?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Gambogic acid (GA), a potent anticancer agent, is limited in clinical administration due to its poor water solubility. The aim of this study was to explore a drug delivery system based on magnetic Fe3O4 nanoparticles (MNP-Fe3O4) conjugated with GA to increase water solubility of the drug and enhance its chemotherapeutic efficiency for pancreatic cancer.
Methods
GA was conjugated with the MNP-Fe3O4 colloidal suspension by mechanical absorption polymerization to construct GA-loaded MNP-Fe3O4, which acted as a drug delivery system.
Results
Combination therapy with GA and MNP-Fe3O4 induced remarkable improvement in anticancer activity, which was demonstrated by optical microscopic observations, MTT assay, and nuclear DAPI staining. Furthermore, the possible signaling pathway was explored by Western blot. In Capan-1 pancreatic cancer cells, our observations demonstrated that this strategy could enhance potential anticancer efficiency by inducing apoptosis. The mechanisms of the synergistic effect may be due to reducing protein expression of Bcl-2 and enhancing that of Bax, caspase 9, and caspase 3.
Conclusion
These findings demonstrate that a combination of GA and MNPs-Fe3O4 represents a promising approach to the treatment of pancreatic cancer.
Introduction
With one of the highest mortality-to-incidence ratios, pancreatic cancer is the eighth leading cause of cancer-related death in men worldwide and the ninth in women.Citation1 The disease is usually detected at an advanced stage, carries a poor prognosis regardless of treatment, and is associated with debilitating symptoms. After initial diagnosis, most patients have a median survival with treatment of about 6 months.Citation1 Even in cases where the cancer is diagnosed at an early resectable stage, 5-year survival is still only 22%.Citation2 Advances in treating pancreatic cancer have been few and modest. Pancreatic cancer is well recognized as an extremely challenging disease on multiple fronts, and the use of chemotherapy has been shown to improve survival.Citation3 There is now more emphasis on early chemotherapy in locally advanced pancreatic cancer and combination chemotherapy in metastatic disease.Citation1
Gambogic acid (GA), a natural compound extracted from gamboges, has recently been identified as a potent anticancer agent. Recent studies have shown that GA can inhibit growth of a wide variety of tumor cells, including hepatoma, pulmonary carcinoma, gastric cancer, and breast cancer.Citation4–Citation7 However, little is known about the effect of GA in pancreatic cancer. In addition, the therapeutic effect of GA is limited due to low water solubility. Therefore, efforts should be made to develop new delivery techniques to increase water solubility which could alter its biodistribution, enhance its deposition in tumor sites, and improve its therapeutic efficacy.Citation8
Various types of nanosized drug carriers, such as liposomes, polymeric micelles, dendrimers, superparamagnetic iron oxide crystals, semiconductor nanomaterials, and colloidal gold, have been investigated in cancer therapy in order to minimize the side effects of anticancer therapy, improve water solubility of drugs, and enhance the antitumoral efficacy of targeted therapies.Citation9,Citation10 The most promising materials are magnetic nanoparticles. Magnetic Fe3O4 nanoparticles (MNP-Fe3O4), a biocompatible and superparamagnetic nanomaterial with satisfactory chemical stability and low toxicity, are widely used as targeted drug carriers with target orientation and sustained-release properties.Citation11–Citation15
In view of this research, we were inspired to explore a drug delivery system for GA based on MNP-Fe3O4 to increase its water solubility and enhance its chemotherapeutic efficiency. To the best of our knowledge, no study to date has been carried out of combination therapy with GA and MNP-Fe3O4 for pancreatic cancer. We have investigated the anticancer efficacy of this combination in pancreatic cancer for the first time. In this study, GA was loaded onto MNPFe 3O4 (GA-MNP-Fe3O4) as a drug delivery system, and we then identified the cytotoxic effects of GA-MNP-Fe3O4 in Capan-1 pancreatic cancer cells, investigated the apoptosis induced, and further measured the expression of apoptosis-related proteins, including caspase 3, caspase 9, Bax, and Bcl-2, to elucidate the possible mechanisms involved.
Materials and methods
Main chemicals and apparatus
Iron (II) chloride tetrahydrate (FeCl2 ·4H2O) and iron (III) chloride hexahydrate (FeCl3 ·6H2O) were obtained from Sinopharm Chemical Reagent Co Ltd (Shanghai, China). Ammonium hydroxide and citric acid were acquired from Shanghai Lingfeng Chemical Reagent Co Ltd (Shanghai, China). GA (Kanion Pharmaceutical Co Ltd, Jiangsu, China) was dissolved in dimethyl sulfoxide (Sigma Aldrich, St Louis, MO), stored at −20°C, and then diluted as needed in RPMI-1640 medium (Gibco/BRL, Carlsbad, CA). Monoclonal antibodies, including caspase 3, Bax, Bcl-2, caspase 9, and β-actin, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The horseradish peroxidase-conjugated IgG antibody was obtained from Nanjing KeyGen Biotech Inc (Nanjing, China). 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and 4, 6-diamidino-2-phenylindole (DAPI) were obtained from Sigma, and stored in the dark. All other reagents were of analytical grade. The transmission electron microscopic images were obtained using a JEM-2100 transmission electron microscope. The fluorescent microscopic images were shot on an Olympus IX51 inverted microscope. The optical density at 492 nm was recorded using a multiwell spectrophotometer reader (Thermo Labsystems, Vantta, Finland).
Synthesis of magnetic Fe3O4 nanoparticles
Magnetic Fe3O4 nanoparticles were prepared by coprecipitation of Fe (III) and Fe (II) with ammonium hydroxide in a nitrogen environment. In a typical synthetic experiment, FeCl3 ·6H2O 2.61 g and FeCl2 ·4H2O 1.04 g were dissolved in 100 mL of deionized water and heated to 80°C, followed by the slow addition of 10 mL of ammonium hydroxide with vigorous stirring for 20 minutes. Black Fe3O4 precipitates were obtained and washed immediately with distilled water five times by magnetic separation. The precipitates were then dispersed in distilled water with 1.25 g citric acid, which acted as a stabilizer of the colloidal nanocrystallites, with vigorous stirring for 90 minutes. The products, ie, citric acid-coated MNP-Fe3O4, were cooled to room temperature and extracted by a magnet. Finally, after being washed with ethanol and finally with deionized water, the products were lyophilized and stored at room temperature.
Preparation of GA-loaded magnetic Fe3O4 nanoparticles
Before being applied in the present experiment, MNP-Fe3O4 were well distributed in RPMI-1640 medium with 10% heated inactivated fetal bovine serum using ultrasound treatment in order to obtain a MNP-Fe3O4 colloidal suspension. As previously reported,Citation16 GA at different concentrations was conjugated with the MNP-Fe3O4 colloidal suspension by mechanical absorption polymerization at 4°C for 48 hours to construct GA-loaded MNP-Fe3O4 (GA- MNP-Fe3O4), which acted as a drug delivery system.
Cell culture
The Capan-1 pancreatic cancer cells, obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37.0°C in humidified air with 5% CO2. The cells were in log phase prior to the following experiments.
Assay of anticancer activity
The cytotoxicity of MNP-Fe3O4, GA, and GA-MNP-Fe3O4 was studied against Capan-1 pancreatic cancer cells with MTT assays. Cells at 1 × 105/mL were seeded in 96-well plates and incubated for 24 hours. The growth medium was then replaced with 200 μL of the prepared medium containing free GA and GA-MNP-Fe3O4, in which the GA concentration was 0, 0.25, 0.5, 1.0, and 2.0 μmol/L. The cells were also treated by MNP-Fe3O4 alone to evaluate cytotoxicity. Cells without any treatment were used as the control group. The cells were further incubated for 48 hours, and the relative anticancer activity was assessed using MTT assays. In brief, MTT solutions were added after the treatments and incubated for an additional 4 hours. Dimethyl sulfoxide was added to solubilize the formazan crystal, and optical density at 492 nm was recorded. The cell viability fraction (%) was calculated as:
DAPI staining
The cells were treated according to the above methods for 48 hours, and then fixed with 4% polyoxymethylene prior to washing with phosphate-buffered saline. The washed cells were then stained with DAPI 1 mg/mL for 15 minutes in the dark. The staining images were recorded using the fluorescent microscope.
Western blot analysis
After the different treatments, expression of apoptosis-related proteins was detected by Western blot. In brief, total protein was isolated and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane. After being blocked, the membrane was incubated with primary polyclonal antibodies, either anti-caspase 3, Bax, Bcl-2, caspase 9, or anti-β-actin overnight at 4°C, and subsequently incubated with horseradish peroxidase-conjugated IgG antibody as the secondary antibody for one hour at room temperature. The protein bands were detected by an enhanced ECL detection system (Amersham, UK). After normalization by the corresponding expression of β-actin, protein expression levels of caspase 3, Bax, Bcl-2, and caspase 9 were determined by densitometry scans.
Statistical analysis
All the data are presented as means ± standard deviations. The F-test was used for significance testing, and P < 0.05 was considered to be statistically significant. All tests were performed using SPSS (v 13.0, SPSS Inc, Chicago, IL).
Results and discussion
Characterization of magnetic Fe3O4 nanoparticles
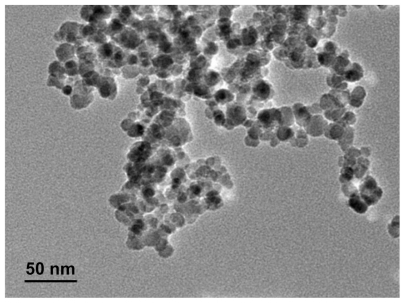
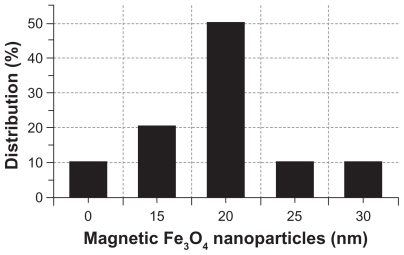
The synthesized MNP-Fe3O4 capped with citric acid were characterized by transmission electron microscopy. As shown in , MNP-Fe3O4 were observed to have a spherical shape, with a diameter of about 20 nm. The size distribution of these MNP-Fe3O4 is shown in . It has suitable dimensions to escape renal rapid excretion, as well as to avoid components of the reticular endothelial system, thus facilitating potentially passive targeting of drugs to tumors via the enhanced permeation and retention effect and active targeting with target orientation of magnetic field, then increasing the accumulation of drugs in tumor cells after endocytosis.Citation17
Anticancer activity in vitro
GA exhibits potent anticancer activity in many kinds of cancer cells.Citation4–Citation7 However, whether or not GA induces apoptosis of Capan-1 pancreatic cancer cells, and the molecular mechanisms involved, is not clear. In addition, the therapeutic effect of GA is limited due to low water solubility (<1 μg/mL).Citation8 Therefore, we sought to identify the potential benefit of combination therapy using GA-MNP-Fe3O4 as a drug carrier for pancreatic cancer and whether MNP-Fe3O4 could promote the apoptosis induced by GA. In our study, no precipitation of GA was noted in the colloidal suspension of the GA-MNPFe 3O4 drug delivery system after 2 months of storage, which indicates that the solubility of GA was improved and drug delivery was stable during storage at 4°C. To explore the anticancer efficiency of the GA-MNP- Fe3O4 drug delivery system, we cultured Capan-1 pancreatic cancer cells with free GA at different concentrations (0, 0.25, 0.5, 1.0, and 2.0 μmol/L), MNP-Fe3O4 loading GA with equivalent GA concentration for 48 hours. The cytotoxicity results were estimated by MTT assay and are shown in . Cytotoxicity testing of a nanomaterial is the first-level evaluation before its biomedical application. When treated by MNP-Fe3O4 20 μg/mL, about 95% of the cells survived (, pink line), which is consistent with our previous report.Citation11–Citation16 The results suggested that the MNP-Fe3O4 capped with citric acid synthesized in this study lack cytotoxicity, thus ensuring a wide potential range of applications in the field of biomedical science and cancer therapy. Compared with GA alone (, red line), the viability of Capan-1 pancreatic cancer cells treated by GA-MNP-Fe3O4 obviously decreased (, green line). Meanwhile, our results also indicate that lethality increased with increasing concentrations of GA, suggesting a dose-dependent effect in vitro. The increased cytotoxicity may be due to improved GA cellular uptake by the GA-MNP-Fe3O4 drug delivery system, which increases the water solubility of GA through the endocytosis pathway and then induces release of GA from the MNP-Fe3O4 to promote efficient cell killing, which is a common characteristic of nanoparticle-based drug delivery systems.Citation18,Citation19
Figure 3 Cytotoxic effect of GA or GA-loaded MNP-Fe3O4 against the Capan-1 pancreatic cancer cells. Inset: Microscopic images of the Capan-1 cells after different treatments for 48 hours. (A) untreated cells as control, (B) MNP-Fe3O4, (C) GA alone, and (D) GA-loaded MNP-Fe3O4.
Notes: The concentrations of GA, MNP-Fe3O4 are 1 μmol/L and 20 μg/mL, respectively. Data are expressed as means ± standard deviations (n − 3).
Abbreviations: GA, gambogic acid; MNP-Fe3O4, magnetic Fe3O4 nanoparticles.
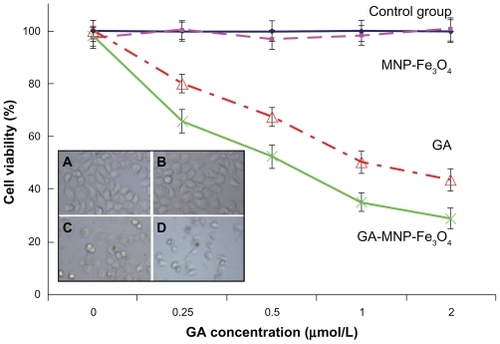
In addition, as shown in the insets of , the optical microscopic observations confirm the MTT results. The Capan-1 pancreatic cancer cells without any treatment attached to the plate with a normal elongated shape. Notably, the cells treated with MNP-Fe3O4 showed no morphological changes. If these cells are in a lethal state, they detach from the plate and assume a smaller and spherical morphology. It is obvious that GA and GA-MNP-Fe3O4 would cause more significant morphological changes, indicating the increasing probability of cell death, and the GA-MNP-Fe3O4 group was even more effective. Cooperation of MNP-Fe3O4 and GA could kill even more cancer cells, indicating their synergistic anticancer activity.
Morphologic characterization of apoptosis
Apoptosis is an important metabolic step in regulating the number and growth of cells. If apoptosis is blocked, metabolism becomes disordered, and tumors develop and grow. Most anticancer agents exert their anticancer effects by inducing apoptosis.Citation16 Recently, MNP-Fe3O4 have been widely used as targeted drug carriers to enhance the efficiency of anticancer drug delivery based on an ability for target orientation and sustained-release properties.Citation20 Our previous studies have demonstrated the synergistic effect between MNP-Fe3O4 and anticancer drugs in terms of intracellular accumulation in cancer cells to induce apoptosis.Citation11–Citation16
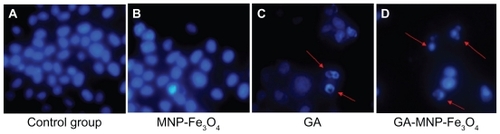
Nuclear DAPI staining was performed in the present study to explore the mechanism of the distinct improvement in anticancer activity induced by synergism between MNPFe 3O4 and GA. To confirm the existence of apoptosis, a study of the morphological changes in the cells was undertaken. Evaluation of normal or apoptotic cells depends on their morphological characterization. Normal nuclei (smooth nuclear) and apoptotic nuclei (condensed or fragmented chromatin) are easily distinguished. As shown in , the nuclear morphology analysis showed characteristic apoptotic changes, such as chromatin condensation, convoluted nuclei with cavitations, fragmentation of the nucleus, and apoptotic bodies in the Capan-1 pancreatic cancer cells after treatment with both GA and GA-MNP-Fe3O4. There was almost no evidence of apoptosis in the control group () and in the MNP-Fe3O4 group (). When the cells were treated with GA-MNP-Fe3O4 (), typical apoptotic morphology was more apparent than in cells treated with GA alone (). These findings strongly indicate that the synergistic effect of MNP-Fe3O4 and GA killed the cancer cells by inducing apoptosis rather than necrosis.
Figure 4 Nuclear morphologic changes of the Capan-1 pancreatic cancer cells after different treatment for 48 hours. (A) untreated cells as control, (B) MNP-Fe3O4, (C) GA alone, and (D) GA-loaded MNP-Fe3O4.
Notes: The concentrations of GA and MNP-Fe3O4 are 1 μmol/L and 20 μg/mL, respectively. Magnification folds ×400. Arrows indicate cells with apoptotic nuclear condensation and fragmentation.
Abbreviations: GA, gambogic acid; MNP-Fe3O4, magnetic Fe3O4 nanoparticles.
Expression of Bax, Bcl-2, caspase 9, and caspase 3 proteins
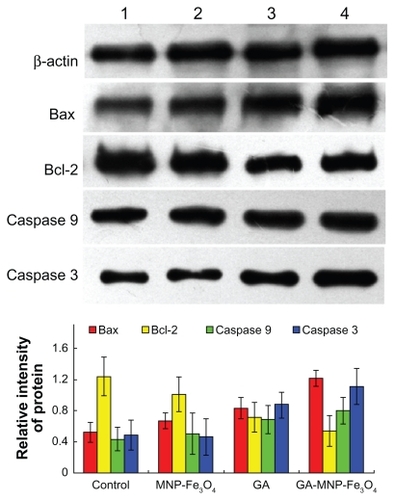
Next, we studied the molecular mechanism of apoptosis induced by the synergistic effect of MNP-Fe3O4 and GA in Capan-1 pancreatic cancer cells. The antiapoptotic protein, Bcl-2, has been associated with inhibition of apoptosis and cell survival mechanisms. The Bax protein is a proapoptotic member of this family, and its increased expression is often associated with increased apoptosis in target cells.Citation21 Apoptosis is the consequence of a series of precisely regulated events that are frequently altered in tumor cells. In general, the sequence of events has been broadly categorized into two pathways, ie, the extrinsic pathway, which involves activation of the tumor necrosis factor/Fas death receptor family, and the intrinsic pathway, which involves the mitochondria. In both pathways, an apoptotic death stimulus results in activation of caspases, the major executioners in this process, either directly or via activation of the mitochondrial death program.Citation16 Therefore, we examined changes in the expression levels of apoptosis-regulating proteins, including caspase 3, caspase 9, Bax, and Bcl-2, by Western blot to explore possible signaling pathways through which GA-MNP-Fe3O4 induced distinct improvement in anticancer activity. As shown in , when the Capan-1 pancreatic cancer cells were treated with GA and GA-MNPFe 3O4 for 48 hours, levels of caspase 3, caspase 9, and Bax protein were significantly upregulated compared with the control group. Meanwhile, upregulated levels in the GA-MNPFe 3O4 group were slightly higher than those in the GA group (P < 0.05). However, they were not obviously altered when the cells were treated with MNP-Fe3O4 alone (P > 0.05). In contrast, compared with the control group, the levels of Bcl-2 protein in cells treated with GA and GA-MNP-Fe3O4 were both significantly downregulated. Furthermore, the level of Bcl-2 in the GA-MNP-Fe3O4 group was lower than that in GA group, and was also not obviously altered when the cells were treated with MNP-Fe3O4 alone (P > 0.05). In our study, the ratio of Bax/Bcl-2 protein expression increased dramatically when the Capan-1 pancreatic cancer cells were treated with GA-MNP-Fe3O4. A large amount of evidence has shown that the sensitivity of cells to the apoptotic stimulus is determined by the relative ratio of proapoptotic and antiapoptotic members of the Bcl-2 family, ie, the mitochondrial-related death switch.Citation22 We deduce that upregulated Bax leads to disruption of the integrity of the mitochondrial membrane and promotes release of cytochrome c from the mitochondria, resulting in caspase 9/caspase 3 activation and DNA fragmentation. Caspase activation is generally considered to be a hallmark of apoptosis, and caspase 3 is the main effector caspase that is involved in apoptosis.Citation23 Thus, the ratio of Bcl-2/Bax might be a critical factor in the cell threshold for undergoing apoptosis induced by GA-MNP-Fe3O4. Collectively, GA combined with MNP-Fe3O4 has been shown to elevate the Bax/Bcl-2 ratio dramatically, enhance caspase 9/caspase 3 activity, and further stimulate the initiation of mitochondrial apoptosis signaling.
Figure 5 Expression of Bax, Bcl-2, caspase 3, and caspase 9 protein in the Capan-1 pancreatic cancer cells by Western blot after treatment of GA and/or MNP-Fe3O4 for 48 hours. Line 1, control; Line 2, incubated with 20 mg/L MNP-Fe3O4; Line 3, incubated with 1 μmol/L GA; Line 4, incubated with 1 μmol/L GA and 20 μg/mL MNP-Fe3O4.
Abbreviations: MNP-Fe3O4, magnetic nanoparticles of Fe3O4; GA, gambogic acid.
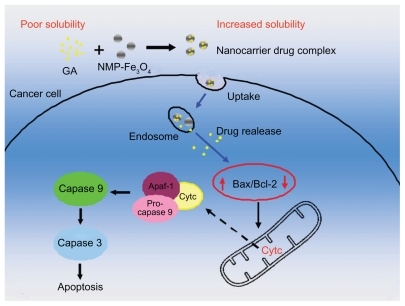
Based on the above studies, schematically illustrates the possible processes by which the GA-MNP-Fe3O4 drug delivery system induces a distinct improvement in anticancer activity. Firstly, GA was conjugated with the MNP-Fe3O4 colloidal suspension by mechanical absorption polymerization to construct GA-MNP-Fe3O4, which acted as a drug delivery system. This drug delivery system increased the water solubility of GA and enhanced its chemotherapeutic efficiency after endocytosis, and so has tremendous potential for application in cancer therapy by inducing apoptosis, a preferred mode for killing cancer cells in cancer therapy, and is induced synergistically, resulting in a distinct improvement in anticancer activity.
Conclusion
A GA-MNP-Fe3O4 drug delivery system was developed to increase the water solubility of GA and enhance its chemotherapeutic efficiency. MNP-Fe3O4 enhanced the anticancer activity of GA in Capan-1 pancreatic cancer cells by inducing apoptosis, and the synergistic effect may be due to regulation of various antiapoptotic and proapoptotic gene products, including Bax, Bcl-2, caspase 9, and caspase 3. All these characteristics demonstrate that combination therapy with GA and MNP-Fe3O4 represents a promising strategy in the treatment of pancreatic cancer.
Acknowledgment
This work was supported by the National Key Basic Research Program (2010CB732404) and the National Nature Science Foundation of China (30740062, 30872970).
Disclosure
The authors report no conflicts of interest in this work.
References
- TemperoMABerlinJDucreuxMPancreatic cancer treatment and research: An international expert panel discussionAnn Oncol2011221500150621199884
- JemalASiegelRXuJQWardECancer Statistics, 2010CA Cancer J Clin20106027730020610543
- NakaiYIsayamaHSasakiTComorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapyCrit Rev Oncol Hematol20117825225920579902
- GuoQLYouQDWuZQYuanSTZhaoLGeneral gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude miceActa Pharmacol Sin20042576977415169630
- YuJGuoQLYouQDGambogic acid-induced G(2)/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cellsCarcinogenesis20072863263817012222
- KasibhatlaSJessenKAMaliartchoukSA role for transferrin receptor in triggering apoptosis when targeted with gambogic acidProc Natl Acad Sci U S A2005102120951210016103367
- WuZQGuoQLYouQDZhaoLGuHYGambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cellsBiol Pharm Bull2004271769177415516720
- ZhuXZhangCWuXLPreparation, physical properties, and stability of gambogic acid-loaded micelles based on chitosan derivativesDrug Dev Ind Pharm2008342918214750
- WangJQSuiMHFanWMNanoparticles for tumor targeted therapies and their pharmacokineticsCurr Drug Metab20101112914120359289
- ZhangHJChenBAJiangHWangCLWangHPWangXMA strategy for ZnO nanorod mediated multi-mode cancer treatmentBiomaterials2011321901914
- ChenBAChengJShenMFMagnetic nanoparticle of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cellsInt J Nanomedicine20094657119421371
- ChenBAMaoPPChengJReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and MDR1 shRNA expression vector in leukemia cellsInt J Nanomedicine2010543744420957165
- WuWWChenBAChengJBiocompatibility of Fe3O4/DNR magnetic nanoparticles in the treatment of hematologic malignanciesInt J Nanomedicine201051079108421170355
- WangJChenBAChengJApoptotic mechanism of human leukemia K562/A02 cells induced by magnetic iron oxide nanoparticles coloaded with daunorubicin and 5-bromotetrandrinInt J Nanomedicine201161027103421720514
- WangJChenBAChengJSynthesis and antitumor efficacy of daunorubicin-loaded magnetic nanoparticlesInt J Nanomedicine2011620321121445276
- ChenBALiangYQWuWWSynergistic effect of magnetic nanoparticles of Fe3O4 with gambogic acid on apoptosis of K562 leukemia cellsInt J Nanomedicine2009425125920011242
- ZhangDWZhangHNieJYangJSynthesis and self-assembly behavior of pH-responsive amphiphilic copolymers containing ketal functional groupsPolym Int201059967974
- BarefordLASwaanPWEndocytic mechanisms for targeted drug deliveryAdv Drug Delivery Rev200759748758
- YooHSLeeKHOhJEParkTGIn vitro and in vivo anticancer activities of nanoparticles based on doxorubicin-PLGA conjugatesJ Control Release20006841943110974396
- LinBLShenXDCuiSApplication of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release propertiesBiomed Mater2007213213418458446
- FeckerLFGeilenCCTchernevGLoss of proapoptotic Bcl-2- related multidomain proteins in primary melanomas is associated with poor prognosisJ Invest Dermatol20061261366137116528364
- YangEKorsmeyerSJMolecular thanatopsis: A discourse on the BCL2 family and cell deathBlood1996883864018695785
- GhavamiSHashemiMAndeSRApoptosis and cancer: Mutations within caspase genesJ Med Genet20094649751019505876