Abstract
Background
Gene therapy provides a novel method for the prevention and treatment of cancer, but the clinical application of gene therapy is restricted, mainly because of the absence of an efficient and safe gene delivery system. Recently, we developed a novel nonviral gene carrier, ie, heparin-polyethyleneimine (HPEI) nanoparticles for this purpose.
Methods and results
HPEI nanoparticles were used to deliver plasmid-expressing mouse survivin-T34A (ms-T34A) to treat C-26 carcinoma in vitro and in vivo. According to the in vitro studies, HPEI nanoparticles could efficiently transfect the pGFP report gene into C-26 cells, with a transfection efficiency of 30.5% ± 2%. Moreover, HPEI nanoparticle-mediated ms-T34A could efficiently inhibit the proliferation of C-26 cells by induction of apoptosis in vitro. Based on the in vivo studies, HPEI nanoparticles could transfect the Lac-Z report gene into C-26 cells in vivo. Intratumoral injection of HPEI nanoparticle-mediated ms-T34A significantly inhibited growth of subcutaneous C-26 carcinoma in vivo by induction of apoptosis and inhibition of angiogenesis.
Conclusion
This research suggests that HPEI nanoparticle-mediated ms-T34A may have a promising role in C-26 colon carcinoma therapy.
Introduction
Manipulation of apoptosis provides new strategies for treating human disease.Citation1 Survivin is one of the proteins that inhibit apoptosis and regulate cell division. Interestingly, survivin is prominently upregulated in most human cancers, with undetectable levels in normal adult tissues.Citation2–Citation4 These features make survivin of interest in targeted cancer therapy.Citation5
Gene therapy holds promise for the treatment of various diseases for which there is little hope of finding a conventional cure.Citation6 Cancer is the leading cause of death in economically developed countries,Citation7 and is a major public health problem throughout the world. Based on GLOBOCAN 2008 estimates, about 12.7 million cancer cases and 7.6 million cancer deaths are estimated to have occurred in 2008. Of these, 56% of cases and 64% of deaths occurred in the developing world.Citation8
Colorectal cancer is the second most common cause of cancer death in men and women, with an estimated incidence in the United States of 145,290 and a mortality rate of 56,290 in 2005.Citation9 Worldwide, colorectal cancer affects more than one million people every year and is responsible for more than 0.5 million cancer-related deaths annually. Presently, only 70% of colorectal tumors are resectable, of which only 75% are curable. However, 25% of resected patients will have recurrent disease, and 19% of patients have advanced disease at the time of diagnosis, of whom the majority present with distal metastases, with some presenting with unresectable tumors.Citation10 Survivin is highly expressed in colon cancer. Downregulating wild-type survivin can inhibit proliferation of colon cancer cells by induction of apoptosis, showing potential application in colon cancer therapy.Citation11,Citation12
Integrity of the survivin pathway is required for viability of cancer cells.Citation13 Molecular antagonists of survivin including antisense or expression of a dominant negative mutant result in spontaneous apoptosis of cancer cells in vitro and in vivo. Survivin phosphorylation on Thr34 may be required to preserve cell viability at the cell division stage, and loss of phosphorylation on Thr34 results in apoptosis of cells.Citation14,Citation15 Recent research has found that transfection of cancer cells with the mouse survivin Thr34 → Ala mutant (ms-T34A) gene led to apoptosis of cancer cells.Citation16–Citation18 Thus, targeting the survivin pathway in cancer with the ms-T34A gene is a novel protocol for cancer gene therapy.Citation19,Citation20
Currently, many cancer-associated genes are being identified. However, the clinical application of gene therapy is restricted, mainly because of the absence of effective and safe gene delivery technology.Citation21–Citation23 Polyethyleneimine (PEI) is one of the most efficient nonviral gene transfection agents. The commercially available branched polyethyleneimine (25,000 kDa, PEI25K) has been used as the “gold standard” to evaluate the transfection efficiency of other newly developed nonviral gene carriers. However, polyethyleneimine is not biodegradable and has severe cytotoxicity.Citation24–Citation26 Coupling short PEI chains into a longer chain using biodegradable linkers can create biodegradable PEI derivates with high transfection efficiency and low toxicity, and this provides an interesting method for developing an advanced PEI-based gene carrier.Citation27,Citation28
Recently, we prepared biodegradable heparin-conjugated PEI2K (HPEI) nanoparticles as a novel nonviral gene carrier with low cytotoxicity and high transfection efficiency.Citation29 In this work, we used these HPEI nanoparticles to deliver the ms-T34A gene to treat colon cancer in vitro and in vivo. The results suggest that the HPEI nanoparticle-mediated ms-T34A gene may be a novel candidate for colon cancer therapy.
Materials and methods
Materials
Polyethyleneimine (molecular weight 2000, PEI2K), polyethyleneimine (molecular weight 25,000, PEI25K), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), 2-(N-morpholino)ethane-sulfonic acid (MES), Dulbecco’s modified Eagle’s medium (DMEM), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO). Heparin (molecular weight 4000–6000) was purchased from Fluka (Milwaukee, WI). The C-26 colon carcinoma cells were obtained from the American Type Culture Collection (Rockville, MD). Female BALB/c mice aged 6–8 weeks were obtained from the West China Experimental Animal Center. The C-26 colon carcinoma cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum and 100 μg/mL amikacin, and were maintained in a humidified chamber at 37°C in a 5% CO2 atmosphere.
Preparation of plasmid DNA
Plasmid pVITRO2 (Invitrogen, San Diego, CA) expressing ms-T34A was constructed in our laboratory.Citation18 Escherichia coli colonies containing ms-T34A and a null colony were cultured in Luria-Bertani broth, with addition of ampicillin 100 μg/mL. The recombinant plasmids were prepared using an Endofree Plasmid Giga kit (Qiagen, Chatsworth, CA). Endotoxin levels of the prepared plasmid DNA were determined by Tachypleus amebocyte lysate. No genomic DNA, small DNA fragments, or RNA were detected in the prepared DNA. The DNA was then dissolved in sterile endotoxin-free water, and the concentration of DNA was determined by ultraviolet spectrophotometry.
Preparation and characterization of HPEI nanoparticles
Preparation
HPEI was prepared according to a method previously described.Citation29 Briefly, 50 mg of heparin was dissolved in MES solution buffer (0.05 M, 100 mL); 20 mg of EDC and 30 mg of NHS were then added to this solution for two hours to activate the carboxylic acid groups of heparin. The activated heparin solution was dropped into PEI2K solution (7.5 mg/mL, 20 mL) while stirring constantly. This reaction was carried out at room temperature overnight. The resulting HPEI nanoparticles were dialyzed in distilled water for three days, filtered by a syringe filter (Millex-LG, Millipore Co, Billerica, MA), adjusted to a concentration of 1 mg/mL, and stored at 4°C for future use.
Characterization
The morphology of the HPEI nanoparticles was observed under a transmission electron microscope (H-6009IV, Hitachi, Japan). The HPEI nanoparticles were then diluted with distilled water and placed on a copper grid covered with nitrocellulose. Samples were negatively stained with phosphotungstic acid. The particle size and zeta potential of the HPEI nanoparticles were determined by dynamic light scattering (Malvern Nano-ZS 90, Worcestershire, UK). The temperature was kept at 25°C during the measuring process. All results were the mean of three test runs.
The DNA-HPEI complexes with different ratios of nitrogen atoms from PEI to the phosphate group from DNA (N/P) were electrophoresed on 1% (w/v) agarose gel for 30 minutes at 100 V. The gel was stained with ethidium bromide 0.5 mg/mL and illuminated on an ultraviolet illuminator to show the location of DNA.
Transfection in vitro
Twenty-four hours prior to transfection, C-26 cells were seeded into a six-well plate (Becton-Dickinson, Franklin Lakes, NJ) at a density of 1 × 105 cells per well in 2 mL of complete DMEM containing 10% fetal calf serum). At the time of transfection, the medium in each well was replaced with 1 mL of fresh serum-free medium. pGFP was used as a report gene. The amount of pGFP was kept at 2 μg/well, while the mass ratios of HPEI/pGFP, PEI25K/pGFP, and PEI2K/pGFP were 5/1, 1/1, and 5/1, respectively. Six hours later, the medium was replaced by fresh medium. After 48 hours, the transfected cells were observed under a fluorescence microscope (Carl Zeiss Microimaging Inc, Thornwood, NJ), and the transfection efficiency was recorded by flow cytometry (Epics Elite ESP, Beckman Coulter, Fullerton, CA).
Preparation of HPEI-DNA complexes
HPEI-DNA complexes were prepared by mixing 5 μg HPEI (5 μL, 1 mg/mL) solution with 1 μg of pVITRO2/GFP plasmid, pVITRO2/null plasmid, or pVITRO2/ms-T34A plasmid, followed by incubation for 30 minutes at room temperature.
In vitro studies
Cytotoxicity assay
C-26 cells were plated at a density of 1 × 104 cells/well into a 96-well plate and incubated at 37°C overnight in DMEM. The medium was then removed, and the cells were then washed with serum-free DMEM without antibiotics, and 200 μL serum-free DMEM without antibiotics was added to the wells. Normal saline (control), HPEI (1 μg), the null HPEI complex (DNA-HPEI, 0.2 μg:1 μg), or ms-T34A-HPEI complex (DNA:HPEI, 0.2 μg:1 μg) was then added to the wells. The plate was incubated for 48 hours at 37°C. Cell viability was then examined using the MTT method.
Morphological analysis
After transfection for 48 hours, C-26 cells were suspended in hypotonic propidium iodide solution containing 50 μg propidium iodide/mL in 0.1% sodium citrate plus 0.1% Triton X-100, incubated in the dark for 10 minutes, and examined by fluorescence microscopy.
Flow cytometric analysis
After the transfected DNA (ms-T34A or null)-HPEI complexes (DNA:HPEI, 1:5) were incubated for 48 hours, the C-26 cells in the six-well plates were washed once with 300 μL of phosphate-buffered solution, detached with 300 μL of trypsin/EDTA, centrifuged (1500 rpm, three minutes), and the supernatant was discarded. The C-26 cells were fixed with 75% alcohol, centrifuged (1500 rpm, three minutes), and resuspended with propidium iodide in an ice bath. Thirty minutes later, these samples were analyzed using a flow cytometer (ESP Elite).
In vivo study
Ten mice were subcutaneously injected with 100 μL of C-26 cell suspension (1 × 106) into the right flank. The β-galactosidase-expressing plasmid pCMV-LacZ was used to evaluate the transfection efficiency of HPEI in vivo. When the mean tumor diameter was 10 mm, the tumor-bearing mice were randomly divided into two groups, and treated with intratumoral injection of null-HPEI (5 μg:40 μg) or Lac-Z-HPEI (5 μg:40 μg), respectively. After 48 hours, an in situ β-galactosidase staining kit (Beyotime, China) was used to detect tumor expression of the Lac-Z gene.
Therapy studies
Female BALB/c mice were injected subcutaneously with 100 μL of C-26 cell suspension (1 × 106) into the right flank. After the tumor mean diameter reached about 6 mm, the tumor-bearing mice were randomly divided into four groups and received the following treatment by intratumoral injection: normal saline, HPEI nanoparticles (25 μg), null-HPEI complexes (5μg plasmid/25μg HPEI), and ms-T34A-HPEI complexes (5μg ms-T34A/25μg HPEI. The mice were treated with five doses at intervals of 3 days. The tumor volume was calculated as 0.52 × length × width2, and was recorded every 3 days. When mice in the control group became very weak, they were sacrificed by dislocation of the cervical vertebra.
Analysis of histology and apoptosis
The tumors were fixed in 4% paraformaldehyde in phosphate-buffered solution for at least 24 hours. Tissues were embedded in paraffin, and at least four cross-sections 3–5 μm thick were taken from each tumor and stained with hematoxylin and eosin. A commercially available TUNEL kit (Promega, Madison, WI) was used to investigate the apoptotic cells in the C-26 tumors. This analysis was performed following the manufacturer’s protocol, and the samples were examined with a fluorescence microscope (400×).
The tumors were stored at −80°C, and to examine microvessel density, were immunostained with an epithelial cell marker goat antimouse CD31 antibody (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Rabbit-antigoat TRITC (dilution 1:100; Santa Cruz Biotechnology) was added, and the tumors were then left in a humidified chamber protected from light at 37°C for one hour and counterstained with Hoechst 33258 for 10 minutes. The microvessel density was determined as the average number of CD31-positive small vessels in a field (400×).
Statistical analysis
The data were evaluated using a two-tailed unpaired Student’s test or compared by one-way analysis of variance, and are expressed as the mean ± standard deviation. P < 0.05 was considered to be statistically significant.
Results and discussion
Use of advanced gene carrier systems is very important for developing a successful gene therapy protocol. Some functional genes associated with disease have been discovered, but the clinical use of gene therapy remains restricted, mainly due to the absence of safe and efficient gene delivery technologies. Nanoparticles have attracted attention as drug delivery systems.Citation30–Citation32 Some nanoparticle-encapsulated drugs are already in clinical trials or marketed.Citation33 Cationic nanoparticles can be used as a nonviral gene vector in vitro and in vivo.Citation34–Citation36 PEI is one of the most efficient nonviral gene transfection agents. However, PEI is not biodegradable and has high cytotoxicity, which greatly restricts its clinical application.Citation37–Citation41 Binding short PEI chains into longer ones using biodegradable linkers can create PEI derivates with high transfection efficiency and low toxicity.Citation42–Citation44 These PEI derivates have more potential in gene therapy than the commercially available PEI25K.Citation45–Citation47 Recently, we used heparin to conjugate low molecular weight PEI (PEI2K), creating novel cationic HPEI nanoparticles with low cytotoxicity and high transfection efficiency.Citation29 Up until now, HPEI nanoparticles have been used to deliver therapeutic genes (such as pVSVMP, pIL-15, and FILIP1L) to treat cancer. The results have shown HPEI nanoparticles to be a novel nonviral gene carrier with promising application in cancer gene therapy.Citation29,Citation48 In this work, we prepared a plasmid-expressing ms-T34A gene and used HPEI nanoparticles to deliver this gene to treat colon cancer in vitro and in vivo. The anticancer effect of HPEI nanoparticle-mediated ms-T34A gene therapy was evaluated, and the relevant anticancer mechanisms are discussed.
Preparation and characterization of HPEI nanoparticles
HPEI nanoparticles were prepared in accordance with our previous description, as schematically represented in . The freshly prepared HPEI nanoparticles were characterized in detail, and found to have a dynamic diameter of 78 nm ± 3.4 nm and a polydispersity index of 0.153, as determined by dynamic light scattering. The transmission electron microscopic image of the HPEI nanoparticles () indicates that these particles had a mean size of about 26 nm. To our knowledge, transmission electron microscopy determines the size of dry particles, while dynamic light scattering determines the hydrodynamic diameter of particles in water. HPEI nano-gels are likely to have high water absorption, because dry HPEI nanoparticles (about 26 nm) can absorb water and swell to become nanogels with a size of about 78 nm. Thus, there is disagreement about particle size measurement by dynamic light scattering and transmission electron microscopy. The zeta potential spectrum for the HPEI nanogels is presented in ; these nanoparticles were cationic and had a zeta potential of 29 ± 0.68 mV.
Figure 1 Preparation and characterization of HPEI nanoparticles. (A) Preparation scheme, (B) transmission electron microscopic image of HPEI nanoparticles, (C) zeta potential spectrum of HPEI nanoparticles, (D) the DNA-binding ability of HPEI nanoparticles determined by gel retardation assay, (E) fluorescent image of transfected C-26 cells, and (F) transfection efficiency determined by cytometry analysis.
Abbreviation: HPEI, heparin-polyethyleneimine.
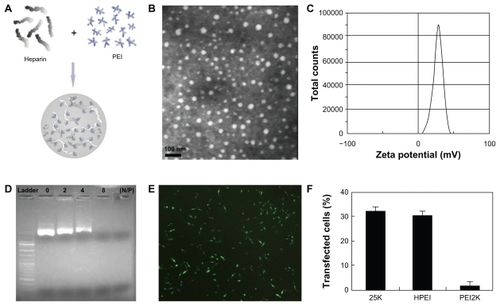
The DNA-binding ability of the HPEI nanoparticles was evaluated by gel retardation assay, and is shown in . When the N/P was 8, complete retardation of DNA was achieved. This suggests that the HPEI nanoparticles could bind DNA completely when the N/P was ≥8.
The transfection efficiency of these HPEI nanoparticles was evaluated in C-26 cells, with GFP plasmid used as the report gene. We observed that many cells had GFP-derived green fluorescence, indicating that HPEI nanoparticles could efficiently transfect the gene into C-26 cells (). Moreover, flow cytometry was used to determine the transfection efficiency of the HPEI nanoparticles. As shown in , HPEI nanoparticles had a transfection efficiency of 30.5% ± 2%, which was comparable with that of PEI25K (32% ± 2.5%), while the transfection efficiency of PEI2K was very low.
Anticancer effect in vitro
The antitumor activity of the HPEI nanoparticle-mediated ms-T34A gene in the C-26 cell line was evaluated in vitro. After transfection with the ms-T34A-HPEI complexes for 48 hours, viability of the C-26 cells was determined by the MTT method, and the results are presented in . The HPEI-mediated ms-T34A gene efficiently inhibited the viability of transfected C-26 cells, while HPEI nanoparticles and the HPEI nanoparticle-mediated null plasmid did not significantly reduce cell viability. This result implies that HPEI nanoparticle-mediated ms-T34A-HPEI has anticancer activity in C-26 colon cancer cells.
Figure 2 Antitumor activity of ms-T34A-HPEI on C-26 cancer cells in vitro. (A) Cell viability was measured by MTT, (B) cellular apoptosis was testified by flow cytometric analysis, and (C) nuclear morphology of C-26 cells, which were treated with the indicated agents for 48 hours and analyzed for nuclear apoptosis by propidium iodide staining.
Abbreviations: HPEI, heparin-polyethyleneimine; ms-T34A, mouse survivin-T34A; NS, normal saline.
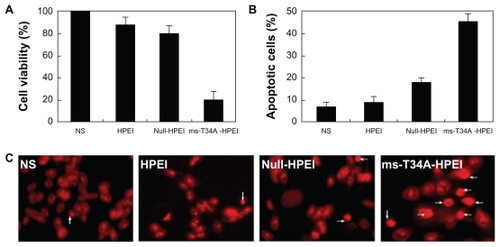
Moreover, we used morphological observations of apoptosis and flow cytometry analysis to evaluate whether induction of apoptosis was one of the reasons why ms-T34A-HPEI complexes are able to kill cancer cells. The results of the flow cytometry are presented in , showing that ms-T34A-HPEI significantly increased the proportion of apoptotic cells (46.8%) compared with other agents (normal saline 7.5%, HPEI 9.5%, null-HPEI 18.2%). According to the morphological observations, there were more apoptosis cells (shown with arrow) in the ms-T34A-HPEI treatment group than in the other treatment groups (). These results suggest that induction of apoptosis was involved in the anticancer mechanism of HPEI nanoparticle-mediated ms-T34A gene therapy.
Antitumor effect of ms-T34A-HPEI in vivo
First, we examined the ability of HPEI nanoparticles to transfect the ms-T34A gene into C-26 carcinoma cells in vivo. Lac-Z gene was used as the report gene. The HPEI-mediated Lac-Z gene was intratumorally injected into the C-26 tumor at a volume of about 500 mm3. Forty-eight hours later, an in situ β-galactosidase staining kit was used to detect expression of β-galactosidase in vivo. As shown in , the blue stain indicates expression of β-galactosidase, revealing that the Lac-Z gene could be efficiently transfected into C-26 cells in vivo. This result confirmed the transfection ability of HPEI nanoparticles in vivo.
Figure 3 Antitumor effect of ms-T34A-HPEI on subcutaneous C-26 tumor models in vivo. (A) Transfection ability of HPEI was evaluated in vivo, while Lac-Z gene was used as the report gene. Blue staining means the gene expression, and (B) tumor volume of each treatment group. Mice bearing C-26 tumors were treated with NS, HPEI, null-HPEI, or ms-T34A-HPEI every three days for five doses (5 μg DNA/dosage).
Note: There was a significant difference in tumor volume between ms-T34A-HPEI and the controls (P < 0.05).
Abbreviations: HPEI, heparin-polyethyleneimine; ms-T34A, mouse survivin-T34A; NS, normal saline.
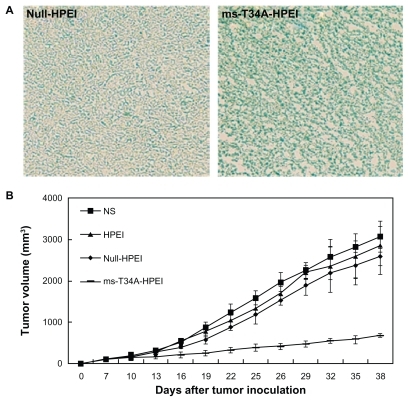
A subcutaneous mouse colon cancer model was used to study the effect of ms-T34A-HPEI on tumor growth inhibition. Tumor-bearing mice were divided into four groups and treated with normal saline, HPEI, null-HPEI, or ms-T34A-HPEI by intratumoral injection. The tumor volume was recorded every three days. Four days after the final dose, the mice were sacrificed, and their tumors were excised and preserved. The ms-T34A-HPEI treatment resulted in regression of tumor growth by 74.8%, 71.2%, and 66.2% compared with the normal saline, HPEI, and null-HPEI groups, respectively (P < 0.05, ). Moreover, according to our previous study, intratumoral injection of low-dose naked ms-T34A plasmid did not significantly inhibit the growth of C-26 carcinoma in vivo, which may be due to the limited expression of naked plasmid in vivo. These results suggest that the HPEI nanoparticle-mediated ms-T34A gene can inhibit growth of C-26 carcinoma in vivo.
To study the mechanism of anticancer activity for HPEI nanoparticle-mediated ms-T34A in vivo, we carried out hematoxylin and eosin staining and TUNEL assays. As shown in , many strongly positive nuclei identified as being in apoptotic cells could be observed in the ms-T34A-HPEI complex-treated tumor tissues, whereas such nuclei were rare in the other groups by TUNEL assay. As shown in , a large number of cells with nuclear condensation were identified as being apoptotic in the tumor tissues treated with ms-T34A-HPEI complexes, whereas nuclear condensation was rare in the other groups on hematoxylin and eosin staining. These results imply that induction of apoptosis may be a mechanism for inhibition of colon cancer by ms-T34A-HPEI complexes in vivo.
Figure 4 Detection of apoptotic tumor cells. (A) TUNEL assay (green fluoresence indicates apoptotic cells) and (B) hematoxylin and eosin assay. ms-T34A-HPEI complexes induced a significant nuclear condensation, implying induction of apoptosis.
Abbreviations: HPEI, heparin-polyethyleneimine; ms-T34A, mouse survivin-T34A; NS, normal saline.
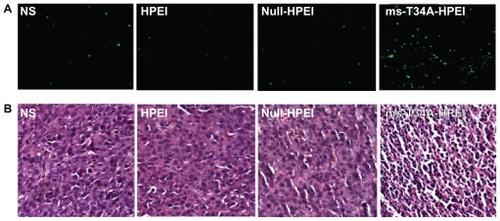
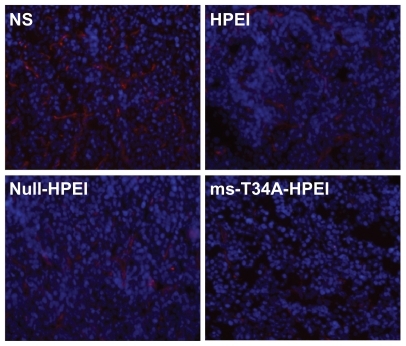
Moreover, tumor sections from each treatment group were stained with CD31 to evaluate microvessel density and counterstained with Hoechst 33258 to mark the cell nucleus. Treatment with ms-T34A-HPEI resulted in dramatic inhibition of angiogenesis in the tumors (). This implies that antiangiogenesis may be another mechanism for inhibition of colon cancer by the ms-T34A-HPEI complexes in vivo.
Figure 5 Angiogenesis of tumor tissues stained by CD31 in different groups. It revealed that ms-T34A-HPEI treatment led to a dramatic inhibition of angiogenesis in tumor tissue.
Abbreviations: HPEI, heparin-polyethyleneimine; ms-T34A, mouse survivin-T34A; NS, normal saline.
Survivin warrants attention as a method for targeted cancer therapy because of its differential expression in tumors versus normal tissues and as a bifunctional protein that acts as a suppressor of apoptosis and plays a central role in cell division.Citation4,Citation16–Citation18 The results of propidium iodide staining and flow cytometric analysis in vitro as well as TUNEL staining in vivo suggested that ms-T34A-HPEI significantly induced apoptosis of cells, more so in the treated group than in controls. Previously, some reports also found that the survivin gene could kill cancer cells by inducing apoptosis,Citation2,Citation4,Citation16–Citation18 which supports our results.
The results of CD31staining in each group indicated that microvessel density in the ms-T34A-HPEI group decreased markedly compared with controls. The generation of new blood vessels (angiogenesis) is important for normal embryonic development and for the development of pathologic conditions such as cancer.Citation49 Previous studies have found that the ms-T34A gene can suppress tumor growth by inhibiting angiogenesis. This may involve upregulated survivin expression during the proliferative phase, with the nonproliferative phase of angiogenesis being a transcriptional target for vascular endothelial growth factors,Citation11,Citation50 indicating that vascular endothelial growth factor protects endothelial cells against apoptosis during angiogenesis by upregulating survivin.Citation12–Citation15 Therefore, in addition to inhibiting tumor cell growth, targeted survivin may also prove to be beneficial in inducing apoptosis in proliferating endothelial cells within the tumor vasculature.Citation17,Citation18
Worldwide, colorectal cancer is responsible for more than 0.5 million cancer-related deaths annually, so new therapeutics for colon cancer are needed.Citation51,Citation52 Gene therapy provides a novel method for cancer treatment. Recently, some gene therapy protocols for colon cancer have been successfully developed, and their positive results are encouraging.Citation53,Citation54 Survivin is upregulated in colon cancer but undetectable in normal adult tissue. Previous work has indicated that the ms-T34A gene can selectively kill tumor cells, but not normal cells in vitro and in vivo,Citation55,Citation56 suggesting a potential therapeutic application for the ms-T34A gene in colon cancer.Citation57 In this work, we used a novel nonviral gene carrier to deliver the ms-T34A gene, and found that ms-T34A/HPEI complexes can inhibit tumor growth efficiently in vitro and in vivo. Moreover, ms-T34A/HPEI complexes did not show strong toxicity to normal cells and tissues. Our results suggest that the HPEI nanoparticle-mediated ms-T34A gene may be an interesting biodrug for colon cancer therapy.
Overall, HPEI nanoparticles can efficiently transfect this gene into C-26 colon cancer in vitro and in vivo. HPEI nanoparticle-mediated ms-T34A can prevent proliferation of C-26 cancer cells by induction of apoptosis. Moreover, HPEI nanoparticle-mediated ms-T34A delivery can inhibit growth of C-26 cancer cells in vivo by induction of apoptosis and antiangiogenesis. These results suggest that HPEI nanoparticle-mediated ms-T34A may be a good candidate for colon cancer therapy.
Conclusion
HPEI nanoparticles are a novel nonviral gene carrier, and can efficiently transfect the ms-T34A gene into C-26 colon cancer cells. HPEI nanoparticle-mediated ms-T34A gene delivery can inhibit growth of C-26 colon cancer cells in vitro and in vivo. Moreover, antiangiogenesis and induction of apoptosis in cancer cells are involved in the anticancer mechanism of HPEI nanoparticle-mediated ms-T34A gene therapy in C-26 colon cancer. Our findings may provide some evidence for targeting survivin in C-26 colon cancer and suggest that ms-T34A-HPEI complexes can be considered as a novel treatment approach for C-26 colon cancer.
Acknowledgment
This work was supported by the National Key Basic Research Program (973 Program) of China (2011CB910703) and the Fund from Science and Technology Department of Sichuan Province (2010SZ0156).
Disclosure
The authors report no conflicts of interest in this work.
References
- DoroudDZahedifardFGholamiECationic solid lipid nanoparticles loaded by cysteine proteinase genes as a novel anti-leishmaniasis DNA vaccine delivery system: characterization and in vitro evaluationsJ Pharm Pharm Sci20101332033521092706
- NagarajSPisarevVKinarskyLDendritic cell-based full-length survivin vaccine in treatment of experimental tumorsJ Immunother20073016917917471164
- IdenoueSHirohashiYTorigoeTA potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteinsClin Cancer Res2005111474148215746049
- ReedJCCancer immunotherapy targeting survivinClin Cancer Res200396523653314695157
- YaoXYoshiokaYMorishigeTSystemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasisGene Ther2009161395140419641532
- Cavazzana-CalvoMThrasherAMavilioFThe future of gene therapyNature200442777978114985734
- World Health OrganizationThe global burden of disease: 2004 updateGeneva, SwitzerlandWorld Health Organization2008
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin20116699021296855
- JemalAMurrayTWardECancer statisticsCA Cancer J Clin200555103015661684
- HuertaSEmilyJEdwardHColon cancer and apoptosisAm J Surg200619151752616531147
- RyanBMO’DonovanNDuffyMJSurvivin: A new target for anti-cancer therapyCancer Treat Rev20093555356219559538
- HarikumarKBAggarwalBBResveratrol: a multitargeted agent for age associated chronic diseasesCell Cycle2008781020103518414053
- YangYXGaoYChenLLDownregulation of survivin expression and enhanced chemosensitivity of MCF-7 cells to adriamycin by PDMAE/survivin shRNA complex nanoparticlesInt J Pharm201140518819521130850
- LiFZAmbrosiniGChuEYControl of apoptosis and mitotic spindle checkpoint by survivinNature19983965805849859993
- SharifiNQiJBaneSSurvivin is not induced by novel taxanesMol Pharm201072216222320863081
- PengXCYangLYangLPEfficient inhibition of murine breast cancer growth and metastasis by gene transferred mouse survivin Thr34→Ala mutantJ Exp Clin Cancer Res2008274618816410
- ShanYWangCTYangLInhibition of human lung adenocarcinoma growth using survivint34a by low-dose systematic administrationJ Biosci20103520921620689177
- PanLPengXCLengFTherapeutic effects of survivin dominant negative mutant in a mouse model of prostate cancerJ Cancer Res Clin Oncol2011137192820217127
- OhlfestJRFreeseABLargaespadaDANonviral vectors for cancer gene therapy: prospects for integrating vectors and combination therapiesCurr Gene Ther2005562964116457652
- ItoTYoshiharaCHamadaKDNA/polyethyleneimine/hyaluronic acid small complex particles and tumor suppression in miceBiomaterials2010312912291820047759
- FujitaTFuruhataMHattoriYHigh gene delivery in tumor by intratumoral injection of tetraarginine-PEG lipid-coated protamine/DNAJ Control Release200812912412718513820
- BrownMDSchätzleinAGUchegbuIFGene delivery with synthetic (non viral) carriersInt J Pharm200122912111604253
- KimTHParkIKNahJWGalactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrierBiomaterials20032537893792
- ParkTGJeongJHKimSWCurrent status of polymeric gene delivery systemsAdv Drug Deliv Rev20065846748616781003
- ZengXSunYXQuWBiotinylated transferrin/avidin/biotinylated disulfide containing PEI bioconjugates mediated p53 gene delivery system for tumor targeted transfectionBiomaterials2010314771478020219242
- Henriksen-LaceyMChristensenDBramwellVWLiposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI responseJ Control Release201014510210820381556
- WaltherWSteinUVossCStability analysis for long-term storage of naked DNA: impact on nonviral in vivo gene transferAnal Biochem200331823023512814626
- LiangBHeMLChanCYThe use of folate-PEG-grafted-hybranched- PEI nonviral vector for the inhibition of glioma growth in the ratBiomaterials2009304014402019427690
- GouMLMenKZhangJAEfficient inhibition of C-26 colon carcinoma by VSVMP gene delivered by biodegradable cationic nanogel derived from polyethyleneimineACS Nano201045573558420839784
- FoillardSZuberGDorisEPolyethylenimine-carbon nanotube nano-hybrids for siRNA-mediated gene silencing at cellular levelNanoscale201131461146421301705
- FengLZhangSLiuZGraphene based gene transfectionNanoscale201131252125721270989
- GouMMenKShiHCurcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivoNanoscale201131558156721283869
- WangXYangLChenZShinDMApplication of nanotechnology in cancer therapy and imagingCA Cancer J Clin2008589711018227410
- KievitFMVeisehOFangCChlorotoxin labeled magnetic nanovectors for targeted gene delivery to gliomaACS Nano201044587459420731441
- PatilMLZhangMMinkoTMultifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencingACS Nano201151877188721322531
- ZhangJSunHMaPXHost-guest interaction mediated polymeric assemblies: multifunctional nanoparticles for drug and gene deliveryACS Nano201041049105920112968
- ItakaKKataokaKRecent development of nonviral gene delivery systems with virus-like structures and mechanismsEur J Pharm Biopharm20097147548318955136
- HashemiMParhizBHHatefiARamezaniMModified polyethyleneimine with histidine-lysine short peptides as gene carrierCancer Gene Ther201118121920930861
- ParkJWBaeKHKimCClustered magnetite nanocrystals cross-linked with PEI for efficient siRNA deliveryBiomacromolecules20111245746521190334
- ChoiYKimCWAntitumor effects of combined granulocyte macrophage colony stimulating factor and macrophage inflammatory protein-3 alpha plasmid DNACancer Sci20101012341235020804501
- GantaSShahiwalaAAmijiMA review of stimuli-responsive nanocarriers for drug and gene deliveryJ Control Release200812618720418261822
- LiSDHuangLNon-viral is superior to viral gene deliveryJ Control Release200712318118317935817
- JoshiMDMullerRHLipid nanoparticles for parenteral delivery of activesEur J Pharm Biopharm20097116117218824097
- CanineBFHatefiADevelopment of recombinant cationic polymers for gene therapy researchAdv Drug Deliv Rev2010621524152920399239
- KlinkDSchindelhauerDLanerAGene delivery systems – gene therapy vectors for cystic fibrosisJ Cyst Fibros2004320321215463959
- BakerAHDesigning gene delivery vectors for cardiovascular gene therapyProg Biophys Mol Biol20048427929914769440
- MorilleMPassiraniCVonarbourgAProgress in developing cationic vectors for non-viral systemic gene therapy against cancerBiomaterials2008293477349618499247
- ZhouXLiXGouMAntitumoral efficacy by systemic delivery of heparin conjugated polyethylenimine-plasmid interleukin-15 complexes in murine models of lung metastasisCancer Sci20111021403140921564417
- WeiYQWangQRZhaoXImmunotherapy of tumors with xenogeneic endothelial cells as a vaccineNat Med200061160116611017149
- O’ConnorDSSchechnerJSAdidaCControl of apoptosis during angiogenesis by survivin expression in endothelial cellsAm J Pathol200015639339810666367
- WilsonAJChuehACTögelLApoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene inductionCancer Res20107060962020068171
- YodkeereeSSungBLimtrakulPAggarwalBBZerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: evidence for an essential role of reactive oxygen speciesCancer Res2009696581658919654295
- Sartore-BianchiAMartiniMMolinariFPIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodiesCancer Res2009691851185719223544
- LiHJEvertsMYamamotoMCurielDTHerschmanHRCombined transductional untargeting/retargeting and transcriptional restriction enhances adenovirus gene targeting and therapy for hepatic colorectal cancer tumorsCancer Res20096955456419147569
- O’ConnorDSGrossmanDPlesciaJRegulation of apoptosis at cell division by p34cdc2 phosphorylation of survivinProc Natl Acad Sci U S A200097131031310711069302
- AltieriDCValidating survivin as a cancer therapeutic targetNat Rev Cancer20033465412509766
- BhatnagarNLiXChenYZhouXGarrettSHGuoB3,3′-diindolylmethane enhances the efficacy of butyrate in colon cancer prevention through down-regulation of survivinCancer Prev Res20092581589