Abstract
In the present work, we prepared silver nanoparticles by laser ablation of pure silver plate in ethanol and then irradiated the silver nanoparticles using a 532 nm Q-switched Nd:YAG pulsed laser. Transmission electron microscopic images of the sample after irradiation clearly showed formation of big structures, such as microrods and microbelts in ethanol. The obtained microbelts had a width of about 0.166 μm and a length of 1.472 μm. The reason for the formation of such a big structure is the tendency of the nanoparticles to aggregate in ethanol before irradiation, which causes fusion of the nanoparticles.
Keywords:
Introduction
Metal nanoparticles with their wide range of applications, such as catalytic systems with optimized selectivity and efficiency, optical components, targeted thermal agents for exploitation in drug delivery and medical therapies, and surface-enhanced Raman spectral probing, have attracted research attention during the last decade. The size-dependent and shape-dependent properties of nanoparticles render them different from their corresponding bulk materials with macroscopic dimensions. Fabrication of metal nanoparticles by laser ablation of bulk plate in liquid media has been investigated intensively in recent research.Citation1–Citation4 The results of these studies have shown that laser beam irradiation onto metal nanoparticles causes fragmentation or fusion of the particles due to photothermal melting.Citation5–Citation9 Therefore, laser irradiation can be used to control the shape and size of particles. On the other hand, there are some reports about fabrication of nanonetworks and nanowire structures by laser ablation of solids in liquid mediaCitation10,Citation11 and laser irradiation of nanocolloids.Citation12 For example, Tsuji et al have found that secondary laser irradiation of a nanocolloid in water can cause formation of a nanowire structure. According to their report, fusion of melted particles as a result of absorption laser beam is an acceptable interpretation of the formation of nanowire. There are reports from further researchers showing that particle aggregation before irradiation is an important factor for particle fusion.Citation13,Citation14 They have found that after laser light irradiation, agglomerated gold colloids become larger, but dispersed colloids become smaller. This means that when colloidal particles are well dispersed in liquid, only fragmentation of particles happens upon laser irradiation. In this study, we report the production of silver nanoparticles in ethanol used as a nonstabilizing medium for preparation of nanoparticles, and subsequent irradiation of the prepared nanoparticles to achieve large structures.
Methods
Silver plate of high purity (99.99%) from Sigma Aldrich (St Louis, MO) was ablated for 15 minutes in 10 mL of distilled water and ethanol in a 1 × 2 × 3 cm3 glass cell by a 1064 nsec-pulsed Q-switched Nd:YAG laser (Brilliant laser system) with a 5 nsec pulse duration, a 10 Hz repetition rate, and 360 mJ/pulse energy to produce silver nanoparticles. The experimental apparatus used for laser ablation of metal plate has been described elsewhere.Citation1–Citation4 The beam was focused onto the target using a 25 cm focal length lens. The plate was then removed from the glass cell, and the silver nanoparticles were irradiated by another Q-switched Nd:YAG pulsed laser (SL400/SL800 system) of wavelength 532 nm, duration 10 nsec, and pulse energy 60 mJ for 15 minutes. This second laser wavelength was used because it had previously been found that silver nanoparticles can effectively interact with this wavelength to become hot compared with 1064 nm.Citation15 The prepared samples were characterized using an ultraviolet-visible double beam spectrometer (Shimadzu, Tokyo, Japan) and a transmission electron microscope (Hitachi H-7100).
Results and discussion
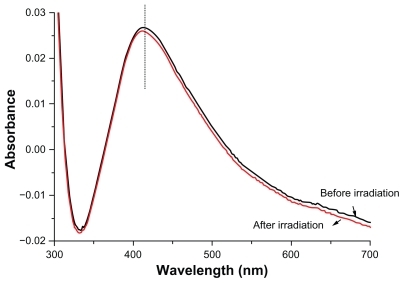
The ultraviolet-visible absorption spectra of silver nanoparticles inside ethanol before and 15 minutes after laser irradiation at a wavelength of 532 nm are presented in . Here it can be seen that the surface plasmon peak shifts a little towards higher energy and the spectra width become a little narrower after laser irradiation. According to Mie theory, this shift corresponds to a decrement in particle size which originates from fragmentation of the nanoparticles by 532 nm laser. On the other hand, the decrease in spectra width is related to a decrease in the particle size distribution within the ethanol after irradiation.
Figure 1 Ultraviolet-visible absorption spectra of silver nanoparticles inside ethanol before and after irradiation.
shows transmission electron microscopic images of silver nanoparticles inside water after 15 minutes of irradiation at 532 nm. In , all the silver nanoparticles are interconnected with each other and form a network structure, while in , silver nanowire was formed. Thus, our results are in agreement with previous work by Tsuji et al,Citation12 who produced nanowire in water by irradiation of silver colloid at 355 nm, and explained that fusion of nanoparticles that had been photothermally melted by laser irradiation was a possible mechanism for formation of the nanowire.
Figure 2 Formation of (A) and (B) network structures and (C) nanowire after irradiation of silver colloids in water.
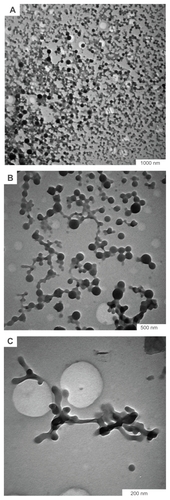
On the other hand, it has also been reported that particle aggregation before irradiation is an important factor causing fusion of nanoparticles.Citation13,Citation14 Previous research has shown that preparation of nanoparticles in ethanol using 1064 nm laser is less stable than when the procedure is performed in water.Citation16 The main reason for this instability is related to the dipole moments of the surrounding molecules. In the case of ethanol, the asymmetric charge distribution on the surface of the nanoparticles causes dipole–dipole interaction and can lead to linear assembly.Citation17 Due to Brownian motion of the particles in solution, the particles attract each other and become aggregated because of weak repulsive force and dipole-dipole interactions. Therefore, in this experiment, we used ethanol as a medium for preparation of silver nanoparticles to increase agglomeration of nanoparticles before irradiation. shows transmission electron microscopic images of silver colloid in ethanol after irradiation at 532 nm.
Figure 3 Formation of (A) bended microrod, (B) microrod, and (C) microbelt structure after irradiation of silver colloids in ethanol.

As is clear from , the structures obtained in ethanol have a different shape to those in water. show formation of large bent nanorods and straight microrods, and formation of microbelts is very clear in . The microbelts obtained had a width of 0.166 μm and a length of 1.472 μm. Therefore, the results show that aggregation of particles before irradiation can cause formation of large structures, such as microbelts.
Conclusion
In summary, we have irradiated silver colloids in water and ethanol by laser with a wavelength of 532 nm. Our results show formation of nanowire in water after irradiation, while in ethanol we obtained larger structures, such as microrods and belts. The formation of such large structures may be due to aggregation of the nanoparticles in ethanol before irradiation, which causes fusion of the nanoparticles.
Acknowledgment
The authors gratefully acknowledge the financial support by Ministry of Higher Education Malaysia through FRGS Project #01-04-10-864FR.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZamiriRAzmiBZDarroudiMPreparation of starch stabilized silver nanoparticles with spatial self-phase modulation properties by laser ablation techniqueAppl Phys A2011102189194
- DarroudiMAhmadMBZamiriRPreparation and characterization of gelatin mediated silver nanoparticles by laser ablationJ Alloys Compd201150913011304
- ZamiriRAzmiBZSadrolhosseiniARZaidanAWMahdiMAPreparation of silver nanoparticles in virgin coconut oil using laser ablationInt J Nanomedicine20106717521289983
- ZamiriRZakariaAAbbastabar AhangarHSadrolhosseiniARMahdiMAFabrication of silver nanoparticles dispersed in palm oil using laser ablationInt J Mol Sci2010114764477021151470
- TakamiAKurataHKodaSLaser-induced size reduction of noble metal particlesJ Phys Chem B199910312261232
- KamatPVFlumianiMHartlandGVPicosecond dynamics of silver nanoclusters. Photoejection of electrons and fragmentationJ Phys Chem B199810231233128
- FujiwaraHYanagidaSKamatPVVisible laser induced fusion and fragmentation of thionicotinamide-capped gold nanoparticlesJ Phys Chem B199910325892591
- AhmadiTSLogunovSLEl-SayedMSPicosecond dynamics of colloidal gold nanoparticlesJ Phys Chem199610080538056
- LinkSMohamedMBNikoobakhtBEl-SayedMALaser photothermal melting and fragmentation of gold nanorods: Energy and laser pulse-width dependenceJ Phys Chem199910311651170
- MafuneFKohnoJYTakedaYKondowTFormation of gold nanonetworks and small gold nanoparticles by irradiation of intense pulsed laser onto gold nanoparticlesJ Phys Chem B20031071258912596
- MafuneFStructure diagram of gold nanoparticles in solution under irradiation of UV pulse laserChem Phys Lett2004397133137
- TsujiTWatanabeNTsujiMLaser induced morphology change of silver colloids: Formation of nano-size wiresAppl Surf Sci2003211189193
- ChandrasekharanNKamatPVHuJJonesGDye-capped gold nanoclusters: Photoinduced morphological changes in gold/rhodamine 6G nanoassembliesJ Phys Chem B20001041110311109
- KamatPVPhotophysical, photochemical and photocatalytic aspects of metal nanoparticlesJ Phys Chem B200210677297744
- EvanoffDDJrChumanovGSize-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sectionsJ Phys Chem B20041081395713962
- TilakiRMIraji ZadAMahdaviSMSize, composition and optical properties of copper nanoparticles prepared by laser ablation in liquidsAppl Phys A200684415419
- LiaoJZhangYYuWLinear aggregation of gold nanoparticles in ethanolColloid Surf A2003223177183