?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Single-walled carbon nanotubes (SWNT) are poorly soluble in water, so their applications are limited. Therefore, aqueous solutions of SWNT, designed by noncovalent functionalization and without toxicity, are required for biomedical applications.
Methods
In this study, we conjugated docetaxel with SWNT via π-π accumulation and used a surfactant to functionalize SWNT noncovalently. The SWNT were then conjugated with docetaxel (DTX-SWNT) and linked with NGR (Asn-Gly-Arg) peptide, which targets tumor angiogenesis, to obtain a water-soluble and tumor-targeting SWNT-NGR-DTX drug delivery system.
Results
SWNT-NGR-DTX showed higher efficacy than docetaxel in suppressing tumor growth in a cultured PC3 cell line in vitro and in a murine S180 cancer model. Tumor volumes in the S180 mouse model decreased considerably under near-infrared radiation compared with the control group.
Conclusion
The SWNT-NGR-DTX drug delivery system may be promising for high treatment efficacy with minimal side effects in future cancer therapy.
Introduction
Due to the unique physical and chemical properties of carbon nanotubes, they are considered to be potential biomedical materials for delivery of biological macromolecules and chemicals into cells and living systems for the purposes of disease diagnosis and therapy.Citation1–Citation3 According to the size of single-walled nanotubes (SWNT) and their uptake by cells, targeted delivery of SWNT-borne pharmaceuticals at the cellular level could be enhanced, and the discomfort associated with current intrusive techniques could be minimized. SWNT is a unique one-dimensional material, and has been explored as a novel drug delivery vehicle in vitro.Citation4,Citation5 SWNT can effectively shuttle various biomolecules including drugs, peptides, proteins, plasmid DNA, and small interfering RNA into cells via endocytosis.Citation6–Citation9 SWNT are not soluble in aqueous solution, which limits their application. However, SWNT with particular design features are able to enter cells without toxicity, giving this nanomaterial wide biomedical applications.Citation10–Citation12
Angiogenic tumor vessels are important elements for tumor growth and metastasis. They are essential for transporting metabolically essential materials to and from tumor cells, and also provide a route for dissemination of tumor cells to distal sites. The Asn-Gly-Arg (NGR) peptide motif has been used as a targeting head for target drugs and drug-containing liposomes to CD13, the tumor vascular antigen,Citation13,Citation14 resulting in improved biodistribution and more effective tumor therapy.Citation15,Citation16 Peptides containing NGR have shown high efficiency in targeted cells and tissues.Citation17,Citation18
Docetaxel (N-debenzoyl-N-tert-butoxy-carbonyl-10-deacetyl) is a new class of taxane, which has achieved higher patient response rates and fewer side effects compared with paclitaxel in the treatment of a wide spectrum of cancers, including breast, ovarian, small and nonsmall cell lung, and head/neck cancer.Citation19–Citation22 Both paclitaxel and docetaxel act by binding to microtubules and inhibiting microtubule depolymerization to free tubulin. As a result, the equilibrium within the microtubule system is disrupted, leading to mitotic arrest in the G2/M phase of the cell cycle and ultimately to cell death.Citation23 In this study, docetaxel was used as a model anticancer agent.
Biological systems are known to be highly transparent under 700–1100 nm near-infrared light. The strong optical absorbance of SWNT can be used for photothermal therapy to afford multifunctional biological nanotube transporters.Citation24–Citation28 Continuous near-infrared irradiation can cause cell death in vitro because of excessive local heating of the SWNT.Citation29
In this study, we developed a SWNT-NGR delivery system conjugated with docetaxel (SWNT-NGR-DTX). In this delivery system, docetaxel was attached through π-π accumulation to SWNT and dispersed in water with PVPk30 and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000)-maleimide (DPM). Because the maleimide group at the end of DPM on the surface of SWNT can react with the double bond in the sulfhydryl groups, the maleimide was reacted with cysteine in CNGRCK2HK3HK11 (C containing sulfhydryl groups) and connected covalently. This delivery system targeted as tumor cells as shown in . It was hypothesized that the docetaxel attached to SWNT would be released from the SWNT-NGR-DTX delivery system in a sustained fashion, and the ability of docetaxel to kill tumor cells would be enhanced through near-infrared irradiation-mediated tumor destruction by the photothermal effect on the carbon nanotubes.
Materials and methods
SWNT produced by chemical vapor deposition (purity > 90%) were purchased from Chengdu Organic Chemicals Co Ltd (Chengdu, China). A PC3 human prostate cancer cell line and S180 mouse ascites tumor cells were obtained from the Chinese Academy of Sciences Cell Bank. NGR peptides (CNGRCK2HK3HK11) were compounded by Shanghai Botai Biotechnology Co Ltd (Shanghai, China). Docetaxel was purchased from Beijing YiHe Biotechnology Co Ltd, DPM and other reagents were all purchased from Sigma (St Louis, MO). Experimental animals were provided by the Henan Laboratory Animal Center.
Preparation of SWNT-DTX
SWNT 5 mg was mixed with docetaxel 20 mg in anhydrous ethanol 0.5 mL using an ultrasonic bath for about 15 minutes while dropwise phosphate-buffered solution 3 mL was added. The mixture was ultrasonicated using an ultrasonic probe (400 W, 10 times). The suspension was centrifuged at 10,000 rpm for 15 minutes until the SWNT were fully precipitated, and the supernatants were discarded. The remaining solids were thoroughly rinsed with anhydrous ethanol and deionized water to remove excess docetaxel.
Conjugation of DPM to SWNT-DTX
The SWNT-DTX were resuspended in an aqueous solution of PVPk30 10 mg/mL and DPM 0.1 mg/mL,Citation30 and the solution was then sonicated using an ultrasonic probe (400 W, 10 times). The suspension was then filtered through a membrane filter (Whatman, Maidstone, UK) with a pore size of 100 nm to remove the excess surfactants, rinsed thoroughly with ultrapure H2O, and then centrifuged at 4000 rpm for 15 minutes and repeated to remove macromolecular particles.
Preparation of SWNT-NGR-DTX
NGR (CNGRCK2HK3HK11) water solution was added to the SWNT-DTX suspension, which was modified by surfactant, and DPM with NGR and maleimide in a molar ratio of 1:20.Citation31 After being left to stand overnight at room temperature, SWNT-NGR-DTX was obtained. The solid was washed with ultrapure H2O through a membrane filter to remove excess NGR.
Thin layer silica gel chromatography using n-butanol-water-acetic acid (4:2:1) as the developing agent and ninhydrin solution 0.5% as the coloring agent were used to test for conjugation of NGR to SWNT-DTX.
Characterization of SWNT-NGR-DTX
Determination of loading efficiency of docetaxel
SWNT-NGR-DTX was diluted with acetonitrile and sonicated to ensure that the docetaxel was dissolved completely, then centrifuged to separate the SWNT and docetaxel to determine the amount of docetaxel bound to the SWNT. The concentration of docetaxel in acetonitrile was determined by high-pressure liquid chromatography. The chromatographic conditions were set as follows: an Eclipse XDB-C18 column (150 mm × 4.6 mm, 5.0 μm); mobile phase acetonitrile/ water 50:50; column temperature 30°C; detection wave-length 231 nm; flow rate 1.0 mL/minute; and injection volume 20 μL. Absorbance at 808 nm (A808) was used to determine the SWNT concentration in the suspension by visible spectrophotometry.Citation29
Determination of size distribution and zeta potential
A 100 μL sample of SWNT-NGR-DTX nanosuspension was diluted with ultrapure H2O to 2 mL. The size distribution and zeta potential of the nanosuspension was determined at 25°C using a Nano ZS-90 (Malvern Instruments, Worcestershire, UK).
Transmission electron microscopy
The SWNT-NGR-DTX nanosuspension was dripped onto the copper line and scanned by transmission electron microscopy (Tecnai G2 20) at 100 kV.
Preparation of SWNT-NGR-DTX-FITC and cell uptake
Fluorescein isothiocyanate (FITC) 0.1 mL in dimethyl sulfoxide 1 mg/mL was added to 5 mL of SWNT-NGR-DTX, and the mixture was then sonicated and protected from light. Gel chromatography was used to remove the excess FITC by loading 1 mL of the solution onto a Sephadex G-25 column (Sigma). When the elution solvent (H2O) was flown through the column, the formation of two separate yellow bands was observed. The fractions were collected, and the absorbance of the various fractions was measured at 488 nm with a spectrophotometer. Fractions from the first elution peak were pooled because they were attributed to the higher molecular weight SWNT-NGR-DTX-FITC conjugate (also confirmed by fluorescence measurement).Citation29
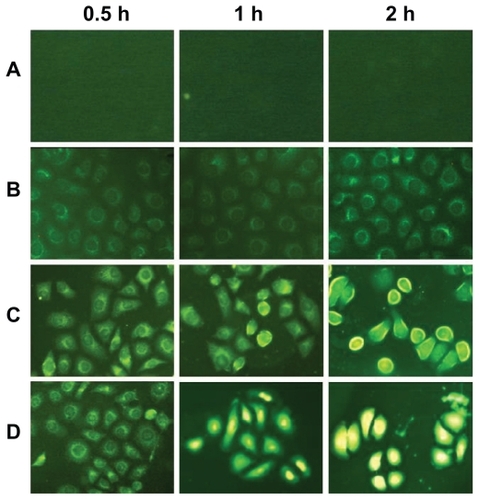
PC3 cells were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum and penicillin-streptomycin 1%. The incubations of SWNT-NGR-DTX-FITC with PC3 cells were carried out on cover glasses in six-well plates, with the cells having been seeded for 24 hours before incubation. SWNT-NGR-DTX-FITC was added to each well at a final SWNT concentration of 2.5–5 μg/mL. The incubations were carried out at 37°C in 5% CO2 for 0.5, 1, and 2 hours, respectively. After incubation, the cover glasses were washed with phosphate-buffered solution followed by soaking for 15 minutes in 4% polymerisatum, then washed with deionized water. The cells were imaged by a fluorescence microscope (Zeiss LSM 510).
Cytotoxicity
PC3 cells were plated in 96-well plates and treated with different concentrations of SWNT-NGR for 72 hours to investigate the cytotoxicity of the delivery system alone, and then SWNT-NGR-DTX, SWNT-DTX, or docetaxel for 24, 48, and 72 hours. The weight ratio of SWNT to docetaxel was 1:1.2. Cell viability was measured using the sulforhodamine B assay.
Pharmacokinetics and biodistribution studies
The concentrations of docetaxel in plasma from the tumor-free healthy mice were measured after intravenous injection of SWNT- NGR-DTX, SWNT-DTX, or docetaxel. The dose of docetaxel used in this study was chosen to approximate the intraperitoneal dosage of docetaxel used in humans (75 mg/m2).
The mice were fasted for 12 hours before injection, but had access to water ad libitum. The experimental groups and the control group were given SWNT-NGR-DTX, SWNT-DTX, and docetaxel, respectively (docetaxel in each group at 25.125 mg/kg). Blood samples were collected at 0.083, 0.25, 0.5, 1, 2, and 4 hours after injection. At hours 0.5, 1, and 2 after administration, the tissues/organs were collected, weighed, and homogenized in buffer (acetonitrile to saline ratio,1:1) for biodistribution analyses. The weight ratio of tissues to buffer was 1:3. The tissues were then sonicated to a homogenate. Docetaxel concentrations in plasma were analyzed by high-pressure liquid chromatography.
Animal model and treatment
All care and treatment of the animals used in this study was performed in accordance with the guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. The S180 tumor models were generated by subcutaneous injection of 2 × 106 S180 cells in 0.2 mL of phosphate-buffered solution into the right shoulder of female BALB/c mice. The mice were used for the study when the tumor volume reached 100 mm3 (7 days after tumor inoculation).
The animals were divided into five groups (n = 6 per group) to receive normal saline (controls), SWNT-NGR, docetaxel, SWNT-DTX, or SWNT-NGR-DTX. The injected doses were normalized to 25.125 mg/kg of docetaxel. The mice were observed daily for clinical symptoms, and the tumor sizes were measured by caliper on alternate days, and the volume calculated as (tumor width)Citation2 × (tumor length)/2.
The study preparations were injected into the mice via the caudal tail vein every 2 days. After seven administrations, the mice were euthanized, and tissues from the heart, liver, spleen, lung, kidney, brain, thymus, and the tumors were harvested and soaked in 10% formalin solution. The tissues collected were embedded in paraffin and mounted on slides stained with hematoxylin and eosin. Morphological changes were observed under a microscope.
Laser radiation at 808 nm
For the ex vitro laser radiation experiments, SWNT-NGR-DTX solutions at high, middle, and low concentrations (SWNT approximately 8.3, 4.2, and 0.9 μg/mL; docetaxel about 10, 5, and 1 μg/mL) were irradiated by 808 nm laser at 1.4 W/cm2, and the temperature was measured.
For the in vitro laser radiation experiments, PC3 were seeded in 96-well plates, and incubated with SWNT-NGR-DTX at high, middle, and low concentrations, respectively. Six hours after treatment, the medium containing the drug was transferred to fresh medium, and the laser irradiation groups were put under an 808 nm laser for 1, 2, and 3 minutes. After 24 hours, a sulforhodamine B assay was performed to determine cell viability and calculate the inhibition rate.
The in vivo laser radiation experiment followed the same steps as for the animal model and treatment section described earlier, except that 808 nm laser irradiation was performed to tumor tissues in the S180 mice model for one minute after each administration.
Statistical analysis
Quantitative data were expressed as the mean ± standard deviation. Means were compared using the Student’s t-test with SPSS 17.0 statistical software (SPSS Inc, Chicago, IL), and P values <0.05 were considered to be statistically significant.
Results and discussion
Preparation of soluble SWNT-DTX
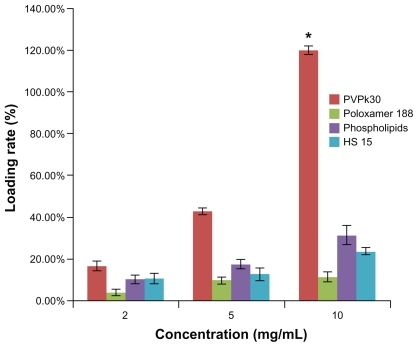
SWNT are not soluble in water, which greatly hinders their application as drug carriers. In this study, the dispersion effect of several surfactants on SWNT was examined. Lecithin High Potency, Poloxamer 188, HS 15, and PVPk30 were used at different concentrations. The loading rate was dose-dependent using PVPk30, and reached a maximum (SWNT to docetaxel ratio about 1:1.2) at 10 mg/mL of PVPk30 ().
Figure 2 Effects of different surfactants on loading rate. Concentrations of PVPk30, Poloxamer 188, phospholipids, and HS 15 were 2, 5, and 10 mg/mL, respectively.
Notes: Data are presented as the mean ± standard deviation (n = 3). *P < 0.01 versus Poloxamer 188, phospholipids, and HS 15 at 10 mg/mL, respectively.
Because SWNT is a new drug delivery carrier, there is not yet a well established method or procedure for its preparation. Our experiment adopts the method of saturated solution crystalization method, which means that docetaxel is dispersed into a molecular state using a soluble organic solvent, SWNT are added, and the water phase is then gradually added under ultrasound to reduce the solubility of docetaxel so that it can be separated out slowly and attached to SWNT with the influence of ultrasonic power. SWNT-DTX is precipitated by high-speed centrifugation, the organic solvent and water phase are removed, SWNT-DTX is dispersed again into aqueous solution with surfactants, and finally the suspension is centrifuged to remove the macromolecular particles.
During the preparation process, docetaxel was added to the SWNT, but they were difficult to adsorb due to their hydrophobic characteristics. Therefore, molecular docetaxel was added to make it fully exposed to the surface. When the phosphate-buffered water phase was dripped into the docetaxel-ethanol solution, docetaxel was gradually precipitated and adsorbed onto the SWNT and, using an ultrasonic probe instrument, more energy was applied to achieve stronger adsorption. Therefore, the power and frequency of the ultrasonic probe had a great impact on drug-loading efficiency.
Compared with zwitterionic surfactant, anionic surfactant has better dispersion and solubilization effects. However, due to its strong hemolytic effect, it cannot be used as an additive in injections. In this study, several surfactants for injection were chosen. Initially, SWNT were dispersed in water, and drugs were then added to the system, but only few were adsorbed to SWNT. The reason was probably that SWNT were wrapped in the surfactants, which prevented the drug from adsorption through π-π stacking interactions. We then adopted the nanoprecipitation method to absorb the drugs onto the surface of SWNT and then disperse the complex with surfactants, which significantly increased the drug loading rate.
Preparation of SWNT-NGR-DTX
With maleimide in the DSPE-PEG-maleimide conjugate, which could undergo an addition reaction with the double bond in sulfhydryl groups at room temperature, the compound reacted with cysteine in CNGRCK2HK3HK11 (C, containing sulfhydryl groups) and connected covalently. Thin layer silica gel chromatography results () showed that there was no free NGR spot after reaction with NGR peptides, while there were visible NGR spots when they were mechanically mixed with SWNT, which demonstrated that NGR had completely attached to the surface of SWNT.
Figure 3 Characterization of SWNT-NGR-DTX. (A) Thin layer silica gel chromatography image of 1-(NGR), 2-(NGR mechanical mixing with the SWNT-DTX), and 3-(SWNT-NGR-DTX); (B) transmission electron microscopic image of SWNT-NGR-DTX; (C) solubility of SWNT-NGR-DTX; (D) particle size of SWNT-NGR-DTX; and (E) zeta potential of SWNT-NGR-DTX.
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.
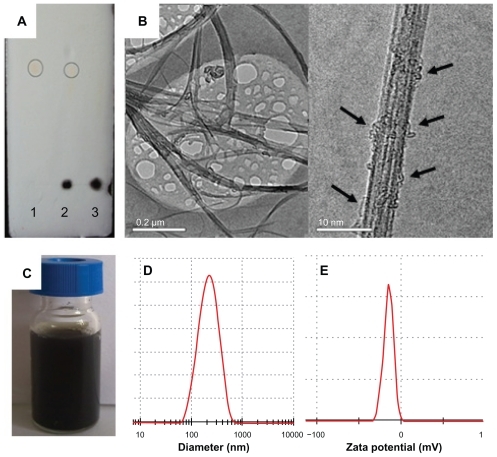
Size distribution is an important parameter for nanopreparation and hugely affects performance in vivo. As electric charges repel each other, potentials on the surface of the particles can prevent them from flocculation and keep the system stable. Charged particle dispersion is measured as the zeta potential, which is closely related to physical stability. The SWNT-NGR-DTX particle size was 182.8 ± 2.8 nm () and the zeta potential was −12.06 ± 0.71 mV (). It is noteworthy that SWNT-NGR-DTX solution can keep a few weeks or even months without aggregation ().
SWNT showed a filamentous distribution, and docetaxel was attached to the surface of the SWNT (), which was reversible. Therefore, when the organism absorbed SWNT, docetaxel could be easily dissociated and take effect.
NGR plays the role of angiogenesis-targeting peptide in the preparation. If NGR is added before the surfactants, it will be adsorbed onto the surface of the SWNT and affect targeting effort, as SWNT also have the adsorbing effect towards protein. Therefore, SWNT-DTX is prepared first, then DSPE-PEG-maleimide is added into it. As in the phospholipids, the DSPE exerts a strong dispersing effect by adsorbing one end to SWNT, the long-chain PEG exposes maleimide, which induces an addition reaction with NGR, and NGR lies at the outer end of the complex, which assures its targeting effect.
Cell uptake by SWNT-NGR-DTX-FITC
The SWNT drug delivery system can effectively deliver drugs into cells and inhibit phagocytic ability. In this study, uptake of FITC, SWNT-DTX-FITC, and SWNT-NGR-DTX-FITC by PC3 cells was examined. With the properties of the SWNT surface, it is easy to adsorb the large π bond and cause a π-π stacking effect. With multibenzenes in the FITC molecular structure, it can easily be adsorbed onto the surface of SWNT.
Fluorescence microscopy was applied to track the location of docetaxel and the fluorescein-labeled SWNT inside cells (). As mentioned earlier, the drug delivery experiment is based on the hypothesis that functionalized SWNT are taken up by PC3 cells through endocytosis. However, the FITC is mainly observed outside the cell. Although drugs can enter cells themselves, nanotubes still play an important role because they enable molecular targeting via the attachment of NGR peptides and are crucial for the delivery of drugs that are not taken up by cells under normal conditions. This experiment showed that uptake of SWNT-NGR-DTX-FITC and SWNT-DTX-FITC by PC3 cells was time-dependent, with uptake of all SWNT-NGR-DTX-FITC into PC3 cells at 2 hours, but only a part of SWNT-DTX-FITC was transferred into the cells at this time point (). Apart from this, our results provide an answer to whether carbon nanotubes enter the nuclei of cells. This study clearly shows the nanotubes located in the cytoplasm under the applied conditions.
Cell viability
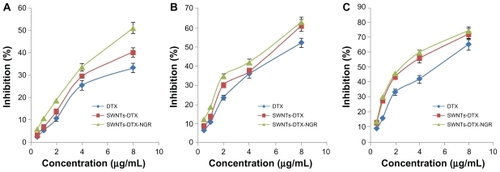
The concentration of docetaxel was 8 μg/mL and for SWNT was about 6.7 μg/mL. When the concentration of SWNT was below 6.7 μg/mL, the SWNT-NGR had no signifcant cytotoxicity to the PC3 cells (), but proliferation of the PC3 cells was inhibited in the docetaxel, SWNT-DTX, and SWNT-NGR-DTX groups, with the inhibition rate being positively correlated with dose and time. The corresponding inhibition rates at each time point for the docetaxel and SWNT-NGR-DTX groups were significantly different (). However, at the time points of 48 hours and 72 hours, as shown in , there is no significant difference between SWNT-NGR-DTX, SWNT-DTX, and docetaxel. This is probably due to the fact that the targeted delivery system can be transferred faster into cells, and as a result, its effect is significantly better than for the other two groups in the early stages. However, when the time periods are prolonged to 48 hours and 72 hours, the docetaxel in all groups is transferred into the cells, so the advantage is weakened.
Figure 5 Cytotoxicity of SWNT-NGR in PC3 cells.
Note: Data are presented as the mean ± standard deviation (n = 3).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide.
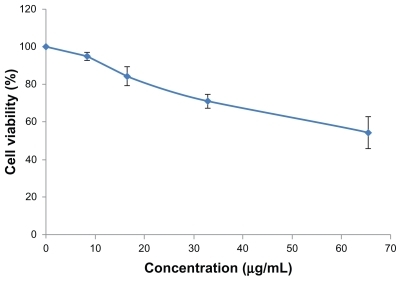
Figure 6 Inhibition rate of docetaxel, SWNT-DTX, and SWNT-NGR-DTX in PC3 cells. SWNT-NGR-DTX was significantly different (P < 0.05) from the other groups at 24 hours (A) but at 48 hours (B) and 72 hours (C) there was no significant difference between SWNT-DTX and SWNT-NGR-DTX, while the SWNT-NGR-DTX group was significantly different from the docetaxel group (P < 0.05).
Note: Data are presented as the mean ± standard deviation (n = 3).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.
Pharmacokinetics and biodistribution
The pharmacokinetics of docetaxel, SWNT-DTX, and SWNT-NGR-DTX were investigated in the blood samples by high-pressure liquid chromatography (), showing that the rate of decrease of docetaxel was faster than for SWNT-DTX and SWNT-NGR-DTX after administration. The blood concentration of docetaxel rapidly declined to the quantitation limit, while the decreasing rate of SWNT-NGR-DTX and SWNT-DTX was evidently slower, the decreasing rates in the SWNT-DTX and SWNT-NGR-DTX groups were not significantly different, but the rates of both were markedly different from that of the docetaxel group.
Figure 7 Mean docetaxel concentration in plasma after intravenous administration of docetaxel, SWNT-DTX, and SWNT-NGR-DTX (mean ± standard deviation, n = 5). The decreasing rates of SWNT-DTX and SWNT-NGR-DTX were not significantly different, but the rate of both were markedly different from that in the docetaxel group (P < 0.05).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.
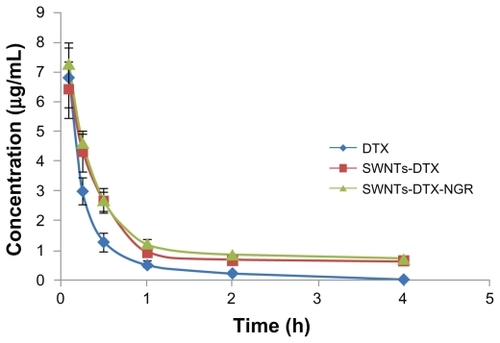
The area under the curve for SWNT-NGR-DTX was about six and 64 times greater than for SWNT-DTX and docetaxel, respectively, while the elimination rate was less () and mean residence time was 1.6 and 2.6 times longer, respectively (). This result is attributed to the high hydrophobicity of conjugated docetaxel, reducing the biological inertness of docetaxel in vivo and shortening the blood circulation time. This shows that SWNT-NGR-DTX significantly increased the blood circulation time of docetaxel.
Table 1 The pharmacokinetic parameters of DTX after intravenous administration of DTX, SWNT-DTX, and SWNTNGR- DTX in mice
Table 2 Pharmacokinetic parameters calculated by statistical moment for mice treated with intravenous DTX, SWNT-DTX, and SWNT-NGR-DTX
To clarify the tumor treatment efficacy of the various docetaxel formulations (SWNT-NGR-DTX, SWNT-DTX, and docetaxel), the biodistribution of docetaxel was investigated in tumors and various organs in the mice. For quantitative evaluation of the targeting characteristics of SWNT-NGR-DTX in vivo, several parameters were applied, including overall targeting efficiency (TE), ie, the percentage of drug distribution in the tissues. The formula, applicable to all tissues, is as follows:
The calculation of AUC0→3h was performed using a trapezoidal method. Significant differences were observed for the biodistribution of docetaxel administered in the three different formulations ().
Figure 8 Targeting efficiency (A) and AUC0→3 h of DTX concentration (B) in tissues of mice after intravenous administration of DTX, SWNT-DTX, and SWNT-NGR-DTX.
Note: Data are presented as the mean ± standard deviation, (n = 6).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel; AUC, area under the concentration versus time curve.
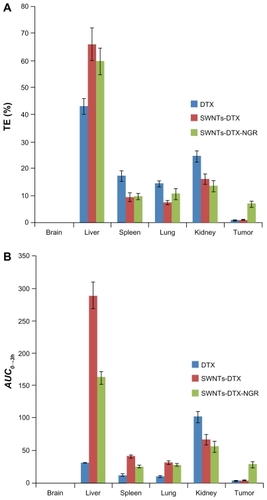
The AUC0→3h of docetaxel in SWNT-NGR-DTX for the liver, spleen, lung, kidney, and tumor tissue was markedly larger than in the docetaxel control group. Compared with the SWNT-DTX group, distribution of SWNT-NGR-DTX in the liver, spleen, lung, and kidney was reduced, while distribution in tumor tissues was significantly increased, and little docetaxel reached the tumor tissues when the drug was administered alone (). It was demonstrated that SWNT functionalized by the PVPk30 method has a particular distribution pattern, so eventually the targeting efficiency of SWNT-NGR-DTX was similar or even less in tumor tissue than in other organs. However, the NGR effect can reduce the distribution of SWNT-DTX in other organs, and increase distribution in the tumor to achieve better treatment of cancer.
The targeting efficiency values for SWNT-NGR-DTX in the liver and tumor were higher than in the docetaxel control group (), indicating good targeting of SWNT-NGR-DTX to the liver and tumor tissues. The concentration of docetaxel in each tissue was increased by SWNT-DTX, which should enhance the therapeutic effect to a certain extent.
Laser radiation
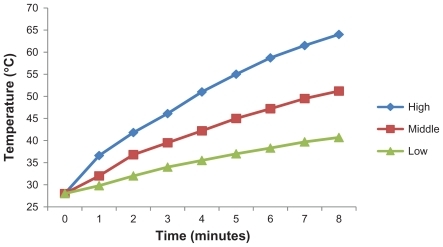
In this study, three different concentrations of SWNT-NGR-DTX were irradiated under laser, and temperature changes with time were measured. The heating efficiency of SWNT-NGR-DTX relies strongly on time and dose, meaning that with increasing concentration and time, the temperature was significantly higher (). Under laser irradiation at 808 nm, SWNT-NGR-DTX showed strong light-heat transfer characteristics.
Figure 9 Temperature evolution of high, middle, and low concentrations of SWNT-NGR-DTX (SWNT approximately 8.3, 4.2, and 0.9 μg/mL; DTX approximately 10, 5, and 1 μg/mL), respectively, during continuous radiation by 808 nm laser at 1.4 W/cm2.
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.
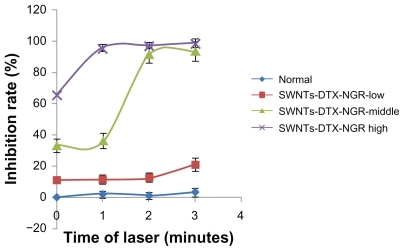
The inhibition rate of the SWNT-NGR-DTX complex towards PC3 cells was significantly increased under 808 nm near-infrared laser radiation (). When the concentration of SWNT was 14.4 μg/mL, the rate of inhibition reached 95.33% after radiation for one minute. When the SWNT-NGR-DTX complex containing SWNT 7.3 μg/mL was irradiated for one minute, the inhibition rate was about 35%, but reduced to 90.73% if irradiated for two minutes. This is probably due to the fact that cell tolerance drops dramatically at a certain temperature during heat treatment, as it does for SWNT of different concentrations over different time periods, resulting in an increased inhibition rate for SWNT-NGR-DTX from one minute to 2 minutes. The results show that SWNT has a significant photothermal effect, and when combined with chemotherapy, ideal antitumor effects could be reached.
Figure 10 Inhibition by SWNT-NGR-DTX of PC3 cells under 808 nm laser irradiation using different concentrations and different laser times. Data presented as the mean ± standard deviation (n = 3).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.
Changes in tumor volume in the S180 mouse model are shown in . Compared with the normal saline group, the tumor volume in the docetaxel, SWNT-DTX, and SWNT-NGR-DTX groups showed a decrease one week after administration, but there were some remarkable differences. Efficacy in the SWNT-NGR-DTX and SWNT-DTX groups was superior to that in the docetaxel group, and both were markedly different from the docetaxel group. However, no significant variation was observed between the SWNT and normal saline groups.
Figure 11 Average tumor volume in a S180 mouse model of treatment without (A) and with (B) laser in vivo. The SWNT-NGR-DTX-laser group shows significant (P < 0.05) suppression of tumor growth compared with the other experimental groups (n = 6).
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel; NS, normal saline.
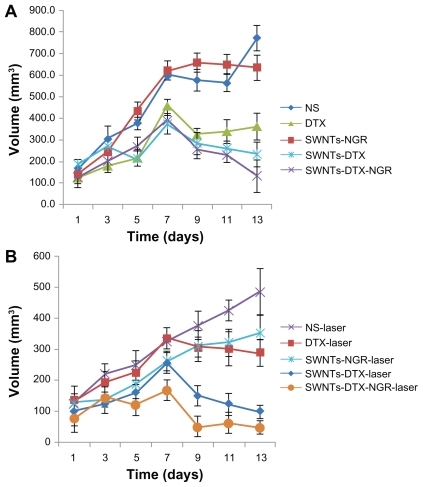
In vivo, the results of the laser irradiation experiment () showed that tumor volumes in the normal saline-laser and docetaxel-laser groups were both decreased when compared with the normal saline and docetaxel groups, respectively. There was a significant difference, indicating that radiation had an antitumor effect. The statistical results indicated a marked difference and better outcome in the SWNT-NGR-laser group than in the normal saline, normal saline-laser, and SWNT-NGR groups. The SWNT-NGR-DTX-laser group showed the best antitumor results and a strong inhibitory effect, even in the early stage of treatment. The results confirmed that radiation at 808 nm can significantly enhance the tumor inhibition effect.
In recent years, photothermal therapy based on nanomaterials has been suggested as a highly efficient therapeutic technique. Moon et al used PL-PEG2000-NH2 to functionalize SWNT, and demonstrated that the photothermal effect of PEG-SWNT exposed to near-infrared irradiation can destroy solid malignant tumors in vivo. SWNT-based photothermal treatment achieved complete eradication of solid malignant tumors without toxicity or recurrence of tumors for a long period of time.Citation27 Using PEG-PMHC18-coated SWNT, Liu et al carried out a pilot in vivo photothermal therapy study and observed promising efficacy in cancer cells. Their results highlight the importance of surface coating for the in vivo behavior of nanomaterials in general, and could provide guidelines for the future design of SWNT bioconjugates for various in vivo applications.Citation25 Yang et al used nanographene sheets with polyethylene glycol (PEG) coating to exploit the strong optical absorbance of nanographene sheets in the near-infrared region for in vivo photothermal therapy, achieving ultraefficient tumor ablation after intravenous administration of nanographene sheets and low-power near-infrared laser irradiation to the tumor.Citation32 SWNT functionalized by PEGylated phospholipids are biologically nontoxic and long-circulating nanomaterials with intrinsic near-infrared photoluminescence.Citation28 All the studies shared the same feature of modifying SWNT with PEG. Our study adopts the method of surfactant modification, whereby DPM is used at a low dose to conjugate with NGR, and the effect of using heat with this method is still significant.
Histological analysis
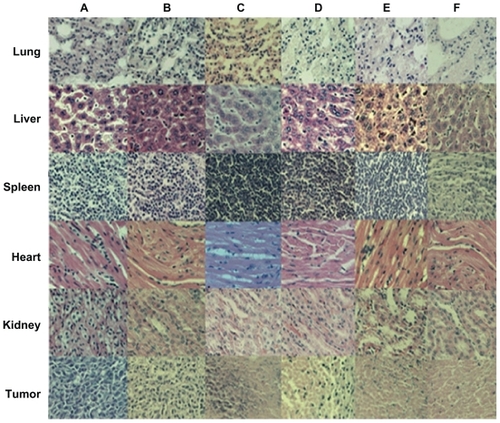
To investigate the influence of SWNT-NGR-DTX on the organs of mice, a histological assay was conducted with paraffin-embedded sections and hematoxylin and eosin staining () which showed that, compared with the control group, there were no significant differences between the various tissues. However, tumor cells in the control group showed vigorous growth, a tight arrangement, a large body and intact shape, while cell necrosis, lysis, and fragmentation occurred to a certain extent in the docetaxel, SWNT-DTX, SWNT-NGR-DTX, and SWNT-NGR-DTX-laser groups. No significant morphological difference was found between the SWNT group and the control group.
Figure 12 Histologic assessments of major organs and tumor tissues with hematoxylin and eosin staining in mice (200×). (A) NS, (B) SWNT, (C) SWNT-DTX, (D) DTX, (E) SWNT-NGR-DTX, and (F) SWNT-NGR-DTX-laser.
Abbreviations: SWNT, single-walled carbon nanotubes; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel; NS, normal saline.
Conclusion
In summary, a new anticancer drug delivery system using docetaxel as a model, ie, SWNT-NGR-DTX, was developed to identify the biodistribution characteristics of SWNT and their effect on cancer cells when mediated by thermal therapy. The targeting efficiency of SWNT-NGR-DTX in tumor tissue was enhanced compared with SWNT-DTX and docetaxel. It is suggested that this SWNT drug delivery system may be promising in terms of treatment efficacy for cancer and with minimal side effects.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- KrajcikRJungAHirschANeuhuberWZolkOFunctionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genesBiochem Biophys Res Commun2008369259560218298946
- JiSRLiuCZhangBCarbon nanotubes in cancer diagnosis and therapyBiochim Biophys Acta201018061293520193746
- MahmoodMKarmakarAFejlehASynergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drugNanomedicine (Lond)20094888389319958225
- MielcarekJSkupinPFunctionalization of carbon nanotubes for multimodal drug deliveryPrzegl Lek2011683167170 Polish21812234
- ChenJShujiCZhaoXFunctionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug deliveryJ Am Chem Soc200813049167781678519554734
- KhazaeiARadMNBorazjaniMKOrganic functionalization of single-walled carbon nanotubes (SWCNTs) with some chemotherapeutic agents as a potential method for drug deliveryInt J Nanomedicine2010563964520856839
- ChenQWangQLiuYCEnergetics investigation on encapsulation of protein/peptide drugs in carbon nanotubesJ Chem Phys2009131101510119586122
- CheungWPontorieroFTaratulaOChenAMHeHDNA and carbon nanotubes as medicineAdv Drug Deliv Rev201062663364920338203
- VarkouhiAKFoillardSLammersTSiRNA delivery with functionalized carbon nanotubesInt J Pharm2011416241942521320582
- CiofaniGRaffaVVittorioOIn vitro and in vivo biocompatibility testing of functionalized carbon nanotubesMethods Mol Biol2010625678320422382
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res200868166652666018701489
- ZhangYBaiYYanBFunctionalized carbon nanotubes for potential medicinal applicationsDrug Discov Today20101511–1242843520451656
- PasqualiniRKoivunenEKainRAminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesisCancer Res200060372272710676659
- OostendorpMDoumaKHackengTMvan ZandvoortMAPostMJBackesWHPharmacokinetics of contrast agents targeted to the tumor vasculature in molecular magnetic resonance imagingContrast Media Mol Imaging20105191720101742
- ZhangZWangBLiHZhouTZhangLThe inhibitory effects of nanosize delivery system for antisense oligonucleotide of hTERT on EC9706 cellsCancer Biol Ther20076332933417438368
- LiHWangJZhouTZhangYZhangZAn investigation of the effects of nanosize delivery system for antisense oligonucleotide on esophageal squamous cancer cellsCancer Biol Ther20087111852185918836295
- ZhaoBJKeXYHuangYThe antiangiogenic efficacy of NGR-modified PEG-DSPE micelles containing paclitaxel (NGR-M-PTX) for the treatment of glioma in ratsJ Drug Target201119538239020677914
- WangXWangYChenXWangJZhangXZhangQNGR-modified micelles enhance their interaction with CD13-overexpressing tumor and endothelial cellsJ Control Release20091391566219470394
- [No authors listed]Docetaxel and adjuvant treatment of breast cancerPrescrire Int20112011714921678701
- De SouzaRZahediPMoriyamaEHAllenCJWilsonBCPiquette-MillerMContinuous docetaxel chemotherapy improves therapeutic efficacy in murine models of ovarian cancerMol Cancer Ther2010961820183020530719
- SaloustrosEGeorgouliasVDocetaxel in the treatment of advanced non-small-cell lung cancerExpert Rev Anticancer Ther2008881207122218699760
- CaponigroFLongoFPerriFIonnaFDocetaxel in the management of head and neck cancerAnticancer Drugs200920863964519639668
- Gueritte-VoegeleinFGuenardDLavelleFLe GoffMTMangatalLPotierPRelationships between the structure of taxol analogues and their antimitotic activityJ Med Chem19913439929981672159
- HuangNWangHZhaoJLuiHKorbelikMZengHSingle-wall carbon nanotubes assisted photothermal cancer therapy: animal study with a murine model of squamous cell carcinomaLasers Surg Med201042963864820949599
- LiuXTaoHYangKZhangSLeeSTLiuZOptimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumorsBiomaterials201132114415120888630
- ZhengXZhouFNoncovalent functionalization of single-walled carbon nanotubes by indocyanine green: Potential nanocomplexes for photothermal therapyJ Xray Sci Technol201119227528421606588
- MoonHKLeeSHChoiHCIn vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubesACS Nano20093113707371319877694
- RobinsonJTWelsherKTabakmanSMHigh performance in vivo near-IR (>1 mm) imaging and photothermal cancer therapy with carbon nanotubesNano Res201031177979321804931
- KamNWO’ConnellMWisdomJADaiHCarbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destructionProc Natl Acad Sci U S A200510233116001160516087878
- ZhangYJeong LeeHBoadoRJPardridgeWMReceptor-mediated delivery of an antisense gene to human brain cancer cellsJ Gene Med20024218319411933219
- LuJJeonELeeBSOnyukselHWangZJTargeted drug delivery crossing cytoplasmic membranes of intended cells via ligand-grafted sterically stabilized liposomesJ Control Release2006110350551316356575
- YangKZhangSZhangGSunXLeeSTLiuZGraphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapyNano Lett20101093318332320684528
![Figure 1 Docetaxel delivery system using noncovalent functionalization of carbon nanotubes.Abbreviations: DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; NGR, (Asn-Gly-Arg) peptide; DTX, docetaxel.](/cms/asset/81064526-15b0-4b2b-a09a-45ed86670468/dijn_a_24167_f0001_c.jpg)