?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To overcome both the dose-limiting side effects of conventional chemotherapeutic agents and the therapeutic failure resulting from multidrug resistance (MDR) and minimize adverse effects of chemotherapy agents, a novel chemotherapy formulation of magnetic nanoparticles co-loaded with daunorubicin and 5-bromotetrandrin (DNR/BrTet-MNPs) was developed, and its effect on MDR leukemic cells was explored. After the DNR and Br were co-loaded onto a pluronic-stabilized and oleic acid-modified magnetic nanosystem, the physical characteristic and drug-loading capacity were evaluated. The cell toxicity of the self-prepared DNR/BrTet-MNPs formulation was then determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay; the cellular uptake of drug was demonstrated by fluorescent microscope. Lastly, the transcription of mdr1 and the expression of P-glycoprotein (P-gp) were detected by the reverse transcription reaction and western blotting assay, respectively. The results showed that the self-prepared DNR/BrTet-MNPs formulation possessed a sustained release of drug and displayed a dose-dependent antiproliferative activity on MDR leukemia K562/A02 cells. It also enhanced the accumulation of intracellular DNR in K562/A02 cells and downregulated the transcription of the mdr1 gene and the expression of P-gp. These findings suggest that the remarkable effect of the novel DNR/BrTet-MNPs formulation, acting as a drug depot system for the sustained release of the loaded DNR and BrTet, on multidrug resistance leukemia K562/A02 cells would be a promising strategy for overcoming MDR.
Introduction
Owing to multidrug resistance (MDR), systematic chemotherapy is limited in leukemia treatment.Citation1 Recent exciting data suggest that the overexpression of adenosine triphosphate-dependent drug efflux pump on the surface of tumor cells, P-glycoprotein (P-gp/ABCB1), is the main cause of MDR.Citation2 P-gp, coded by the mdr1 gene, effluxes drug and decreases intracellular drug dose, which reduces cytotoxicity of antitumor drugs and thus induces MDR.Citation3 Therefore, exploiting P-gp inhibitors has the potential of overcoming MDR and enhancing chemotherapy efficacy.Citation4 Unfortunately, these compounds are often nonspecific and have low efficiency and/or high toxicity; as such, Phase 3 clinical trials of these agents are largely disappointing.Citation5–Citation9 Therefore, searching for more efficient and lower toxic MDR reversal agents is our main purpose in refractory tumor therapy.
Tetrandrine (Tet), a bis-benzylisoquinoline alkaloid, is the extract component from the root of Stephania tetrandra S. Moore (or Fenfangji) in the Menispermaceae family of traditional Chinese herbs.Citation10 A number of accumulated evidences indicate that Tet has been shown to be a Ca2+ channel antagonist, and it has reversal effect on P-gp-mediated MDR.Citation11,Citation12 Moreover, 5-bromotetrandrine (BrTet) is a bromized derivative of Tet, which is more efficient than Tet at modulating MDR in vitro.Citation13 Therefore, co-administration of conventional chemotherapy with an MDR reversal agent enhances their antitumor efficacy and reduces their side effects. However, it is necessary to minimize the exposure of normal cells to inhibitor and anticancer drugs, and to co-localize both of them in the tumor cells. Currently, nano-technology has shown tremendous progress in target-specific delivery of drugs in the body.Citation14 The targeting drug-delivery system is far more effective than conventional chemotherapy, benefiting from decreasing toxicity of drugs to normal tissues, prolonging circulation half-life in plasma, enhancing drug stability, improving aqueous solubility, and increasing tumor site selectivity.Citation15,Citation16 Due to their biocompatibility and biodegradability, superparamagnetic iron oxide nanoparticles have been widely used in various biomedical applications,Citation17 such as a carrier for anticancer drugs. Daunorubicin (DNR) is one of the anthracyclines and a substrate for P-gp,Citation18 which is the essential part of first-line chemotherapy in the treatment of many solid and hematological malignancies,Citation19 but the clinical use of DNR has been impeded by its serious side effects, such as cardiotoxicity and drug resistance.Citation20 To overcome both the dose-limiting side effects of conventional chemotherapy and the therapeutic failure of MDR, a novel drug-delivery formulation, magnetic iron oxide nanoparticles co-loaded with DNR and BrTet (DNR/BrTet-MNPs) was developed, and its reversal efficacy on K562/A02 cells was evaluated in vitro.
Materials and methods
Main drugs and chemicals
The following materials were purchased: DNR hydrochloride (DNR·HCl) (Huifengda Chemical Co, Ltd, Jinan, China); BrTet (Kanghong Phamaceuticals Inc, Chengdu, China); doxorubicin (Hisun Phamaceutical Co, Zhejiang, China); iron chloride hexagydrate (FeCl3·6H2O), iron sulfate heptahydrate (FeSO4·7H2O), ammonium hydroxide (NH3·H2O), oleic acid (OA), and absolute ethanol (Sinopharm Chemical Reagent Co, Ltd, Shanghai, China); Pluronic® F-127, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma Aldrich, St Louis, MO); 4′, 6-diamidino-2-phenylindole (DAPI) (Beyotime Institute of Biotechnology, Shanghai, China); randomly primed polymerase chain reaction (RP-PCR) kit (Takara Biotechnology, Dalian, China); mouse monoclonal anti-human P-gp and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA).
Synthesis of DNR/BrTet-MNPs
According to the authors’ previously described method,Citation21 OA-coated and pluronic-stabilized iron oxide nanoparticles were synthesized, both hydrophobic DNR and hydrophobic BrTet were prepared by adding triethylamine into DNR or BrTet hydrochloride methanolic solution, and a combination of DNR/BrTet (w/w = 1:1) was added dropwise into the above mentioned aqueous dispersion of MNPs, which was being stirred vigorously on a magnetic stirring plate overnight to make the drug participate into OA layer.Citation22 Finally, the product (DNR/BrTet-MNPs) was washed twice with deionized water to remove the drug adsorbed on the surface of the MNPs. A magnet was put outside of the vial for 5 hours, which attracted the drug-loaded MNPs, and then the liquid was removed carefully. The drug-loaded MNPs were then resuspended in deionized water and washed twice to obtain the synthesized DNR/BrTet-MNPs.
Physical characteristic measurements
The sample was suspended in water and dropped on a carbon-coated copper grid to analyze the morphology of OA-coated and pluronic-stabilized iron oxide nanoparticles under transmission electron microscope (JEM-2100; JEOL, Tokyo, Japan). Meanwhile, the hydrodynamic diameter and zeta potential of DNR/BrTet-MNPs were determined by dynamic light scattering (ZS90; Malvern Instruments, Worcestershire, UK) and zeta plus potential analyzer (Brookhaven Instruments, Holtsville, NY), respectively. Furthermore, the magnetic properties of drug-loaded MNPs were measured using a vibration sample magnetometer (VSM) integrated in a physical property measurement system (PPMS-9; Quantum Design, San Diego, CA) up to 50,000 Oe at 300 K.
Studying drug loading and release
To determine DNR loading, less than 2 mg lyophilized drug-loaded MNPs were dispersed in 2 mL methanolic solution (12.5%) in chloroform (v/v). The solution was shaken for 24 hours and then centrifuged at 16,000 rpm for 10 minutes at 4°C. Volumes of 100 μL supernatant were diluted to 2 mL methanolic-chloroform solution and analyzed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) at detection/ excitation wavelengths of 480/560 nm. To determine BrTet loading, the lyophilized drug-loaded MNPs were dispersed in 2 mL methanolic solution and shaken as above; 1 mL supernatant was diluted to 4 mL methanolic solution and measured at wavelength of 281 nm using an ultraviolet spectrophotometer (UV-3600; Shimadzu). The DNR or BrTet loading capacity (LC) and encapsulation efficiency (EE) of the nanoparticles were calculated as:
and
where We is the amount of DNR or BrTet encapsulated in nanoparticles, Wp is the weight of drug-loading MNPs, and Wt is the total amount of DNR or BrTet added.
For the in vitro drug release study, a certain amount of lyophilized drug loaded-MNPs were dispersed in 2.5 mL Tween®-PBS (phosphate buffered saline) (pH = 7.4, containing 0.1% w/v Tween-80), and then placed in a dialysis membrane bag with a molecular weight cutoff of 8000–14,000 Da. The bag was bathed in a tube with 2.5 mL Tween-PBS solution, and the tube was incubated in a shaking water bath at 37°C. The buffer was withdrawn at different time intervals and complemented with the fresh buffer. The amount of DNR and that of BrTet in buffer were analyzed as above.
Cell culture
Human chronic myeloid leukemia resistant cell line to doxorubicin, K562/A02, was received as a gift from the Institute of Hematology, Chinese Academy of Medical Sciences. Cells were cultured in RPMI (Roswell Park Memorial Institute) 1640 medium containing 10% heat-inactivated newborn bovine serum, 10 mM HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 incubator. For maintaining MDR phenotype, K562/A02 was cultured in the medium containing 1 μg/mL doxorubicin at least 1 week before use.
Cytotoxicity assay
K562/A02 cells were plated at a density of 20,000 cells per well in 96-well plates. Different concentrations of DNR alone or its combination with MNPs (0.1–50.0 μg/mL), and co-administration of DNR and BrTet (DNR:BrTet = 1:1 w/w) or MNPs (0.05–20.00 μg/mL) were added. After treatment for 48 hours, 20 μL MTT solution (5 mg/mL) was added to each well and incubated for another 4 hours at 37°C, the formazan salts were dissolved with 150 μL dimethyl sulfoxide, and the absorbance at 450 nm was measured using a microplate reader (Multiskan MK3; Thermo Scientific, Boston, MA). The cell viability was calculated as (ODtreated group/ODcontrol group) × 100%, and half-maximal inhibitory concentration (IC50) values were calculated.
Fluorescence microscope
K562/A02 cells were collected and smeared after being cultured as above. The cells were fixed with methanol for 15 minutes and then stained with fluorochrome dye DAPI. Morphologic characteristics were observed under a fluorescence microscope (X51; Olympus, Tokyo, Japan), with a peak excitation wavelength of 340 nm.
Intracellular DNR accumulation with rhodamine 123 staining
Rhodamine 123 (R123), cellular accumulation of P-gp substrate, was examined to evaluate the function of P-gp of K562/A02 cells. In brief, after being treated as above, K562/A02 cells were washed with ice-cold PBS and then incubated with 100 μg/L R123 for 60 minutes. After being washed and suspended in 500 μL PBS, each sample was determined using a flow cytometer at excitation/emission wavelengths of 488/575 nm. The relative R123 fluorescence intensity (FI) was calculated as FItreated group/FIcontrol group. Those cells treated with RPMI 1640 alone were used as negative control.
RT-PCR analysis of mdr1 mRNA
The K562/A02 cells were incubated as above. Total RNA extraction was conducted with TRIzol® (Invitrogen, Carlsbad, CA), and RT-PCR was performed according to manufacturer’s instructions. The reverse transcription reactions were performed using TaKaRa RNA PCR Kit as previously described.Citation23 The designed PCR primers included the mdr1 primers (forwards 5′-TGGTTTGATGTGCACGATGTTGGG-3′, reverse 5′-AGATCAGCAG GAAAGCAGCACCTA-3′) and the GAPDH primers (forwards 5′-GCCACATCGC TCAGACAC- 3′, reverse 5′-CATCACGCCACAGTTTCC-3′). The amplified PCR products were 436 bp for mdr1 and 614 bp for GAPDH. The newly synthesized cDNA was amplified by PCR, and the conditions were 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute. There was pre-denaturing at 94°C for 2 minutes and final extension at 72°C for 7 minutes. The obtained PCR products were visualized on 1.5% agarose gels with ethidium bromide staining, and densitometry analysis was performed using ScnImage software (Scion Corporation, Frederick, MD).
Western blotting assay
K562/A02 cells were incubated as above. The harvested cells were lysed in lysis buffer containing 25 mM Tris-HCl (pH = 7.4), 150 mM NaCl, 1% Triton® X-100, 5 mM EDTA (ethylenediaminetetraacetic acid), 5 mM EGTA (ethylene glycol tetraacetic acid), 10 mM NaF, 1 mM phenylmethanesulfonylfluoride, 0.5% NP-40, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM pepstatin for 15 minutes at 4°C, then centrifuged at 10,000 rpm for 5 minutes. The supernatant was collected and the amount of protein was measured using Bio-Rad protein assay (Bio-Rad, Hercules, CA). Samples containing 25 μg protein were separated by 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and then electro-transferred onto nitrocellulose filter. Five percent nonfat milk was used to block the nonspecific binding sites for 1 hour. Mouse monoclonal anti-human P-gp (1:200) or GAPDH (1:400) antibodies were incubated to the membrane overnight at 4°C, followed by horseradish peroxidase-labeled rabbit-mouse immunoglobulin G (1:5000) as a secondary antibody. The blots were calculated by densitometry scans (ECL system, Amersham, UK) according to manufacturer’s instructions.
Statistical analysis
All the data were expressed as mean ± standard deviation, and analyzed using SPSS software (v 13.0; SPSS Inc, Chicago, IL). Differences among various groups were evaluated using one-way analysis of variance. All the reported P values were two-tailed, and those <0.05 were considered to be statistically significant.
Results
Physical characteristics of drug-loaded magnetic nanoparticles
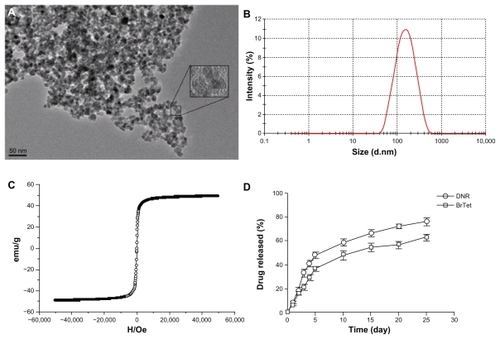
The range of magnetic iron oxide nanoparticles was 10–20 nm, and the clusters of nanoparticles resulting from dehydration were also observed in local fields under transmission electron microscopy. The lattice structure of iron oxide nanoparticles under high-resolution electron microscope demonstrated that the nanoparticles were composed of magnetite (Fe3O4) (). The synthesized DNR/BrTet-MNPs yielded a hydrodynamic average size of 135 nm with polydispersity index of 0.185 by dynamic light scattering (). The zeta potential of DNR/BrTet-MNPs in water was −27.02 ± 0.75 mV, and the drug-loaded MNPs were superparamagnetic with a saturation magnetization (Ms) of 51.31 emu/g magnetic and coercivity (Hc) of 15.67 Oe by VSM ().
Figure 1 Physical characteristic of drug-loaded magnetic nanoparticles. (A) The nanoparticles under the transmission electron microscope. (B) Hydrodynamic particle size distribution of DNR/BrTet-loaded MNPs by Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK). (C) Magnetizaion as a function of field drug-loaded iron oxide nanoparticles by a vibration sample magnetometer. (D) Release of DNR and BrTet from MNPs under in-vitro conditions.
Abbreviations: DNR, daunorubicin; BrTet, 5-bromotetrandrin; MNP, magnetic nanoparticle.
Drug loading and release
Drug-loading capacity and encapsulation efficiency are shown in . The released behavior of DNR/BrTet-MNPs in Tween-PBS solution at 37°C was shown in . The common burst release of DNR and BrTet were both observed in the first 5 days, of which about 48% was for DNR and 37% for BrTet. Interestingly, the drug-loaded MNPs were able to release drugs sustainably until 25 days.
Table 1 Drug loading of DNR and BrTet in MNPs (n = 3, mean ± standard deviation)
In vitro cytotoxicity on K562/A02 cells of drug-loaded MNPs
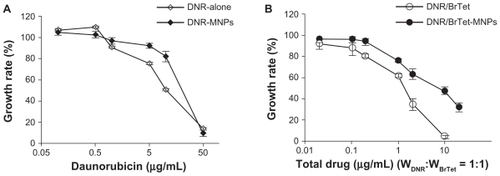
When incubated with MNPs (less than 100 μg/mL) or BrTet (less than 2 μg/mL) for 48 hours, obvious cytotoxic effects on K562/A02 cells were not observed (). Both DNR alone and in combination with MNPs significantly inhibited the growth of K562/A02 cells, and the IC50 values were 19.11 ± 1.05 μg/mL and 10.71 ± 0.62 μg/mL, respectively. Notably, when DNR was co-administrated with BrTet (DNR: BrTet = 1:1 w/w), the cytotoxicity to K562/ A02 cells was significantly enhanced and the IC50 value was 1.01 ± 0.17 μg/mL. DNR/BrTet-co-loaded MNPs also displayed a dose-dependent antiproliferative activity, and the IC50 value was 8.40 ± 1.26 μg/mL ().
Table 2 Effect of MNPs and BrTet on K562/A02 cells for 48 hours (n = 3, mean ± standard deviation)
Fluorescent microscope for cellular uptake of drug
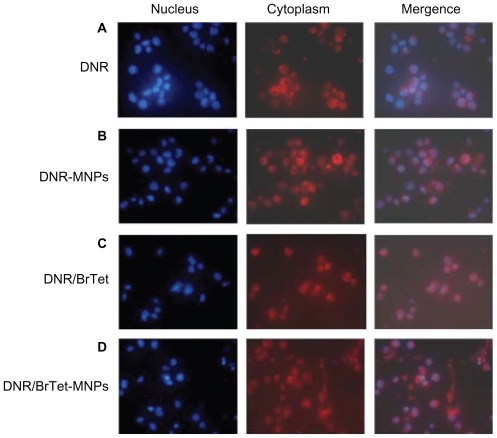
After incubation for 48 hours, high red fluoresce from DNR was seen localized in the cytoplasm of cells treated with DNR alone or DNR-MNPs, indicating that cells endocytosed and/or pinocytosed free DNR or DNR-MNPs (). When being combined with BrTet, a prominent increased red fluorescence was observed in the nucleus (). The nuclear and cytoplasm localization of DNR was observed in cells treated with DNR/BrTet-MNPs ().
Function of P-gp in K562/A02 cells
R123 uptake was increased in K562/A02 cells treated with either BrTet alone or DNR/BrTet compared with DNR alone, and the increase was also observed in cells treated with either BrTet-MNPs or DNR/BrTet-MNPs compared with DNR-MNPs (P < 0.05). In addition, there were significant differences among BrTet, DNR/BrTet, BrTet-MNPs, and DNR/BrTet-MNPs (P < 0.05) ().
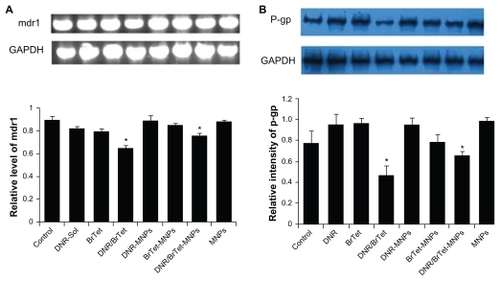
Expression of mdr1/P-gp in K562/A02 cells
Compared with control group, the transcriptions of mdr1 mRNA were significantly downregulated by nearly 26% in the combination of DNR with BrTet (DNR/BrTet l) group and by 13% in the DNR/BrTet-MNPs group (P < 0.05) (). The levels of P-gp were significantly downregulated by nearly 42% in the DNR/BrTet group and by 32% in DNR/BrTet-MNPs group (P < 0.05) ().
Figure 4 Function of P-gp in K562/A02 cells. (A) Intracellular fluorescence intensity (FI) associated with R123 in treated cells: (a) control; (b) DNR; (c) BrTet; (d) DNR/ BrTet; (e) DNR-MNPs; (f) BrTet-MNPs; (g) DNR/BrTet-MNPs; (h) MNPs. (B) Relative R123 accumulation in K562/A02 cells. Data are presented as means ± standard deviation (n = 3) and analyzed using one-way analysis of variance.
Note: *P < 0.05, when compared with control group.
Abbreviations: BrTet, 5-bromotetrandrin; DNR, daunorubicin; FITC-H, heavy chain fluorescein isothiocyanate; MNP, magnetic nanoparticle; P-gp, P-glycoprotein; R123, rhodamine 123; RT-PCR, reverse transcriptase polymerase chain reaction.
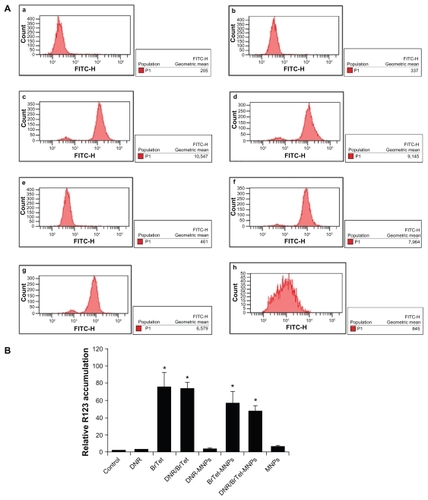
Figure 5 Transcription of mdr1 and expressions of P-gp in K562/A02 cells. (A) Transcription of mdr1 mRNA by RT-PCR; (B) expression of P-gp by western blotting assay.
Note: *P < 0.05, when compared with control group.
Abbreviations: BrTet, 5-bromotetrandrin; DNR, daunorubicin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MNP, magnetic nanoparticle; P-gp, P-glycoprotein; RT-PCR, reverse transcriptase polymerase chain reaction.
Discussion
Currently, systemic chemotherapy is still the main treatment option for hematologic malignancies, but the disturbing fact is that drug-resistant cells usually lead to therapeutic failure. MDR is a multifactorial problem involving several genes acting either alone or in concert with other factors. For example, the increased expression of MDR1 drug transporter gene coding for P-gp pump, MDR-related protein, and topoisomerase II has been associated with the MDR phenotype.Citation24 A lot of chemotherapeutic agents such as anthracyclines or paclitaxel have been proved to be capable of inducing drug resistance via overexpression mdr1/P-gp pathway,Citation25 which is one of the major mechanisms of MDR. Although several MDR reversal agents have been applied in laboratory studies and clinical trials, there is still no significant improvement. Notably, BrTet, an excellent reversal of P-gp mediated MDR,Citation23,Citation26 significantly enhanced the anticancer activity of doxorubicin in vitro by inhibiting the overexpression of P-gp.Citation27
To overcome drug resistance and reduce the side effects during chemotherapy, nanotechnology holds promising potential, utilizing targeted drug delivery. In this study, the ability of a novel chemotherapy formulation (DNR/BrTet-MNPs) to overcome P-gp-mediated drug resistance in K562/ A02 cells was investigated, and the efficacy evaluated. First, an anticancer drug and a MDR reversal agent were encapsulated in the pluronic-OA-iron oxide nanoparticles, which are roughly spherical from 135 nm and have superparamagnetic with Ms of 51.31 emu/g magnetic and Hc of 15.67 Oe. In this formulation, DNR and BrTet are stored in the OA shell and released sustainably, where OA acts as a drug depot and Pluronic F-127, an amphiphilic synthetic polymer, is used to improve aqueous dispersity of the formulation, which makes the nanoparticles disperse well in water and prolongs the systemic circulation time and increases eventual localization efficiency of the drug-delivery system to the tumor site.Citation28 In addition, it also acts as a drug depot system for the sustained release of drug and ensures effective dose in targeted tissue and reduces damage to other organs. Compared with co-administration of a P-gp inhibitor and an anticancer drug,Citation29 in this present study the adoption of nanoparticles in the drug-delivery system made it possible for two drugs with different pharmacokinetics and tissue disposition to play roles at the same time and same place.
Next, the effects of the formulation of a nanoparticle delivery system for overcoming drug-resistant K562/A02 in vitro was tested. In the present study, only about 19% of DNR and 16% of BrTet are released from MNPs in the first 2 days. The efficacy of DNR/BrTet-MNPs on K562/A02 cells at 48 hours was lower than that of DNR-BrTet, which may be down to the sustained release of drugs. Meanwhile, it is found that DNR/BrTet-MNPs show significant antiproliferative activity compared with DNR-MNPs, which is due to the reserve effect of BrTet. It is reasonable to see that red fluorescence intensity was primarily accumulated in the cytoplasm of K562/A02 cells treated with either DNR or DNR-MNPs, once DNR was co-administered with BrTet; red fluorescence intensity was increased significantly in the nucleus of K562/ A02 cells, indicating BrTet makes the increase of intracellular DNR accumulation through inhibiting the drug efflux pumps on the cell membrane, such as P-gp, and then DNR further diffuses into the nucleus. Interestingly, red fluorescent light both in the cytoplasm and nucleus was observed in K562/ A02 cells treated with DNR/BrTet-MNPs, suggesting that DNR and BrTet are released slowly from the nanoparticles in the cytoplasm after being taken up by cells, and then DNR slowly diffuses into the nucleus. Thus, the authors considered that DNR/BrTet-MNPs act as an intracellular depot system and promote sustained drug retention.
P-gp has been demonstrated to significantly reduce the toxicity of anticancer drugs by influencing their pharmacokinetics and pharmacodynamics.Citation13 The present study clearly revealed that the transcription of mdr1 and expression of P-gp were both downregulated in K562/A02 cells after incubation with either DNR/BrTet-MNPs or DNR/BrTet for 48 hours. The results of R123 uptake suggested that BrTet obviously inhibited the efflux of R123 by P-gp both in solution and in MNPs. Moreover, the accumulation of R123 in both BrTet and BrTet-MNPs groups was found to be lower than that in both DNR/BrTet-MNPs and DNR/BrTet. The possible explanation is that the decrease of R123 accumulation means cells treated with DNR/BrTet-MNPs or DNR/BrTet are undergoing apoptosis, for R123 is a fluorescent probe for monitoring the membrane potential of mitochondria.Citation30 In summary, these results indicate that a combination of DNR and BrTet with or without MNPs prevent the development of mdr1 phenotype in K562/A02 cells in vitro. But the effect has not been observed by DNR in nanoparticles, which is to some extent controversial to that reported by Jain et al; in their study, doxorubicin loaded with nanoparticles showed antitumor efficacy on tumor cells.Citation30,Citation31 These opposing results suggest that there may be fundamental differences between cell types and the characteristic of sustained release of the formulation used in this study. Importantly, this novel magnetic iron oxide nanoparticle formulation co-loading DNR and BrTet with the excellent property of sustained release is superior to the prior polymerized MNPs with DNR and BrTet.Citation23 All of these findings suggest the novel magnetic iron oxide nanoparticle formulation co-loading DNR and BrTet may be a successful platform to overcome MDR.
Conclusion
The remarkable effect of the novel DNR/BrTet-MNPs formulation, acting as a drug depot system for the sustained release of the loaded DNR and BrTet on multidrug resistance leukemia K562/A02 cells would be a promising strategy for overcoming MDR.
Acknowledgments
This work was financially supported by National Key Basic Research Program 973 of China (No 2010CB732404) and National Nature Science Foundation of People’s Republic of China (No 30740062 and No 30872970).
Disclosure
The authors report no conflicts of interest in this work.
References
- SzakácsGPatersonJKLudwigJABooth-GentheCGottesmanMMTargeting multidrug resistance in cancerNat Rev Drug Discov2006521923416518375
- GottesmanMMFojoTBatesSEMultidrug resistance in cancer: role of ATP dependent transportersNat Rev Cancer20022485811902585
- LooTWClarkeDMRecent progress in understanding the mechanism of P-glycoprotein-mediated drug effluxJ Membr Biol2005206173185
- FoxEBatesSETariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitorExpert Rev Anticancer Ther2007744745917428165
- KlopmanGShiLMRamuAQuantitative structure activity relationship of multidrug resistance reversal agentsMol Pharmacol1997523233349271356
- SikicBIFisherGALumBLHalseyJBeketic-OreskovicLChenGModulation and prevention of multidrug resistance by inhibitors of P-glycoproteinCancer Chemother Pharmacol199740S13199272128
- KolitzJEGeorgeSLMarcucciGP-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808Blood20101161413142120522709
- AbrahamJEdgerlyMWilsonRA phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbineClin Cancer Res2009153574358219417029
- RuffPVorobiofDAJordaanJPA randomized, placebo-controlled, double-blind Phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimenCancer Chemother Pharmacol20096476376819241078
- DaiCLXiongHYTangLFTetrandrine achieved plasma concentrations capable of reversing MDR in vitro and had no apparent effect on doxorubicin pharmacokinetics in miceCancer Chemother Pharmacol20076074175017273824
- WangGLemosJRTetrandrine: a new ligand to block voltage-dependent Ca-2+ and Ca-2+-activated K+ channelsLife Sci1995562953067837929
- FuLLiangYDengLCharacterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistanceCancer Chemother Pharmacol20045334935614666379
- JinJWangFPWeiHLiuGTReversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrineCancer Chemother Pharmacol20055517918815378274
- HuYLFuYHTabataYGaoJQMesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapyJ Control Release201014715416220493219
- KimKYNanotechnology platforms and physiological challenges for cancer therapeuticsNanomedicine2007310311017442621
- SahooSKLabhasetwarVNanotech approaches to drug delivery and imagingDrug Discov Today200381112112014678737
- MahmoudiMSimchiAImaniMMilaniASStroevePOptimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imagingJ Phys Chem B2008112144701448118729404
- EndicottJALingVThe biochemistry of P-glycoprotein-mediated multidrug resistanceAnnu Rev Biochem1989581371712570548
- LothsteinLIsraelMSweatmanTWAnthracycline drug targeting: cytoplasmic versus nuclear – a fork in the roadDrug Resist Updat2001416917711768330
- GuoJZhouJYingXEffects of stealth liposomal daunorubicin plus tamoxifen on the breast cancer and cancer stem cellsJ Pharm Pharmaceut Sci201013136151
- WangJChenBAChengJSynthesis and antitumor efficacy of daunorubicin-loaded magnetic nanoparticles in vitroInt J Nanomedicine2010620321121445276
- XuJPJiJChenWDShenJCNovel biomimetic polymersomes as polymer therapeutics for drug deliveryJ Control Release200510750251216154659
- ChengJWuWWChenBAEffect of magnetic nanoparticles of Fe3O4 and 5-bromotetrandrine on reversal of multidrug resistance in K562/A02 leukemic cellsInt J Nanomedicine2009420921619918367
- LingVMultidrug resistance: molecular mechanisms and clinical relevanceCancer Chemother Pharmacol199740S389272126
- HorwitzSBCohenDRaoSRingelIShenHJYangCPTaxol: mechanisms of action and resistanceJ Natl Cancer Inst Monogr19931555617912530
- ChenBAChengJWuYNReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-miceInt J Nanomedicine20094737819421372
- ChenBAWangJDingJHReversal of P-glycoprotein-dependent resistance to adriamycin by 5-bromotetrandrine in K562/A02 cell line [abstract]J Clin Oncol200826Suppl13557
- IyerAKKhaledGFangJMaedaHExploiting the enhanced permeability and retention effect for tumor targetingDrug Discov Today20061181281816935749
- MiettinenSGrenmanSYlikomiTInhibition of P-glycoprotein-mediated docetaxel efflux sensitizes ovarian cancer cells to concomitant docetaxel and SN-38 exposureAnticancer Drugs20092026727619262372
- SusaMIyerAKRyuKDoxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcomaBMC Cancer2009939919917123
- JainTKMoralesMASahooSKIron oxide nanoparticles for sustained delivery of anticancer agentsMol Pharm2005219420515934780