie L, Lin D, Luo Y, Li H, Yang X, Wei G. Effects of hydroxylated carbon nanotubes on the aggregation of Aβ16–22 peptides: a combined simulation and experimental study. Biophys J. 2014;107(8):1930–1938. doi:10.1016/j.bpj.2014.08.03425418174
- Li H, Luo Y, Derreumaux P, Wei G. Carbon nanotube inhibits the formation of β-sheet-rich oligomers of the Alzheimer’s amyloid-β (16–22) peptide. Biophys J. 2011;101(9):2267–2276. doi:10.1016/j.bpj.2011.09.04622067167
- Jana AK, Sengupta N. Adsorption mechanism and collapse propensities of the full-length, monomeric Aβ1–42 on the surface of a single-walled carbon nanotube: a molecular dynamics simulation study. Biophys J. 2012;102(8):1889–1896. doi:10.1016/j.bpj.2012.03.03622768945
- Mohammadi S, Nikkhah M, Hosseinkhani S. Investigation of the effects of carbon-based nanomaterials on A53T alpha-synuclein aggregation using a whole-cell recombinant biosensor. Int J Nanomedicine. 2017;12:8831–8840. doi:10.2147/IJN.S14476429276384
- Pang R, Li M, Zhang C. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: diffusional limitation investigation. Talanta. 2015;131:38–45. doi:10.1016/j.talanta.2014.07.04525281070
- Shvedova AA, Kisin ER, Murray AR, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221(3):339–348. doi:10.1016/j.taap.2007.03.01817482224
- Singh RP, Sharma G, Sonali, et al. Vitamin E TPGS conjugated carbon nanotubes improved efficacy of docetaxel with safety for lung cancer treatment. Colloids Surf B Biointerfaces. 141;2016:429–442. doi:10.1016/j.colsurfb.2016.02.01126895505
- Ono K, Yamada M. Vitamin A potently destabilizes preformed α-synuclein fibrils in vitro: implications for Lewy body diseases. Neurobiol Dis. 2007;25(2):446–454. doi:10.1016/j.nbd.2006.10.01017169566
- Yoosefian M, Jahani M. A molecular study on drug delivery system based on carbon nanotube for the novel norepinephrine prodrug, Droxidopa. J Mol Liq. 2019;15(284):258–264. doi:10.1016/j.molliq.2019.04.016
- Yoosefian M, Rahmanifar E, Etminan N. Nanocarrier for levodopa Parkinson therapeutic drug; comprehensive benserazide analysis. Artif Cells Nanomed Biotechnol. 2018;46(sup1):434–446. doi:10.1080/21691401.2018.143058329378432
- Yoosefian M, Pakpour A, Etminan N. Nanofilter platform based on functionalized carbon nanotubes for adsorption and elimination of Acrolein, a toxicant in cigarette smoke. Appl Surf Sci. 2018;444:598–603. doi:10.1016/j.apsusc.2018.03.108
- Yoosefian M, Etminan N. Leucine/Pd-loaded (5, 5) single-walled carbon nanotube matrix as a novel nanobiosensors for in silico detection of protein. Amino Acids. 2018;50(6):653–661. doi:10.1007/s00726-018-2552-429536267
- Yoosefian M. A high efficient nanostructured filter based on functionalized carbon nanotube to reduce the tobacco-specific nitrosamines, NNK. Appl Surf Sci. 2018;15(434):134–141. doi:10.1016/j.apsusc.2017.10.166
- Yoosefian M, Etminan N. The role of solvent polarity in the electronic properties, stability and reactivity trend of a tryptophane/Pd doped SWCNT novel nanobiosensor from polar protic to non-polar solvents. RSC Adv. 2016;6(69):64818–64825. doi:10.1039/C6RA14006H