Abstract
Background and methods
In this study, gelatin was blended with polyglycolic acid (PGA) at different ratios (0, 10, 30, and 50 wt%) and electrospun. The morphology and structure of the scaffolds were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, and differential scanning calorimetry. The mechanical properties were also measured by the tensile test. Furthermore, for biocompatibility assessment, human umbilical vein endothelial cells and human umbilical artery smooth muscle cells were cultured on these scaffolds, and cell attachment and viability were evaluated.
Results
PGA with 10 wt% gelatin enhanced the endothelial cells whilst PGA with 30 wt% gelatin increased smooth muscle cell adhesion, penetration, and viability compared with the other scaffold blends. Additionally, with the increase in gelatin content, the mechanical properties of the scaffolds were improved due to interaction between PGA and gelatin, as revealed by Fourier transform infrared spectroscopy and differential scanning calorimetry.
Conclusion
Incorporation of gelatin improves the biological and mechanical properties of PGA, making promising scaffolds for vascular tissue engineering.
Introduction
The extracellular matrix complex, composed of proteoglycans, collagens, elastin, and various glycoproteins, is central to the maintenance of vessel wall cell integrity and appropriate signal transduction during important biological functions such as adhesion, differentiation, migration, induction of inflammatory responses, and wound healing through the integrin superfamily of receptors.Citation1 Methods influencing cellular functions using electrospun scaffolds remain a challenge because the scaffolds need to mimic some of the components of the natural extracellular matrix, whilst providing appropriate biochemical and mechanical inputs for the cellular environment.Citation2 Previous studies have attempted to develop thromboresistant and long-lasting synthetic fibrous scaffolds for tissue engineering in the field of vascular grafts. Subsequently, due to poor antithrombogenicity and inconsistent material properties,Citation3 clinical outcomes of synthetic vascular grafts have not always proven satisfactory.Citation4 In addition, the bio-compatibility of tissue-engineered scaffolds is of primary concern because this affects cell attachment, proliferation, differentiation, and growth.Citation5
Electrospinning has been used effectively to generate biomimetic nonwoven scaffolds for tissue engineering purposes.Citation6–Citation8 Various synthetic biodegradable polymers have been electrospun into thin fibers for generating fibrous scaffolds, including poly(3-caprolactone),Citation9–Citation11 polyethylene oxide,Citation11 poly(L-lactide-co-3-caprolactone) (PLCL),Citation12 polylactic acid,Citation13 poly(lactideco-glycolide),Citation14,Citation15 and polyglycolic acid (PGA).Citation16 All these scaffolds have been reported to be bio-compatible and to enhance cell functions in vitro. PGA is one of a group of biodegradable aliphatic polyesters currently exploited in a variety of medical applications. The successful clinical use of PGA sutures has demonstrated that PGA-containing polymers can be used safely in soft tissue applications.Citation17 PGA has also been found to be useful in the engineering of many types of tissuesCitation18–Citation23 and is also used in vascular tissue engineering.Citation24–Citation27
Gelatin is a polymer derived from partial hydrolysis of native collagen, with uses in the pharmaceutical and medical fields, including as sealants for vascular prostheses,Citation28 carriers for drug delivery,Citation29 and wound dressings.Citation30 Furthermore, it is known that gelatin contains many integrin binding sites for cell adhesion, migration, and differentiation, which are found in natural collagen and other extracellular matrix proteins.
In this study, gelatin was added to PGA and electrospun with different weight ratios of gelatin to develop novel electrospun PGA/gelatin fibrous scaffolds which are noncytotoxic, support cell attachment, maintain viability of the major cellular constituents of the vasculature, namely endothelial and smooth muscle cells, and have suitable biomechanical properties.
Materials and methods
Materials and cells
The PGA and gelatin (type A, porcine skin) were purchased from Sigma-Aldrich (St Louis, MO). For electrospinning, PGA and gelatin were dissolved in 1,1,1,3,3,3-hexafluoro- 2-propanol (Merck, Darmstadt, Germany). Cell culture media, growth factors, and supplements were purchased from Invitrogen (Carlsbad, CA) and disposable tissue culture supplies from Orange Scientific (Brussels, Belgium). Tetrazolium salt (MTT) was purchased from Sigma-Aldrich. Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cords according to the method reported in our previous work.Citation1 Human umbilical artery smooth muscle cells (HUASMCs) were obtained from the Pasteur Institute (Tehran, Iran).
Electrospinning
A series of PGA/gelatin solutions with different mixing ratios were prepared by dissolving them in hexafluoroisopropanol at a concentration of 10 wt%. The gelatin contents were 10, 30, and 50 wt%. The PGA/gelatin solutions were delivered by a syringe pump at a constant flow rate of 5 mL/hour. A blunt-ended 18-gauge needle was clamped to the positive electrode of a high-voltage power supply generating 25 kV of electric field, and the negative electrode was connected to an Al foil collector with an air gap distance of 20 cm. The PGA/gelatin solutions were electrospun under the same conditions, producing fibers of varied diameters. All the electrospun PGA/gelatin fibers were vacuum-dried for three days at room temperature and stored in desiccators for subsequent use.
Scanning electron microscopy
The morphology and pore structure of the electrospun PGA/gelatin blend fibers were observed using a scanning electron microscope. Prior to observation, platinum was coated by ion sputtering for a few seconds. The average diameter, diameter distribution, area of porosity between fibers, and distribution of fiber orientation angles were obtained by analyzing scanning electron microscopy images using a custom code image analysis program.
Fourier transform infrared spectroscopy
Chemical analysis of PGA, gelatin, and the PGA/gelatin nanofibrous scaffolds was performed by attenuated total reflectance-Fourier transform infrared spectroscopy over a range of 4000–400 cm−1. Attenuated total reflectance-Fourier transform infrared spectra of PGA and PGA/gelatin nanofibrous scaffolds were obtained on a Bruker spectrometer system (Vertex 80).
Differential scanning calorimetry
A Perkin-Elmer differential scanning calorimeter was used for studying the thermal behavior of the samples. The temperature was raised from room temperature to 350°C using a linear programmer at a heating rate of 10°C per minute.
Mechanical properties
The mechanical properties of the electrospun PGA/gelatin fiber sheets, approximately 60 mm × 10 mm × 0.1–0.2 mm (width × length × thickness), were measured using a uniaxial testing machine (Santam, STM-20) with a 10 N load cell under a cross-head speed of 1 mm/minute and gauge length of 30 mm (n = 3). From the stress–strain curves, Young’s modulus, tensile strength, and elongation at break were obtained.
Cell culture
PGA/gelatin fibers produced by electrospinning were examined as scaffolds for growth of HUVECs and HUASMCs. Cells were grown in Dulbecco’s modified Eagle’s medium with 4.5 g/L glucose supplemented by 4 mM L-glutamine, 25 units/mL penicillin/streptomycin, and 10% fetal bovine serum under standard culture conditions (37°C, 5% CO2). The electrospun fiber sheets were cut into small circular pieces and rinsed with sterilized phosphate-buffered solution and then transferred to 96-well tissue culture polystyrene (TCPS). Scaffolds were sterilized with 70% ethanol and ultraviolet irradiation, followed by washing with phosphate-buffered solution. Fiber scaffolds were soaked in Dulbecco’s modified Eagle’s medium overnight. The cells were seeded at a density of 1 × 104 cells/well onto the sheets, and cultured with regular replacement of culture medium every two days. Cell adhesion and viability were assayed by a colorimetric MTT assay after days 1, 3, and 5, respectively. The morphology of cell attachment on the scaffolds after one day of cell culture was observed by scanning electron microscopy.
MTT assay
The MTT assay is based on reduction of the yellow tetrazolium salt to purple formazan crystals by dehydrogenase enzymes secreted from the mitochondria of metabolically active cells. The amount of purple formazan crystals formed is proportional to the number of viable cells. First, each sample was incubated at 37°C for four hours with MTT solution 0.5 mg/mL without phenol red. After incubation, the MTT solution was removed. A buffer solution containing dimethylsulfoxide was added into the wells to dissolve the formazan crystals. After 30 minutes of rotary agitation, the solutions were transferred into a cuvette and placed in a spectrophotometer, and absorbance was measured at 570–630 nm.
Scanning electron microscopy
To study the morphology of cell attachment, the cell-cultured fiber sheets were washed with phosphate-buffered solution three times and then fixed with 2.5% glutaraldehyde for three hours, followed by formalin fixation (10%) overnight. After washing three times with distilled water, the fiber sheets were dehydrated through a series of graded ethanol solutions (50%, 60%, 70%, 80%, 90%, and 100%). Completely dried samples were sputter-coated with platinum and observed using scanning electron microscopy.
Statistical analysis
The results are presented as the mean ± standard deviation. Statistical significance was tested using SPSS software (SPSS Inc, Chicago, IL). Values of P < 0.05 were considered to be statistically significant.
Results
Scaffold characterization
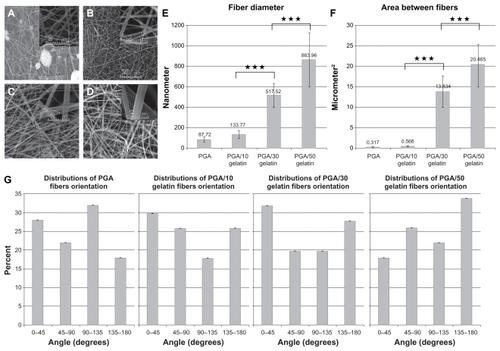
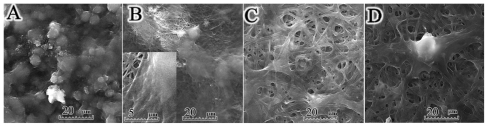
Morphological images of the electrospun PGA/gelatin fibers by scanning electron microscopy are shown in . The scanning electron microscopy images of electrospun pure PGA showed a bead-on-string formation (), while PGA with 10 wt% gelatin fibers was a bead-free and homogeneous structure (). As seen in , an increase in the concentration of gelatin in the solution was accompanied with an increase in fiber size. shows a quantitative analysis of at least 50 fibers from different blended samples, indicating that the mean fiber diameter increased from 87.72 ± 23.34 nm for pure PGA fibers to 863.96 ± 265.09 nm for PGA/50 gelatin fibers. Statistical analysis revealed that the fiber sizes with different weight ratios of gelatin were significantly different from each other (P < 0.001). As depicted in , the biggest increment in fiber size occurred when the gelatin weight ratio was increased from 10 wt% to 30 wt%. The mean area of porosity between the fibers is displayed in . As seen in , the area between the fibers increased with a higher gelatin concentration similar to fiber diameter. represents the distribution of fiber orientation angles with respect to the horizontal axis, demonstrating that the electrospun nanofiber orientation appeared to be random.
Figure 1 Scanning electron microscopic morphology of electrospun PGA/gelatin nanofibers (2000× and 20,000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin. (E) Mean diameter (± standard deviation) variations of electrospun PGA/gelatin fibers, (F) mean area (± standard deviation) between fibers, and (G) distribution of fiber orientation angles (n = 50).
Notes: ***P < 0.001, significantly different from the previous group.
Abbreviation: PGA, polyglycolic acid.
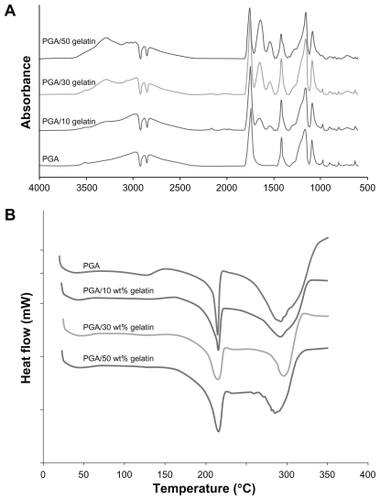
Fourier transform infrared spectroscopy
The infrared absorbance spectra for the scaffolds are shown in . The typical characteristic features observed in the infrared spectra of all scaffolds are reported here. Pure PGA was typically around 2960 cm−1 (γ–CH), 2882 cm−1, 2820 cm−1 (–CH2), 1417 cm−1 (δ–CH), 1163 cm−1, and 1090 cm−1 (γ–C–O), and the group of bands in the region of 1000–800 cm−1 was possibly due to a mix of vibrational modes of [–C–C–]n repeat units and –CH twist. The strong band around 1744 cm−1 is characteristic of [C=O] groups. In the PGA/gelatin scaffolds, the bond groups of PGA are presented; in addition, the 3290 cm−1 region is donated by N–H bond-stretching mode of hydrogen-bonded amide A groups. Common bands of protein appeared at approximately 1642 cm−1 (amide I) and 1539 cm−1 (amide II), corresponding to the stretching vibrations of C=O bond, and coupling of the bending of the N–H bond and stretching of the C–N bonds, respectively.
Differential scanning calorimetry
The differential scanning calorimetry results of the samples are displayed in . As shown in this figure, the first endothermic peaks are at about 210°C–220°C, representing the melting point of PGA. When comparing the PGA curve and the PGA/gelatin curves, in the presence of gelatin, the first peak becomes broader and shifts slightly to a higher temperature.
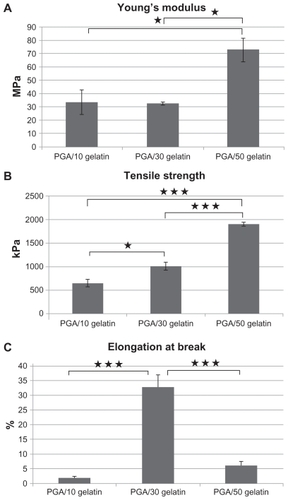
Mechanical properties
The tensile properties of electrospun PGA/gelatin fiber sheets were characterized. Young’s modulus, tensile strength, and elongation at break were calculated from stress–strain curves, as shown in . The pure PGA fiber sheet showed very rigid and brittle characteristics, so no further studies were done. The PGA with 10 wt% gelatin sheets showed a tensile strength of 650 kPa. The PGA with 30 wt% gelatin sheet showed very soft and flexible characteristics, with a Young’s modulus of 32 MPa and a high elongation at break of 32%. PGA/gelatin 50 wt% showed an enhanced tensile strength of 1907 kPa, which was increased about three-fold when compared with that of PGA with 10 wt% gelatin. It also showed a high Young’s modulus of 72 MPa and an elongation break of 6%. It was observed that the addition of gelatin significantly increased tensile strength and the Young’s modulus of the structures.
Figure 3 (A) Young’s modulus of PGA/gelatin scaffolds, mean ± standard deviation (n = 3), (B) tensile strength of PGA/gelatin scaffolds, mean ± standard deviation (n = 3), and (C) elongation at break of PGA/gelatin scaffolds, mean ± standard deviation (n = 3).
Notes: *P < 0.05; ***P < 0.001, significantly different compared with the previous group.
Abbreviation: PGA, polyglycolic acid.
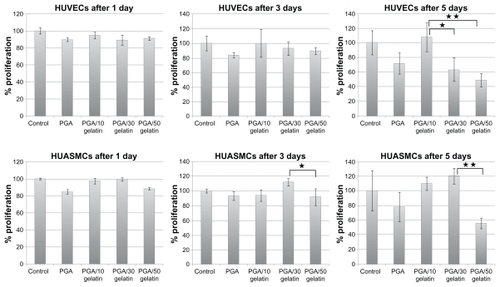
Cell study
HUVECs and HUASMCs were seeded at a density of 10,000 cells/well in triplicate for each experiment. The cells were allowed to proliferate for up to five days. Over the five-day course, relative viable cell numbers were determined continuously on days 1, 3, and 5 using the MTT assay. shows the number of viable cells on electrospun PGA/gelatin fiber and control (TCPS). HUVECs and HUASMCs cells proliferated similarly on all substrates at day 1 of culture period, albeit with some slight differences. On day 1, the number of viable cells on the fibrous substrates was slightly lower than on the controls (P > 0.05). Conversely, on days 3 and 5, there were more endothelial cells on the PGA with 10 wt% gelatin (P < 0.05) substrates. Moreover, there was a high number of smooth muscle cells on the PGA with 30 wt% gelatin substrate, indicating a higher degree of cell viability (P < 0.05).
Figure 4 Cell viability (MTT results) on PGA/gelatin scaffolds after days 1, 3, and 5. Mean ± standard deviation (n = 3).
Notes: *P < 0.05; **P < 0.01.
Abbreviation: PGA, polyglycolic acid.
The enhanced cell adhesion on PGA/gelatin fibers was also confirmed by the scanning electron microscopic images ( and ). The endothelial cell density on the PGA with 10 wt% gelatin increased significantly and enough to cover the electrospun sheets confluently. It also had high cell adhesion and penetration into the fibers (), whereas adherent cells on substrates above this surface displayed flattened morphologies. shows a spheroid cell with some filapodial extensions onto the scaffold due to high concentrations of gelatin. This kind of structure may reduce cell spreading, migration, and viability compared with the cell culture achieved using the lower concentration of gelatin. As shown in , smooth muscle cells can attach to the PGA/gelatin fibers, and the larger space between fibers aids high cell penetration into the PGA with 30 wt% gelatin. The scanning electron microscopy results confirm the data obtained by MTT assay.
Figure 5 Scanning electron micrographs of human umbilical vein endothelial cells cultured on PGA/gelatin scaffolds (2000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin.
Figure 6 Scanning electron micrographs of human umbilical artery smooth muscle cells cultured on PGA/gelatin scaffolds (2000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin.
Abbreviation: PGA, polyglycolic acid.

Abbreviation: PGA, polyglycolic acid.

Discussion
Vascular tissue engineering has emerged as one of the most promising approaches for the development of ideal substitutes for native tissue with similar properties. The design of these scaffolds must meet several physical and chemical criteria of the normal vascular system, such as being biocompatible and biodegradable, and with mechanical properties close to that of native blood vessels.
In the electrospinning process, there are a number of parameters affecting fiber morphology and fiber diameter, including polymer concentration/viscosity, voltage applied, needle diameter, and the delivery rate of polymer solution.Citation6 Pure PGA solutions of high concentrations have been found to be less viscous than PGA/gelatin solutions. Low-viscosity solutions are more prone to produce beads, and bead formation reduces as viscosity is increased by addition of gelatin. Kim et al investigated the electrospinning of polyurethane and gelatin, and reported that addition of gelatin also increased fiber diameter.Citation31 Gu et al also showed that when the gelatin concentration was increased to 10%, uniform fibers were obtained, suggesting a change from beads-on-string structures to smooth fibers.Citation32 Therefore, the addition of gelatin to PGA has a significant effect on the spinnability of a polymer solution and the fiber morphology, resulting in bead-free nanofibers ().
Normally, in the Fourier transform infrared spectra of gelatin, a free N–H stretching vibration occurs in the range of 3400–3440 cm−1. It has been shown that when the N–H group of a peptide is involved in a hydrogen bond, the position is shifted to lower frequencies.Citation33 At the amide A region, a lower amplitude, as well as a lower wave number, was found in the PGA/gelatin structure, compared with gelatin, indicating that the N–H group of shorter peptide fragments in PGA/gelatin was involved in hydrogen bonding. Gelatin might also form new covalent intermolecular cross-links during electrospinning. The amide A also tends to join with the –CH2 stretch peak when carboxylic acid groups exist in a dimeric intermolecular interaction.Citation34
In the current work, the appearance of an amide group in the Fourier transform infrared spectra of PGA/gelatin nanofibrous scaffolds indicates that the PGA chains were chemically bonded to the gelatin side chains, leading to introduction of functional groups, such as –NH2 and –COOH, on the surface of the PGA/gelatin scaffolds. Moreover, the combination of PGA and gelatin with a ratio of 30 wt% gave rise to a strong and flexible structure, which confirms appropriate interaction between PGA and gelatin.
As shown in the differential scanning calorimetry results (), the area under the first peak in the differential scanning calorimetry curves was determined as the enthalpy of the melting of samples (ΔHm).Citation35 The broadening of the melting peak in the samples containing gelatin shows that these samples consume more energy for melting than pure PGA. Therefore, the presence of gelatin molecules in the scaffolds stabilizes the PGA molecules, based on molecular interaction between gelatin and PGA. These results are indicative of interactions between PGA and gelatin, thus confirming the Fourier transform infrared spectra results.
The mechanical properties of the blended fibrous scaffolds that are produced from natural extracellular matrix proteins, namely collagen and other byproducts, are not sufficient for tissue-engineering applications due to their low stability, but nanofibrous membranes made of a polymeric blend system have the advantage of suitable mechanical strength of both natural and synthetic polymers.Citation31 In this study, it was found that PGA nanofibers showed inferior mechanical properties with beads present. Nanofibers with beads have a lower Young’s modulus, tensile strength, and elongation at break. This is due to stress concentrations induced by the beads as the nanofibers are stretched. One study showed that addition of a natural polymer like collagen or gelatin to a synthetic polymer such as PLCL decreased the strength of the polymer,Citation36 whereas Zhang et al showed that Young’s modulus of electrospun poly(3-caprolactone) fiber increased with the addition of gelatin, but the tensile strength decreased to some extent.Citation37 Wang et al tested the mechanical properties of a normal human saphenous vein and showed a Young’s modulus of around 15 MPa and a tensile strength of around 1000 kPa for a natural vessel.Citation24 Our results demonstrate that electrospun PGA with 10 wt% and 30 wt% gelatin scaffolds are mechanically suitable for vascular tissue engineering, with tensile strength values approximating natural vein values and a higher Young’s modulus of 30 MPa, which is about twice as high and, therefore, stiffer than natural veins.
Matsuda et al studied the cell adhesion and proliferation of HUVECs on 0.3 μm, 1.1 μm, and 7 μm PLCL fibers. The dense surface of the small fibers (0.3 μm, 1.1 μm) provided good cell adhesion, spreading, and proliferation, while on the large diameter (7 μm) fibers, cells were sparsely distributed with less proliferation.Citation12 Similar to our observations, they also reported that addition of collagen (5 wt% or 10 wt%) enhanced cell adhesion/proliferation, but the effects were diminished for fibers with a higher collagen content.Citation38
It is well known that basement membranes and their interaction with endothelial cells play a major role in vascular development. Endothelial cells can produce a variety of basement membrane components. Fibronectin and interstitial collagens seem to promote migration and proliferation, whereas basement membrane collagen and laminin stimulate attachment and differentiation.Citation39 Reactivity of the synthetic peptide analogs of adhesive proteins with regard to the interaction of human endothelial cells with the extracellular matrix is also well described in the literature.Citation40 As an example, endothelial cell attachment on uncoated vascular prostheses is very weak, but the vascular graft surface with cell adhesion promoting Arg-Gly-Asp (RGD)-containing synthetic peptides significantly improves this important step in endothelial cell seeding of vascular grafts.Citation4,Citation41 Gelatin also has many integrin binding sites (such as RGD) for cell adhesion, migration, and differentiation, which are found in natural collagen and other extracellular matrix proteins. As expected, blending of PGA and gelatin improves cell attachment and proliferation compared with cell growth on pure PGA nanofibrous scaffolds.
Cell penetration is highly important for obtaining cell-cell contacts and also the outcome of engineered tissue. In two-dimensional systems, contact inhibition in a monolayer culture caused a decrease in cell proliferation when cultured on TCPS as control. In three-dimensional scaffold systems, cells can penetrate into the scaffold structure, but due to the incorporation of high concentrations of gelatin, cell penetration may be hampered even in the presence of adhesion molecules, due to the physical obstruction posed by the dense matrix.Citation42 Mann et al showed that a low concentration of cell adhesion ligand (RGD) aids cell migration, while a higher concentration impedes cell migration, and cells growing on the surfaces with a greater RGD produce less matrix.Citation43,Citation44 Neff et al demonstrated that an intermediate RGD concentration provides maximum proliferation of fibroblasts, indicating that excessive peptide levels on the surface causes a decrease in proliferation.Citation45 Other studies have shown that maximal migration of cells on surfaces coated with matrix proteins, such as RGD, collagen and fibronectin, occurs at an intermediate level of cell-substrate adhesiveness.Citation46–Citation49 These results reflect the fact that cells require cell adhesion ligands for migration, but they also demonstrate that there may be an optimal ligand concentration for migration and proliferation, and indicate that cell viability decreases in scaffolds with a high concentration of gelatin after five days. Therefore, it can be concluded that only a certain amount of gelatin (10 wt% for endothelial cells and 30 wt% for smooth muscle cells) in the PGA/gelatin-blended fiber scaffolds can improve cell viability significantly, and show better vascular cell compatibility than PGA fiber scaffolds and controls.
Conclusion
In this study, gelatin was added to PGA and electrospun with different weight ratios. The results demonstrate that the properties of nanofibrous scaffolds were strongly influenced by the concentration of gelatin in the scaffolds. Given the suitable mechanical properties of these PGA/gelatin scaffolds and favorable biocompatibility with vascular cells, it is suggested that tubular scaffolds with an inner layer of PGA/10 wt% gelatin and an outer layer of PGA/30 wt% gelatin are promising scaffolds for vascular tissue engineering and regeneration in vivo.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShahgasempourSWoodroffeSBSullivan-TailyourGGarnettHMAlteration in the expression of endothelial cell integrin receptors α5β1 and α2β1 and α6β1 after in vitro infection with a clinical isolate of human cytomegalovirusArch Virol19971421251389155877
- NisbetDRForsytheJSShenWFinkelsteinDIHorneMKReview paper: A review of the cellular response on elecrospun nanofibers for tissue engineeringJ Biomed Appl200924729
- NeremRMEnsleyAEThe tissue engineering of blood vessels and the heartAm J Transplant20044364214871272
- MeinhartJGDeutschMFischleinTHowanietz FroschlAZillaPClinical autologous in vitro endothelialization of 153 infrainguinal ePTFE graftsAnn Thorac Surg200171327331
- WilliamsDFOn the mechanisms of biocompatibilityBiomaterials2008292941295318440630
- NukavarapuSPKumbarSGMerrellJGLaurencinCTElectrospun polymeric nanofiber scaffolds for tissue regenerationLaurencinCTNairLSNanotechnology and Tissue Engineering: The ScaffoldBoca Raton, FLCRC Press2008
- VasitaRKattiDSNanofibers and their applications in tissue engineeringInt J Nanomedicine20061153017722259
- ZhangYZSuBVenugopalJRamakrishnaSLimCTBiomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibersInt J Nanomedicine2007262363818203429
- ZongXBienHChungCElectrospun fine-textured scaffolds for heart tissue constructsBiomaterials2005265330533815814131
- BakhshandehHSoleimaniMHosseiniSSPoly (∑caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial corneaInt J Nanomedicine201161509151521845040
- NevesNMCamposRPedroACunhaJMacedoFReisRLPatterning of polymer nanofiber meshes by electrospinning for biomedical applicationsInt J Nanomedicine2007243344818019842
- KwonIKKidoakiSMatsudaTElectrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potentialBiomaterials2005263929393915626440
- ZengJChenXXuXUltrafine fibers electrospun from biodegradable polymersJ Appl Polym Sci20035910851092
- LiWJLaurencinCTCatersonEJTuanRSKoFKElectrospun nanofibrous structure: a novel scaffold for tissue engineeringJ Biomed Mater Res20026061362111948520
- SeilJTWebsterTJSpray deposition of live cells throughout the Electrospinning process produces nanofibrous three-dimensional tissue scaffoldsInt J Nanomedicine201161095109921698076
- BolandEDWnekGESimpsonDGPawlowskiKJBowlinGLTailoring tissue engineering scaffolds by employing electrostatic processing techniques: a study of poly(glycolic acid)J Macromol Sci2001A3812311243
- PerrinDEEnglishJPPolyglycolide and polylactideDombAJKostJWisemanDMHandbook of Biodegradable PolymersBoca Raton, FLCRC Press1997
- XuLCaoDLiuWZhouGZhangWJCaoYIn vivo engineering of a functional tendon sheath in a hen modelBiomaterials2010313894390220170958
- CuiLWuYCenLRepair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid meshBiomaterials2009302683269319217157
- AbbushiAEndresMCabrajaMRegeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degenerationSpine2008331527153218520635
- KimWSKimHKTissue engineered vascularized bone formation using in vivo implanted osteoblast-polyglycolic acid scaffoldJ Korean Med Sci20052047948215953873
- KojimaKBonassarLJRoyAKMizunoHCortiellaJVacantiCAA composite tissue-engineered trachea using sheep nasal chondrocyte and epithelial cellsFASEB J20031782382812724341
- CooperMLHansbroughJFSpielvogelRLCohenRBaxtelRLNaughtmGIn vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin meshBiomaterials1991122432481652296
- WangCCenLYinSA small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cellsBiomaterials20103162163019819545
- XuZCZhangWJLiHEngineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactorBiomaterials2008291464147218155136
- IwasakiKKojimaKKodamaSBioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactorCirculation2008305257
- RashidSTSalacinskiHJHamiltonGSeifalianAMThe use of animal models in developing the discipline of cardiovascular tissue engineering: a reviewBiomaterials2004251627163714697864
- GuiRMarceauDRaoTJIn vitro and in vivo characterization of an impervious polyester arterial prosthesis: the Gelseal triaxial graftBiomaterials198784334413427141
- TabataYHijikataSIkadaYEnhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogelsJ Control Release199431189199
- ChoiYSHongSRLeeYMSongKWParkMHNamYSStudy on gelatin-containing artificial skin: 1. Preparation and characteristics of novel gelatin alginate spongeBiomaterials19992040941710204983
- KimSEHeoDNLeeJBElectrospun gelatin/polyurethane blended nanofibers for wound healingBiomed Mater2009444106
- GuSYWangZMRenJZhangCYElectrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressingMater Sci Eng C Mater Biol Appl20092918221828
- DoyleBBBloutERBenditEGInfrared spectroscopy of collagen and collagen like polypeptidesBiopolymers1975149379571156652
- KempWOrganic Spectroscopy2nd edHampshire, UKMacmillan Education Ltd1987
- PungorEA Practical Guide to Instrumental AnalysisBoca Raton, FLCRC Press1995
- LeeJTaeGKimYHParkISKimSHKimSHThe effect of gelatin incorporation into electrospun poly(L-lactide-co-3-caprolactone) fibers on mechanical properties and cytocompatibilityBiomaterials2008291872187918234330
- ZhangYOuyangHLimCTRamakrishnaSHuangZMElectrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffoldsJ Biomed Mater Res B200572156165
- KwonIKMatsudaTCo-electrospun nanofiber fabrics of poly (L-lactideco-3-caprolactone) with type I collagen or heparinBiomacromolecules200562096210516004450
- OlsenBRMatrix molecules and their ligandsLanzaRPLangerRChickWLPrinciples of Tissue EngineeringGeorgetown, TXRG Landes1997
- ChenCSHawigerJReactivity of synthetic peptide analogs of adhesive proteins in regard to the interaction of human endothelial cells with extracellular matrixBlood199177220022061709375
- WalluscheckKPSteinhoffGKelmSHaverichAImproved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD-containing peptidesEur J Vasc Endovasc Surg1996123213308896475
- PeytonSRRaubCBKeschrumrusVPPutnamAJThe use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cellsBiomaterials2006274881489316762407
- MannBKWestJLCell adhesion peptides alter smooth muscle adhesion, proliferation, migration and matrix protein synthesis on modified surfaces and in polymer scaffoldsJ Biomed Mater Res200260869311835163
- MannBKTsaiATScott-BurdenTWestJLModification of surfaces with cell adhesion peptides alters extracellular matrix depositionBiomaterials1999202281228610614934
- NeffJATrescoPACaldwellKDSurface modification for controlled studies of cell-ligand interactionsBiomaterials1999202377239310614943
- PalecekSPLoftusJCGinsbergMHLuffenburgerDAHorwitzAFIntegrin-ligand binding properties govern cell migration speed through cell-substratum adhesivenessNature19973855375409020360
- BurgessBTMylesJLDickinsonRBQuantitative analysis of adhesion-mediated cell migration in three-dimensional gels of RGD-grafted collagenAnn Biomed Eng20002811011810645794
- DiMillaPAStoneJAQuinnJAAlbeldaSMLauffenburgerDAMaximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strengthJ Cell Biol19931227297378335696
- OlbrichKCAndersenTTBlumenstockFABiziosRSurfaces modified with covalently-immobilized adhesive peptides affect fibroblast population motilityBiomaterials1996177597648730959