Abstract
Objective
To investigate the effect of magnetic nanoparticles (MNPs) of Fe3O4 combined with cyclosporin A (CsA) on acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in murine models.
Methods
BALB/c mice preconditioned with total-body irradiation generated aGVHD and then were followed with allo-HSCT from allogeneic C57BL/6. Recipient mice were randomly divided into five groups and then given different supportive care and followed up. The physical signs and median survival time (MST) were recorded, peripheral blood cell counts were assessed, and histological changes of the main tissues were evaluated with hematoxylin-eosin staining. Furthermore, fluorescence polarization immunoassay was used to monitor the concentration of CsA.
Results
The irradiated-only mice developed typical aGVHD, and the typical signs of aGVHD in the skin, liver, and intestine were observed by histopathological examination. Both CsA alone and in combination with Fe3O4 MNPs significantly prolonged the MST of recipient mice compared with both the control and the Fe3O4 MNPs groups. Notably, a combination of CsA with Fe3O4 MNPs can elevate the peripheral white blood cells and alleviate the symptoms of GVHD and the pathological damage after allo-HSCT. In addition, the concentration of CsA was higher in plasma, heart, liver, and spleen of recipient mice with supporting care of the combination of CsA with Fe3O4 MNPs than with CsA alone.
Conclusion
Taken together, Fe3O4 MNPs may be used as a carrier of immunosuppressive agents to alleviate GVHD after allo-HSCT in murine models.
Introduction
Hematopoietic stem cell transplantation (HSCT) was originally one of the most effective treatments for various malignant and nonmalignant hematological diseases.Citation1 With the development of research skills, HSCT has become one of the important means in the treatment of benign blood diseases, solid tumors, autoimmune disease, genetic disease, heart disease, neurological diseases, and other diseases. When attention was paid to this problem, graft-versus-host disease (GVHD) in allogenetic bone marrow transplantation (allo-HSCT) became a major complication; the incidence rate was as high as 40%–60%, and the mortality rate was almost 50%. The current main measures to prevent and treat GVHD are the application of cytotoxic drugs, immunosuppressive agents, or renovating of T cells in graft before and after transplantation. Cytotoxic drugs and immunosuppressive agents can decrease the recipient immune function and induce secondary infection and the occurrence of other tumors. The removal of T cells in grafts not only reduces the incidence of GVHD, but also weakens graft-versus-leukemia effect or graft-versus-tumor effect, which leads to leukemia or cancer recurrence and graft failure.Citation2
Cyclosporine A (CsA), which has a moderate effect on GVHD, has become the basis for many of the immunosuppressive regimens; it has been especially successful when used for the treatment of GVHD following allogenetic bone marrow transplantation, but it has been associated with drawbacks, such as hepatotoxicity, neurotoxicity, and nephrotoxicity,Citation3 so it is necessary to administer frequent doses of CsA to patients to prolong medium survival time (MST). Research has been proposed for stearic acid nanoparticles or solid lipid nanoparticles as a carrier to increase the oral absorption and relative bioavailability of cyclosporine.Citation4,Citation5 Notably, the solid lipid nanoparticle in situ gel for bioadhesive CsA can reduce its dosage and side effects.Citation6 The unique ability of magnetic nanoparticles (MNPs) to be functionalized with bioactive agents and respond to magnetic fields has made them useful for their stable performance, hardness, strong magnetism, simple preparation, and good biocompatibility. Despite MNPs having numerous advanced applications, the potential effects of pure MNPs on mammals’ transplantation have rarely been reported. The present study aimed to investigate the immunosuppressive role of CsA loaded with Fe3O4 MNPs in murine models after allo-HSCT.
Materials and methods
Animals
Pure inbred female BABL/c(H-2d) and male C57BL/6(H-2b, B6) mice (aged 10–12 weeks), weighing 18.5 ± 1.5 g were purchased from Shanghai National Center for Laboratory Animals. Mice were kept with a 12-hour light/dark cycle and given sterile water of erythromycin (250 mg/L) and gentamicin (320 mg/L) from 1 week prior to 2 weeks after bone marrow graft transplant to reduce intestinal microflora. All animal experiments were carried out in adherence with the Guidelines for the Care and Use of Laboratory Animals of the National Institute of Health.
Main reagents
Roswell Park Memorial Institute (RPMI) 1640 medium was supplied by GIBCO BRL, (Gaithersburg, MD). Cyclosporin A was supplied by North China Pharmaceuticals, Shijiazhuang, China. Fetal bovine serum (FBS) was supplied by Gibco Chemical Co (Carlsbad, CA). Anti-CsA mAb was supplied by Abbott Laboratories (North Chicago, IL).
Preparation of CsA-loaded Fe3O4 MNPs
Fe3O4 MNPs were produced by the electrochemical deposition under oxidizing conditions in a 0.1 M tetraheptylammonium 2-propanol solution as described previously.Citation7,Citation8 The deposited clusters were capped with tetraheptylammonium, which acts as a stabilizer of the colloidal nanocrystallites.Citation9 Before being applied in this experiment, the prepared Fe3O4 MNPs were well distributed in RPMI 1640 medium containing 10% (v/v) heat-inactivated FBS by using ultrasound treatment in order to obtain the Fe3O4 MNPs colloidal suspension. CsA was conjugated with Fe3O4 MNPs at the weight ratio of 1:400 by mechanical absorption polymerization at 4°C for 12 hours, as previously reported.Citation8
Transplantation
To prepare for allo-HSCT, bone marrow cells were harvested from femurs, tibias, and spleen of donor C57BL/6 mice according to the methods described by Dobson and colleagues.Citation10 Briefly, the epiphysis ends of femur and tibia were removed, the bone marrow cells in the bone marrow cavity were quickly removed, and the spleen was cut into small pieces, ground with a syringe needle core, and then flushed with RPMI 1640 medium containing 5% FBS to prepare for single cell suspension using a 200 mesh wire mesh filter. The erythrocyte was lysed, and the nucleated cells were washed with serum free RPMI 1640 medium twice. The cells were adjusted to 1 × 108/mL, and cell activity was assessed by trypan blue staining. Thereafter, 0.2 mL mixed bone marrow (1 × 108/mL) per mouse was prepared for transplantation.
Recipient BABL/c (H-2d) mice were subjected to total body irradiation (TBI) using a megavoltage linear accelerator with 60Coγ-rays (total dose: 8Gy) on day 0 to establish bone marrow graft transplant models in the radiotherapy centers of the Cancer Hospital of Jiangsu Province. The mice then received 0.2 mL donor mixed bone marrow cells via tail vein on the day of irradiation.
Following transplant, recipient mice were housed in micro-isolator cages containing autoclaved bedding. Supportive care was identical for the recipient mice, and different prevention programs were used for acute GVHD (aGVHD) prophylaxis in three groups. Mice in the first group were given an intraperitoneal injection of CsA (1.5 mg ·kg−1 ·d−1) on the first day until bowel function was normal, and then the dose of CsA was changed to 5 mg ·kg−1 ·d−1; mice in the second group were given CsA (1.5 mg ·kg−1 ·d−1) combined with Fe3O4 MNPs (600 mg ·kg−1 ·d−1); and mice in the third group were given only Fe3O4 MNPs (600 mg ·kg−1 ·d−1). Meanwhile, irradiated mice without controlling GVHD after allo-HSCT and irradiated-only mice were regarded as control groups. Each supportively cared group was randomly divided into five subgroups (three mice each) for study on days 5, 10, 15, 20, and 25.
Evaluation of engraftment
The diagnosis and grading of aGVHD were established according to the previous definable criteria.Citation11 Engraftment was evaluated by checking the animals daily for MST, peripheral blood cell counts, and signs of GVHD including hunched posture, wasting, skin lesions, and diarrhea.
At each time point, blood was collected from the retroorbital sinus into an EDTA (ethylenediaminetetraacetic acid) tube, and then diluted 1:10 in phosphate buffered saline. The number of white blood cells (WBCs) was assessed with Sysmex XE-2100 hematology analyzer using standard protocols; once the absolute neutrophil count was more than 0.5 × 109/L, the neutrophils were considered engrafted.
Hematoxylin and eosin staining
The recipient mice were killed, and specimens of liver, small intestine, and ear skin were taken immediately. All tissues were initially fixed with 10% formalin, dehydrated in a graded alcohol series, and then embedded in paraffin. Slices (0.5 mm thick) were cut and stained with hematoxylin and eosin for microscopic examination. All sections were observed in a blinded manner and photomicrographed under a confocal microscope. The tissues from the irradiated but nontransplanted mice were treated in a similar fashion as control.
FPIA procedure
The level of CsA in plasma and tissues was measured using a fluorescence polarization immunoassay (FPIA) as previously described.Citation12 In brief, 700 μL solubilization reagent and 100 μL precipitation reagent were added to each sample, vortex-mixed and allowed to stand for 5 minutes; the supernatant was transferred into their respective reaction cells and then measured using a fluorescence spectrophotometer at the excitation and emission wavelengths of 492/514 nm. The compounds were quantified using the ratio of their peak height to the internal standard, based on the calibration curve of CsA.
Statistical analysis
All experiments were repeated at least three times, data were expressed as mean ± standard deviation and analyzed using SPSS (v 11.5; SPSS Inc, Chicago, IL). Survival analysis was performed using the Kaplan–Meier method. Differences among various groups were evaluated using one-way analysis of variance. A P-value of 0.05 was considered statistically significant.
Results
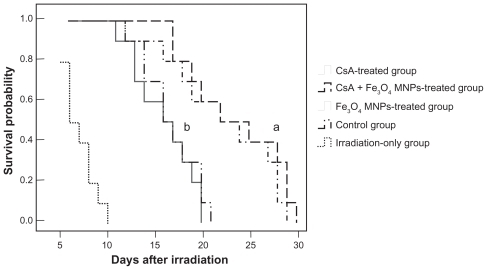
MST after transplantation
The prevention of aGVHD with either CsA alone or in combination with Fe3O4 MNPs prolonged the survival of recipient mice compared with other groups (P < 0.05) (), but there was no significant difference between CsA alone (22.3 ± 5.6 days) and the combination of CsA with Fe3O4 MNPs (23.6 ± 4.9 days) (P > 0.05). During the 25 days’ observation, the MST of the irradiation-only mice (8.0 ± 1.6 days) was shorter than that of both the Fe3O4 MNPs-treated group and the control group (P < 0.05), but there was no significant difference between the Fe3O4 MNPs-treated group (16.1 ± 3.0 days) and the control group (16.8 ± 2.8 days) (P > 0.05).
Figure 1 Survival curve of mice after prevention of aGVHD program.
Notes: a,b<0.05, compared with the irradiation-only group; a<0.05, compared to other groups.
Abbreviations: aGVHD, acute graft-versus-host disease; MST, medium survival time; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4 magnetic nanoparticles.
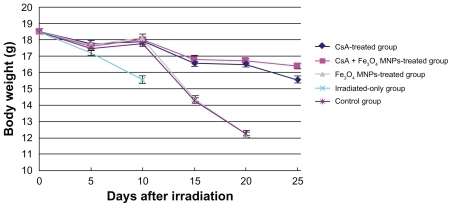
Physiologic observation after transplantation
The weight of mice decreased in the first week. The survival recipients, except the irradiated-only mice, began to gain weight again from day 5, and then fell quickly from day 10, but there were no obvious weight changes in the Fe3O4 MNPs-treated and control groups (P > 0.05). It is noteworthy that from day 10, the body weight decrease was less in the group of immunosuppressive CsA than in both the Fe3O4 MNPs-treated and control groups, but there was no obvious difference between the CsA-only group and the CsA + Fe3O4 MNPs-treated group. Notably, mice in the irradiated-only group developed clinical symptoms of aGVHD, including hair loss, diarrhea, and anal irritation; thereafter, all died before day 10. The mice without supporting care of CsA also developed aGVHD, and the symptoms of aGVHD were not relieved. The symptoms of aGVHD in the mice with supporting care of CsA progressively disappeared, and no obvious changes were observed during the follow-up after the supporting care of CsA ().
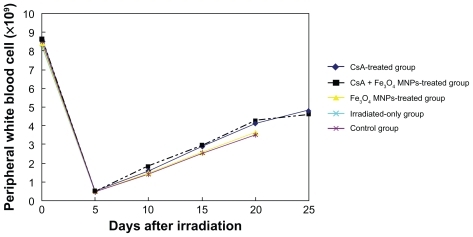
Peripheral blood cell counts
WBC count in peripheral blood of the mice was from 8.0 × 109/L to 9.0 × 109/L before allo-HSCT and decreased from 0.5 × 109/L to 5.0 × 109/L after allo-HSCT, suggesting that WBC count of mice is lower after allo-HSCT than that prior to transplantation. After transplantation for 5 days, WBC count in all recipient mice was less than 0.5 × 109/L. With the hematopoietic recovery, WBC increased gradually and reached from 4.0 × 109/L to 5.0 × 109/L in recipient mice with the supporting care of CsA alone or in combination with Fe3O4 MNPs and reached from 3.0 × 109/L to 4.0 × 109/L in the mice without supporting care of CsA (). Unluckily, the WBC count of the irradiated-only mice was low and they all died of aplastic crisis or infection after irradiation for less than 1 week.
Concentration of cyclosporine
Concentration of cyclosporine in blood, heart, liver, and spleen in CsA-treated mice was all lower than that of mice treated with the combination of CsA and Fe3O4 MNPs, and there were statistically significant differences (P < 0.05); on the other hand, there was no significant difference in the tissues of lung, kidney, and brain between the two groups (P > 0.05), suggesting partly that Fe3O4 MNPs can increase the concentration of cyclosporine in blood and tissue of heart, liver, and spleen ().
Table 1 Concentration of CsA in different tissues on days 5, 10, 15, 20, and 25 (mean ± SD)
Histopathology
A reduced number of hair follicles and little sebaceous glands appended to the hair follicles was observed, and a diffuse and moderate lymphocytic infiltration was found around hair follicles and extending to the lower surface epidermis in the irradiated-only mice (). Inflammatory cells were also observed, but the hair follicles and sebaceous glands were not influenced in the mice treated with Fe3O4 MNPs alone after allo-HSCT (). No noticeable structural change was observed in the skin of mice with supported care of CsA, but the number of follicles was less in the CsA-only treated mice than in the combination of CsA with Fe3O4 MNPs ().
Figure 4 Histopathology of skin tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.
Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4. magnetic nanoparticles.
![Figure 4 Histopathology of skin tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4. magnetic nanoparticles.](/cms/asset/3e2a9dec-0871-42e6-9929-2d97b4a6deda/dijn_a_12187813_f0004_c.jpg)
In liver, a severe, mixed inflammatory response and various degrees of focal degeneration and necrosis were noted near the central veins, and the liver tissue structure was unclear in the irradiation-only mice when compared with that of CsA-only or its combination with Fe3O4 MNPs (). On the other hand, local minor inflammatory response was also observed in liver tissue of mice in the Fe3O4 MNPs-treated () and control group (). It is noteworthy that the focal degeneration and necrosis were inconspicuous in both CsA and its combination with Fe3O4 MNPs, and fewer inflammatory cells were presented when CsA was loaded with Fe3O4 MNPs ().
Figure 5 Histopathology of liver tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.
Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4 magnetic nanoparticles.
![Figure 5 Histopathology of liver tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4 magnetic nanoparticles.](/cms/asset/a1d6f6a6-4605-4e70-8a9a-bab246a67ed5/dijn_a_12187813_f0005_c.jpg)
In histological sections of the intestinal tissues, those of coadministration of CsA with Fe3O4 MNPs were significantly thick in submucosal-serosa compared with other groups. Numbers of inflammatory cells per image in fibrous thickening areas of either CsA or its combination with Fe3O4 MNPs were less than that of both irradiation-only and Fe3O4 MNPs-only group, but very few inflammatory cells appeared when CsA was loaded with Fe3O4 MNPs (). No obviously changes were observed, suggesting that Fe3O4 MNPs are able to enhance the effect of CsA on alleviating intestinal damage after allo-HSCT.
Figure 6 Histopathology of intestine tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.
Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4 magnetic nanoparticles.
![Figure 6 Histopathology of intestine tissues of mice after allo-HSCT [hematoxylin and eosin, magnification ×4 (objective lens)]. (A) Control group; (B) irradiation-only group; (C) Fe3O4 MNPs-treated group; (D) CsA-treated group; (E) CsA + Fe3O4 MNPs-treated group.Abbreviations: allo-HSCT, allogenetic hematopoietic stem cell transplantation; CsA, cyclosporine A; Fe3O4 MNPs, Fe3O4 magnetic nanoparticles.](/cms/asset/8f813f5c-20ed-4a41-8c25-71d0884a04e0/dijn_a_12187813_f0006_c.jpg)
Discussion
Allo-HSCT is an important means for treating hematologic malignancies and certain solid tumors.Citation13 Models for CsA-induced autologous GVHD have been developed in rats and mice.Citation14 Several studies have shown that susceptibility to autologous GVHD varied greatly from one strain to another; DBA/2 and C3H/He but not BALB/c mice are able to develop clinical symptoms following autologous stem cell transplantation.Citation15 In this study, the shapes of survival curves of recipients of allogenetic bone marrow cells suggest that CsA or its combination with Fe3O4 MNPs pretreatment inhibited aGVHR. The GVHD symptoms and survival in the Fe3O4 MNPs group compared with the irradiation-only group showed no significant difference, suggesting that low concentration of Fe3O4 MNPs has no obvious prevention of the incidence of GVHD, though Fe3O4 MNPs could influence immune functions of normal imprinting control region mice in a dose-dependent manner in the authors’ previous studies.Citation16 The decreased weight, with other physical characteristics of less activity, ruffled fur, and arched back in murine models, were improved after allo-HSCT. However, the successful rate and long survival time of allogenetic bone marrow transplantation have been limited by transplantation-related mortality. GVHD normally develops in clinical settings when allogenetic tissues containing immunocompetent cells are transferred to an immunocompromised host.Citation17 The occurrence of this frequent scenario in allo-HSCT results from the graft in the antigen-specific lymphocytes recognizing the host tissue antigens. Citation18 According to its pathogenesis, a traditional method for prevention and treatment of GVHD is to be given high doses of immunosuppressive drugs after allo-HSCT.Citation19
Immunotherapy, which may offer a non-cross-resistant anticancer mechanism compared with chemotherapy, represents a potential therapeutic approach for reducing the risk of relapse after allo-HSCT.Citation20 CsA, as one of the prevention drugs in clinical organ transplantation, can inhibit transplant rejection and GVHD and is widely applied and has achieved remarkable success. However, the side effects of CsA, include neurological damage, gingival hypertrophy, urinary system damage, digestive system damage, skin damage, respiratory damage, blood system damage, unexplained fever, unexplained increase in blood pressure, and other adverse reactions.
Based on previous studies,Citation7,Citation8 Fe3O4 MNPs were used as a carrier in this present study to increase the concentration of CsA tissues and to decelerate the clearance of CsA in murine models.Citation18 Subsequent experiments have demonstrated that the measured blood parameters in the present study were not significantly affected by supporting care of CsA alone or in combination with Fe3O4 MNPs, and the irradiated-only mice all died of aplastic crisis or infection in 1 week. This may be related to the impact of irradiation on the behavioral phenotype of the transplanted BALB/c mice with slower reconstitution of blood leukocytes.Citation21
Another important finding of this present study is that the concentrations of CsA in blood, heart, liver, and spleen were all lower in the mice with supporting care of CsA than that with coadministration of CsA with Fe3O4 MNPs; otherwise, there were no differences in lung, kidney, and brain, suggesting that Fe3O4 MNPs can increase the concentration of CsA in blood and part tissues, such as heart, liver, and spleen. One may argue that the cytostatic effect exerted by CsA might be more important in humans than in mice. Histopathology with hematoxylin and eosin staining indicated that a reduced number of hair follicles and little sebaceous glands appended to the hair follicles existed, and a diffuse and moderate lymphocytic infiltration was also found around hair follicles and extending to the lower surface epidermis in the irradiated-only mice, but no noticeable structural changes were observed in the mice with supported care of CsA, suggesting that Fe3O4 MNPs enhance the CsA on the occurrence of a diffuse immune response in the skin of recipient mice. Furthermore, focal degeneration and necrosis were inconspicuous in both CsA and its combination with Fe3O4 MNPs, and fewer inflammatory cells were presented in the tissue of liver when CsA was loaded with Fe3O4 MNPs. Similarly, few inflammatory cells and no obvious changes were observed in the intestinal tissues of mice after allo-HSCT when the supporting care of CsA was loaded with Fe3O4 MNPs, suggesting that Fe3O4 MNPs are able to enhance the effect of CsA on alleviating the liver and intestinal damage after allo-HSCT. Fe3O4 MNPs are able to enhance the effect of CsA on alleviating the tissue damage in murine models.
Conclusion
Fe3O4 MNPs may be used as a carrier of an immunosuppressive agent to alleviate GVHD after allo-HSCT. Although there is a considerable anatomical difference between human bone and mouse bone, the basic mechanisms of immunosuppressive activity is similar, so further research is needed to clarify the most effective CsA dose in combination with Fe3O4 MNPs to achieve maximal effects on GVHR.
Disclosures
The authors have no conflicts of interest to report in this work.
Acknowledgments
This work was supported by 973 National Key Fundamental Research Project of China (No 2006 CB933205), National Nature Science Foundation of People’s Republic of China (No 30740062 and No 30872970), and Special-Purpose Science Research Foundation for High School (No 20070286042).
References
- TabbaraIAAllogeneic bone marrow transplantationSouth Med J1996898578688790307
- Vargas-DiezEGarcia-DiezAMarinAFernandez-HerraraJLife-threatening graft-vs-host diseaseClin Dermatol20052328530015896544
- BachFHAuchellonclossHJrTransplantation ImmunologyNew YorkWiley-Liss19951346
- ZhangQYieGLiYStudies on the cyclosporin A loaded stearic acid nanoparticlesInt J Pharm200020015315910867245
- UgazioECavalliRGascoMRIncorporation of cyclosporin A in solid lipid nanoparticles (SLN)Int J Pharm200224134134412100861
- CuiFNiuMSolid lipid nanoparticle in situ gel for bioadhesive cyclosporin A comprises cyclosporin A, lipid material, emulsifier, coating material, gel base material, and purified waterUniv Shenyang Medical (UYSH-Non-standard)2008M6538454
- ChenBASunQWangXMReversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/AO2 leukemic cellsInt J Nanomedicine2008327728618686787
- JiangZChenBAXiaGHThe reversal effect of Fe3O4-magnetic nanoparticles loaded with cisplatin on the SKOV3/DDP ovarian carcinoma cellsInt J Nanomedicine2009410711419516889
- WangXMZhangRYWuCHThe application of Fe3O4 nanoparticles in cancer research: a new strategy to inhibit drug resistanceJ Biomed Mater Res A200780A85286017072850
- DobsonKRReadingLHabereyMMarineXScuttACentrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femuraeCalcif Tissue Int19996541141310541770
- PrzepiorkaDWeisdorfDMartinP1994Consensus Conference on Acute GVHD GradingBone Marrow Transplant1995158258287581076
- SalmPWarnholtzCBoydJArabshahiLMarbachPTaylorPJEvaluation of a fluorescent polarization immunoassay for whole blood everolimus determination using samples from renal transplant recipientsClinical Biochemistry20063973273816725133
- AppelbaumFRFisherLDThomasEDChemotherapy v marrow transplantation for adults with acute nonlymphocytic leukemia: a five-year follow-upBlood1988721791843291979
- HequetOVocansonMSaint-MezardPKaiserlianDNicolasJFBernardFCD4+T cells prevent skin autoimmunity during chronic autologous graft-versus-host-diseaseAm J Transplant2004487287815147420
- BrysonJSJenningsCDCaywoodRIKaplanAMStrain specificity in the induction of syngeneic graft-versus-host disease in miceTransplantation1991519119132014553
- ChenBAJinNWangJThe effect of magnetic Fe3O4 nanoparticle on immune function in normal ICR miceInt J Nanomedicine2010559359920856834
- FerraraLMTeshimaTUnderstanding the alloresponse: new approach to graft-versus-host disease preventionSemin Hematol200239152211799525
- BillinghamREThe biology of graft-versus-host reactionsHarvey Lect1966–1967622178
- NeippMExnerBGIldstadSTA nonlethal conditioning approach to achieve engraftment of xenogeneic rat marrow in mice and to induce donor-specific toleranceTransplantation1998669699759808477
- KlineJSubbiahSLazarusHMvan BesienKAutologous graft-versus- host disease: harnessing anti-tumor immunity through impaired self-tolerance bone marrowTransplantation200841505513
- Duran-StruuckRHartiganAClouthierSGDifferential susceptibility of C57BL/6NCr and B6.Cg-Ptprca mice to commensal bacteria after whole body irradiation in translational bone marrow transplant studiesJ Transl Med200861018307812