Abstract
Background
The ABC phenomenon is described as a syndrome of accelerated clearance of polyethylene glycol (PEG)-modified liposomes from the bloodstream when repeatedly injected, with their increased accumulation in the liver and spleen.
Methods
To clarify this immune response phenomenon, we evaluated a novel modified pH-sensitive liposome with a cleavable double smart PEG-lipid derivative (mPEG-Hz-CHEMS).
Results
The ABC phenomenon in mice was brought about by repeated injection of conventional PEG-PE liposomes and was accompanied by a greatly increased uptake in the liver. However, a slight ABC phenomenon was brought about by repeated injection of mPEG-CHEMS liposomes and was accompanied by only a slightly increased uptake in the liver, and repeated injection of mPEG-Hz-CHEMS liposomes did not induce the ABC phenomenon and there was no increase in liver accumulation. This finding indicates that the cleavable mPEG-Hz-CHEMS derivative could lessen or eliminate the ABC phenomenon induced by repeated injection of PEGylated liposomes.
Conclusion
This research has shed some light on a solution to the ABC phenomenon using a cleavable PEG-Hz-CHEMS derivative encapsulated in nanoparticles.
Introduction
Many liposome studies have been undertaken to develop a wide variety of amphiphilic-associated conjugates containing a polyethylene glycol (PEG) segment as a modifier, such as PEG bonded to dimyristoyl phosphatidylethanolamine (DMPE-PEG)Citation1 or to distearoyl phosphatidylethanolamine/methoxypolyethylene glycol (DSPE-PEG).Citation2 PEG enhances blood levels of the conjugate, but when the drug carrier is intended for intracellular penetration, PEG often prevents normal interaction of the conjugated drug with cells.Citation3 According to the recent literature, PEGylated liposomes have also been shown to induce a significant immune response, known as the accelerated blood clearance (ABC) phenomenon, when repeatedly injected into the same animal. The ABC phenomenon is described as a syndrome of accelerated blood clearance whereby repeated injection of PEGylated nanoparticles can cause their rapid clearance from the bloodstream, with increased accumulation in the liver and spleen.Citation4–Citation6
The ABC phenomenon represents a barrier in research and the clinical use of PEGylated nanocarriers, such as liposomes, nanoparticles, microemulsions, or even PEGylated proteins. Lu et alCitation7 found that cationic bovine serum albumin-conjugated polyethyleneglycol-polylactide (PEG-PLA) nanoparticles could induce the ABC phenomenon with repeated injection of PEGylated nanoparticles. Ishihara et alCitation8 also found that the ABC phenomenon could appear when PEG-PLA-modified nanoparticles were administered.
Many researchers have studied the ABC phenomenon, but there has not yet been any feasible solution to avoid or alleviate this problem. Judge et alCitation9 and Semple et alCitation10 used PEGylated lipids with smaller C14 lipid anchors in modified liposomes and found that the PEG dissociating from the particles increased and the ABC phenomenon was avoided. Some researchers have made efforts to alter the linkage between the lipids and PEG and use them in the liposome. Romberg et alCitation11 have replaced PEG-modified liposomes with polyamino acid polyhydroxyethyl-L-asparagine (PHEA). PHEA liposomes have shown favorably long circulation times compared with PEGylated liposomes, and a second dose of PHEA liposomes given seven days after the first injection is more slowly cleared from the blood circulation than a second dose of PEG liposomes.
Esterase belongs to the group of plasma functionality enzymes that include carboxylesterase and cholinesterase.Citation12 Mammalian esterases distribute widely in the plasma and many organs and tissues. They efficiently catalyze the breakage of ester bonds or thioester bonds to free acids from endogenous or exogenous materials.Citation13,Citation14 Xu et alCitation15,Citation16 synthesized two PEG-lipid derivatives that linked via a single ester band so that PEG could be cleaved by esterase in plasma. The vesicles or liposomes modified by different PEG-lipid derivates (PEG-CHEMS or PEG-CHMC) could reduce or avoid occurrence of the ABC phenomenon. However, the capacity of PEG-CHEMS with a single ester bond is still not strong and requires further investigation.
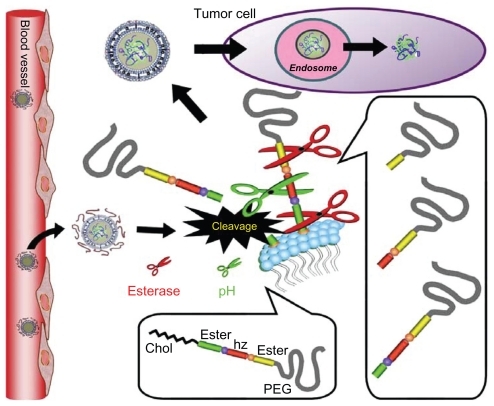
In this study, we prepared modified pH-sensitive liposomes with mPEG-Hz-CHEMS, a cleavable PEG-lipid derivative and control mPEG-CHEMS.Citation17–Citation19 A schematic diagram of the strategy is shown in . The modified pH-sensitive liposomes accumulate in tumor via the enhanced permeability and retention effect. We linked PEG to cholesterol by two ester bonds and one pH-sensitive hydrazone, with the expectation that the chemical bond would gradually be cleaved in serum and at lower pH conditions in vivo. The PEG was then detached from the surface of the liposome, allowing the liposome to associate with the tumor cell surface, followed by cellular uptake. We assume that because PEG dissociates from the surface of the particle, the ABC phenomenon is avoided. Compared with mPEG-CHEMS, mPEG-Hz-CHEMS could have novel dual features, both at a lower pH and as an esterase. In addition, cleavable mPEG-Hz- CHEMS-modified liposomes have a long circulation time. Therefore, the purpose of this study was to design double smart mPEG-Hz-CHEMS-modified liposomes to reduce the likelihood of the ABC phenomenon.
Materials and methods
Soybean phosphatidylcholine (S100PC) was purchased from Dongshang Co Ltd (Shanghai, China). mPEG-DSPE was obtained from Lipoid (Ludwigshafen, Germany). Cholesterol (Chol) was obtained from China Medicine Shanghai Chemical Reagent Corporation (Shanghai, China). Paclitaxel (PTX) was supplied by Taihua Natural Plant Pharmaceutical Co Ltd (Xi’an Shaanxi, China). PEG2000-CHEMS and PEG2000-Hz-Chol were synthesized in our laboratory. All other chemicals were of analytical grade.
Animals
Five-week-old female BALB/c mice were obtained from Shanghai SLAC Laboratory Animal Co Ltd (Shanghai, China). The animals had free access to water and rat chow. All the animal experiments were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Preparation of liposomes
Conventional PEG-DSPE liposomes composed of S100PC/ Chol/PEG-DSPE/PTX (90:10:3:3), mPEG-CHEMS liposomes composed of S100PC/Chol/PEG-CHEMS/PTX (90:10:3:3), and pH-sensitive mPEG-Hz-CHEMS liposomes composed of S100PC/Chol/ mPEG2000-Hz-CHEMS/PTX (90:10:3:3) were prepared by the thin-film hydration method. Briefly, the hydrophobic excipients, PTX, cholesterol, and lipids were dissolved in chloroform and transferred into a suitable conical flask. The flask was evaporated under reduced pressure. The dry lipid formed was hydrated with phosphate-buffered saline (pH 7.4). The liposomal suspension was filtered through 0.2 μm polycarbonate filters and stored at 4°C until use.
Pharmacokinetic and tissue distribution study
To assess the effect of different liposomes on the pharmacokinetic and tissue distribution of PTX, BALB/c mice (18–22 g) were used in following formulations () after intravenous administration via the caudal vein at the dose of 20 mg/kg bodyweight. The first injection used empty PEGylated liposomes, which were injected via the tail vein. Control animals received phosphate-buffered saline instead of the liposomes. The interval between the two injections was three days. For the second injection, liposomes were injected intravenously via the tail vein. Injection protocols for the liposomes are presented in . A-01 represents the first injection using empty mPEG-PE liposomes instead of phosphate-buffered saline, while the second injection comprised mPEG-PE liposomes via the tail vein. A-02 represents the first injection using mPEG-PE liposomes, and the second injection also comprised mPEG-PE liposomes. B-01 denotes the first injection using empty mPEG-CHEMS liposomes instead of phosphate-buffered saline, while the second injection was of mPEG-CHEMS liposomes via the tail vein. B-02 denotes the first injection using mPEG-PE liposomes, while the second injection was also of mPEG-CHEMS liposomes. C-01 represents the first injection using empty mPEG-Hz- CHEMS liposomes instead of phosphate-buffered saline, while the second injection was of mPEG-Hz-CHEMS liposomes via the tail vein. C-02 denotes the first injection using mPEG-Hz-CHEMS liposomes, while the second injection also contained mPEG-PE liposomes.
Table 1 Injection protocols for liposomes
At hours 0.25, 0.5, 1, 2, 4, 6, 8, and 12 following injection, blood samples were collected from the eyes of six mice in each group, after which the mice were sacrificed by cervical dislocation. Plasma was obtained by centrifugation at 5000 rpm for 10 minutes. The liver and spleen were removed and washed twice with physiological solution (0.9% NaCl), weighed, and homogenized. The plasma and tissue samples were kept at −20°C until analysis.
Analytical procedure
A 140 μL aliquot blood sample, 10 μL of internal standard (Diazepam in methanol), and 290 μL of acetonitrile were added to a protein precipitation tube. The mixture was vortexed for five minutes and centrifuged at 8000 rpm for 10 minutes. Then 20 μL of the solution were injected into a high-pressure liquid chromatography (HPLC) system. A 150 μL aliquot of homogenate from the liver and spleen, 10 μL of internal standard (Diazepard in methanol) and 290 μL of acetonitrile were added. The mixture was vortexed for five minutes and centrifuged at 15,000 rpm for 10 minutes, and 20 μL of the supernatant was then injected into the HPLC system.
The concentrations of PTX in plasma and tissue samples were assayed by an HPLC method. The HPLC was equipped with a reverse-phase column (4.6 mm × 250 mm, Hanbon, Jiangsu, China) at 35°C and an ultraviolet spectrophotometer (Agilent Technologies, Palo Alto, CA). The mobile phase was a mixture of methanol and water (72:28 v/v). The samples were delivered at a flow rate of 1.0 mL/min and detected at a wavelength of 227 nm.
Statistical analysis
Statistical comparisons were performed using the Student’s t-test for the two groups, and one-way analysis of variance for multiple groups. P < 0.05 was considered to be statistically significant.
Results and discussion
Effect of repeated injections on pharmacokinetics of PEGylated liposomes
The structure and 1H-NMR spectrum of mPEG-Hz-CHEMS is shown in . The pharmacokinetics of PEG-lipid-modified liposomes in mice were evaluated after the first and second injections. There was an obvious ABC phenomenon with the conventional mPEG-PE modified liposomes. The plasma PTX concentration decreased significantly (P < 0.05) after repeated injections of mPEG-PE liposomes (-02).
Table 2 Physicochemical parameters of different liposomes
Figure 2 Structure and 1H-NMR spectra of mPEG-Hz-CHEMS in CDCl3. Mean particle size of different liposomes ranged in size from 100 to 200 nm with no significant differences. The mean zeta potential of the different liposomes were also similar. The entrapment efficiency of the different liposomes was determined to be above 90% (see ).
Abbreviation: mPEG-Hz-CHEMS, methoxy polyethylene glycol 2000-hydrazone-cholesteryl hemisuccinate.
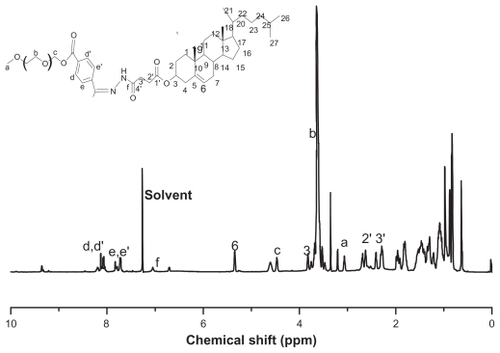
Figure 3 Blood clearance of paclitaxel in mice after a first and second injection of mPEG-PE liposomes (n = 6).
Abbreviation: mPEG-PE, methoxy polyethylene glycol 2000-distearoyl phosphatidylethanolamine.
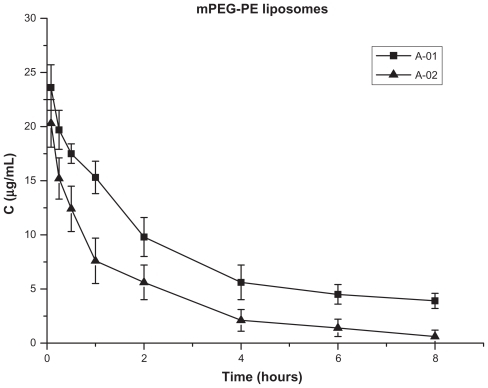
There was a sharp increase in plasma clearance rate after repeated injections of the mPEG-PE liposomes ( and ). However, the cleavable PEG-lipid derivative-modified liposomes showed different pharmacokinetics after the repeat injection compared with the conventional mPEG-PE liposomes. The plasma PTX concentration did not decrease significantly after repeated injections, either in the mPEG-CHEMS liposomes and pH-sensitive mPEG-Hz- CHEMS liposomes ( and , ).
Table 3 Main pharmacokinetic parameters of different liposomes
Figure 4 Blood clearance of paclitaxel in mice after a first and second injection of mPEG-CHEMS liposomes (n = 6).
Abbreviation: mPEG-CHEMS, methoxy polyethylene glycol 2000-cholesteryl hemisuccinate.
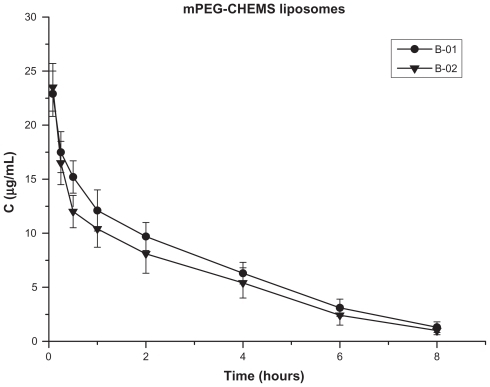
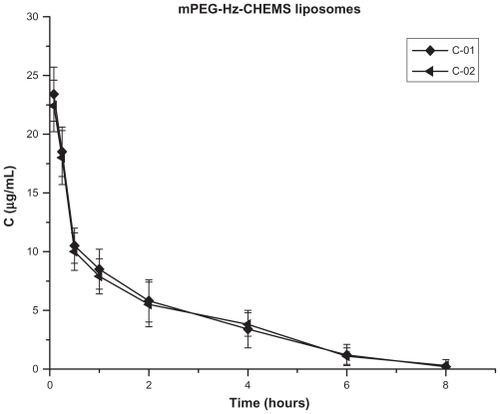
Figure 5 Blood clearance of paclitaxel in mice after a first and second injection of mPEG-Hz-CHEMS liposomes (n = 6).
Abbreviation: mPEG-Hz-CHEMS, methoxy polyethylene glycol 2000-hydrazone-cholesteryl hemisuccinate.
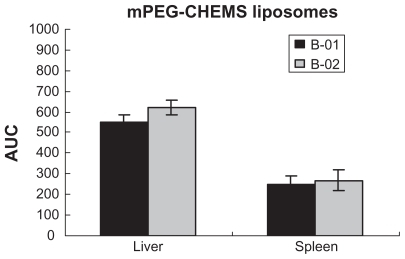
As shown in , the plasma PTX concentration in the B-01 group at each time point was slightly higher than that in the B-02 group (mPEG-CHEMS liposomes). The different PEGylated liposomes have different characteristics. mPEG-Hz-CHEMS have double pH-sensitive hydrazone and esterase-sensitive ester, which could be easily cleavable. mPEG-CHEMS has only esterase-sensitive ester, while mPEG-PE is not steady under lower pH and esterase conditions. The main pharmacokinetic parameters of different liposomes also indicate this phenomenon ().
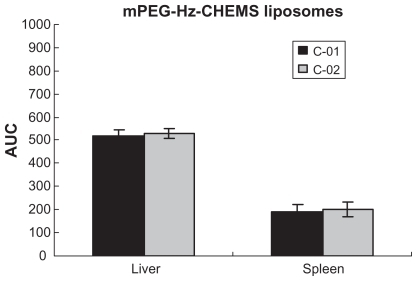
However, as shown in and , there was no significant difference between the C-01 and C-02 groups (mPEG-Hz-CHEMS liposomes). Blood clearance curves and plasma clearance rate in the C groups were basically the same. That is, mPEG-CHEMS-modified liposomes could alleviate the ABC phenomenon, while mPEG- Hz-CHEMS-modified liposomes could avoid the ABC phenomenon.
Effect of repeated injections on biodistribution of PEGylated liposomes
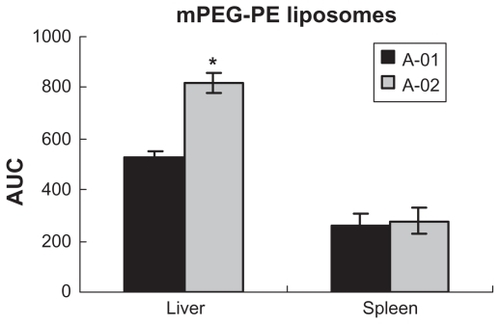
With regards to biodistribution of the mPEG-PE liposomes, there was a markedly increased uptake in the liver after the second dose, but no significant difference in the spleen. As shown in , the accumulated amount of PTX in the liver increased after the second dose (P < 0.05). However, compared with mPEG-PE liposomes, the accumulated amount of PTX in the liver and spleen was not changed in mPEG-CHEMS liposomes and mPEG-Hz-CHEMS liposomes. As shown in , mPEG-CHEMS modified liposomes only induced a slight ABC phenomenon which could alleviate the ABC phenomenon. As shown in , repeated injection of mPEG-Hz-CHEMS liposomes caused no increase in liver and spleen accumulation. From the results above, mPEG-Hz-CHEMS-modified liposomes could avoid the ABC phenomenon.
Figure 6 Biodistribution of paclitaxel in mice after a first and second injection of mPEG-PE liposomes (n = 6).
Abbreviation: mPEG-PE, methoxypolyethylene glycol 2000-distearoyl phosphatidylethanolamine.
Discussion
The ABC phenomenon is important for the development of drug delivery systems, especially for nanoparticles in the case of repeated injection of nanomedicines. Researchers have used different animal models to investigate the mechanism of the ABC phenomenon, including rats, mice, rhesus monkeys, and rabbits, among others. Dams et al demonstrated that rhesus monkeys and rats could bring out the ABC phenomenon after repeated injection of PEGylated liposomes, whereas mice could not.Citation20 Ishida et al have studied the first liposomal characteristics that affect the ABC phenomenon and demonstrated that an intense accelerated clearance can be induced in mice.Citation21
Although the conclusion from animal experiments might not be fully consistent with the clinical manifestations of liposomes, when there is a need for repeated injections, thorough study of their distribution in the body and their pharmacokinetics is necessary. Many researchers have sought a possible solution for the ABC phenomenon. Xu et al have investigatedCitation16 vesicles or liposomes modified by different PEG-lipid derivatives (PEG-CHEMS or PEG-CHMC) that could reduce or avoid the ABC phenomenon. In our studies, pH-sensitive liposomes modified by PEG-lipid derivatives (PEG-Hz-CHEMS) showed better PEG cleavage rates when incubated in serum. The PEG-Hz-CHEMS was easily degraded at pH 5.5 (half-life 6.7 hours), while it has higher stability at physiological pH (half-life 40.9 hours).Citation17
Due to the pH-sensitive hydrazone bond, the degree of PEG cleavage from PEG-Hz-CHEMS was higher than that from PEG-CHEMS, based on pH-insensitive ester bands and noncleavable PEG-CHOL and PEG-DSPE. This is consistent with the conclusion reached by Xu et al.Citation16 When rats received repeated injections of PEG-CHEMS-modified liposomes, there were minor occurrences of the ABC phenomenon, which disappeared with PEG-Hz-CHEMS. However, PEG-PE and PEG-CHOL-modified liposomes produced a significant ABC phenomenon. Our results showed that the phenomenon was more obvious with lower degrees of PEG cleavage (PEG-Hz- CHEMS > PEG-CHEMS > PEG-CHOL > PEG-DSPE). Therefore, our hypothesis was that a first injection of cleavable PEG-lipid derivative-modified liposomes could reduce or avoid the ABC phenomenon.
When we adopted the cleavable pH-sensitive PEG-Hz- CHEMS to modify liposomes, the ABC phenomenon was almost eliminated. In studies of the ABC phenomenon, the second injected liposomes have usually been tracer-labeled with technetium (99 mTc-PEG)Citation6,Citation10 or 3H-CHE. However, because this method needs to be carried out by skilled personnel and with strict security precautions, its applications are limited. Xu et alCitation17 used calcein, a water-soluble fluorescence probe, to mark the inner water phase of liposomes and vesicles to investigate the ABC phenomenon. Compared with radioimmunoassay, fluorescence labeling has no radioactive contamination and is easy to perform. Moreover, the calcein marker can reflect overall changes in liposomes in the body, while a tracer marker can only express the changes in membrane components. However, the calcein marker also has some limitations, especially in that it does not reflect the characteristics of a poorly water soluble drug, as is the case with many antitumor drugs. PTX, the first of the microtubule-stabilizing agents, is one of the most successful anticancer drugs and shows potency against a broad spectrum of cancers, especially carcinomas of the breast, ovary and lung.Citation22,Citation23 It is currently marketed in a vehicle composed of Cremophor® EL (polyethoxylated castor oil) and ethanol due to its low aqueous solubility.Citation24,Citation25 Comparing 3H-CHE labeling and fluorescence labeling, the PTX marker could better reflect its real pharmacokinetic behavior in terms of the ABC phenomenon. In this study, we studied the effect on the ABC phenomenon of novel pH-sensitive liposomes with cleavable pH-sensitive PEG-Hz-CHEMS liposomes containing PTX.
Conclusion
We modified liposomes by mPEG-Hz-CHEMS via cleavable pH-responsive hydrazone and esterase-catalyzed ester linkages so that the PEGylated coating can be efficiently removed by the lower pH and esterase in the serum. This is the first time hydrazone and ester esterase-cleavable linkers have been utilized simultaneously as an effective strategy for PEG-lipid cleavage. The ABC phenomenon was induced by repeated injection of PEGylated liposomes into the rat and was accompanied by substantially increased uptake into the liver. Repeated injection of the conventional and noncleavable PEG-lipid derivative-modified microparticles induced the ABC phenomenon. We used cleavable esterase-catalyzed and pH-responsive double smart mPEG-Hz-CHEMS to modify liposomes and showed that the ABC phenomenon became weaker or disappeared. Therefore, cleavable esterase-catalyzed and pH-responsive double smart mPEG-Hz-CHEMS can not only prolong the circulation time of liposomes, but also avoid or weaken the ABC phenomenon. These studies have paved the way for a solution to the ABC phenomenon using cleavable PEG-lipid derivatives in nanoparticles.
Acknowledgments
This work was supported by Yantai University Research Program and Enterprise state key laboratory of 973 Research Program (China, 2010CB735600). The authors also thank Professor Qineng Ping, Can Zhang, and Changli Wang for their efforts and collaboration in this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- GeorgievGASarkerDKAl-HanbaliOGeorgievGDLalchevZEffects of poly (ethylene glycol) chains conformational transition on the properties of mixed DMPC/DMPE-PEG thin liquid films and monolayersColloids Surf B Biointerfaces200759218419317587556
- LasicDDMartinFJGabizonAHuangSKPapahadjopoulosDSterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation timesBiochim Biophys Acta1991107011871921751525
- ShinJShumPThompsonDHAcid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipidsJ Control Release2003911–218720012932651
- DamsETLavermanPOyenWJAccelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomesJ Pharmacol Exp Ther200029231071107910688625
- LavermanPCarstensMGBoermanOCFactors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injectionJ Pharmacol Exp Ther2001298260761211454922
- IshidaTAtobeKWangXKiwadaHAccelerated blood clearance of PEGylated liposomes upon repeated injections: Effect of doxorubicin-encapsulation and high-dose first injectionJ Control Release2006115325125817045355
- LuWWanJSheZJiangXBrain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticleJ Control Release20071181385317240471
- IshiharaTTakedaMSakamotoHAccelerated blood clearance phenomenon upon repeated injection of PEG modified PLA- nanoparticlesPharm Res200926102270227919633820
- JudgeAMcClintockKPhelpsJRMacLachlanIHypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomesMol Ther200613232833716275098
- SempleSCHarasymTOClowKAAnsellSMKlimukSKHopeMJImmunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acidJ Pharmacol Exp Ther200531231020102615525796
- RombergBOussorenCSnelCJCarstensMGHenninkWEStormGPharmacokinetics of poly(hydroxyethyl-L-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administrationBiochim Biophys Acta20071768373774317223070
- MungerJSShiGPMarkEAChinDTGerardCChapmanHAA serine esterase released by human alveolar macrophages is closely related to liver microsomal carboxylesterasesJ Biol Chem19912662818832188381918003
- MaxwellDMBrechtKMO’NeillBLThe effect of carboxylesterase inhibition on interspecies differences in soman toxicityToxicol Lett198739135423672554
- MaxwellDMLenxDFGroffWAKaminskisAFroehlichHLThe effect of blood flow and detoxification on in vivo cholinesterase inhibition by soman in ratsToxicol Appl Pharmacol198788166763564032
- XuHDengYChenDHongWLuYDongXEsterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivativesJ Control Release2008130323824518657874
- XuHWangKQDengYHChen daWEffects of cleavable PEG-cholesterol derivatives on the accelerated blood clearance of PEGylated liposomesBiomaterials2010311747574756320303164
- ChenDJiangXHuangYZhangCanPingQpH-Sensitive mPEG-Hz-cholesterol conjugates as a liposome delivery systemJ Bioact Compat Polym2010255552755242
- ChenDJiangXLiuJJinXZhangCPingQIn vivo evaluation of novel pH-sensitive mPEG-Hz-Chol conjugate in liposomes: Pharmacokinetics, tissue distribution, efficacy assessmentArtif Cells Blood Substit Immobil Biotechnol201038313614220337549
- LiuJLiHChenDIn vivo evaluation of novel chitosan graft polymeric micelles for delivery of paclitaxelDrug Deliv201118318118920942638
- DamsETLavermanPOyenWJAccelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomesJ Pharmacol Exp Ther200029231071107910688625
- IshidaTIchikawaTIchiharaMSadzukaYKiwadaHEffect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in miceJ Control Release200495340341215023452
- PanchagnulaRPharmaceutical aspects of paclitaxelInt J Pharm1998172115
- SinglaAKGargAAggarwalDPaclitaxel and its formulationsInt J Pharm20022351–217919211879753
- GelderblomHVerweijJNooterKSparreboomACremophorELthe drawbacks and advantages of vehicle selection for drug formulationEur J Cancer200137131590159811527683
- ZhangJAAnyarambhatlaGMaLDevelopment and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEPETU) formulationEur J Pharm Biopharm200559117718715567316