Abstract
Rationale and objective
Receptor-targeted delivery of imaging and therapeutic agents can lead to enhanced efficacy for both. Multimodality imaging offers unique advantages over traditional single modality imaging. Tumor marker folate receptor (FR)-targeted fluorescent paramagnetic bimodal liposomes were synthesized to co-deliver paramagnetic and fluorescence agents for magnetic resonance (MR) and optical bimodal imaging contrast enhancement.
Materials and methods
Fluorescent and paramagnetic bimodal liposomes were synthesized with a mean diameter of 136 nm and a low polydispersity index. The liposomes incorporated folate-PEG3350-CHEMS for FR targeting, Gd(III)[N,N-Bis-stearylamidomethyl-N’-amidomethyl]diethylenetriamine tetraacetic acid (Gd-DTPA-BSA) for MR contrast, and calcein for fluorescence. To determine the specificity and efficiency of delivery, the liposomes were evaluated in FR-positive KB and HeLa cells and FR-negative A549 cells, which were analyzed by fluorescence microscopy, magnetic resonance imaging (MRI), and flow cytometry (FCM).
Results
FR-specific and efficient cellular uptake of the FR-targeted bimodal liposomes was confirmed by fluorescence microscopy and by FCM. The mean fluorescence intensity (MFI) of KB cells treated with FR-targeted liposomes was 45× that of cells treated with nontargeted liposomes, and 18× that of cells treated with FR-targeted liposomes and excess folic acid (FA). The MFI of HeLa cells treated with targeted liposomes was 33× that of nontargeted liposomes, and was 16× that of the mixture of targeted liposomes and free FA. In contrast, the MFI of A549 cells treated with FR-targeted liposomes was nearly the same as those treated with nontargeted liposomes. The T1-weighted MR images of HeLa and KB cells incubated with FR-targeted liposomes had much higher signal intensity than those treated with nontargeted liposomes or free Gd-DTPA. Furthermore, the FR-targeting effect could be blocked by excess free FA.
Conclusion
FR-targeted fluorescent paramagnetic bimodal liposomes provided a novel platform for bimodal tumor imaging and theranostic delivery.
Introduction
Noninvasive diagnostic imaging techniques such as magnetic resonance imaging (MRI) and optical tomography are becoming increasingly important in cancer diagnosis and therapy. Imaging contrast agents can facilitate tumor detection at tissue, cellular and molecular levels.Citation1 MRI enables the visualization of the three-dimensional structure of tissues. It has a high degree of spatial resolution but suffers from a relatively low sensitivity. Contrast agents can offer useful signal enhancement to MRI.Citation2 Gadoliniumdiethylenetriamine pentaacetic acid/dimeglumine (Gd-DTPA/dimeglumine) (Magnevist®, Bayer HealthCare, Leverkusen, Germany) is the first US Food and Drug Authority–approved clinical contrast agent. It is a low molecular weight compound without tissue specificity that is cleared rapidly from the circulation. Several contrast agents have been developed to prolong the circulation time and improve the tissue specificity of the magnetic resonance (MR) contrast agent.Citation3–Citation6 For example, Gd(III) [N,N-Bis-stearylamidomethyl-N′-amidomethyl] diethylenetriamine tetraacetic acid (Gd-DTPA-BSA), an amphipathic Gd complex, has been synthesized for incorporation into liposomes.Citation7 The resulting paramagnetic liposomes effectively enhanced T1-imaging. Optical imaging is another useful technology platform. Fluorescent probes can be used as optical contrast agents. However, optical imaging is hampered by the very limited depth of light penetration.Citation8 Combining MRI and fluorescence imaging would enable visualization of tumors by MRI and analysis of histological samples by fluorescence imaging.Citation9,Citation10 In addition, Kircher et al reported the delineation of tumors both by preoperative MRI and by intraoperative optical imaging.Citation11
Lipid-based nanoparticles, such as liposomes, micelles, and microemulsions, have been evaluated as carriers of MRI contrast and fluorescent agents for noninvasive imaging.Citation9,Citation12–Citation20 There are several recent reviews on lipid-based paramagnetic nanoparticlesCitation21,Citation22 and on paramagnetic and fluorescent nanoparticles.Citation3,Citation10,Citation23,Citation24 Kostarelos and Miller described a modular design for targeted paramagnetic liposomes carrying nucleic acids, termed Gd-ABCD.Citation25 These are Gd liposomes, in which “A” is condensed nucleic acids. “B” is a lipid bilayer, “C” is the PEG polymer, and “D” is a suitable ligand.Citation25
Folate receptor (FR) is a well-known tumor marker. It has limited expression in normal tissues, and is greatly overexpressed in a variety of carcinomas.Citation26 Folate conjugates of imaging agents have high binding affinity for cell-surface FRs, which allows for selective targeting of tumor cells.Citation27 Kamaly et al showed that FR-targeted fluorescent paramagnetic bimodal liposomes have increased accumulation in a FR + murine tumor model.Citation13
In this study, the authors report the synthesis and evaluation of FR-targeting bimodal liposomes for MRI and fluorescent imaging based on the previously reported Gd-ABCD design.Citation25 Gd-DTPA-BSA was incorporated into the bilayer of the liposomes as a paramagnetic agent, whereas calcein, a fluorescent dye, was encapsulated in the aqueous core of liposomes. Folate-polyethylene glycol3350-cholesteryl hemisuccinate (folate-PEG3350-CHEMS)Citation28 was used as a ligand for FR targeting. FR-mediated tumor cell targeting by these bimodal liposomes was demonstrated both by fluorescence imaging and MRI.
Materials and methods
Materials
Hydrogenated soy phosphatidylcholine (HSPC) and monomethoxy polyethylene glycol2000-distearoylphosphatidylethanolamine (mPEG2000-DSPE) were purchased from Avanti Polar Lipids Inc (Alabaster, AL). Cholesterol and octadecylamine were obtained from J&K Chemical Ltd (Beijing, China). Sepharose CL-4B chromatography media was purchased from Sigma Aldrich (St Louis, MO). Folic acid was purchased from Huixing Biological and Chemical Reagents Co, Ltd (Shanghai, China). Gadolinium oxide was purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). Diethylenetriaminepentaacetic acid (DTPA) was purchased from Tianjin Yuanhang Chemical Reagents Co, Ltd (Tianjin, China). Calcein was purchased from Aladdin Reagent Co (Shanghai, China). Folate-PEG3350-CHEM was synthesized in the authors’ laboratory, as described previously.Citation28 All reagents were of analytical grade and were used without any further purification.
L-glutamine, trypsin (1:250), and EDTA-Na2 were purchased from Amresco (Solon, OH). Penicillin-streptomycin (100,000 units/mL) was supplied by Hyclone (Logan, UT). Fetal bovine serum (FBS) was a product of Hangzhou Sijiqing Biological Engineering Materials Co, Ltd (Hangzhou, China). Roswell Park Memorial Institute medium (RPMI)–1640 10× medium without folic acid was purchased from Sigma Aldrich.
The human nasopharyngeal epidermal carcinoma KB cell line, which has been identified as being derived from the human cervical cancer HeLa cell line, was obtained from the Cell Bank at the Chinese Academy of Science (Shanghai, China). The human cervical carcinoma HeLa cell line was provided by the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, China. A549, a human lung carcinoma cell line, was provided by the Union Hospital, Tongji Medical College.
Synthesis of Gd-DTPA-BSA
[N,N-Bis-stearylamidomethyl-N′-amidomethyl]diethylenetriamine tetraacetic acid (DTPA-BSA) was prepared using the method outlined by Kamaly et al.Citation14 Briefly, a solution of octadecylamine dissolved in chloroform was added to a solution of DTPA bis-anhydride previously dissolved in dimethylformamide. The reaction was stirred at 40°C for 2 hours. The white precipitate formed was collected by filtration and dried under vacuum overnight. The white solid was stirred in water to dissolve any excess DTPA and stirred in boiling chloroform to dissolve any excess DTPA bis-anhydride and octadecylamine. Gd3+ was then added to synthesize Gd-DTPA-BSA according to the procedure of Kabalka et al.Citation7 The concentrations of Gd were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (OPTIMA 4300DV, Perkin Elmer Ltd, Waltham, MA). The phase-transition temperature of Gd-DTPA-BSA was determined by differential scanning calorimetry on a Diamond DSC (Perkin Elmer Ltd, Waltham, MA).
Bimodal liposome preparation
The FR-targeted liposomes were prepared by hydration of a thin lipid film composed of HSPC/mPEG2000-DSPE/cholesterol/Gd-DTPA-BSA/folate-PEG3350-CHEMS at 30/40/25/4/1 (mole/mole). Nontargeted liposomes composed of HSPC/mPEG2000-DSPE/Cholesterol/Gd-DTPA-BSA 30/40/25/5 (mole/mole) were synthesized by the same method. Briefly, the lipids (0.05 mmol total) were dissolved in chloroform/methanol (1/1), followed by evaporation to dryness to form a lipid film. The lipid film was subsequently hydrated in 2 mL 2.5 mM calcein solution for 40 minutes at 60°C and then sonicated for 10 minutes. The resulting lipid dispersion was extruded sequentially five times through polycarbonate membrane filters with a pore diameter of 200 nm and 100 nm (Lipex Extruder, Northern Lipids Inc, Vancouver, Canada). Liposomes and free calcein were separated by size exclusion chromatography on a Sepharose CL-4B gel (Sigma Aldrich).
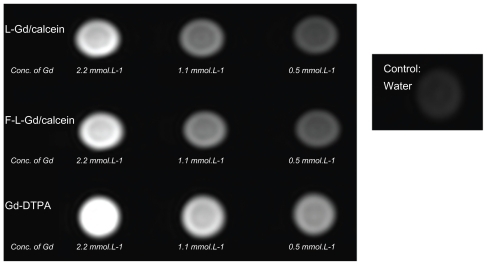
The size and zeta potential of the liposomes were determined by dynamic light scattering on a Zetasizer (nanoseries) Nano-ZS 90 (Malvern Instruments Ltd, Malvern, Worcestershire, UK). Fluorescence measurements were carried out on a fluorescence spectrophotometer (LS55 fluorescence spectrophotometer, Perkin Elmer Ltd). The incorporation of Gd in the liposome preparations was confirmed by ICP-AES. For MR imaging, nontargeted liposomes (L-Gd/calcein), FR-targeted liposomes (F-L-Gd/calcein), and Gd-DTPA were prepared at three different concentrations. The MR images of liposome suspensions were collected, along with the MR image of water. The T1-weighted image was acquired by 3.0T MRI on a Signa HDxt Hdi 3.0T MRI (General Electric, Fairfield, CT).
In vitro targeting experiments
Fluorescence imaging of cells
KB cells were plated in a six-well plate at 37°C under 5% CO2 in folate-free RPMI 1640 medium containing 10% fetal bovine serum. The cells were grown until 80% confluence was reached. The medium was then removed and replaced with fresh medium (without serum). F-L-Gd/calcein, L-Gd/calcein, and F-L-Gd/calcein plus 1 mM folic acid (FA) were added. FA was added to FR-targeted liposomes prior to use in cellular treatment. All liposomes contained calcein at a concentration of 15.2 μM. One mM FA was used as an FR blocking agent. After incubation at 37°C for 1 hour, the cells were examined on an Olympus IX71 fluorescence microscope (Perkin Elmer Ltd).
Quantitative analysis of cell uptake
KB, HeLa, and A549 cells were seeded into six-well plates and separately incubated with F-L-Gd/calcein, L-Gd/calcein, and F-L-Gd/calcein plus 1 mM FA. The final concentration of calcein in the incubation media was 12μM. After incubation at 37°C for 1 hour, the cells were washed 3× with cold phosphate-buffered saline (PBS) and treated with trypsin. The fluorescence intensity of cells was analyzed by flow cytometry (FCM) on a BD-LSR cytometer (BD Biosciences, Franklin Lakes, NJ). Each study was repeated 3–4 times.
MR imaging of cells
HeLa and KB cells were grown in folate-free RPMI 1640 medium containing 10% fetal bovine serum. The cells were grown to 80% confluence and were then suspended by treatment with trypsin. Aliquots of the cells were transferred to Eppendorf tubes and were incubated with fresh media containing FR-targeted or nontargeted liposomes. The final concentration of Gd in the incubation media was 18.5 mg/L. After incubation at 37°C for 1 hour, cells were washed 3× with cold PBS. The tubes were imaged on a 3.0T MRI with a standard spin-echo sequence (time of repetition = 20, 40, 100, 200 ms; time of echo = 9.0 ms/Fr; number of excitation: 2.0).
Results
Synthesis of Gd-DTPA-BSA
The identity of Gd-DTPA-BSA product was confirmed by its melting point and IR spectrum (data not shown). Based on ICP-AES determination, the complex contained 13.3% Gd. The phase-transition temperature of Gd-DTPA-BSA was found to be 65.32°C.
Characterization of the bimodal liposomes
Size and zeta potential of the liposomes
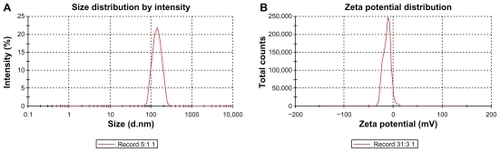
Liposomes had a mean diameter of <136 nm with a low polydispersity index. The preparations were stable for at least 3 months at 4°C (). The zeta potential of liposomes was <–13.6 mV ().
The incorporation of calcein and Gd
The maximum fluorescence emission wavelength of calcein did not change when being encapsulated in liposomes (Ex-max was at 491 nm, Em-max was at 511 nm). The encapsulation efficiency for calcein was low, ranging from 1.2% to 4.7%, as determined by UV absorption at 491 nm. Meanwhile, the encapsulation efficiency for Gd was substantially higher, ranging from 20% to 27%, as determined by ICP-AES.
MR imaging of liposomes
Compared with water, the Gd liposomes and Gd-DTPA all showed brighter MR signals in T1-weighted images (). Therefore, the paramagnetic liposomes were demonstrated to be MR detectable. The intensity of the signal was concentration dependent. A decrease in the concentration of the Gd liposomes was associated with a reduction in signal intensity in the MR image (). The MR images of Gd-DTPA were slightly brighter than those of liposomes. This might be due to easier proton exchange between the bulk water and the Gd-DTPA.
In vitro targeting experiments
Fluorescence imaging of cells
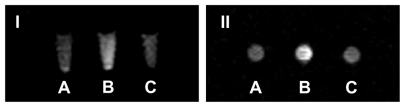
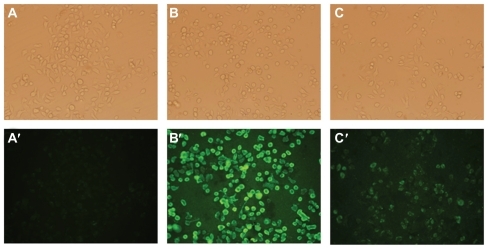
Uptake of liposomes by FR positive KB cells was analyzed by fluorescence microscopy. Green fluorescence was associated with liposomal calcein. As shown in , F-L-Gd/calcein () was taken up by the KB cells more efficiently than nontargeted liposomes (), and the uptake could be blocked by 1 mM free FA (). Additionally, FR-targeted liposomes were primarily located on the surface of cell membrane. This phenomenon was consistent with previous observations,Citation29,Citation30 which showed extensive surface binding of FR-targeted liposomes to the FR on the cell surface.
Figure 3 Uptake of liposomal calcein by KB cells. The cells were incubated at 37°C for 1 hour with L-Gd/calcein (A, A′), F-L-Gd/calcein (B, B′), or F-L-Gd/calcein + 1 mM FA (C, C′). Top panels show cells visualized in the phase-contrast mode (A, B, C); bottom panels show the same fields in the fluorescence mode (A′, B′, C′)
Abbreviation: FA, folic acid.
Quantitative analysis of cell uptake
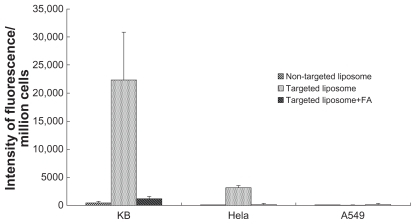
Targeted and nontargeted liposomes were incubated with FR positive tumor cells (KB and HeLa) and FR negative tumor cells (A549). Free FA, used as a blocking agent, was added to FR-targeted liposomes prior to use in cellular treatment. The FR specificity of cellular uptake of targeted and nontargeted liposomes was quantitatively analyzed by FCM. The experiments were repeated at least three times. (, ). The MFI of KB cells treated with F-L-Gd/calcein was 45 times higher than that of cells treated with nontargeted L-Gd/calcein, and 18 times higher than that of cells treated with the F-L-Gd/calcein plus 1 mM free FA. The MFI of HeLa cells treated with F-L-Gd/calcein was 33 times higher than that of cells treated with nontargeted liposomes L-Gd/calcein, and 16 times higher than that of cells treated with the F-L-Gd/calcein plus 1 mM free FA. In contrast, the MFI of A549 cells treated with F-L-Gd/calcein was nearly identical with that of cells treated with L-Gd/calcein, or F-L-Gd/calcein plus free FA. In summary, the FR-targeted liposomes F-L-Gd/calcein showed delivery specificity in FR positive cells but not in FR negative cells, and the FR targeting effect could be blocked by 1 mM free FA.
Table 1 Uptake of liposomal calcein by KB, HeLa, and A549 cells determined by flow cytometry
MRI of cells
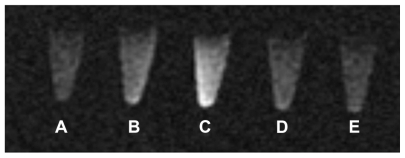
For MRI, HeLa cells were incubated with (1) blank liposome, (2) L-Gd/calcein, (3) F-L-Gd/calcein, (4) F-L-Gd/calcein + 1 mM FA, or (5) Gd-DTPA for 1 hour. From T1-weighted images, the tube containing HeLa cells incubated with F-L-Gd/calcein () had a much higher signal intensity than that of the tubes of cells treated with blank liposomes or nontargeted liposomes (). This was probably due to FR-mediated uptake by the FR-positive HeLa cells. The MR signal was diminished when the cells were incubated with F-L-Gd/calcein + 1 mM FA. This showed the blocking of FR-dependent uptake by free FA (). The MR image of HeLa cells incubated with F-L-Gd/calcein was also brighter than that of HeLa cells incubated with Gd-DTPA (). This may be due to the fact that Gd-DTPA was unable to enter cells by diffusion due to its hydrophilicity and was therefore removed during washing of the cells. Meanwhile, the liposomes were retained on the cell surface via FR binding and therefore enhanced the T1 signal.
Figure 5 T1-weighted image of HeLa cells after incubated with contrast agents (coronal scan). (A) L. (B) L-Gd/calcein. (C) F-L-Gd/calcein. (D) F-L-Gd/calcein + 1 mM FA. (E) Gd-DTPA.
Abbreviations: FA, folic acid; DTPA, diethylenetriamine pentaacetic acid.
KB cells were also incubated with (1) L-Gd/calcein, (2) F-L-Gd/calcein, or (3) F-L-Gd/calcein + 1 mM FA for 1 hour at 37°C followed by washing with PBS. MRI showed that cells incubated with F-L-Gd/calcein had the highest signal intensity (), and that this could be blocked by 1 mM free FA (). These data further confirmed FR-dependent cellular uptake of F-L-Gd/calcein in another FR-positive cell line.
Discussion
Liposomes are long-circulating vehicles that are capable of encapsulation of water-soluble agents and incorporation of amphiphilic and hydrophobic agents in the lipid bilayer. Two types of liposomal MRI contrast agents have been described.Citation22,Citation23 The first is based on liposomes entrapping paramagnetic agents, typically Gd-DTPA, in the aqueous lumen.Citation31,Citation32 A drawback of these liposomes is that encapsulation reduces the relaxivity of the paramagnetic agent, because of the limited access to exchange with bulk water by the contrast agents. A second type of liposomal contrast agents are based on liposomes carrying amphiphilic paramagnetic molecules in the lipid bilayer, which makes them an integral part of the liposomal surface, therefore providing greater access to proton exchange with water compared with the liposomes of the first type. Lipid chain length and degree of saturation affect the particle relaxivity of the second type of liposomes.Citation33
In this study, a combination of an amphiphilic Gd-DTPA-BSA and a fluorescent dye, calcein, was used to prepare fluorescent paramagnetic bimodal liposomes. Gd-DTPA-BSA has been used in previous studies.Citation9,Citation19 The amount of Gd-DTPA-BSA used in this study, which was 25 mole % of total lipids (50 μmol), was consistent with previous reports.Citation9 Calcein is a hydrophilic fluorescent marker that has been used to investigate membrane permeability. Citation9 Calcein can be replaced by quantum dots, other fluorescent probes, or a hydrophilic therapeutic drug in further work. Effective FR targeting was achieved by incorporation of folate-PEG3350-CHEM at 1 mole% of the total lipids.
The small size, the slightly negative zeta potential of the liposomes (), along with surface PEGylation are expected to produce a relatively long circulation time in vivo.Citation21,Citation34 The liposomes were shown to be paramagnetic (). When the concentration of the contrast agents decreased, the contrast of MR images was also decreased. The T1 image intensity of Gd-liposomes and Gd-DTPA were much higher than that of water. In addition, the T1 images of Gd-DTPA were slightly brighter than those of Gd-liposomes at the same Gd concentration, presumably due to greater access to proton exchange with environmental water.
The uptake of F-L-Gd/calcein by KB and HeLa cells was FR specific, which was also confirmed qualitatively by fluorescence microscopy () and MRI (), and then quantitatively by FCM (, ). FR-targeted liposomes were primarily located on the surface of the cell membrane (), due to binding to FR. Uptake of the liposomes was greater in KB cells than in HeLa cells. This can be explained by the higher FR expression in the former.Citation35
Conclusion
FR-targeted fluorescent paramagnetic bimodal liposomes were synthesized. Both MRI and fluorescence imaging showed FR-specific uptake of these liposomes in FR-positive but not FR-negative cells. Further work will determine the plasma clearance kinetics and the biodistribution of the liposomes in vivo. In addition, the authors will explore loading the liposomes with therapeutic agents to enable image-guided therapy.
Acknowledgments
This work was supported by National Natural Science Foundation of China grant (No. 81072596), Program for New Century Excellent Talents in University (No. NCET-08-0226), and the Fundamental Research Funds for the Central Universities (HUST: M2009044).
Disclosure
The authors report no conflicts of interest in this work.
References
- SumerBGaoJTheranostic nanomedicine for cancerNanomedicine (Lond)20083213714018373419
- FriasJCMaYWilliamsKJFayadZAFisherEAProperties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imagingNano Lett20066102220222417034087
- JacquesVDesreuxJNew Classes of MRI Contrast AgentsKrauseWContrast Agents I221Berlin/HeidelbergSpringer Verlag2002123
- FrullanoLRohovecJPetersJGeraldesCStructures of MRI Contrast Agents in SolutionKrauseWContrast Agents I221Berlin/HeidelbergSpringer Verlag200225
- TóthÉHelmLMerbachARelaxivity of MRI Contrast AgentsKrauseWContrast Agents I221Berlin/HeidelbergSpringer Verlag200261
- BrücherEKinetic Stabilities of Gadolinium(III) Chelates Used as MRI Contrast AgentsKrauseWContrast Agents I221Berlin/HeidelbergSpringer Verlag2002103
- KabalkaGWDavisMAMossTHGadolinium-labeled liposomes containing various amphiphilic Gd-DTPA derivatives: targeted MRI contrast enhancement agents for the liverMagn Reson Med19911924064151881329
- SchnallMRosenMPrimer on imaging technologies for cancerJ Clin Oncol200624203225323316829646
- MulderWJStrijkersGJGriffioenAWA liposomal system for contrast-enhanced magnetic resonance imaging of molecular targetsBioconjug Chem200415479980615264867
- FrullanoLMeadeTJMultimodal MRI contrast agentsJ Biol Inorg Chem200712793994917659368
- KircherMFMahmoodUKingRSWeisslederRJosephsonLA multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineationCancer Res200363238122812514678964
- GianellaAJarzynaPAManiVMultifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancerACS Nano20115644224433
- KamalyNKalberTThanouMBellJDMillerADFolate receptor targeted bimodal liposomes for tumor magnetic resonance imagingBioconjug Chem200920464865519368341
- KamalyNKalberTAhmadABimodal paramagnetic and fluorescent liposomes for cellular and tumor magnetic resonance imagingBioconjug Chem200819111812917985841
- KluzaEvan der SchaftDWHautvastPASynergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesisNano Lett2010101525819968235
- van TilborgGAMulderWJChinPTAnnexin A5-conjugated quantum dots with a paramagnetic lipidic coating for the multimodal detection of apoptotic cellsBioconjug Chem200617486586816848390
- MulderWJStrijkersGJHabetsJWMR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticleFASEB J200519142008201016204353
- MulderWJvan der SchaftDWHautvastPAEarly in vivo assessment of angiostatic therapy efficacy by molecular MRIFASEB J200721237838317202248
- MulderWJKooleRBrandwijkRJQuantum dots with a paramagnetic coating as a bimodal molecular imaging probeNano Lett2006611616402777
- Van SchooneveldMMVucicEKooleRImproved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: a multimodality investigationNano Lett2008882517252518624389
- SunCLeeJSZhangMMagnetic nanoparticles in MR imaging and drug deliveryAdv Drug Deliv Rev200860111252126518558452
- MulderWJStrijkersGJvan TilborgGAGriffioenAWNicolayKLipid-based nanoparticles for contrast-enhanced MRI and molecular imagingNMR Biomed200619114216416450332
- KamalyNMillerADParamagnetic liposome nanoparticles for cellular and tumour imagingInt J Mol Sci20101141759177620480040
- MulderWJStrijkersGJvan TilborgGACormodeDPFayadZANicolayKNanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imagingAcc Chem Res200942790491419435319
- KostarelosKMillerADSynthetic, self-assembly ABCD nanoparticles; a structural paradigm for viable synthetic non-viral vectorsChem Soc Rev2005341197099416239997
- XiaWLowPSFolate-targeted therapies for cancerJ Med Chem201053196811682420666486
- SegaEILowPSTumor detection using folate receptor-targeted imaging agentsCancer Metastasis Rev200827465566418523731
- XiangGWuJLuYLiuZLeeRJSynthesis and evaluation of a novel ligand for folate-mediated targeting liposomesInt J Pharm20083561–2293618258394
- YooHSParkTGFolate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugateJ Control Release2004100224725615544872
- LeamonCPReddyJAFolate-targeted chemotherapyAdv Drug Deliv Rev20045681127114115094211
- AyyagariALZhangXGhaghadaKBAnnapragadaAHuXBellamkondaRVLong-circulating liposomal contrast agents for magnetic resonance imagingMagn Reson Med20065551023102916586449
- UngerENeedlemanPCullisPTilcockCGadolinium-DTPA liposomes as a potential MRI contrast agent Work in progressInvest Radiol198823129289323203995
- StrijkersGJMulderWJvan HeeswijkRBRelaxivity of liposomal paramagnetic MRI contrast agentsMAGMA200518418619216155762
- MaruyamaKPEG-liposome in DDS and clinical studiesNippon Rinsho19985636326379549348
- MullerCSchubigerPASchibliRIn vitro and in vivo targeting of different folate receptor-positive cancer cell lines with a novel 99 mTc-radiofolate tracerEur J Nucl Med Mol Imaging200633101162117016721570