?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to investigate lecithin-chitosan nanoparticles as a topical delivery system for quercetin.
Methods
Tocopheryl propylene glycol succinate was chosen to be the surfactant for the nanosystem. The mean particle size of the nanoparticles was 95.3 nm, and the entrapment efficiency and drug loading for quercetin were 48.5% and 2.45%, respectively. Topical delivery in vitro and in vivo of the quercetin-loaded nanoparticles was evaluated using quercetin propylene glycol solution as the control.
Results
Compared with quercetin solution, the quercetin-loaded nanoparticles showed higher permeation ability, and significantly increased accumulation of quercetin in the skin, especially in the epidermis. Microstructure observation of the skin surface after administration indicated that the interaction between ingredients of the nanoparticles and the skin surface markedly changed the morphology of the stratum corneum and disrupted the corneocyte layers, thus facilitating the permeation and accumulation of quercetin in skin.
Conclusion
Lecithin-chitosan nanoparticles are a promising carrier for topical delivery of quercetin.
Introduction
Skin is the largest organ of the human body and is directly exposed to the environment. Therefore, skin often suffers oxidative stress caused by environmental factors, such as ultraviolet irradiation, ionizing radiation, and toxic chemicals.Citation1 Although the skin does provide a certain degree of self-protection, it can be overpowered by reactive oxygen species, sustaining severe damage, such as lipid peroxidation, enzyme inactivation, and DNA breakage. Superoxide radicals and hydrogen peroxide are precursors to reactive oxygen species.Citation2,Citation3 Developing effective methods to combat reactive oxygen species has been a subject of intense research. Quercetin, a topical antioxidant, is known to have the ability to delay ultraviolet radiation-mediated oxidant injury and cell death by scavenging oxygen radicals, protecting lipids against peroxidation to terminate the chain-radical reaction, and chelating metal ions to form inert complexes that prevent conversion of superoxide radicals and hydrogen peroxide into hydroxyl radicals.Citation4–Citation8 Quercetin has superior antioxidant potency compared with many other well known antioxidant molecules, owing to the optimized number and distinctive positions of the free hydroxyl groups in this molecule.Citation9,Citation10 However, insolubility of quercetin in water hinders its permeation into skin, and its protection for skin is limited. Therefore, development of novel nanovehicles that are capable of transporting quercetin across the skin to exert its bioactivity in inhibiting ultraviolet B-induced cutaneous oxidative stress and inflammation is of great significance.
Nanoparticles have considerable potential in topical delivery of drugs due to their distinctive properties. Because of their increased surface area, nanoparticles have greater efficiency in enhancing the permeation of drugs into skin than many other vehicles. Several studies have shown that nanosized particles tend to reside in the outer layers of the stratum corneum and epidermis, with negligible penetration into the dermis.Citation11–Citation13
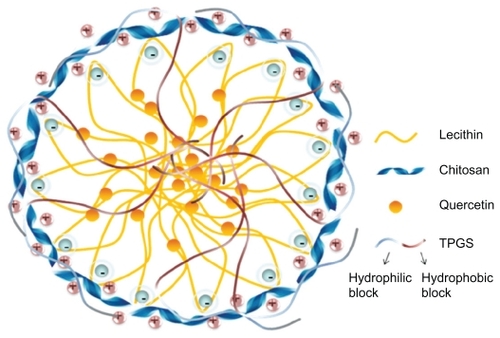
Lecithin is a natural lipid mixture of phospholipids and is frequently used for the preparation of various nanosystem delivery vehicles, such as microemulsions, liposomes, micelles, and nanoparticles, and is considered to be a safe and biocompatible excipient.Citation14–Citation19 Chitosan, a polycationic polymer comprised mainly of glucosamine units, is a N-deacetylated derivative of chitin. It has been reported that chitosan has antioxidantCitation20,Citation21 and anti-inflammatory properties,Citation22,Citation23 as well as many other attractive biopharmaceutical properties, including biocompatibility, biodegradability, bioadhesion, penetration enhancement, and antimicrobial activity.Citation24–Citation27 This polycationic polymer can interact with negatively charged substances to form a core shell nanostructure which has been proved to be a promising carrier for drugs.Citation18,Citation19,Citation28 Chitosan has been used in many topical preparations to enhance drug uptake in nasal, buccal, and intestinal epithelia, and skin.Citation29–Citation33 It has also been reported that lecithin-chitosan nanoparticles obtained through the supramolecular self-organizing interaction between negatively charged lecithin and positively charged chitosan can become strongly attached to mammalian skin, which is slightly negatively charged, and offers better coverage for topical delivery of active compounds.Citation17–Citation19,Citation34
In this study, quercetin-loaded lecithin-chitosan nanoparticles containing D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) were prepared and optimized, and the physicochemical properties of the nanoparticles were characterized. In vitro and in vivo drug distribution in the epidermis and dermis of mice after topical administration of quercetin-loaded lecithin-chitosan nanoparticles was evaluated, and the effect of lecithin-chitosan nanoparticles on the skin surface of the mice was investigated.
Materials and methods
Materials
Quercetin was purchased from Xi’an Senmu Biological Technology Co Ltd (Xi’an, China). Soya lecithin was obtained from Shanghai Taiwei Pharmaceutical Co Ltd (Shanghai, China). Chitosan of purity 95% (molecular weight 200 kDa, 85% deacetylation) was obtained from Haidebei Marine Bioengineering Co Ltd (Jinan, China) and used without further purification. TPGS was supplied by Wuhan Yuancheng Co Ltd (Wuhan, China). Tween80 and propylene glycol were purchased from Yongda Chemical Reagent Co Ltd (Tianjin, China). Ethanol and methanol of high-pressure liquid chromatography (HPLC) grade were purchased from Tianjin Guangcheng Chemical Agent Co Ltd (Tianjin, China). Other chemicals and solvents were of analytical reagent grade.
Preparation of nanoparticles
Lecithin-chitosan nanoparticles were prepared according to an established method.Citation18 Lecithin 5% (w/v) was dissolved in a 96% ethanol solution. Quercetin was then dissolved in the ethanol solution containing lecithin to obtain a weight ratio of lecithin to quercetin of 10:3. The chitosan solutions were prepared by dissolving 10 mg of chitosan in 50 mL of distilled water with 0.1% acetic acid. TPGS was subsequently dissolved in the chitosan solution, and then 4 mL of the aforementioned mixed ethanol solution was injected into 46 mL of chitosan solution through a plastic needle tubing (internal diameter 0.75 mm, injection rate 2 mL/minute) under mechanical agitation at 1000 rpm. After stirring for half an hour, the pH of the nanoparticle suspension was adjusted to 4.5 with 0.5 M sodium hydroxide solution. After the resulting suspension was filtered through a filter membrane (0.8 μm) to remove the nonincorporated drug, the prepared nanoparticle system was obtained. The lecithin-chitosan ratio was set at 20:1 (w/w) in the nanoparticles.
Physicochemical characterization of nanoparticles
Particle size and zeta potential
The mean diameter of the quercetin-loaded nanoparticles was measured by photon correlation spectroscopy using a particle sizer (Zetasizer 3000 HAS; Malvern Instruments Ltd, Worcestershire, UK) at a fixed angle of 90° with a He-Ne laser of 633 nm at 25°C. Particle size was evaluated using volume distribution. The zeta potential was analyzed using a microscopic electrophoresis system (DXD-II; Jiangsu Optics Co Ltd, Jiangsu, China) at 25°C.
Morphology
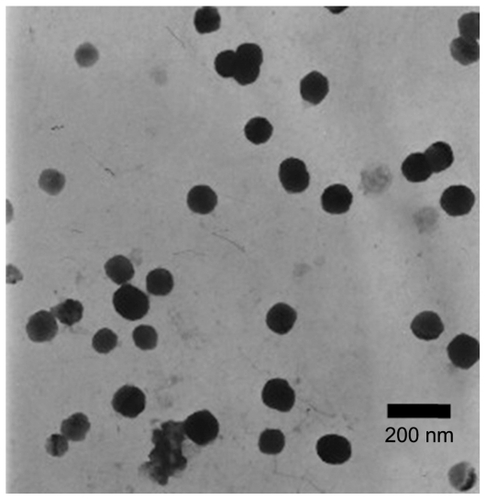
The morphology of the quercetin-loaded nanoparticles was observed under transmission electron microscopy (JEM-1200EX; JEOL, Tokyo, Japan). One drop of diluted quercetin-loaded nanoparticle suspension was deposited on a film-coated copper grid and stained with one drop of 2% (w/v) aqueous solution of phosphotungstic acid, and then allowed to dry for contrast enhancement. The sample was then examined by transmission electron microscopy.Citation35
Entrapment efficiency and drug loading
The prepared nanoparticles were separated from the free drug using a Sephadex G-50 minicolumn centrifugation technique for measurement of entrapment efficiency.Citation36,Citation37 Briefly, 0.3 mL of the prepared nanoparticle suspension was placed in a Sephadex G-50 minicolumn (presaturated with empty nanoparticles prepared using the same composition and methods) and centrifuged at 1000 rpm for 1 minute, and the column was then washed seven times with 0.1 mL of distilled water. Elutes containing drug-loaded nanoparticles were collected and adjusted to a volume of 25 mL by ethanol, and the elute-ethanol solution was then vortexed for 3 minutes to break down the nanoparticles and dissolve the quercetin. The mixture was then centrifuged at 12,000 rpm for 10 minutes to precipitate the crystallized salt and chitosan. Thereafter, the amount of entrapped drug in the supernatant liquid was detected using an ultraviolet-visible spectrophotometer (Lambda 15; Perkin-Elmer, Schwerzenbach, Switzerland) at a wavelength of 373 nm. The entrapment efficiency and drug-loading of quercetin in the nanoparticles were calculated according to the following equations:Citation38
where Winitial drug represents the initially added amount of drug and Wentrapped drug represents the amount of drug entrapped in the nanoparticles. WNP represents the total weight of all components in the nanoparticles.
In vitro permeation studies
Preparation of skins
The male Kunming mice (20 ± 2 g) used in the permeation studies were purchased from the Experimental Animal Center of Shandong University. The study protocol was approved by the ethics committee of Shandong University. The mice were sacrificed and their dorsal hair was carefully removed with a razor. The skins were obtained from the hairless regions of the mice, and the subcutaneous fat and connective tissue were trimmed. The excised skins were washed and examined to ensure their integrity, and were then stored in a refrigerator at −20°C overnight for later use.Citation39
In vitro study in mice
The in vitro permeation experiment was carried out using Franz diffusion cells with an effective diffusion area of 3.14 cm2. The excised skin was thawed and mounted between the donor and receptor chamber of the Franz diffusion cell, with the stratum corneum side facing upwards. A quercetin-loaded nanoparticle suspension (0.5 mL) or control solution with the same quercetin concentration was applied to the surface of the stratum corneum. The receptor chamber was filled with 20 mL of phosphate-buffered saline (pH 7.2) containing 1% Tween80 which can increase quercetin solubility to 155.8 μg/mL and keep a sink condition for quercetin penetrated through the skin. The receptor medium was kept at 32°C ± 0.5°C and stirred at 300 rpm throughout the experiment. At designated time intervals (hours 0.5, 1, 2, 4, 6, 8, 10, 12, or 24 following application) 0.5 mL of receptor solution was taken out and 0.5 mL of blank medium at the same temperature was added into the receptor compartment to keep the volume constant. Acetic ether 3 mL was added to the sample solution and vortexed for 3 minutes, and then centrifuged at 4000 rpm for 10 minutes. The supernatant was collected and air-dried in nitrogen, and the solid obtained was redissolved in 250 μL of methanol, then the amount of quercetin in the methanol solution was analyzed by HPLC.
At hours 3, 6, 9, and 12 after application of drug-loaded nanoparticles and quercetin propylene glycol solution, the skin surfaces were thoroughly washed with distilled water to remove excess formulation, and were then placed above an aqueous bath (60°C) for 60 seconds to separate the epidermis and dermis according to the established method.Citation40 After that, the epidermis and dermis were cut into pieces and put into a plastic test tube (10 mL). Physiological saline solution 1 mL was added to each of them, and after being homogenized, extraction of quercetin from the homogenized skin tissue was performed using the same steps as those for the receptor solution described earlier. The amount of quercetin was analyzed by HPLC.
In vivo study in mice
All the experimental mice were housed in cages, with access to food and water until use. Twenty-four hours prior to the experiment, the dorsal hair was removed with a razor and the bare skins were washed with physiological solution. Quercetin-loaded nanoparticle suspension 0.5 mL (drug content 0.5%, w/w) or control solution was applied to the dorsal surface (3.14 cm2). At hours 3, 6, 9, and 12 thereafter, the mice were killed by cervical dislocation, and the treated skin area was dissected. Further steps for skin sample treatment and extraction of quercetin from skin tissues were performed using the same methods as those described earlier. The amount of quercetin in the epidermis and dermis was analyzed by HPLC.
Effect of nanoparticles on surface of skin
The aim of the experiment was to determine the topical effect of quercetin-loaded nanoparticle suspension on mouse skin using a previously reported method.Citation41 The nanoparticle suspension and quercetin propylene glycol solution were applied to the hairless dorsal skin of mice for 24 hours. The mice were then killed by cervical dislocation, and the treated skin area was dissected and collected in 4% formalin for histopathological studies. About 3–4 μm of paraffin tissue sections were cut and subjected to hematoxylin and eosin staining. Microscopic pictures were taken by Nikon Alphaphot-2 YS2 microscope (Tokyo, Japan).
HPLC analysis of quercetin
The samples were analyzed using an HPLC system (1200 series; Agilent Technologies, Palo Alto, CA) and an HPLC column (C18, 4.6 × 250 mm, 5 μm). The mobile phase was a mixture of methanol-water at a ratio of 50:50 (v/v) containing 3% acetic acid. The flow rate was set at 1 mL/minute and quercetin was detected at 373 nm. Aliquots of 20 μL of each sample were injected into the column, and all operations were carried out at ambient temperature. The peak area correlated linearly with the quercetin concentration in the range of 1.5–150 μg/mL and the lowest detection limit at 0.5 μg/mL.
Statistical analysis
Data are shown as means ± standard deviation (n = 5). Statistical data were analyzed by the Student’s t-test at the level of P = 0.05.
Results and discussion
Preparation of quercetin-loaded nanoparticles
Injection of an alcohol solution containing lecithin into chitosan aqueous solution is a well established method for the preparation of nanoparticles, and based on previous studies, nanoparticles made from lecithin and chitosan exhibited well rounded shapes and better stability when the weight ratio of lecithin to chitosan was 20:1.Citation19,Citation24 This ratio was adopted in the present study. TPGS was chosen for its known ability to increase drug solubility, stabilize nanosystems,Citation42–Citation44 and improve drug absorption.Citation45,Citation46 The visual observation of prepared quercetin-loaded nanoparticles and crude quercetin in water is shown in . Crude quercetin was hardly dissolved in water and precipitated from the suspension in a short time, whereas the quercetin-loaded nanoparticle suspension showed a translucent state with visible opalescence.
Figure 1 Visual observation of crude quercetin in water (A) and quercetin-loaded nanoparticle suspension (B).
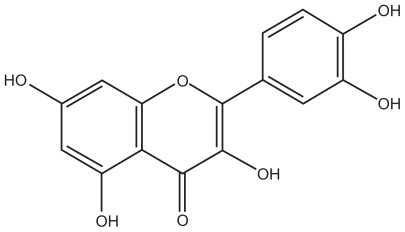
shows the structure of quercetin, and shows the possible structure of nanoparticles prepared in this work. According to previous studies, lecithin and chitosan can form perfect round nanoparticles by the electrostatic interaction between the polycationic chitosan and negatively charged lecithin, in which lecithin molecules form the core and chitosan molecules form the hydrophilic shell layer to protect the inner structure.Citation18,Citation19 In this study, the hydrophobic groups in the TPGS and lecithin molecules intertwine with each other to form the hydrophobic core of the nanoparticles. Also, the hydrophilic polyethylene glycol chain in the molecular structure of TPGS and the hydrophilic parts of the lecithin molecules might participate in the formation of the hydrated shell layer with chitosan. Because of the good lipophilicity of quercetin and the hydrated structure of the shell layer, quercetin should dissolve or disperse in the hydrophobic core, and be protected by the outer shell layer. However, the exact structure of quercetin-loaded nanoparticles still needs further investigation. The effect of TPGS on entrapment efficiency and drug-loading was investigated, as shown in . Drug-loading was clearly influenced by the concentration of TPGS and increased by more than five-fold when the concentration of TPGS changed from 0% to 2% (w/v). However, with increasing concentrations of TPGS, drug-loading decreased slightly, indicating that drug-loading reached a maximum level when the TPGS concentration was 2%. Therefore, based on drug-loading, the optimized formulation for the nanoparticles was 0.02% chitosan, 0.4% lecithin, and 2% TPGS.
Table 1 Influence of the concentration of TPGS on entrapment efficiency and drug-loading of nanoparticles
Characterization of nanoparticles
The morphology of the nanoparticles was observed under transmission electron microscopy. As shown in , the optimized nanoparticles were spherical. The particle size of both the drug-loaded nanoparticles and the blank nanoparticles was 95.3 nm and 168.0 nm, respectively, and the polydispersity index was 0.441 and 0.331. The relatively large values for the polydispersity index may be due to the small particle size fraction of formed micelles of TPGS. After quercetin was loaded into particles, the particle size became smaller. A possible reason for this was that the loaded drug enhanced the cohesive force of the hydrophobic interaction and thus caused the decrease in size.Citation47
The zeta potential of the drug-loaded nanoparticles and blank nanoparticles was 10.85 ± 0.05 mV and 24.78 ± 0.05 mV, respectively. It has been reported that the electrostatic attraction between the most acidic phenolic OH groups of quercetin and the positively charged particles influences the charge distribution, thus causing a decrease in zeta potential of the whole system.Citation48 Although the measured zeta potential was relatively low, the nanoparticle suspension still showed good stability, and there was no notable change in morphology or particle size of the quercetin-loaded nanoparticles during 2 weeks stored at 4°C. The result was consistent with previous reports of TPGS used as a stabilizer for nanoparticles.Citation49–Citation51
In vitro and in vivo percutaneous delivery study
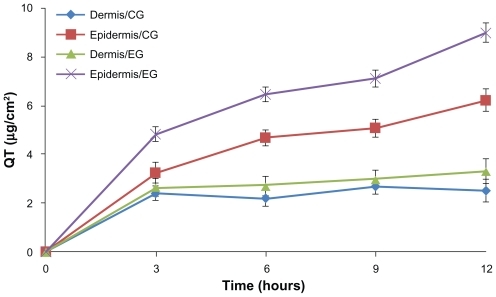
In the in vitro percutaneous study, quercetin was barely detectable in the receptor cell for 12 hours after administration of quercetin-loaded nanoparticles or quercetin control solution. The distribution of quercetin in the excised dorsal skins of mice is shown in . Compared with the quercetin propylene glycol solution, nanoparticles could markedly enhance the cumulative amounts of quercetin in the dermis and epidermis within 12 hours of application (P < 0.05), and the amounts of quercetin in epidermis and dermis were 9.00 ± 0.40 μg and 3.31 ± 0.51 μg, respectively, which were 1.45 and 1.32 times these of the control formulation.
Figure 5 Time course of in vitro skin permeation of quercetin incorporated into nanoparticles or control formulation.
Note: Results are represented by means ± standard deviation (n = 3).
Abbreviations: CG, control group; EG, experimental group.
In vivo, quercetin extracted from the epidermis and dermis was analyzed by HPLC at every sampling point. shows the amounts of quercetin remaining in the epidermis and dermis at hours 3, 6, 9, and 12 after application of the nanoparticle and control formulations. For the nanoparticles, the amounts of quercetin in the epidermis and dermis were 8.4 ± 1.53 μg and 2.21 ± 0.01 μg, respectively, which were 2.3 and 1.2 times those of the control formulation. The permeation behavior of quercetin in vivo was similar to that in vitro (P < 0.05).
Figure 6 Amount of quercetin in the (A) dermis and (B) epidermis after hours 3, 6, 9, and 12, respectively.
Note: Results are represented by means ± standard deviation (n = 3).
Abbreviations: CG, control group; EG, experimental group.
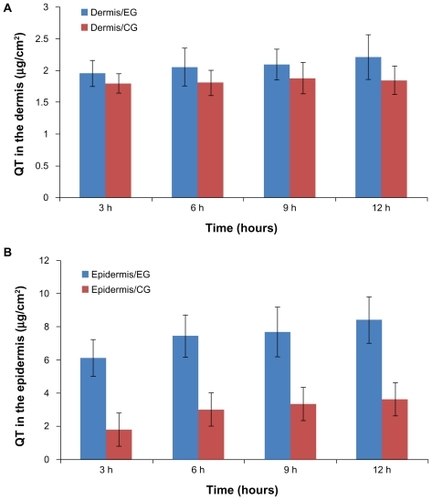
The statistical difference between the experimental group and the control group in the accumulation of quercetin in the epidermis could be due to the unique characteristics of the prepared nanoparticles, as well as their positive charge and large surface area, ensuring an excellent contact surface between the vehicles and skin over the entire application area and promoting permeation and accumulation of drug in the skin.Citation11,Citation42 In addition, the nanoparticles tended to suppress penetration (transdermal delivery) through the skin while enhancing permeation (dermal delivery) of the drug into the upper skin layers.Citation12,Citation13,Citation41,Citation52–Citation54 In , the amounts of quercetin in the dermis were similar in the control and experimental groups but were clearly different in the epidermis. Because of the highly lipophilic properties of lecithin and quercetin, the carrier cannot transport the drug through the inner more hydrophilic skin layers. In contrast, the alcohol vehicle was relatively more likely to facilitate penetration of the drug into the dermis.Citation55 In accordance with the experimental results, under the influence of multiple factors, the gap of drug retention between the experimental group and control group narrowed. Such peculiarity of lecithin-chitosan nanoparticles could improve accumulation of quercetin in the epidermis and avoid its permeation through the skin, which indicates that the lecithin-chitosan nanoparticles are an ideal carrier for the cutaneous delivery of quercetin to exert its antioxidant and anti-inflammatory effects in the skin. Moreover, the structure of lecithin-chitosan nanoparticles could influence the distribution of quercetin in skin tissue. A solid matrix in the core of the nanoparticles could immobilize quercetin molecules and show controlled release for quercetin, which resulted in a low concentration gradient, and thus quercetin stored in nanoparticles could hardly penetrate into the dermis and only distributed in the epidermis, especially in the stratum corneum.Citation19,Citation54
On the other hand, TPGS played a very important role in the formulation by disrupting the lipid structure of the stratum corneum to reduce the barrier of skin, and providing better wetting to the skin, which might be essential for bioadhesion and facilitating diffusion of drug through the barrier layer, furthermore TPGS could also increase the diffusion coefficient of the drug between the skin and the vehicle.Citation46,Citation56,Citation57
To sum up, the results demonstrate that, compared with the control formulation, nanoparticles could significantly promote the accumulation of quercetin in skin, especially in the epidermis. Because the efficacy of quercetin in delaying ultraviolet radiation-mediated oxidant injury and cell death mainly occurs in the epidermis, it is of great significance for the nanoparticles to make quercetin remain in skin, especially in the epidermis.
Effect of nanoparticles on the surface of mice skin
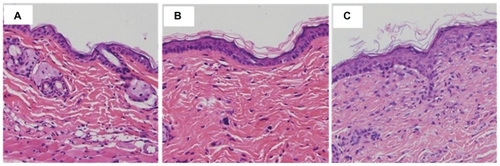
Microscopic pictures of untreated skin, skin treated with quercetin propylene glycol solution, and quercetin-loaded nanoparticles are shown in . Untreated skin () had a compact stratum corneum, with the corneocyte layer closely conjugated. Application of quercetin propylene glycol solution could slightly change the tight structure of the stratum corneum (). The skin treated with the nanoparticle suspension appeared swollen, with increased overall thickness of the stratum corneum and, furthermore, loose cell junctions and increased intercellular space could also be observed (). The change in the skin surface resulting from the effect of the nanoparticles might be related to their composition. The positive charge of chitosan ensured close contact between the nanoparticles and the skin. The hydrated outer environment provided by chitosan and the active surface nature of TPGS provided better wetting to the skin, which might significantly alter the barrier properties and reduce or prevent skin dehydration. Also, increased hydration of the stratum corneum could facilitate permeation of drug into the epidermis. Following interaction between the skin and the nanoparticles, the exposed lecithin could mix and fuse with lipids in the skin to loosen the structure of the skin and disturb the lamellar arrangement of the lipids to increase the thickness of the stratum corneum.Citation38,Citation58 The effects of the nanoparticles and their ingredients, including chitosan, TPGS, and lecithin, on the skin weakened the stratum corneum barrier and facilitated drug permeation.
Conclusion
Quercetin-loaded chitosan-lecithin nanoparticles were prepared in this study for topical administration. Addition of TPGS into the nanosystem resulted in increased entrapment efficiency and drug-loading of quercetin. Compared with the quercetin propylene glycol solution, the quercetin-loaded nanoparticles could notably enhance the skin permeation of quercetin in vitro and in vivo, and effectively promote the retention of quercetin in the epidermis. The interaction between nanoparticles and the skin surface changed the morphology of the stratum corneum and broke the close conjugation of the corneocyte layers, which resulted in the increased permeation of quercetin into the skin. It can be concluded that the obtained formulation of chitosan-lecithin nanoparticles could be a promising vehicle for topical delivery of quercetin.
Acknowledgment
This work was supported by a research grant from the Science and Technology Department of Shandong University, People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- SaijaaATomainoaATrombettaaDGiacchibMPasqualeaADBoninaFInfluence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoidsInt J Pharm199817518594
- SkaperSDFabrisMFerrariVCarbonarMDLeonAQuercetin protects cutaneous tissue-associated cell types including sensory neurons from oxidative stress induced by glutathione depletion: cooperative effects of ascorbic acidFree Radic Biol Med19962246696789013129
- WisemanHHalliwellBDamage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancerBiochem J1996313117298546679
- BorsWHellerWMichelCSaranMFlavonoids as antioxidants: determination of radical-scavenging efficienciesMethods Enzymol199018634355
- BorsWHellerWMichelCSaranMFlavonoid antioxidants: rate constants for reactions with oxygen radicalsMethods Enzymol19942344204297808314
- LaughtonMJEvansPJMoroneyMAHoultJRSHalliwellBInhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing abilityBiochem Pharmacol1991429167316811656994
- DecharneuxTDuboisFBeauloyeCWattiaux-De ConinckSWattiauxREffect of various flavonoids on lysosomes subjected to an oxidative stressBiochem Pharmacol1992447124312481417948
- TorelJCillardJCillardPAntioxidant activity of flavonoids and reactivity with peroxy radicalPhytochemistry1986252383386
- CaoGSoficEPriorRLAntioxidant and prooxidant behavior of flavonoids: structure-activity relationshipsFree Radic Biol Med19972257497609119242
- KumariAYadavSKPakadeYBSinghBYadavSCDevelopment of biodegradable nanoparticles for delivery of quercetinColloids Surf B Biointerfaces201080218419220598513
- ChoksiANPoonawallaTWilkersonMGNanoparticles: a closer look at their dermal effectsJ Drugs Dermatol20109547548120480790
- GamerAOLeiboldEvan RavenzwaayBThe in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skinToxicol in Vitro200620330130716182508
- CrossSEInnesBRobertsMSTsuzukiTRobertsonTAMcCormickPHuman skin penetration of sunscreen NPs: in-vitro assessment of a novel micronized zinc oxide formulationSkin Pharmacol Physiol200720314815417230054
- KelmannaRGKuminekaGTeixeirabHFKoesteraLSCarbamazepine parenteral nanoemulsions prepared by spontaneous emulsification processInt J Pharm20073421–223123917582711
- PavelićZSkalko-BasnetNFilipović-GrcićJMartinacAJalsenjakIDevelopment and in vitro evaluation of a liposomal vaginal delivery system for acyclovirJ Control Release20051061–2344315979189
- CerauloLFanaraSTurco LiveriVRuggirelloAPanzeriWMeleAOrientation and molecular contacts of melatonin confined into AOT and lecithin reversed micellar systemsColloids Surf A Physicochem Eng Asp20083161–3307312
- SchubertMAHarmsMMüller-GoymannCCStructural investigations on lipid NPs containing high amounts of lecithinEur J Pharm Sci2006272–322623616298113
- SonvicoFCagnaniARossiAFormation of self-organized NPs by lecithin/chitosan ionic interactionInt J Pharm20063241677316973314
- ŞenyiǧitTSonvicoFBarbieriSÖzerÖSantiPColomboPLecithin/chitosan NPs of clobetasol-17-propionate capable of accumulation in pig skinJ Control Release2010142336837319932722
- LiuHTLiWMXuGChitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cellsPharmacol Res200959316717519121394
- ParkPJJeJYKimSKFree radical scavenging activity of chito oligosaccharides by electron spin resonance spectrometryJ Agric Food Chem200351164624462714705887
- YoonHJMoonMEParkHSImSYKimYHChitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cellsBiochem Biophys Res Commun2007358395495917512902
- QiaoYBaiXFDuYGChitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stressInt Immunopharmacol201111112112721059391
- FakhryASchneiderGBZahariasRSenelSChitosan supports the initial attachment and spreading of osteoblasts preferentially over fibroblastsBiomaterials200425112075207914741622
- SinglaAKChawlaMChitosan: some pharmaceutical and biological aspects – an updateJ Pharm Pharmacol20015381047106711518015
- IllumLChitosan and its use as a pharmaceutical excipientPharm Res1998159132613319755881
- AgnihotriSAMallikarjunaNNAminabhaviTMRecent advances on chitosan-based micro- and NPs in drug deliveryJ Control Release2004100152815491807
- SungHWChenMCLinYHLiangHFTuHGP Medical Inc, National Tsing Hua University, Assignee. Nanoparticles for protein drug deliveryUS Patent 7919293452011
- HammesMSinghAEffect of polycations on permeability of glomerular epithelial cell monolayers to albuminJ Lab Clin Med199412334374467510767
- MeiDMaoSSunWWangYKisselTEffect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in ratsEur J Pharm Biopharm200870387488118656537
- LangothNKahlbacherHSchöffmannGThiolated chitosans: design and in vivo evaluation of a mucoadhesive buccal peptide drug delivery systemPharm Res200623357357916411147
- SchipperNGOlssonSHoogstraateJAdeBoerAGVårumKMArturssonPChitosans as absorption enhancers for poorly absorbable drugs. 2: mechanism of absorption enhancementPharm Res19971479239299244151
- OzcanIAbaciOUztanAHEnhanced topical delivery of terbinafine hydrochloride with chitosan hydrogelsAAPS Pharm Sci Tech200910310241031
- WuXLandfesterKMusyanovychAGuyRHDisposition of charged nanoparticles after their topical application to the skinSkin Pharmacol Physiol201023311712320051712
- ZhuWYuAWangWDongRWuJZhaiGFormulation design of microemulsion for dermal delivery of penciclovirInt J Pharm20083601–218419018541394
- SorensenENWeismanGVidaverGAA Sephadex column procedure for measuring uptake and loss of low molecular weight solutes from small, lipid-rich vesiclesAnal Biochem1977822376384562078
- SinghBMehtaGKumarRBhatiaAAhujaNKatareOPDesign, development and optimization of nimesulide-loaded liposomal systems for topical applicationCurr Drug Deliv20052214315316305415
- LvQYuAXiYDevelopment and evaluation of penciclovir-loaded solid lipid NPs for topical deliveryInt J Pharm20093721–219119819429280
- ZhaoXLiuJPZhangXLiYEnhancement of transdermal delivery of theophylline using microemulsion vehicleInt J Pharm20063271–2586416926077
- PugliaCBlasiPRizzaLLipid NPs for prolonged topical delivery: An in vitro and in vivo investigationInt J Pharm20083571–229530418343059
- FangJYFangCLLiuCHSuYHLipid NPs as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC)Eur J Pharm Biopharm200870263364018577447
- MuLSeowPHApplication of TPGS in polymeric nanoparticulate drug delivery systemColloids Surf B Biointerfaces2006471909716412619
- GaoYLiLBZhaiGPreparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecinColloids Surf B Biointerfaces200864219419918325744
- GaoLZhangDChenMStudies on pharmacokinetics and tissue distribution of oridonin nanosuspensionsInt J Pharm20083551–232132718242896
- RepkaMAMcGinityJWBioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusionJ Control Release200170334135111182204
- ChangTBenetLZHebertMFThe effect of water-soluble vitamin E on cyclosporine pharmacokinetics in healthy volunteersClin Pharmacol Ther19965932973038653992
- YuanHLuLJDuYZHuFQStearic acid-g-chitosan polymeric micelle for oral drug delivery: in vitro transport and in vivo absorptionMol Pharm20118122522238
- LiuWGuoYInteraction between flavonoid, quercetin and surfactant aggregates with different chargesJ Colloid Interface Sci2006302262563216870199
- MishraPRAl ShaalLMüllerRHKeckCMProduction and characterization of hesperetin nanosuspensions for dermal deliveryInt J Pharm20093711–218218919162147
- Van EerdenbrughBVan den MooterGAugustijnsPTop-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid productsInt J Pharm20083641647518721869
- BaertLvan’t KloosterGDriesWDevelopment of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatmentEur J Pharm Biopharm200972350250819328850
- LiuJHuWChenHNiQXuHYangXIsotretinoin-loaded solid lipid nanoparticles with skin targeting for topical deliveryInt J Pharm2007328219119516978810
- ChenHChangXDuDPodophyllotoxin-loaded solid lipid nanoparticles for epidermal targetingJ Control Release2006110229630616325954
- Lombardi BorgiaSRegehlyMSivaramakrishnanRLipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopyJ Control Release2005110115116316297487
- ReifenrathWGHawkinsGSKurtzMSPercutaneous penetration and skin retention of topically applied compounds: an in vitro-in vivo studyJ Pharm Sci19918065265321941541
- ZhuWGuoCYuaAGaoYCaoFZhaiGMicroemulsion-based hydrogel formulation of penciclovir for topical deliveryInt J Pharm20093781–215215819463929
- El MaghrabyGMTransdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactantsInt J Pharm20083551–228529218243604
- El MaghrabyGMBarryBWWilliamsACLiposomes and skin: from drug delivery to model membranesEur J Pharm Sci2008344–520322218572392