Abstract
Parietaria pollen is one of the major causes of allergic reaction in southern Europe, affecting about 30% of all allergic patients in this area. Specific immunotherapy is the only treatment able to modify the natural outcome of the disease by restoring a normal immunity against allergens. The preparation of allergen-solid lipid nanoparticles as delivery vehicles for therapeutic proteins, P. judaica major allergen Par j 2, was investigated. The Par j 2 allergen was expressed in a large amount in Escherichia coli and purified to homogeneity. Its immunological properties were studied by western blotting and enzyme-linked immunosorbent assay inhibition. Solid lipid nanoparticles were obtained by water-in-oil-in-water multiple emulsion method and characterized in terms of mean size and surface charge. These systems (approximately 250 nm diameter and negative surface charge) incorporated recombinant Par j 2 with 40% or greater efficiency. Moreover, the endotoxin level and anaphylactic activity of the empty solid lipid nanoparticles and recombinant Par j 2-loaded solid lipid nanoparticles were evaluated by looking at the overexpression of CD203c marker on human basophils. These results demonstrate that recombinant Par j 2-nanoparticles could be proposed as safe compositions for the development of new therapeutic dosage forms to cure allergic reactions.
Introduction
Allergy is an immunoglobulin E (IgE)-mediated hypersensitivity reaction affecting the respiratory tract (rhinitis, rhinoconjunctivitis, urticaria, and asthma). It is a global health problem affecting more than 25% of the population living in industrialized countriesCitation1 with significant worldwide increases in the prevalence of asthma and allergic rhinitis since 1960.Citation2 This pathological status is characterized by the presence of IgE antibodies towards molecules from different sources (eg, pollen, animal dander, dust) capable of triggering the release of inflammatory substances from effector cells of the immune system (mast cells, basophils). Parietaria is a genus of dicotyledonous weeds belonging to the Urticaceae family which is composed of several cross-reactive allergenic species representing a common cause of pollinosis in the Mediterranean area.Citation3–Citation5 Using DNA recombinant technology, the major allergens of P. judaica (Par j 1 and Par j 2) were isolated and characterized in the authors’ labs.Citation3 So far, the Par j 2 allergen has been a well established marker for the diagnosis of Parietaria pollinosis and, for this reason, it represents the primary target to tackle for the development of new therapeutic strategies.Citation6
Specific immunotherapy is the only treatment capable of modifying the natural immune response in order to ameliorate symptoms, thereby reducing the consumption of drugs and arresting the natural progression of the disease.Citation7 For these reasons, the development of new pharmaceutical products that can improve the safety and efficacy of novel forms of vaccine is of utmost importance in the clinic.
Recently, recombinant allergens have also been used in clinical trials, which shows that these products may represent new formulations of tailored immunotherapy vaccines.Citation8 In addition, different routes for the administration of vaccines have recently been exploited.Citation9
Solid lipid nanoparticulate systems such as solid lipid nanoparticles (SLN) have been sought as vehicles for therapeutic peptides, proteins, and antigens.Citation10,Citation11 Taking into consideration that peptide or protein antigens are ineffective for mucosal immunization due to proteolytic degradation at mucosal sites, encapsulation into particulate carriers is a potential strategy to improve vaccine efficacy after administration by parenteral routes or upon uptake at mucosal sites.Citation11 Lipid-based particles have already been successfully obtained for the intranasal immunization against hepatitis B, and a recent report describes oral immunization in mice with SLN containing a Japanese encephalitis antigen.Citation12,Citation13 Despite the important efforts dedicated to the design of peptide delivery systems, the administration of these sensitive molecules remains a challenge.
SLN are excellent drug carriers thanks to many advantages associated with their use. In fact, they are quite easy to produce without necessarily using organic solvents, and large-scale production is possible with qualif ied production lines.Citation14–Citation16 They exhibit good stability during long-term storage and are amenable to both lyophilization and steam sterilization.Citation11 Moreover, SLN consist of physiologically well-tolerated ingredients often already approved for pharmaceutical applications in humans and are generally recognized as safe.Citation15 Under optimized conditions, they can be produced to incorporate therapeutic peptides and proteins. In fact, formulation in SLN confers improved protein stability and avoids proteolytic degradation, granting sustained release of the incorporated molecules. Important peptides have been incorporated into SLN and several therapeutic applications may be foreseen, such as immunization with protein antigens.Citation11
This study focuses on the production, purity estimation, and allergenic activity of recombinant Par j 2 (rPar j 2) allergen. The preparation and chemical-physical characterization of empty and rPar j 2-loaded SLN are described in detail and the endogenous content in lipopolysaccharides (LPS) into empty SLN and rPar j 2-loaded SLN is analyzed. The study culminates in the evaluation of the anaphylactic activities of rPar j 2-loaded SLN and rPar j 2 allergen.
Materials and methods
Induction and purification of rPar j 2 allergen
The recombinant allergen was expressed as a histidine-tagged protein (pQE-30 Vector; QIAGEN Ltd, Crawley, United Kingdom) in the M15 strain (Escherichia coli Host Strain M15[pREP4]; QIAGEN). The recombinant clones were grown overnight at 37°C in 2YT broth (bacto tryptone 16 g/L, bacto yeast 10 g/L, sodium chloride 5 g/L, pH 7.0). A 1:40 dilution was taken for 2 hours at 37°C and then induced with 1 mM isopropylthio-β-galactoside for 4 hours at 37°C. Cells were harvested by centrifugation (5000 rpm for 15 minutes at 4°C) and disrupted by using a sonicator device (Bandelin Sonoplus Ultrasonic homogenizer HD 2200, Berlin, Germany) in a buffer containing 10 mM of sodium phosphate at pH 7.4. Cell debris was removed by centrifugation at 10,000 rpm for 30 minutes at 4°C. Supernatant was filtered through a disposable sterile 0.8 μm syringe disc-type filter (Corning Life Sciences, Lowell, MA) to disrupt genomic DNA and loaded on an ion exchange CM Sephadex® C-25 column (GE Healthcare, Amersham, United Kingdom). The recombinant proteins were eluted using a discontinuous sodium chloride gradient (0–2 M sodium chloride, 10 mM sodium phosphate at pH 7.4). The outcoming fractions were analyzed by 16% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on nitrocellulose membrane (Amersham™ ECL™ Western Blotting System; GE Healthcare, Hertfordshire, UK). Membranes were incubated with an antihistidine specific probe (INDIA™ His-Probe HRP, EuroClone SpA, Milan, Italy) in order to detect fractions containing rPar j 2 protein. Fractions containing the allergens were collected and loaded on a HisTrap™ FF Column (GE Healthcare, Hertfordshire, UK) equilibrated in starting buffer (10 mM sodium phosphate at pH 7.4, 1 M sodium chloride, 10 mM imidazole). The column was washed in wash buffer (10–50 mM imidazole in starting buffer) and the histidine-tagged proteins were eluted by using a discontinuous imidazole gradient (50–500 mM imidazole in starting buffer). Then, fractions were analyzed on a 16% SDS-PAGE gel, and aliquots containing the recombinant allergens were pooled, diluted 1:25 in starting buffer, and reloaded on a second HisTrap column and eluted using an elution buffer (10 mM sodium phosphate at pH 7.4, 1 M sodium chloride, 500 mM imidazole). Eluted fractions containing rPar j 2 were desalted using a Sephadex® G-25 Superfine column (GE Healthcare, Hertfordshire, UK) equilibrated in 1× phosphate buffered saline (PBS). Its concentration was determined by Coomassie Brilliant Blue staining and densitometric analysis (Quantity One® 1-D Analysis Software; BioRad Laboratories, Hercules, CA). Finally, rPar j 2 was further purified by using Detoxi-Gel Endotoxin Removing Gel (Thermo Fisher Scientific, Rockford, IL) following manufacturer’s instructions.
Preparation of empty and rPar j 2-loaded SLN
Empty and rPar j 2-loaded SLN were prepared by double emulsion-solvent evaporation method (water-in-oil-in-water). Briefly, to prepare the lipid phase, cutin (mixture of glycerol monostearate and diglyceride, 100 mg; Cognis, Dusseldorf, Germany) was heated at 5°C–10°C above its melting point and an organic phase of dichloromethane solution (1.5 mL; Sigma-Aldrich Corporation, St Louis, MO) containing Epikuron™ 200 (20 mg; Cargill Deutschland GmbH, Krefeld, Germany) was added. Then, an aqueous phase (100 μL Milli-Q™ water; EMD Millipore, Billerica, MA) or an aqueous dispersion of rPar j 2 (100 μL, 100 ng/μL) was added to this organic solution under stirring by Ultra-Turrax® T125 (IKA Labortechnik, Staufen, Germany) at 13,500 rpm in order to obtain empty or rPar j 2-loaded SLN. After that, a first aqueous solution of Tween 80® (3 mL at 2%; Sigma-Aldrich, Milan, Italy) and a second one (6 mL at 1%) were added and stirred again by Ultra-Turrax at 13,500 rpm. The organic solvent was evaporated under vacuum for 30 minutes and the obtained aqueous dispersion of nanoparticles was centrifuged at 45,000 rpm (Beckman Optima XL-90 Ultracentrifuge; Beckman Coulter, Inc, Brea, CA) for 1 hour at 4°C. Finally, the samples were freeze-dried (Modulyo Freeze Dryer; Edwards Ltd, Crawley, United Kingdom) and stored for successive characterization. In order to obtain sterile samples, Milli-Q water was substituted with sterile water (clinical grade pyrogen-free water; EuroClone); glass and metallic tools were sterilized in oven at 180°C for 48 hours.
Preliminary studies were performed by high-performance liquid chromatography (HPLC) analysis (Jupiter® 5 μm C5 250 × 4.6 mm internal diameter; Phenomenex, Inc, Torrance, CA) to ensure rPar j 2 stability above the lipid melting point so nanoparticles could be obtained. No degradation process occurred on the protein at tested conditions (data not shown).
SLN characterization: mean size and zeta potential
The mean diameter and width of distribution (polydispersity index, PDI) of each sample were determined by photon correlation spectroscopy (Zetasizer Nano ZS; Malvern Instruments Ltd, Malvern, United Kingdom) that utilized noninvasive back-scattering technique. Each dried sample was appropriately suspended with filtered (0.45 μm) bidistilled water, isotonic aqueous solution (sodium chloride 0.9% weight-in-weight), or PBS (at pH 7.4), and the reading was carried at a 173° angle in respect to the incident beam. Each reported value was the average of three measurements. The deconvolution of the measured correlation curve to an intensity size distribution was accomplished by using a nonnegative least-squares algorithm.
Zeta potential values were measured according to the principles of laser Doppler velocimetry and phase analysis light scattering (M3-PALS technique). In support of that, a Zetasizer Nano ZS (Malvern) was used. Freeze-dried SLN samples were dispersed in the same media utilized for size measurements. Each sample was analyzed in triplicate.
rPar j 2 encapsulation efficiency
The encapsulation efficiency was determined directly and indirectly. In order to determine rPar j 2 encapsulation efficiency directly on rPar j 2-loaded SLN, an HPLC method was developed. HPLC analysis was performed by using a C5 column (Phenomenex); the mobile phase was a mixture of acetonitrile/trifluoroacetic acid (0.1% volume to volume [v/v]) and water/trifluoroacetic acid (0.1% v/v) with a flow rate of 1 mL/minute, reading at γ 280 nm, and utilizing a gradient method with an increase of organic phase from 10% to 60% in 1-hour period. For this purpose, 10 mg of rPar j 2-loaded SLN were dissolved in 1 mL of dichloromethane and then 1 mL of water was added in order to extract the allergen. The latter was repeated until exhaustive extraction. Results were expressed as weight percent ratio between loaded rPar j 2 and total amount of rPar j 2 used to prepare the nanoparticles. For indirect analysis, the amount of protein entrapped into SLN was calculated by the difference between total amount of rPar j 2 used to prepare the nanoparticles and amount of protein remaining in the aqueous phase (supernatant) after SLN separation. Protein concentration in the supernatant was determined by HPLC analysis as reported above.
Storage stability
Both lyophilized empty and rPar j 2-loaded SLN were stored at 4°C for 4 months and 10 months in the dark. After this period of storage, samples were dispersed in bidistilled water and characterized in terms of mean size, PDI, zeta potential, and drug stability.
Western blotting
IgE-binding activity of the purified recombinant allergen was studied by western blotting by using ten sera from clinically characterized Parietaria allergic patients. In particular, rPar j 2 was fractionated by 16% SDS-PAGE and blotted to nitrocellulose membranes (Hybond™ECL™ [GE Healthcare]). Sera were diluted 1:5 in buffer A (1× PBS, 0.1% Tween 20® [Sigma-Aldrich], and 0.25% bovine serum albumin) and incubated with blotted membranes overnight at room temperature. IgE complexes were detected by using a BioSource™ goat antihuman IgE-horseradish peroxidase conjugate (Invitrogen, Monza, Italy) and visualized by autoradiography as previously described.Citation22
Enzyme-linked immunosorbent assay inhibition
Microtiter plates (Greiner Bio-One, Frickenhausen, Germany) were coated with the P. judaica extract (20 mg/mL) in carbonate/ bicarbonate buffer (pH 9.6) overnight at room temperature. Plates were washed with PBS and 0.05% v/v Tween 20. Nonspecific binding sites were blocked with PBS and 1% weight-in-volume bovine serum albumin for 1 hour at room temperature. Inhibition assay was performed by adding serum samples (diluted 1:5) in PBS, 0.5% weight-in-volume bovine serum albumin, and 0.05% v/v Tween 20 and preincubated overnight at room temperature with increasing concentrations of the allergen used as inhibitor (P. judaica extract: from 100 to 0.16 mg/mL; rPar j 2: from 5 to 0.04 mg/mL) in dilution buffer.
Plates were washed between each incubation step with PBS and 0.05% v/v Tween 20. Bound antibodies were detected by using a goat antihuman IgE-horse radish peroxidase conjugate (Invitrogen, Milan, Italy) as previously described.Citation17 The percentage of inhibition of human IgE binding was calculated as follows: 100 – (ODI/ODP × 100), where ODI and ODP represent the absorbance after preincubation with treated serum and untreated serum, respectively.
Endotoxin content
LPS contamination of rPar j 2 allergen and SLN particles was evaluated using the multitest limulus amebocyte lysate QCL-1000® (Cambrex Corporation, East Rutherford, NJ) (endogenous endotoxin sensitivity ≤0.03 EU/mL). In order to determine the endotoxin concentration of samples, serial twofold dilutions of each sample were tested until an endpoint was reached. Lysate sensitivity was calculated by determining the geometric mean of the endpoint, each endpoint value being converted to log10. The individual log10 values were averaged and lysate sensitivity was taken as the antilog10 of this average log value.
CD203c basophil activation assay
Heparinized peripheral blood was obtained from nonallergic subjects (n = 2). Aqueous suspensions of empty SLN (10 ng/mL and 100 ng/mL in 1× PBS) and dilutions of the free allergen and rPar j 2-loaded SLN (10 ng/mL and 100 ng/mL in 1× PBS) were incubated with 100 μL of blood for 15 minutes at 37°C; 1× PBS was used as a negative control. AntiIgE antibodies were used as positive control (1 μg/mL). After incubation, cells were resuspended in 100 of μL FACS™ buffer (Becton, Dickinson and Company, Franklin Lakes, NJ) and incubated with 20 μL/ test of phycoerythrin-labeled antiCD203c mAb 97 A6 (Immunotech, Marseille, France) for 20 minutes at 4°C in the dark. Samples were subjected to erythrocyte lyses, washed twice in ice cold FACS buffer, and analyzed by means of flow cytometry on FACSCalibur™ flow cytometer (Becton Dickinson). Basophils were detected on the basis of side-scatter characteristics and expression of CD203c. For each sample, 100,000–200,000 cells were analyzed. Analysis gates were set on lymphocytes by forward and side-scatter profile and on CD3+ cells. Results were analyzed by using WinMDI 2.8 software (Scripps Research Institute, Jupiter, FL).
Results and discussion
This work aimed to set up and standardize the preparation of a recombinant allergenic protein encapsulated within SLN for the realization of new forms of stable and safe vaccines for the treatment of allergic disorders. Moreover, a protocol for the expression and purification at homogeneity of P. judaica major allergen Par j 2 in E. coli was standardized. Furthermore, the immunological activity of rPar j 2, empty SLN, and rPar j 2-loaded SLN was evaluated.
Purification and IgE-binding activity of the obtained rPar j 2 allergen
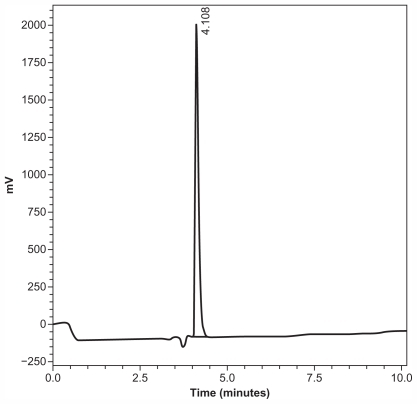
The rPar j 2 allergen was purified at homogeneity using independent chromatography techniques. Yield of the recombinant protein was about 0.5 mg/L of E. coli culture (average of three preparations). Protein concentration and purity were estimated on the basis of spectrophotometry analysis (280 nm), SDS-PAGE, and Coomassie Brilliant Blue staining (, lane C). In addition, chromatography of the purified rPar j 2 on reversed phase HPLC resulted in a well-defined single peak ().
Figure 1 Western blot analysis of recombinant Par j 2 with ten sera from Parietaria judaica allergic patient (lanes 1–10). Lane NA shows incubation with a nonallergenic control serum. Lane C displays a Coomassie Brilliant Blue stained sodium dodecyl sulfate polyacrylamide gel electrophoresis of the purified recombinant Par j 2. Molecular weights are indicated in lane M.
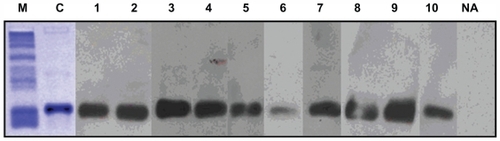
Figure 2 Reversed phase high-performance liquid chromatography elution profile of recombinant Par j 2.
As shown in , no other component was detected. The IgE-binding activity of the Par j 2 allergen was evaluated by western blot analysis to confirm the allergenic activity of the recombinant protein. Immunoblot experiments performed on ten individual sera from allergic patients revealed that the purified recombinant allergen displayed IgE-binding activity in all tested subjects (, lane 1–10). A nonallergic serum was used as negative control showing no unspecific IgE binding (, lane NA).
Furthermore, P. judaica extract was coated in enzymelinked immunosorbent assay plates and incubated with sera from four P. judaica allergic patients pretreated with the rPar j 2 allergen (). This assay showed that the recombinant allergen was capable of inhibiting the majority of human IgE against the whole Parietaria extract raised during the course of natural sensitization. This analysis reinforced the observation that rPar j 2 resembles the majority of B cell epitopes against the P. judaica pollen and it can be used as a bona fide major Parietaria allergen.
Table 1 Enzyme-linked immunosorbent assay inhibition assay performed on plate-bound Parietaria judaica extract
Preparation and characterization of empty and peptide-loaded SLN
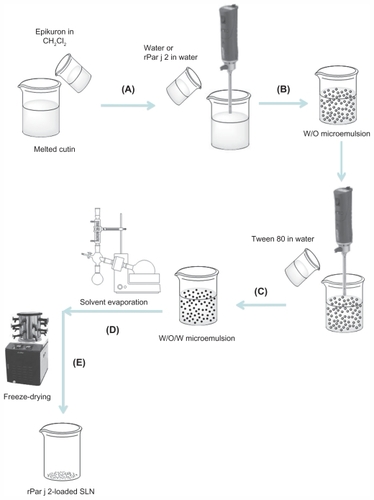
Empty and rPar j 2-loaded SLN were successfully produced by double emulsion (water-in-oil-in-water) solvent evaporation method by using cutin as lipid matrix, and Tween 80 and Epikuron 200 as surfactants.Citation18,Citation19 In , the schematic representation of this method is reported.
Figure 3 Schematic diagram of solid lipid nanoparticle (SLN) preparation using double emulsion/evaporation method. Lipid heated to ~10°C of its melting point and organic solution of dichloromethane (CH2Cl2) containing Epikuron™ 200 added. Aqueous phase or aqueous dispersion of recombinant Par j 2 (rPar j 2) added under stirring by Ultra-Turrax® T125 at 13,500 rpm (A), water in oil (w/o) emulsion formed (B), aqueous solution of Tween 80® at 2% and second one at 1% added under stirring to obtain double emulsion water-in-oil-in-water (w/o/w) (C), organic solvent removed by evaporation (D), and freeze-dried (E).
After proper purification, obtained samples were characterized in terms of mean size and zeta potential measurements in bidistilled water. Results are reported in .
Table 2 Chemical-physical characterization of empty and recombinant Par j 2-loaded solid lipid nanoparticles
The average diameter of empty SLN was 242.7 nm and 252.2 nm for rPar j 2-loaded SLN in bidistilled water. There were slightly lower diameters in the other investigated media; these small differences can be attributed to their different ionic strength of media. The zeta potential values of these structures, also reported in , were −44.9 mV for empty SLN and −38.3 mV for rPar j 2-loaded SLN in bidistilled water, which decreased when they were determined in PBS and sodium chloride 0.9% weight-in-volume aqueous solution for the potential screening effect of solution ions.
Moreover, an adequate method was developed to determine the encapsulation efficiency of rPar j 2 in cutin-based SLN, which resulted in 40% weight-in-weight. It was also demonstrated that the protein was mainly entrapped into SLN by an indirect method and it was stable after its incorporation into the SLN, as proven by the lack of degradation peaks in the HPLC chromatogram (data not shown).
The stability of empty and rPar j 2-loaded SLN after storage, for 4 months and 10 months at 4°C, was evaluated in terms of size, PDI, zeta potential, and drug stability. Results (reported in ) show that both empty and rPar j 2-loaded SLN were stable during storage in tested conditions comparable to that of fresh samples. HPLC analysis also shows that the protein was stable under these storage conditions.
Obtained data (mean size and zeta potential values) support the potential application for sublingual administration of these particles, including after storage, suitable for intravenous circulation. Once into the blood stream, these carriers may be recognized by macrophages as the immune response towards allergens during specific immunotherapy, crucial for the induction of specific T cell tolerance or anergy against allergens and for the generation of allergen-specific blocking IgG subclasses of antibodies.Citation8,Citation20
Endotoxin content
Parenteral pharmaceutical products for animal and human use have to be free from pyrogen contamination. While a pyrogen may in general be defined as any substance causing fever, pyrogens that almost invariably contaminate pharmaceuticals are bacterial endotoxins (LPS) from Gram-negative bacteria. For these reasons, empty SNL and rPar j 2 were analyzed for their endogenous content in LPS. Using standard purification procedures, empty SLN resulted in high LPS contamination (mean 2 EU/μg of particles, average of two evaluations). On the other hand, SNL produced under sterile conditions displayed no LPS contamination. A negative limulus amebocyte lysate test was also observed for the rPar j 2 allergen (average of two evaluations) after purification of the protein on endotoxin removing gel (data not shown).
Basophil activation assay
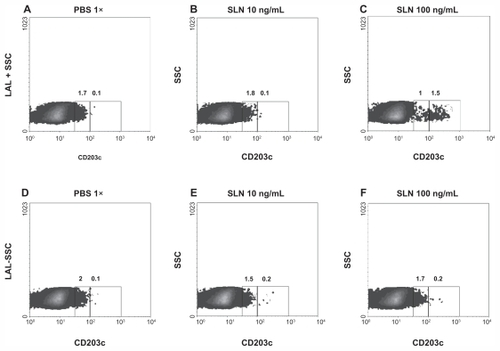
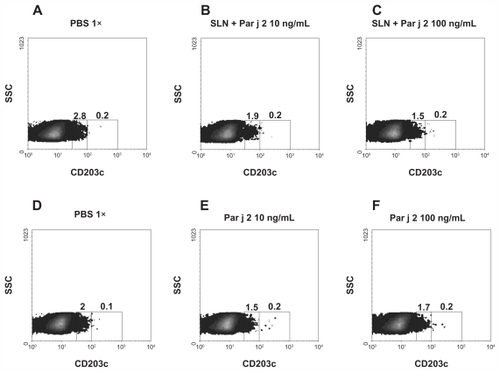
At first attempt, the anaphylactic activity of the endotoxin-free SLN was compared to the activity of SLN produced under standard conditions. Their activities were studied by detecting overexpression of the CD203c marker on human basophils. The assay showed that standard preparations of SLN can activate overexpression of the CD203c marker on human basophil (, panel C). LPS-free SLN did not show this pattern of activation when tested in the same assay (, panels E and F).
Figure 4 Basophil activation assay. Basophils from one representative healthy subject were stimulated with an increasing concentration of solid lipid nanoparticles produced under standard procedures (panels B and C) and with an equimolar concentration of SLN-pyrogen free particles (panels E and F). Panels A and D show the unstimulated sample.
Note: Numbers inside the graph express the percentage of cells with upregulated CD203c.
Abbreviations: LAL, limulus amebocyte lysate; PBS, phosphate buffered saline; SLN, solid lipid nanoparticles; SSC, side-scatter characteristics.
The anaphylactic activity of rPar j 2-loaded SLN and allergen obtained in sterile conditions was studied by using blood from two healthy subjects. shows a representative plot from one out of two individuals stimulated with increasing concentrations of rPar j 2-loaded SLN (panels B and C) and the free rPar j 2 allergen (panels E and F). Panels A and D display the untreated negative controls. This analysis demonstrated that rPar j 2 and rPar j 2-loaded SLN obtained in sterile conditions did not show any anaphylactic activity, which makes them potential candidates for new formulations of stable and safe vaccines.
Figure 5 Basophil activation assay. Basophils from one representative Parietaria judaica healthy subject were stimulated with increasing concentration of solid lipid nanoparticles (SLN)-recombinant Par j 2 (panels B and C) and with an equimolar concentration of the recombinant Par j 2 (panels E and F). Panels A and D show the unstimulated sample.
Note: Numbers inside the graph express the percentage of cells with upregulated CD203c.
Abbreviations: PBS, phosphate buffered saline; SSC, side-scatter characteristics.
Conclusion
This paper has described a new protocol for the preparation of highly purified rPar j 2 allergen and its incorporation into SLN in order to obtain new potential dosage forms of stable and safe vaccines for the treatment of allergic disorders by sublingual route. In fact, extract-based specific immunotherapy currently used in Parietaria allergy vaccination presents several major disadvantages. Generally speaking, extracts are usually poorly characterized mixtures containing nonallergenic and allergenic components with concentration not easily assessable in terms of absolute concentration of allergens and relative quantification of major and minor allergens.Citation21–Citation23 In addition, it has been demonstrated that pollen extracts contain proinflammatory substances acting as adjuvantsCitation24,Citation25 and proteolytic activities which can alter the epithelial integrity contributing to the sensitization and pathogenesis of allergy.
The use of highly purified recombinant allergens can overcome all these problems, allowing standardization of pharmacological treatment. Furthermore, recombinant allergens have been used in clinical trials showing that such products may represent new formulation of tailored immunotherapy vaccines.Citation8
Recently, several published papers have reported the characterization of nanoparticles containing allergenic extracts with adjuvant activityCitation26,Citation27 and ovalbumin.Citation28,Citation29 These studies showed protection from anaphylactic shock and allergenspecific interleukin-10 production from T cells in animal models suggesting that such formulations may represent sophisticated allergen-delivery systems in allergen specific immunotherapy.
The SLN described in this paper showed average diameters in the colloidal size range, good loading capacity, and highly negative zeta potential. Moreover, they were stable during storage in the used experimental conditions. Furthermore, the anaphylactic activity of rPar j 2-loaded SLN was evaluated demonstrating the safety of the formulation.
In conclusion, this paper has described a novel approach based on the production of Par j 2-loaded SLN carrying the major allergen of the Parietaria pollen produced by DNA recombinant technology. Their chemical-physical and immunological properties were evaluated in vitro, which shows promising characteristics for the set up of immunological studies using animal models.
Acknowledgments
The authors acknowledge Francesco Mineo for contributing to the language revision of this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- HolgateSTBroideDNew targets for allergic rhinitis – a disease of civilizationNat Rev Drug Discov200321190291414668811
- DevereuxGThe increase in the prevalence of asthma and allergy: food for thoughtNat Rev Immunol200661186987417063187
- ColomboPBonuraACostaMThe allergens of ParietariaInt Arch Allergy Immunol2003130317317912660421
- AyusoRCarreiraJPoloFQuantitation of the major allergen of several Parietaria pollens by an anti-Par 1 monoclonal antibody-based ELISA. Analysis of cross-reactivity among purified Par j 1, Par o 1 and Par m 1 allergensClin Exp Allergy199525109939998556571
- ScalaEAlessandriCBernardiMLCross-sectional survey on immunoglobulin E reactivity in 23,077 subjects using an allergenic molecule-based microarray detection systemClin Exp Allergy201040691192120214663
- StumvollSWestritschnigKLidholmJIdentification of crossreactive and genuine Parietaria judaica pollen allergensJ Allergy Clin Immunol2003111597497912743560
- BousquetJLockeyRMallingHJAllergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paperJ Allergy Clin Immunol19981024 Pt 15585629802362
- ValentaRFerreiraFFocke-TejklMFrom allergen genes to allergy vaccinesAnnu Rev Immunol20102821124120192803
- CompalatiERogkakouAVillaEPassalacquaGCanonicaGWEmerging sublingual immunotherapy drugsExpert Opin Pharmacother201011182963297220958111
- EylesJECarpenterZCAlparHOWilliamsonEDImmunological aspects of polymer microsphere vaccine delivery systemsJ Drug Target2003118–1050951415203919
- AlmeidaAJSoutoEBSolid lipid nanoparticles as a drug delivery system for peptides and proteinsAdv Drug Deliv Rev200759647849017543416
- SarafSMishraDAsthanaAJainRSinghSJainNKLipid microparticles for mucosal immunization against hepatitis BVaccine2006241455616122855
- PichayakornWKusonwiriyawongCLakornrachTThirapakpoomanuntSLipipunVRitthidejGCSolid lipid microparticles for oral Japanese encephalitis vaccine delivery: in vitro study in Caco-2 cells and human intestinal M-cells models and in vivo immunization in micePaper presented at: 33rd International Symposium on Controlled Release of Bioactive MaterialsJuly 22–26. 2006Vienna, Austria
- SoutoEBMüllerRHDombATobataYKumarRFarberSLipid nanoparticles (SLN and NLC) for drug deliveryNanoparticles for Pharmaceutical ApplicationsValencia, CAAmerican Scientific Publishers2007103122
- MüllerRHRadtkeMWissingSANalwaHSSolid lipid nanoparticles and nanostructured lipid carriersEncyclopedia of Nanoscience and NanotechnologyValencia, CAAmerican Scientific Publishers20044356
- DoktorovovaSGokceEOzyaziciMSoutoEBLipid matrix nanoparticles: pharmacokinetics and biopharmaceuticsCurr Nanosci200953358371
- BonuraAAmorosoSLocorotondoGHypoallergenic variants of the Parietaria judaica major allergen Par j 1: a member of the nonspecific lipid transfer protein plant familyInt Arch Allergy Immunol20011261324011641604
- Garcia-FuentesMTorresDAlonsoMJNew surface-modified lipid nanoparticles as delivery vehicles for salmon calcitoninInt J Pharm20052961–212213215885464
- Garcia-FuentesMTorresDAlonsoMJDesign of lipid nanoparticles for the oral delivery of hydrophilic macromoleculesColloids Surf B Biointerfaces2003272–3159168
- LarcheMAkdisCAValentaRImmunological mechanisms of allergen-specific immunotherapyNat Rev Immunol200661076177116998509
- BrunettoBTinghinoRBraschiMCAntonicelliLPiniCIacovacciPCharacterization and comparison of commercially available mite extracts for in vivo diagnosisAllergy201065218419019796217
- FockeMMarthKFlickerSValentaRHeterogeneity of commercial timothy grass pollen extractsClin Exp Allergy20083881400140818564332
- FockeMMarthKValentaRMolecular composition and biological activity of commercial birch pollen allergen extractsEur J Clin Invest200939542943619302561
- Traidl-HoffmannCMarianiVHochreinHPollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarizationJ Exp Med2005201462763615728240
- MarianiVGillesSJakobTImmunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attractionJ Immunol2007178127623763117548598
- GomezSGamazoCSan RomanBA novel nanoparticulate adjuvant for immunotherapy with Lolium perenneJ Immunol Methods20093481–21819545572
- BroosSLundbergKAkagiTImmunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapyVaccine201028315075508520478343
- PandeyRSSahuSSudheeshMSMadanJKumarMDixitVKCarbohydrate modified ultrafine ceramic nanoparticles for allergen immunotherapyInt Immunopharmacol201111892593121333772
- GómezSGamazoCSan RomanBNanoparticles as an adjuvant for oral immunotherapy with allergensVaccine200725295263527117576025