Abstract
Background
Hybrid liposomes can be prepared by simply sonicating a mixture of vesicular and micellar molecules in buffer solutions. In this study, we investigated the effects of hybrid liposomes on the growth of human colon cancer cells in vitro.
Methods
Hybrid liposomes (HL-n, n = 21, 23, 25) composed of L-α-dimyristoylphosphatidylcholine (DMPC) and polyoxyethylene(n) dodecyl ethers (C12(EO)n, n = 21, 23, 25) were prepared by the sonication method and their inhibitory effects on growth of human colon cancer HCT116 cells were examined in vitro.
Results
Significant growth inhibition of HCT116 cells was observed in the presence of HL-n. The fifty percent inhibitory concentration (IC50) of HL-n was less than half that of DMPC liposomes. Furthermore, fluorescence microscopic and flow cytometric analyses indicated that the markedly inhibitory effects of HL-n on the growth of HCT116 cells could be attained through the induction of cell cycle arrest at the G0/G1 phase along with apoptotic cell death.
Conclusion
It was found for the first time that HL-n can induce both cell cycle arrest and apoptosis in colon cancer cells. The findings in this study should contribute to novel chemotherapy for colon cancer.
Introduction
Colorectal cancer is one of the most malignant neoplasms in the world and develops in the cecum, colon and rectum.Citation1 Generally, patients with tumoral tissues confined within the intestinal wall are curable by enucleation of the local lesions. However, when the tumors are enlarged and advanced into the deeper regions, it is difficult to cure cases with surgical treatment.Citation2 Although chemotherapy with anticancer drugs, such as 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin, can prolong survival, this therapy may not effect a complete cure for the metastatic carcinoma.Citation2,Citation3 Therefore, a new remedy for cancer is urgently needed.
Hybrid liposomes, first developed by Ueoka et al,Citation4 can be prepared by simply sonicating a mixture of vesicular and micellar molecules in buffer solutions. Inhibitory effects of hybrid liposomes composed of L-α-dimyristoylphosphatidylcholine (DMPC) and polyoxyethylene(20) sorbitan monolaurate (Tween 20) including antitumor drugs such as 1,3-bis(2-chloroethyl)-1-nitrosourea on the growth of glioma cells in vitro and in vivo have been observed.Citation5 On the other hand, hybrid liposomes composed of DMPC and polyoxyethylene(n) alkyl ethers without any drugs demonstrated remarkable inhibitory effects on the growth of various tumor cells in vitroCitation6–Citation8 and in vivo.Citation9–Citation12 In addition, it has been demonstrated that hybrid liposomes could induce apoptotic cell death in leukemia,Citation13 hepatoma,Citation7 lung cancer,Citation14 breast cancer,Citation8 and primary effusion lymphoma cells.Citation15 With respect to colon cancer cell lines, hybrid liposomes induced apoptosis in human colon adenocarcinoma WiDrCitation16 and mouse colon carcinoma Colon26 cells in vitro.Citation17 Furthermore, we also reported that hybrid liposomes distinguished between normal colon CCD33Co cells and tumor colon WiDr cells, then fused and accumulated into the plasma membranes of tumor cells, leading to apoptosis. More recently, it was observed that hybrid liposomes could inhibit growth of cholangiocarcinoma cells through induction of cell cycle arrest.Citation18 However, research concerning the effects of hybrid liposomes on the cell cycle of cancer cells is very limited.
In this study, we investigated the effects of hybrid liposomes (HL-n, n = 21, 23, 25) composed of DMPC and polyoxyethylene(n) dodecyl ethers (C12(EO)n, n = 21, 23, 25) on the growth of human colon cancer (HCT116) cells in vitro, and found significant inhibitory effects on growth of HCT116 cells through induction of cell cycle arrest at the G0/G1 phase along with apoptotic cell death.
Materials and methods
Preparation of hybrid liposomes
Hybrid liposomes (HL-n, n = 21, 23, 25) were prepared by the following methods.Citation14 DMPC (NOF, Tokyo, Japan) and polyoxyethylene(n) dodecyl ethers (C12(EO)n, n = 21, 23, 25) (C12(EO)21 and C12(EO)25; Nikko Chemicals, Tokyo, Japan, C12(EO)23; Sigma Chemicals, St Louis, MO) were mixed in 5% glucose solution and sonicated with a bath type sonicator (VS-N300, Velvo-Clear, Tokyo, Japan) at 45°C under a nitrogen atmosphere with 300 W, followed by filtration with a 0.20 μm filter. The liposomes composed of DMPC were prepared in the same manner as described above.
Dynamic light scattering method
The size of the HL-n was measured with an electrophoretic light scattering spectrophotometer (ELS-8000, Otsuka Electronics, Hirakata, Japan).Citation13 Using a He-Ne laser as the light source, a 633 nm laser line with 10 mW power was applied with a scattering angle of 90°. The hydrodynamic diameter (dhy) of the HL-n was calculated by the Stokes-Einstein equation (dhy = kT/(3πηD), where k is Boltzmann’s constant, T is the absolute temperature, η is the viscosity of solvent, and D is the diffusion coefficient).
Cell culture
Human colon cancer HCT116 cell lines were purchased from the American Type Culture Collection (Manassas, VA). HCT116 cells were maintained in RPMI-1640 medium (Gibco, Gaithersburg, MD) supplemented with penicillin 100 U/mL, streptomycin 50 μg/mL, and 10% fetal bovine serum (HyClone Laboratories, Logan, UT). The cells were cultured in a 5% CO2 humidified incubator at 37°C.
WST-1 assay
The inhibitory effects of HL-n on the growth of HCT116 cells were examined on the basis of a WST-1 (2-methoxy-4-nitrophenyl- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) assay.Citation12 HCT116 cells were seeded at a density of 2.0 × 103 cells per well in 96-well plates (Sumitomo Bakelite, Tokyo, Japan) and incubated in a humidified atmosphere of 5% CO2 at 37°C. After 24 hours, HL-n were added into each well and the plates were incubated for 48 hours. The viable cell number was measured with a Cell Counting Kit (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions, and the IC50 of HL-n was determined from the concentration-dependence of the viable cell number.
Annexin-V labeling assay
Phosphatidylserines exposed on the outer plasma membranes of apoptotic HCT116 cells were detected by Annexin-V labeling assay.Citation19 HCT116 cells were seeded at a density of 4.0 × 103 cells in glass bottom dishes (Mat Tek, Flint, MI) in a humidified atmosphere of 5% CO2 at 37°C. After 24 hours, HL-n were added at the IC50 values and the dishes were incubated for 3 hours. Subsequently, the cells were washed with phosphate-buffered saline and dyed with an Annexin-V-FLUOS staining kit (Roche Diagnostics, Basel, Switzerland). Briefly, the cells were treated with 2 μL of FLUOS-conjugated Annexin-V and 2 μL of propidium iodide stock solutions. After incubation for 10 minutes at room temperature, the cells were observed using a confocal laser microscope (TCS-SP, Leica, Germany) with a 75 mW Ar laser (Annexin-V, excitation/detection = 488 nm/500–550 nm; propidium iodide, excitation/detection = 488 nm/620–720 nm).
TUNEL method
DNA fragmentations in apoptotic HCT116 cells were detected by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) method.Citation10 HCT116 cells were seeded at a density of 4.0 × 103 cells in glass bottom dishes in a humidified atmosphere of 5% CO2 at 37°C. After 24 hours, HL-n were added at the IC50 and the dishes were incubated for 48 hours. The cells were then fixed with a 4% paraformaldehyde solution and stained using an in situ cell death detection kit (Roche Diagnostics) according to the manufacturer’s recommendations. The stained cells were observed using a confocal laser microscope with an Ar laser (TUNEL, excitation/detection = 488 nm/500–550 nm) and a He-Ne laser (TOPRO-3, excitation/detection = 633 nm/650–740 nm).
Flow cytometry
Cell cycle analysis of HCT116 cells was performed with a flow cytometer (Epics XL system II, Beckman Coulter, Fullerton, CA).Citation13,Citation16 HCT116 cells were seeded at a density of 2.0 × 103 cells per well in 6-well plates (Sumitomo Bakelite) and incubated in a humidified atmosphere of 5% CO2 at 37°C. After 24 hours, HL-n were added into each well and the plates were incubated for 48 hours. After treatment with trypsin, the cells were centrifuged at 200 × g for 5 minutes, washed with phosphate-buffered saline, and resuspended in phosphate-buffered saline containing 40 μg/mL propidium iodide, 1 mg/ mL RNase, and 0.1% Triton X-100 in a dark room. The DNA contents of the cells were then analyzed using a flow cytometer with a single excitation 488 nm of 15 mW Ar laser. The propidium iodide signal was detected by FL3 sensor at 605–635 nm and the data were analyzed on WinMDI (v 2.8; The Scripps Research Institute, Flow Cytometry Core Facility, La Jolia, CA) software.
Enzyme immunometric assay
Expression of p21 WAF1/CIP1 in HCT116 cells was analyzed by an enzyme immunometric assay.Citation20 HCT116 cells were seeded at a density of 2.0 × 103 cells per well in 6-well plates and incubated in a humidified atmosphere of 5% CO2 at 37°C. After 24 hours, HL-23 were added at 200 μM and the plates were incubated for 48 hours. After treatment with trypsin, the cells were centrifuged at 200 × g for 5 minutes, washed with phosphate-buffered saline, and resuspended in cell lysis buffer solution containing 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate. Then, p21 WAF1/CIP1 in the cell lysates was assayed using a TiterZyme® EIA human p21 enzyme immunometric assay kit (Assay Designs, Ann Arbor, MI) according to the manufacturer’s recommendations.
Results and discussion
The hybrid liposomes were prepared by sonication of a mixture containing 90 mol% DMPC and 10 mol% C12(EO)n (n = 21, 23, 25) in 5% glucose solutions, and the morphology of HL-n was measured by the dynamic light scattering method. The results are shown in . The diameters (dhy) of the HL-n were about 40 nm with a narrow range in the size distribution and remained stable for more than 1 month, whereas that of liposomes composed of only DMPC were about 200 nm in diameter. This suggested that HL-n less than 100 nm in diameter could avoid clearance by the reticular endothelial system in vivo.Citation21
Figure 1 Time course of dhy change (A) and the size distribution (B) of HL-n. HL-n (n = 21, 23, 25) were prepared by sonication of a mixture containing DMPC and C12(EO)n using a bath type sonicator in 5% glucose solution. The diameter (dhy) of HL-n was measured by a dynamic light scattering method using an electrophoretic light scattering spectrophotometer at 25°C. Data are the mean ± standard error of the mean (n = 3) from three independent experiments. [DMPC] = 10 mM, [C12(EO)n] = 1.1 mM.
Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes.
![Figure 1 Time course of dhy change (A) and the size distribution (B) of HL-n. HL-n (n = 21, 23, 25) were prepared by sonication of a mixture containing DMPC and C12(EO)n using a bath type sonicator in 5% glucose solution. The diameter (dhy) of HL-n was measured by a dynamic light scattering method using an electrophoretic light scattering spectrophotometer at 25°C. Data are the mean ± standard error of the mean (n = 3) from three independent experiments. [DMPC] = 10 mM, [C12(EO)n] = 1.1 mM.Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes.](/cms/asset/13674121-28e7-43d8-888c-d07755369217/dijn_a_24160_f0001_b.jpg)
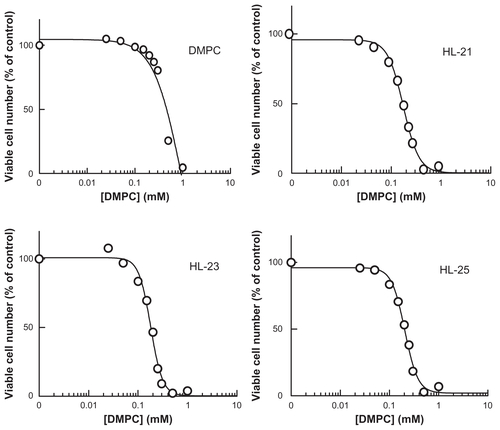
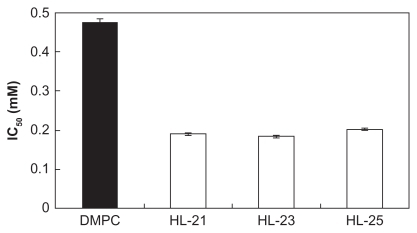
First, we examined the inhibitory effects of HL-n (n = 21, 23, 25) on the growth of colon cancer HCT116 cells in vitro on the basis of WST-1 assay. The number of viable HCT116 cells was evaluated in the absence or in the presence of different concentrations of HL-n by WST-1, and the IC50 of HL-n was determined from the concentration-dependence of the viable cell number (). In , the IC50 value is the concentration of HL-n necessary to inhibit the growth of HCT116 cells by half. The IC50 values were 0.190 mM DMPC for HL-21, 0.183 mM DMPC for HL-23, and 0.202 mM DMPC for HL-25. On the other hand, the IC50 of DMPC liposomes was 0.477 mM and the value was more than twice those of HL-n. These results indicate that HL-n should be effective for inhibiting the growth of HCT116 cells as well as in the case of other colon cancer cell lines.Citation16,Citation17 Hybrid liposomes being more fluid as compared with DMPC liposomes showed strong inhibitory effects on the growth of human colon cancer (WiDr) cellsCitation16 and human leukemia cells.Citation12 The strong inhibitory effects on the growth of HCT116 cells should be closely related to the membrane fluidity of HL-n.
Figure 2 IC50 of HL-n on the growth of HCT116 cells in vitro. Inhibitory effects of HL-n (n = 21, 23, 25) on the growth of HCT116 cells were examined on the basis of WST-1 assay. Fifty percent inhibitory concentration (IC50) of HL-n was determined from the concentration-dependence for the viable cell number of HCT116 cells incubated in the presence of HL-n for 48 hours. Data are the mean ± standard error of the mean (n = 3) from three independent experiments.
Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes; IC50, 50 percent inhibitory concentration.
Second, we examined the induction of apoptosis by HL-n in HCT116 cells with a confocal laser microscope. It is well known that the redistribution of phosphatidylserines from the inner to outer leaflet of the plasma membrane is a hallmark of early apoptotic cells. Therefore, HCT116 cells were treated with HL-n for 3 hours and observed by the Annexin-V/propidium iodide dual staining method. The double staining assay with Annexin-V and propidium iodideCitation19,Citation22 detects early apoptotic and necrotic cells as green and red fluorescent cells, respectively. The fluorescence micrographs of HCT116 cells treated with HL-n are shown in . Green fluorescence was observed at the plasma membranes of HCT116 cells after treatment with HL-n, although no green fluorescence was observed in the case of DMPC liposomes. No red fluorescent cells were observed in HCT116 cells treated with HL-n, suggesting that HL-n did not induce necrosis in HCT116 cells. On the other hand, the latter apoptotic cells are characterized by increased plasma membrane permeability and fragmentation of nuclear DNA. Using the TUNEL method, we observed the DNA fragmentation of HCT116 cells treated with HL-n for 48 hours. The fluorescence micrographs are shown in . The nuclei of all cells were stained by TOPRO-3 and exhibited red fluorescence. As regards TUNEL staining, green or orange (overlay) fluorescence was observed in cells treated with HL-n, indicating the presence of nuclear condensation and fragmentation in apoptotic cells. In contrast, green (or orange) fluorescence in HCT116 cells was not observed in the case of DMPC liposomes. These observations demonstrated induction of apoptotic cell death toward HCT116 cells by HL-n. It has been already elucidated that hybrid liposomes induce apoptosis in various tumor cells, with activations of caspases-3, -8, and -9, and reduction of mitochondria membrane potential.Citation8,Citation11–Citation13,Citation16 HL-n could probably fuse and accumulate into HCT116 cells, and induce apoptosis through activation of caspase cascades.
Figure 3 Induction of apoptosis in HCT116 cells by HL-n. (A) Fluorescence micrographs of HCT116 cells stained with FLUOS-conjugated Annexin-V and propidium iodide after the treatment with HL-n (n = 21, 23, 25) for 3 hours. (B) Fluorescence micrographs of HCT116 cells stained with TUNEL and TOPRO-3 after the treatment with HL-n (n = 21, 23, 25) for 48 hours. In both experiments, HCT116 cells were treated with HL-n at the IC50 (DMPC liposomes; [DMPC] = 0.477 mM, HL-21; [DMPC] = 0.190 mM, HL-23; [DMPC] = 0.183 mM, HL-25; [DMPC] = 0.202 mM).
Note: Scale bar; 20 μm.
Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes; IC50, 50 percent inhibitory concentration.
![Figure 3 Induction of apoptosis in HCT116 cells by HL-n. (A) Fluorescence micrographs of HCT116 cells stained with FLUOS-conjugated Annexin-V and propidium iodide after the treatment with HL-n (n = 21, 23, 25) for 3 hours. (B) Fluorescence micrographs of HCT116 cells stained with TUNEL and TOPRO-3 after the treatment with HL-n (n = 21, 23, 25) for 48 hours. In both experiments, HCT116 cells were treated with HL-n at the IC50 (DMPC liposomes; [DMPC] = 0.477 mM, HL-21; [DMPC] = 0.190 mM, HL-23; [DMPC] = 0.183 mM, HL-25; [DMPC] = 0.202 mM).Note: Scale bar; 20 μm.Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes; IC50, 50 percent inhibitory concentration.](/cms/asset/2ffc0088-bd6b-4323-822c-ec564c761d95/dijn_a_24160_f0003_c.jpg)
In order to gain further insight into the mechanism of inhibitory effects of HL-n on growth of HCT116 cells, we performed a cell cycle analysis of HCT116 cells treated with HL-n by flow cytometry. The results are shown in . After treatment with HL-n, G0/G1 populations of HCT116 cells gradually increased with the decrease of S and G2/M populations in the lower concentration range ([DMPC] = 0–0.2 mM). Interestingly, G0/G1 populations of HCT116 cells gradually decreased with the increase in sub-G1 populations in the higher concentration range ([DMPC] = 0.2–0.5 mM). These results indicate that HL-n should arrest the cell cycle progression of HCT116 cells at the G0/G1 phase at the lower concentrations and induce apoptosis of HCT116 cells at the higher concentrations. In regard to induction of cell cycle arrest by HL-n, we examined the regulatory protein p21 WAF1/CIP1, which play a key role in regulating entry of cells at the G1/S transition check point,Citation23 in HCT116 cells by an enzyme immunometric assay. As shown in , a significant increase in p21 WAF1/CIP1 was observed in HCT116 cells at 48 hours after the treatment with HL-23 ([DMPC] = 0.2 mM). This result suggests that hybrid liposome-mediated G0/G1 arrest of HCT116 cells was associated with upregulation of p21 WAF1/CIP1 protein. With respect to cell cycle regulation, some studies have reported that cell cycle arrest is closely associated with metabolic events in plasma membranes.Citation24–Citation27 Although the mechanistic details are not yet clear, it seems that HL-n could accumulate in plasma membranes of HCT116 cells, change the membrane characteristics related to cell cycle progression, and induce G0/G1 phase arrest. On the other hand, G0/G1 phase arrest was also observed for HCT116 cells treated with DMPC liposomes, that is, the G0/G1 population of HCT116 cells increased with the decrease in S and G2/M populations in a dose-dependent manner. However, the sub-G1 population of HCT116 cells was not detected in the whole concentration range ([DMPC] = 0–0.5 mM) in this study. These results in relation to the cell cycle were in good agreement with the observations for apoptotic HCT116 cells by fluorescence microscopy. In our previous study, we reported that HL-n elicited G1 phase cell cycle arrest of human cholangiocarcinoma cells, although HL-n did not induce apoptosis toward cholangiocarcinoma cells.Citation18 In this study, we have found for the first time that inhibitory effects of HL-n on the growth of HCT116 cells could be caused by induction of cell cycle arrest at the G0/G1 phase along with apoptotic cell death.
Figure 4 Cell cycle analysis of HCT116 cells treated with HL-n. (A) Cell cycle analysis of HCT116 cells was performed by flow cytometry. HCT116 cells were incubated in the presence of HL-n (n = 21, 23, 25) at the IC50 for 48 hours. DNA contents in HCT116 were analyzed using a flow cytometer.a (B) Expression of p21 WAF1/CIP1 in HCT116 cells treated with HL-23. HCT116 cells were incubated in the presence or absence (control) of HL-23 ([DMPC] = 0.2 mM) for 48 hours and p21 expression was determined by enzyme immunometric assay.b (C) Schematic representation of cell cycle arrest and apoptosis in cancer cells induced by HL-n.
Notes: *Significant difference (P < 0.05) compared with the controls (Student’s t-test); adata are the mean ± standard error of the mean (n = 3) from three independent experiments; bdata are the mean ± standard error of the mean (n = 6) from two independent experiments.
Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes.
![Figure 4 Cell cycle analysis of HCT116 cells treated with HL-n. (A) Cell cycle analysis of HCT116 cells was performed by flow cytometry. HCT116 cells were incubated in the presence of HL-n (n = 21, 23, 25) at the IC50 for 48 hours. DNA contents in HCT116 were analyzed using a flow cytometer.a (B) Expression of p21 WAF1/CIP1 in HCT116 cells treated with HL-23. HCT116 cells were incubated in the presence or absence (control) of HL-23 ([DMPC] = 0.2 mM) for 48 hours and p21 expression was determined by enzyme immunometric assay.b (C) Schematic representation of cell cycle arrest and apoptosis in cancer cells induced by HL-n.Notes: *Significant difference (P < 0.05) compared with the controls (Student’s t-test); adata are the mean ± standard error of the mean (n = 3) from three independent experiments; bdata are the mean ± standard error of the mean (n = 6) from two independent experiments.Abbreviations: DMPC, dimyristoylphosphatidylcholine; HL, hybrid liposomes.](/cms/asset/19ca4bbd-fa18-4e17-ad16-b6fe930968c2/dijn_a_24160_f0004_c.jpg)
As mentioned above, markedly inhibitory effects of HL-n on growth of human colon cancer HCT116 cells in vitro were obtained in this study. It is noteworthy that induction of cell cycle arrest along with apoptosis with HL-n could play an important role in growth inhibition of HCT116 cells (). Deviation from the normal cell cycle and the resistance to apoptosis lie at the heart of tumor development.Citation28 This study suggests that HL-n could be an effective chemotherapeutic agent for colon cancer in the near future.
Conclusion
In conclusion, we have clearly demonstrated the inhibitory effects of hybrid liposomes composed of DMPC and C12(EO)n on growth of human colon cancer HCT116 cells in vitro. The noteworthy aspects are as follows. The diameters of the HL-n were less than 100 nm and remained stable for more than 1 month. The markedly inhibitory effects of HL-n on growth of HCT116 cells were obtained. IC50 values of HL-n were less than half of that of DMPC liposomes. The inhibitory effects of HL-n on the growth of HCT116 cells were attained through induction of cell cycle arrest at G0/G1 phase along with apoptotic cell death. It is worthy of note that HL-n can induce both cell cycle arrest and apoptosis in colon cancer cells. The results of this study should contribute to novel chemotherapy for colon cancer.
Supplementary figure
Acknowledgments
The technical assistance of Ms Yoko Tomita and Dr Mamiko Yukihara with this research was appreciated. This work was supported in part by a Grant-in-Aid for Science Research from the Ministry of Education, Science and Culture of Japan.
Disclosure
The authors have no conflicts of interest in this work.
References
- DanaeiGVan der HoornSLopezADMurrayCJLEzzatiMThe comparative risk assessment collaborating group (cancers), causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factorsLancet200536694991784179316298215
- ScheeleJAltendorf-HofmannAResection of colorectal liver metastasesLangenbeck’s Arch Surg19993844313327
- GoldbergRMSargentDJMortonRFA randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancerJ Clin Oncol2004221233014665611
- UeokaRMatsumotoYMossRAMembrane matrix for the hydrolysis of amino acid esters with marked enantioselectivityJ Am Chem Soc1988110515881595
- KitamuraIKochiMMatsumotoYUeokaRKuratsuJUshioYIntrathecal chemotherapy with 1,3-bis(2-chloroethyl)-1-nitrosourea encapsulated into hybrid liposomes for meningeal gliomatosis: An experimental studyCancer Res19965617398639928752168
- MatsumotoYImamuraCItoTTaniguchiCUeokaRSpecific hybrid liposomes composed phosphatidylcholine and polyoxethlenealkyl ether with markedly enhanced inhibitory effects on the growth of tumor cells in vitroBiol Pharm Bull19951810145614588593457
- NakanoKIwamotoYTakataWMatsumotoYUeokaRSpecific accumulation and growth inhibitory effects of hybrid liposomes to hepatoma cells in vitroBioorg Med Chem Lett200212223251325412392725
- NagamiHMatsumotoYUeokaRInduction of apoptosis by hybrid liposomes for human breast tumor cells along with activation of caspasesBiol Pharm Bull200629238038116462050
- UeokaRMatsumotoYIchiharaHKiyokawaTChemotherapy with hybrid liposomes composed of dimyristoylphosphatidylcholine and polyoxyethylenealkyl ether without drugsMartenMRParkTHNagamuneTBiological Systems Engineering (ACS Symposium Series)Washington, DCAmerican Chemical Society2002
- IchiharaHNagamiHKiyokawaTMatsumotoYUeokaRChemotherapy using hybrid liposomes along with induction of apoptosisAnticancer Res2008282B1187119518505055
- ShimodaSIchiharaHMatsumotoYUeokaRChemotherapy with hybrid liposomes for human breast tumors along with apoptosis in vivoInt J Pharm20093721–216216819429276
- MatsumotoYIwamotoYMatsushitaTUeokaRNovel mechanism of hybrid liposomes-induced apoptosis in human tumor cellsInt J Cancer2005115337738215700314
- IwamotoYMatsumotoYUeokaRInduction of apoptosis of human lung carcinoma cells by hybrid liposomes containing polyoxyethylenedodecyl etherInt J Pharm20052921–223123915725570
- TowataTKomizuYSuzuSMatsumotoYUeokaROkadaSHybrid liposomes inhibit the growth of primary effusion lymphoma in vitro and in vivoLeuk Res201034790691120074798
- TowataTKomizuYSuzuSUeokaROkadaSHighly selective fusion and accumulation of hybrid liposomes into primary effusion lymphoma cells along with induction of apoptosisBiochem Biophys Res Commun2010393344544820138834
- KomizuYMatsumotoYUeokaRMembrane targeted chemotherapy with hybrid liposomes for colon tumor cells leading to apoptosisBioorg Med Chem Lett200616236131613417005395
- FunamotoKIchiharaHMatsushitaTMatsumotoYUeokaRMarked therapeutic effects of hybrid liposomes on the hepatic metastasis of colon carcinomaYakugaku Zasshi20091294464473 Japanese
- TowataTKomizuYSuzuSHybrid liposomes inhibit the growth of cholangiocarcinoma by induction of cell cycle arrest in G1 phaseBioorg Med Chem Lett201020123680368220494578
- Van EngelandMNielandLJWRamakerssFCSSchutteBReutelingspergerCPMAnnexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposureCytometry1998311199450519
- HsuYLKuoPLChiangLCLinCCIsoliquiritigenin inhibits the proliferation and induces the apoptosis of human non-small cell lung cancer A549 cellsClin Exp Pharmacol Physiol200431741441815236626
- HuangSKLeeKDHongKFriendDSPapahadjopoulosDMicroscopic localization of sterically stabilized liposomes in colon carcinoma-bearing miceCancer Res19925219513551431394121
- VermesIHanenCSteffens-NakkenHReutelingspergerCA novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression early apoptotic cells using fluorescein labelled Annexin VJ Immunol Methods1995184139517622868
- MassaguéJG1 cell-cycle control and cancerNature2004432701529830615549091
- HattenMEHorwitzAFBurgerMMThe influence of membrane lipids on the proliferation of transformed and untransformed cell linesExp Cell Res197710713134862677
- JackowskiSCell cycle regulation of membrane phospholipid metabolismJ Biol Chem19962713420219202228702749
- AlbinoAPJuanGTraganosFCell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: Association with decreased pRb phosphorylationCancer Res200060154139414510945621
- ZhangXHZhaoCMaZAThe increase of cell-membranous phosphatidylcholines containing polyunsaturated fatty acid residues induces phosphorylation of p53 through activation of ATRJ Cell Sci2007120Pt 234134414318032786
- EvanGIVousdenKHProliferation, cell cycle and apoptosis in cancerNature2001411683534234811357141