Abstract
Background
Bacteria from the hospital environment, including linens and curtains, are often responsible for hospital-associated infections. The aim of the present study was to evaluate the bactericidal effects of fabrics coated with the hydroxyapatite-binding silver/titanium dioxide ceramic nanocomposite “Earth-plus”.
Methods
Bactericidal activities of woven and nonwoven fabrics coated with Earth-plus were investigated by the time-kill curve method using nine bacterial strains, including three Staphylococcus aureus, three Escherichia coli, and three Pseudomonas aeruginosa strains.
Results
The numbers of viable S. aureus and E. coli cells on both fabrics coated with Earth-plus decreased to below 2 log10 colony-forming units/mL in six hours and reached the detection limit in 18 hours. Viable cell counts of P. aeruginosa on both fabrics coated with Earth-plus could not be detected after 3–6 hours. Viable cells on woven fabrics showed a more rapid decline than those on nonwoven fabrics. Bacterial cell counts of the nine strains on fabrics without Earth-plus failed to decrease even after 18 hours.
Conclusion
Woven cotton and nonwoven polypropylene fabrics were shown to have excellent antibacterial potential. The woven fabric was more bactericidal than the nonwoven fabric.
Introduction
Prevention of infections associated with health care is extremely important, not only for the safety of health care institutions but also for financial reasons. There are many immunocompromised patients in hospitals and adequate infection control measures are necessary. Many pathogenic microorganisms including bacteria, fungi, and viruses, cause hospital-related infections. Among them, methicillin-resistant Staphylococcus aureus, multidrug resistant Pseudomonas aeruginosa, and bacteria with extended-spectrum β-lactamase are significant problems.Citation1–Citation5
The prevention of cross-infection is a serious issue in medical care and welfare institutions. Although the standard precautions and maximal barrier precautions are very important and performed to diminish cross-infections, these procedures are not sufficient to completely prevent the incidence of infection. Reduction of pathogenic bacteria in the patient environment, which may exist on door knobs, handrails, faucets, tables, beds, linen, clothes, curtains, and the like, is an important medical practice to prevent hospital-related infections and health care-associated infections.Citation6–Citation13
Medical devices pose a great risk of cross-infection because bacteria easily attach to these devices and can therefore be transmitted to the patients.Citation14–Citation19 Metals such as silver (Ag), copper, and titanium dioxide (TiO2) are known to have an antimicrobial effect, and Ag is commonly used to prevent bacterial contamination in urinary tract catheters and endotracheal tubes.Citation7,Citation8,Citation15,Citation20 However, the antimicrobial effect of Ag is poor in an environment devoid of water. TiO2, on the other hand, is a photocatalyst in the oxidation of harmful substances such as microbes, molds, odors, and soils, and TiO2 is effective in both wet and dry environments.Citation9,Citation21,Citation22
The new hydroxyapatite-binding Ag/TiO2 ceramic nanocomposite, “Earth-plus”, was developed by the Shinshu Ceramics Co Ltd, Nagano, Japan. Earth-plus can be coated on various materials, including fabrics (curtains, linen, clothes, and socks), metals, and plastics commonly found in the health care environment and in welfare institutions. Several products using Earth-plus are available on the market, ie, an air purifier with Earth-plus-coated filters,Citation23 a water-purifier tank with Earth-plus-coated plates, and a mask coated with Earth-plus. Citation24 Earth-plus obtained a Japanese patent in 1998.Citation25
The objective of the present study was to evaluate the bactericidal properties of fabrics coated with Earth-plus against S. aureus, Escherichia coli, and P. aeruginosa strains, which are commonly encountered as causative pathogens of hospital-related infections.
Materials and methods
Woven cotton fabric and nonwoven polypropylene fabric were used in the study. The woven and nonwoven fabrics were coated with Earth-plus (4.5 g/m2 and 6.0 g/m2, respectively) using the dipping method developed by Shinshu Ceramics (Nagano, Japan). In summary, fabrics were soaked in a processing liquid containing Earth-plus. Although the concentrations of Earth-plus in the liquid are determined by the processed fabrics and the planned adhesion capacity, the final amounts used were 7 wt% of Earth-plus for woven fabrics and 10 wt% of Earth-plus for nonwoven fabrics. After a specific period of immersion in the liquid, the extra liquid was squeezed out by pressing the fabrics with a roller, and the fabrics were then dried at 160°C for three minutes for woven fabrics and 130°C for one minute for nonwoven fabrics. Woven and nonwoven fabrics without Earth-plus coating and fabrics coated only with hydroxyapatite were also examined as controls. The fabrics were cut into 3 cm squares and then sterilized by autoclaving at 121°C for 20 minutes.
A total of nine strains, which consisted of three strains of S. aureus, three of E. coli, and three of P. aeruginosa, were used for evaluating the bactericidal activities of the woven and nonwoven fabrics coated with Earth-plus. The bacteria used were three standard strains of S. aureus ATCC29213, E. coli ATCC25922, and P. aeruginosa ATCC9027 and six clinical strains, including methicillin-susceptible S. aureus, methicillin-resistant S. aureus, extended-spectrum β-lactamase-producing and -nonproducing E. coli, and metallo-β-lactamase producing and nonproducing P. aeruginosa isolates. The latter six strains were clinically isolated from patients at Shinshu University Hospital. They were stored in Micro-Bank vials (Pro-Lab Diagnostics, Ontario, Canada) at −83°C in a deep freezer after identification by the MicroScan WalkAway (Dade Behring Inc, Deerfield, IL) system.
The preparation of bacterial suspensions was performed following the procedures recommended by the Japanese Industrial Standards L1902.Citation26,Citation27 Prior to the examinations, the nine strains were grown on heart infusion agar (Eiken Chemical Co, Osaka, Japan) plates at 35°C for 24 hours. The colonies on the plates were then inoculated into heart infusion broth (Eiken Chemical Co) media and incubated with gentle agitation at 35°C for 18 hours. The bacterial suspensions were diluted with sterilized physiological saline solution to adjust to a final density of approximately 1 × 105 colony-forming units (CFU)/mL.
The time-kill curve procedure recommended by the Japanese Industrial Standards L1902Citation26,Citation27 was used in this study. Each fabric was aseptically placed into sterilized glass containers with 50 mL capacity. A bacterial suspension of nine isolates (100 μL, approximately 104 CFU/mL) was dropped onto each fabric and incubated at 35°C under ambient air. Bacterial solutions were extracted from each fabric by heavy shaking 30 times and used for viability measurements at various time points, namely, hours 0, 1, 3, 6, and 18. Viability was determined by the plate-colony count method. After 10-fold serial dilutions with sterilized physiological saline solution, 100 μL of each sample was plated onto heart infusion agar plates. Colonies were counted after incubation for 24 hours at 35°C. Measurements were performed in triplicate.
Morphology of bacterial cells on the fabrics
After 18 hours of incubation, the fabrics were cut into small pieces and placed in 1% glutaraldehyde solution for scanning electron microscopy. The specimens were stored in 1% cacodylate buffer containing 1% glutaraldehyde and 4% form-aldehyde at 4°C. The fixation solutions were rinsed out with cacodylate buffer and the samples were immersed in 1% osmium tetroxide in cacodylate buffer for two hours. After rinsing, the specimens were dehydrated by increasing concentrations of acetone. After placing the specimens on electron microscopy plates, they were coated with 200 Å gold (Bio-Rad, SEM coating system Hercules, CA), and electron microscopy was performed using a JSM-6360 LV scanning electron microscope (JEOL, Tokyo, Japan) at 15 kV of accelerating voltage.
Silver ion concentration in the suspensions
The Earth-plus-coated woven and nonwoven fabrics were cut into 5 cm squares, and then ten pieces of each fabric were immersed in 350 mL of ultrapure water at room temperature. After a standing time of 120 hours, the incubation water was centrifuged (10,000 rpm for 60 minutes), and the supernatant was analyzed to determine the amounts of Ag derived from the fabrics using inductively coupled plasma mass spectrometry with the SPQ9200 analyzer (Seiko Instruments Inc, Chiba, Japan).Citation28,Citation29
Statistical analysis
Data were plotted as the mean ± standard error of mean. Viable bacterial cell counts were analyzed by the Mann–Whitney U test. A P value of <0.05 was considered statistically significant.
Results
Evaluation of bactericidal activities
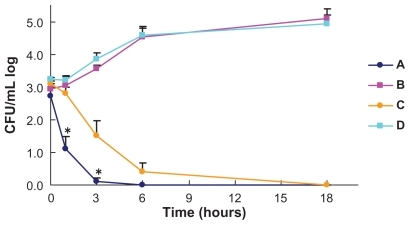
Time-kill curves of the average counts of three S. aureus strains on the woven and nonwoven fabrics are shown in ; the three strains showed similar curves. Live bacterial cell counts on both the woven and nonwoven fabrics coated with Earth-plus gradually decreased to below 2-log10 CFU/mL in six hours and reached the detectable limits of the method in 18 hours. The counts on the woven fabric coated with Earth-plus decreased more rapidly than those on the nonwoven fabric without Earth-plus (P = 0.036 in one hour and P = 0.0140 in three hours). The counts on the woven and nonwoven fabrics without Earth-plus did not decrease during the 18 hours of incubation.
Figure 1 Bactericidal activity against Staphylococcus aureus of woven and nonwoven fabrics with and without Earth-plus. Three S. aureus strains were incubated with the woven cotton fabric coated with Earth-plus (A), woven cotton fabric without Earth-plus (B), nonwoven polypropylene fabric coated with Earth-plus (C), and nonwoven polypropylene fabric without Earth-plus (D). Samples were taken at the indicated times, and viability was determined by colony counting. The bactericidal activities of the woven fabrics with Earth-plus were significantly more rapid than those of the nonwoven fabrics.
Note: *P < 0.05; Mann–Whitney U test.

Time-kill curves of the average counts of three E. coli strains on the woven and nonwoven fabrics are shown in ; the three strains showed similar curves. Live bacterial cell counts on both woven and nonwoven fabrics coated with Earth-plus gradually decreased to below 2-log10 CFU/ mL six hours after inoculation and reached detectable limits in 18 hours. The counts on the woven fabric with Earth-plus showed a more rapid decrease than those on the nonwoven fabric, but this difference did not reach statistical significance. The counts on the woven and nonwoven fabrics without Earth-plus did not decrease during 18 hours of incubation.
Figure 2 Bactericidal activity against Escherichia coli of woven and nonwoven fabrics with and without Earth-plus. Three E. coli strains were incubated with woven cotton fabric coated with Earth-plus (A), woven cotton fabric without Earth-plus (B), nonwoven polypropylene fabric coated with Earth-plus (C), and nonwoven polypropylene fabric without Earth-plus (D). Samples were taken at the indicated times, and viability was determined by colony counting. The bactericidal activities of the woven fabrics with Earth-plus were significantly more rapid than those of the nonwoven fabrics.
Note: *P < 0.05; Mann–Whitney U test.
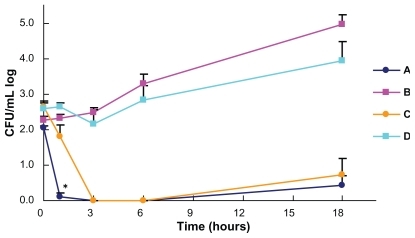
Time-kill curves of three strains of P. aeruginosa on the woven and nonwoven fabrics are shown in . Live bacterial cell counts on both woven and nonwoven fabrics coated with Earth-plus gradually decreased and reached detectable limits six hours after the inoculation. However, the time-kill curves showed an upward slope at 6–18 hours due to the increase in cell counts of two of the three strains, namely, the ATCC strain and the strain without metallo-β-lactamase. The counts on the woven fabric with Earth-plus decreased more rapidly than those on the nonwoven without Earth-plus (P = 0.0011 in one hour). The counts on the woven and nonwoven fabrics without Earth-plus showed a gradual increase during the incubation period. E. coli and P. aeruginosa were killed more rapidly than S. aureus on both fabrics coated with Earth-plus. Preliminary examination of bactericidal activities of fabrics coated only with hydroxyapatite showed no changes in the bacterial counts of S. aureus ATCC29213, E. coli ATCC25922, and P. aeruginosa ATCC9027 after six and 18 hours of incubation compared with the fabrics without Earth-plus.
Figure 3 Bactericidal activity against Pseudomonas aeruginosa of woven and nonwoven fabrics with and without Earth-plus. Three P. aeruginosa strains were incubated with woven cotton fabric coated with Earth-plus (A), woven cotton fabric without Earthplus (B), nonwoven polypropylene fabric coated with Earth-plus (C), and nonwoven polypropylene fabric without Earth-plus (D). Samples were taken at the indicated times, and viability was determined by colony counting. The bactericidal activities of the woven fabrics with Earth-plus were significantly more rapid than those of the nonwoven fabrics.
Note: *P < 0.05; Mann–Whitney U test.
Concentration of silver ions
Silver ions derived from both Earth-plus-coated fabrics were eluted at a concentration of around 0.2 parts per billion.
Scanning electron microscopy analysis
Morphological observations of the three species after six hours of exposure to the woven fabrics coated with Earth-plus are shown in –. S. aureus ATCC29213 cells attached to the Earth-plus granules () mostly showed a normal round shape. However, some cells showed alterations in shape and an irregular surface.
Figure 4 Staphylococcus aureus, scanning electron microscope. S. aureus cells attached to the Earth-plus granules showed a round shape with a partially depressed surface.
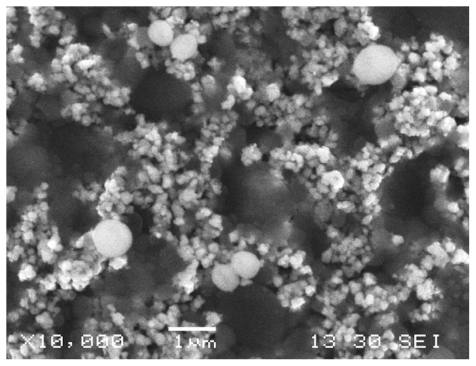
E. coli ATCC25922 cells also attached to the Earth-plus granules and exhibited alterations in shape, with a partially depressed surface (). The cells of P. aeruginosa ATCC25922 () showed similar alterations in shape as E. coli. Cell debris or crushed cells were not observed.
Discussion
Woven cotton and nonwoven polypropylene fabrics coated with the hydroxyapatite-binding Ag/TiO2 ceramic nano-composite, Earth-plus, showed bactericidal effects against S. aureus, E. coli, and P. aeruginosa, which are frequent causative pathogens in hospital-related and health care-associated infections. Earth-plus consists of fine granules that can be used to coat many materials, including fabrics (curtains, linen, clothes, and socks), metals (door knobs, faucets, and bed rails), and plastics (keyboards, television controllers, and switches). The use of Earth-plus in hospital rooms and wards can potentially reduce the number of bacterial pathogens in the patient environment, thus preventing cross-infection among patients and health care workers.
Several composite materials consisting of hydroxyapatite-binding Ag and TiO2 have been developed and evaluated for their antimicrobial activities.Citation22,Citation30–Citation32 However, these materials have been difficult to convert into a commercially available product. We developed Earth-plus as a hydroxyapatitebinding Ag and TiO2 composite and showed that it can be used for coating different materials such as woven and non-woven fabrics by using a dipping method. Several products with the Earth-plus technology are currently available on the market, including an air purifier, a water-purifier tank, and a mask.Citation23,Citation24 Earth-plus obtained a Japanese patent in 1998.Citation25 Curtains coated with Earth-plus have been used in Nagano Kiso Hospital in Japan for three years, and the properties of Earth-plus on these curtains have not shown changes after washing (data not shown). The cost of curtains coated with Earth-plus was estimated to be 10%–50% higher than that of uncoated curtains.
Slight to moderate morphological changes in the bacterial cells were observed on the Earth-plus-coated fabrics using scanning electron microscopy, but severely damaged cells and cell debris could not be found. Although we initially assumed that bacterial cells in various stages of cell death would be detected on the fabric, only cells showing early stages of damage were imaged. This could be due to the detachment of cells from the Earth-plus during the early stages of cell damage.Citation33,Citation34
Among the three species investigated, the Gram-negative pathogens E. coli and P. aeruginosa were killed more rapidly than the Gram-positive S. aureus pathogens, which could be due to differences in cell structure between Gram-negative and Gram-positive bacteria,Citation35,Citation36 namely, the increased thickness of the cell wall of Gram-positive bacteria compared with that of Gram-negative bacteria. This could result in a longer period of time required for the Earth-plus to kill the Gram-positive bacteria. Moreover, the negative charge on the surface of Gram-negative bacterial cells could have resulted in a stronger attraction to the Earth-plus material.
Interestingly, the time-kill curves corresponding to the P. aeruginosa strains showed an upward slope during 18 hours of incubation. In particular, P. aeruginosa ATCC9027 and a clinical metallo-β-lactamase-nonproducing strain slightly and gradually proliferated in 18 hours, while the metallo-β-lactamase-producing clinical isolate failed to regrow. This is likely due to the fact that P. aeruginosa ATCC9027 and the clinical strain are capable of forming biofilms, which may have been protected from the effect of Earth-plus. Drug-resistant metallo-β-lactamase-producing clinical P. aeruginosa isolates with plasmids show a reduced ability to form biofilms. Resistant bacteria that have acquired extracellular drug-resistance genes generally show weaker viability than sensitive strains.Citation37–Citation39 The ability of the P. aeruginosa ATCC9027strain to form biofilms is therefore thought to be one of the reasons for the regrowth. These data have implications for the control of infection in hospitals, where drug-resistant bacteria are commonly found.
The Ag ion concentration measured after washing fabrics coated with Earth-plus was approximately 0.2 ppb, which is below the antimicrobial concentration of 50 ppb.Citation29 The bactericidal effect of Earth-plus itself was therefore not affected by Ag ions eluted from the material. The antimicrobial activity of Ag is dependent on Ag cations that can be eluted in water and strongly bind to electron donor groups in biological molecules containing sulfur, oxygen, or nitrogen atoms.Citation29,Citation40–Citation42 Although the antimicrobial activity of Ag has been utilized extensively in medicine, one major problem is its poor performance in environments devoid of water, in addition to the gradual decrease in the activity of Ag over time.
TiO2, on the other hand, generates strongly oxidizing substances against microbes, moulds, or odors under visible-light irradiation, and it degrades or decomposes them into carbon dioxide, water, and/or other smaller molecules.Citation9,Citation21,Citation22 The energy from photons generates an electron-hole pair on the TiO2 surface, which reacts with hydroxide ions (OH−) in water to yield a hydroxyl radical (OH+). In addition, the electron in the conduction band can reduce O2 to produce superoxide anions (O2−). Both the OH+ and the O2− are extremely reactive against organic compounds and promptly transform them into nontoxic materials. TiO2 is one of the most promising photocatalysts, not only in water environments but also under dry conditions.Citation43–Citation45 The TiO2 materials only behave as an accelerator without altering their structure, and theoretically, their catalytic activities should last indefinitely.
TiO2 requires ultraviolet light at wavelengths of less than 385 nm to acquire a strong antimicrobial effect, and it therefore has no activity in the human body in the absence of visible-light illumination,Citation7,Citation46,Citation47 which hinders its practical application. TiO2 can photochemically decompose not only microorganisms but also organic materials such as organic paint, textiles, and paper.Citation22 The antimicrobial activity of TiO2 requires specific conditions in the environment.
Apatite has been used in chromatographic columns to selectively adsorb protein and in respirators for selective adsorption of pollen.Citation22,Citation48–Citation50 Hydroxyapatite is responsible for the chemical reaction by which microorganisms are adsorbed and trapped.Citation25,Citation37,Citation38,Citation40 The adsorption and immobilization of viruses or bacteria on hydroxyapatite facilitates their decomposition through a photocatalytic process mediated by TiO2. Microorganisms adsorbed by hydroxyapatite are not released except through strong shaking or hard vibration. We confirmed that heavy shaking with an excess of 30 repetitions was necessary to release the bacterial cells trapped on the hydroxyapatite (data not shown). Decomposition through the TiO2 photocatalytic process is only possible with the excitation of the Ag ion, which enhances the electron-hole separation and interfacial charge transfer.Citation32,Citation35,Citation41–Citation43
The photocatalytic activity of TiO2 becomes weak when it coexists with hydroxyapatite.Citation31 Therefore, the activity of TiO2 can be adjusted by modifying the amount of hydroxyapatite. The bactericidal activity of Earth-plus can therefore be explained as follows. Once microorganisms are adsorbed by hydroxyapatite, they cannot be released and are destined to be killed by decomposition through the TiO2 photocatalytic and Ag bactericidal processes.
Earth-plus can be used to coat most materials found in hospital rooms and wards, and various species of bacteria are killed without antibiotics and disinfectants simply by coming into contact with Earth-plus on doorknobs, tables, walls, and linen, which makes it an effective agent for the control of bacterial pathogens found in dry environments. The effect of Earth-plus was found to have a time lag of several hours, which indicated a weak effect. However, the attenuation of its effect by adjustment of the proportion of hydroxyapatite is necessary to make Earth-plus harmless to humans.
The effective control of causative bacteria in the proximity of patients will reduce cross-infections in hospitals and health care institutions. Causative bacteria are directly transmitted between patients and by medical workers through hands and/or medical instruments, and they are also spread to patients and medical workers via the patient environment. Earth-plus can decrease the latter course of bacterial transmission. A hospital room with Earth-plus-coated equipment, including curtains, linen, and wallpaper, and air cleaners with Earth-plus-coated filters will be built in Japan to test the antimicrobial effects of this material.
Disclosure
The authors have no conflicts of interest to disclose in relation to this work.
References
- RogersSSvan der WalleCWaighTAMicrorheology of bacterial biofilms in vitro: Staphylococcus aureus and Pseudomonas aeruginosaLangmuir200824135491355518980352
- PeacockJEJrMarsikFJWenzelRPMethicillin-resistant Staphylococcus aureus: introduction and spread within a hospitalAnn Intern Med1980935265326904159
- AryaSCAgarwalNAgarwalSComment on “Antimicrobial susceptibility and molecular epidemiological analysis of clinical strains of Pseudomonas aeruginosa”J Infect Chemother20081444544619089561
- YujiKOisoGMatsumuraTMurashigeNKamiMPolice investigation into multidrug-resistant Acinetobacter baumannii outbreak in JapanClin Infect Dis20115242221217192
- MoriguchiNItahashiYTabataNOutbreak of CTX-M-3-type extended-spectrum beta-lactamase-producing Enterobacter cloacae in a pediatric wardJ Infect Chemother20071326326617721690
- CollierMSilver dressings: more evidence is needed to support their widespread clinical useJ Wound Care200918777819418785
- OhkoYUtsumiYNiwaCSelf-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: a preclinical workJ Biomed Mater Res2001589710111153004
- TarquinioKMKothurkarNKGoswamiDYSandersRCJrZaritskyALLeVineAMBactericidal effects of silver plus titanium dioxide-coated endotracheal tubes on Pseudomonas aeruginosa and Staphylococcus aureusInt J Nanomedicine2010517718320463933
- KangwansupamonkonWLauruengtanaVSurassmoSRuktanonchaiUAntibacterial effect of apatite-coated titanium dioxide for textiles applicationsNanomedicine2009524024919223243
- BorkowGGabbayJBiocidal textiles can help fight nosocomial infectionsMed Hypotheses20087099099417959322
- HamiltonDFosterABallantyneLPerformance of ultramicrofibre cleaning technology with or without addition of a novel copperbased biocideJ Hosp Infect201074627119819583
- Bischof VukusicSFlincec GrgacSBudimirAKalenicSCotton textiles modified with citric acid as efficient anti-bacterial agent for prevention of nosocomial infectionsCroat Med J201152687521328723
- CollinsASPreventing health care-associated infections: patient safety and qualityHughesRGAn Evidence-Based Handbook for NursesRockville MDAgency for Healthcare Research and Quality2008
- ScalesKReducing infection associated with central venous access devicesNurs Stand201125495621702355
- WangHHuangTJingJEffectiveness of different central venous catheters for catheter-related infections: a network meta-analysisJ Hosp Infect20107611120638155
- TidswellECRockwellJWrightMOReducing hospital-acquired infection by quantitative risk modeling of intravenous bag preparationPDA J Pharm Sci Technol201064829121502008
- CoelloRBranniganELawsonWWickensHHolmesAPrevalence of healthcare device-associated infection using point prevalence surveys of antimicrobial prescribing and existing electronic dataJ Hosp Infect20117826426821652112
- TutuncuEEGurbuzYSencanIOzturkBSenturkGCKilicAUDevice-associated infection rates and bacterial resistance in the intensive care units of a Turkish referral hospitalSaudi Med J20113248949421556470
- UnekeCJIjeomaPAThe potential for transmission of hospital-acquired infections by non-critical medical devices: the role of thermometers and blood pressure cuffsWorld Health Popul20111251221677524
- HardesJvon EiffCStreitbuergerAReduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcomaJ Surg Oncol201010138939520119985
- SunadaKWatanabeTHashimotoKStudies on photokilling of bacteria on TiO2 thin filmJ Photochem Photobiol A Chem2003156227233
- NonamiTHaseHFunakoshiKApatite-coated titanium dioxide photocatalyst for air purificationCatalysis Today200496113118
- TsukasaSAir cleaner Available from: http://www.shincera.co.jp/pdf_catalog/earthplus/SA-807J_cat_e.pdf
- TsukasaSShinshu Ceramics Co Ltd http://www.shincera.co.jp/english/index.htmlAccessed August 26, 2011
- TsukasaSYoshiharuYoshidaJapanese Patent 29636571998
- Japanese Industrial Standards CommitteeJIS L1902 Available from: http://www.jisc.go.jp/index.htmlAccessed August 26, 2011
- SwensonLMHindlerJFJorgensenJHAssessment of Bactericidal Activity by the Time-Kill MethodManual of Clinical Microbiology8th edWashington, DCAmerican Society of Microbiology2003
- HuangHIShihHYLeeCMYangTCLayJJLinYEIn vitro efficacy of copper and silver ions in eradicating Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii: implications for on-site disinfection for hospital infection controlWater Res200842738017655912
- KumarRMunstedtHSilver ion release from antimicrobial polyamide/ silver compositesBiomaterials2005262081208815576182
- Pratap ReddyMVenugopalASubrahmanyamMHydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalystWater Res20074137938617137613
- ElahifardMRRahimnejadSHaghighiSGholamiMRApatite-coated Ag/AgBr/TiO2 visible-light photocatalyst for destruction of bacteriaJ Am Chem Soc20071299552955317630741
- ManjubalaISampath KumarTSEffect of TiO2-Ag2O additives on the formation of calcium phosphate based functionally graded bioceramicsBiomaterials2000211995200210941921
- GreenwoodDO’GradyFScanning electron microscopy of Staphyloccus aureus exposed to some common anti-staphylococcal agentsJ Gen Microbiol1972702632704483215
- DidenkoLVGerasimenkoDVKonstantinovaNDUltrastructural study of chitosan effects on Klebsiella and staphylococciBull Exp Biol Med200514035636016307058
- DahlTAMiddenWRHartmanPEComparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygenJ Bacteriol1989171218821942703469
- LiuLBarfordJYeungKLNon-UV germicidal activity of fresh TiO2 and Ag/TiO2J Environ Sci (China)20092170070620108675
- YoshimuraFNikaidoHDiffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12Antimicrob Agents Chemother19852784922580479
- SawaiTHirumaRKawanaNKanekoMTaniyasuFInamiAOuter membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacaeAntimicrob Agents Chemother1982225855926758687
- RussellADDayMJAntibacterial activity of chlorhexidineJ Hosp Infect1993252292387907620
- RussellADHugoWBAntimicrobial activity and action of silverProg Med Chem1994313513708029478
- KimJYLeeCChoMYoonJEnhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiationWater Res20084235636217692890
- AhmedIReadyDWilsonMKnowlesJCAntimicrobial effect of silver-doped phosphate-based glassesJ Biomed Mater Res A20067961862616826601
- SokmenMCandanFSumerZDisinfection of E. coli by the Ag-TiO2/ UV system: lipid peroxidationJ Photochem Photobiol A Chem2001143241244
- WeiCLinWYZainalZBactericidal activity of TiO2 photocatalyst in aqueous-media – toward a solar-assisted water disinfection systemEnviron Sci Technol199428934938
- ManessPCSmolinskiSBlakeDMHuangZWolfrumEJJacobyWABactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanismAppl Environ Microbiol1999654094409810473421
- SunadaKWatanabeTHashimotoKBactericidal activity of copper-deposited TiO2 thin film under weak UV light illuminationEnviron Sci Technol2003374785478914594392
- YaoYOhkoYSekiguchiYFujishimaAKubotaYSelf-sterilization using silicone catheters coated with Ag and TiO2 nanocomposite thin filmJ Biomed Mater Res B Appl Biomater20088545346018098205
- NonamiTTaodaHHueNTApatite formation on TiO2 photocatalyst film in a pseudo body solutionMater Res Bull199833125131
- TsuruSShinomiyaNKatsuraYUwabeYNoritakeMRokutandaMAdsorption and preparation of human viruses using hydroxyapatite columnBiomed Mater Eng199111431471668797
- TiseliusAHjerténSLevinÖProtein chromatography on calcium phosphate columnsArch Biochem Biophys19566513215513373414

