Abstract
Background
The independent role of the surface chemistry of titanium in determining its biological properties is yet to be determined. Although titanium implants are often in contact with muscle tissue, the interaction of muscle cells with titanium is largely unknown. This study tested the hypotheses that the surface chemistry of clinically established microroughened titanium surfaces could be controllably varied by coating with a minimally thin layer of TiO2 (ideally pico-to-nanometer in thickness) without altering the existing topographical and roughness features, and that the change in superficial chemistry of titanium is effective in improving the biological properties of titanium.
Methods and results
Acid-etched microroughened titanium surfaces were coated with TiO2 using slow-rate sputter deposition of molten TiO2 nanoparticles. A TiO2 coating of 300 pm to 6.3 nm increased the surface oxygen on the titanium substrates in a controllable manner, but did not alter the existing microscale architecture and roughness of the substrates. Cells derived from rat skeletal muscles showed increased attachment, spread, adhesion strength, proliferation, gene expression, and collagen production at the initial and early stage of culture on 6.3 nm thick TiO2-coated microroughened titanium surfaces compared with uncoated titanium surfaces.
Conclusion
Using an exemplary slow-rate sputter deposition technique of molten TiO2 nanoparticles, this study demonstrated that titanium substrates, even with microscale roughness, can be sufficiently chemically modified to enhance their biological properties without altering the existing microscale morphology. The controllable and exclusive chemical modification technique presented in this study may open a new avenue for surface modifications of titanium-based biomaterials for better cell and tissue affinity and reaction.
Introduction
Because of their chemical stability, biocompatibility, and their excellent mechanical properties, titanium materials have been used as implantable devices in various fields of medicine, such as dental and orthopedic endosseous implants and plates, endovascular stents, and scaffolds and frameworks for tissue generation and regeneration. In addition, titanium-based materials in various modified or processed forms have been demonstrated to exert advantageous biological effects as anti-inflammatory,Citation1–Citation3 antioxidant,Citation4 and antitumor,Citation5 and gene transfer agents,Citation6 as well as stimulation of nerve growth and signal transduction.Citation7–Citation9
Recent studies have demonstrated noncontact biological effects of titanium, ie, cellular and systemic effects where cells, tissues, and whole body are not in direct contact with titanium. Synaptic plasticity, resting membrane potential, and firing rate of pyramidal neurons are significantly altered in culture dishes surrounded by titanium. Citation10 A recent animal study showed that housing mice in titanium-containing cages significantly reduced light stimulation- triggered spontaneous motor activity.Citation11 Based on heart rate variability assessment, it was suggested that titanium promotes rest by suppressing neuronal excitability and regulating autonomic nerve activity. Despite these suggested relaxant effects of titanium at neuronal levels, its effects on muscle cells are largely unknown. There may be an effect of titanium on muscle cells that plays a role in relaxed and soothed motor activity. A human clinical study also showed that titanium may improve the joint range of motion probably because of modulated physiology and biomechanics in the muscle-tendon complex.Citation12 Although these studies carried out a noncontact mode of experiments on the effect of titanium, testing the effect of titanium on muscle cells when they are in direct contact with titanium may be the first important step toward identifying the mechanisms underlying the above-mentioned findings.
In orthopedic reconstructive and repair surgery involving titanium plates and screws, particularly in osteosynthesis, titanium devices are in direct contact with skeletal muscles.Citation13 Possible adverse or unfavorable effects of these titanium materials on surrounding soft tissues have been a concern.Citation13–Citation17 For instance, the interaction between titanium and muscle cells/tissues may affect the process of local wound healing, and if the interaction is unfavorably reactive or inflammatory, may delay the process of bone-titanium implant integration or degrade once integration is established.Citation13 Therefore, improving the biological reaction of muscle cells with titanium-based materials is an effective measure for a successful titanium implant/device therapy.
Many endovascular stents are made of titanium-based materials. The primary purpose of currently used endovascular stents is to restore the blood vessels physically and mechanically and secure blood flow. However, modern tissue engineering approaches allow for exploring the regeneration of vascular walls by improving the interaction between titanium and vascular cells.Citation18–Citation20 The vascular wall consists partially of smooth muscle cells. Smooth muscle cells show biological, physiological, and structural features in common with skeletal muscle cells, eg, both cell types form actin-myosin filaments. Understanding and improving the interaction between titanium and skeletal muscle cells would provide crucial information and cues to help improve the interaction between titanium and vascular smooth muscle cells.
There are two primary factors responsible for determining the biological properties of titanium, ie, surface chemistry and morphology. Despite considerable progress in titanium implant research, there are still critical gaps in knowledge as to how surface properties determine the biological properties of titanium. The most fundamental question of which surface chemistry or morphology is more important remains unanswered. In fact, the surface chemistry and morphology of titanium are mutually influenced, and it is a technical challenge to alter one property exclusively without altering the other, which makes the isolation of the actual effects of surface chemistry or morphology extremely difficult.Citation21–Citation23
Clinically used titanium implant products have an individual surface morphology, ie, specific roughness features and topography. Surface morphology significantly affects osteogenic cell behavior and phenotypes, and thereby the speed and quality of peri-implant osteogenesis, and eventually the establishment and maintenance of bone-implant integration.Citation24–Citation27 Depending on the purpose of the implant device and therapeutic indications, a specific surface morphology may be chosen on site with an established clinical evidence and treatment rationale. Therefore, if we could enhance the surface chemistry of titanium implants without altering their biologically proven surface morphology/topography, this could provide a promising strategy to improve further the biological capability of titanium. However, as already mentioned, it has been a difficult challenge to achieve this goal, in particular, for roughened or textured implant surfaces, which have been a choice of surface types in modern implant therapy.
This study tested the hypotheses that the surface chemistry of microroughened titanium surfaces could be controllably varied without altering the existing topographical and roughness features by coating with a minimally thin layer of TiO2 and that the change in superficial chemistry of titanium improves the biological properties of titanium substrates. Using slow-rate sputter deposition of molten TiO2 nanoparticles, acid-etched microroughened titanium surfaces were successfully coated with a pico-to-nanometer thickness layer that controllably increases the oxygen component of the titanium surface but does not alter the existing microscale morphology. The objective of this study was to compare the behavior, response, and function of rat femoral muscle-derived cells on acid-etched microroughened titanium surfaces with and without super-thin TiO2 coating to isolate the actual effect of the surface chemistry of titanium.
Materials and methods
Titanium samples, TiO2 super-thin coating, and surface characterization
Titanium discs (20 mm in diameter and 1 mm in thickness) of grade 2 commercially pure titanium were prepared by machining. To create microroughened morphology with peaks and valleys, titanium samples were acid-etched with 66% H2SO4. To alter the surface chemistry, titanium discs were coated with molten TiO2 nanoparticles having an average diameter of 120 nm (AquaTi, Phiten Co Ltd, Kyoto, Japan) using a sputter deposition system (CFS-36PV-120-2D, Shibaura Mechatronics Inc, Yokohama, Japan). Deposition time was varied at five, 10, 15, and 30 minutes (DC 1 kW; 1.00 × 10−2 Pa). The titanium discs were autoclave-sterilized and stored under dark ambient conditions for four weeks, which allowed sufficient aging of the surfaces and helped to standardize the surface energy.Citation28 The surface energy was evaluated by the level of hydrophilicity, defined as a contact angle of a 10 μL ddH2O drop placed on the titanium disc. The morphology of these surfaces was examined using a scanning electron microscope (XL30, Philips, Eindhoven, The Netherlands) and a laser profile microscope (VK-8500, Keyence, Osaka, Japan) to determine the average roughness, peak-to-valley roughness, and inter-irregularities space. The elemental composition of the titanium surfaces was examined using an energy dispersive x-ray spectroscope (JSM-5900LV, Joel Ltd, Tokyo, Japan).
Measurement of TiO2 coating thickness
To measure the thickness of the TiO2 coating optically, a 2 mm thick, transparent acrylic plate was coated with TiO2 using the abovementioned protocol for five, 10, and 15 minutes. Transmissivity of light at various wavelengths was measured for these coated plates along with a control plate without TiO2 coating using a spectrophotometer (U-4100, Hitachi, Tokyo, Japan). Based on the transmissivity of light at 633 nm, the thickness of the TiO2 coating was determined from the standard transmissivity-thickness curve (refraction factor N = 2.15; absorption index K = 2.92).
Cell culture
Skeletal muscle cells were isolated from bilateral femoral muscles of eight-week-old male Sprague-Dawley rats. The muscle tissue dissected carefully from the femoral bone and skin was minced to a slurry with a razor blade in the culture dish. The tissues were incubated at 37°C for one hour in Dulbecco’s modified Eagle’s medium containing 0.1% collagenase. The tissues were further incubated with 0.25% trypsin-ethylenediamine tetra-acetic acid for 30 minutes until the mixture was a fine slurry. The supernatant was centrifuged for four minutes at 1400 rpm. The pellet obtained was resuspended in 2–4 mL of Dulbecco’s modified Eagle’s medium and inoculated onto titanium discs at a density of 3 × 104 cells/cm2. The culture medium was renewed every three days. The University of California at Los Angeles Chancellor’s Animal Research Committee approved this protocol, and all experiments were performed in accordance with the United States Department of Agriculture guidelines for animal research.
Cell attachment, density, and proliferation assays
Initial attachment of cells was evaluated by measuring the number of cells attached to the titanium discs after three and 24 hours of incubation. The density of propagated cells was quantified at day 2 of culture. These quantifications were performed using a tetrazolium salt (WST-1)-based colorimetric assay (WST-1, Roche Applied Science, Mannheim, Germany). A culture well was incubated at 37°C for four hours with 100 μL of WST-1 reagent. The amount of formazan product was measured at 420 nm using an enzyme-linked immunosorbent assay reader (Synergy HT, BioTek Instruments, Winooski, VT). The proliferative activity of cells was measured by incorporating BrdU during DNA synthesis. At day 2 of culture, 100 μL of 100 mM BrdU solution (Roche Applied Science) was added to the culture wells and incubated for 10 hours. After trypsinizing the cells and denaturing DNA, cultures were incubated with an anti-BrdU antibody conjugated with peroxidase for 90 minutes and reacted with tetramethylbenzidine for color development. Absorbance was measured at 370 nm using an enzyme-linked immunosorbent assay reader.
Morphology and spreading behavior of cells
The spreading behavior and cytoskeletal arrangement of muscle cells seeded onto the titanium surfaces were examined by confocal laser scanning microscopy. At three and 24 hours after seeding, cells were fixed in 10% formalin and stained with the fluorescent dye, rhodamine phalloidin (actin filament, red color; Molecular Probes, Eugene, OR). A confocal laser scanning microscope (Carl Zeiss LSM 310, Jena, Germany) was used to scan 1 μm layers of each culture specimen. Individual sections were then digitally reconstructed. The area, perimeter, and Feret’s diameter of the cells were quantified using an image analyzer (ImageJ, NIH, Bethesda, ML). To observe the intracellular expression and localization of vinculin, a focal adhesion protein, the cells were additionally stained with a mouse antivinculin monoclonal antibody (Abcam, Cambridge, MA), followed by a FITC-conjugated antimouse secondary antibody (Abcam). The level of actin and vinculin expression was quantified as a pixel-based density using an image analyzer (ImageJ, NIH). The density was calculated in two different ways, ie, cell-based expression (total pixels/cell number) and cell area-based expression (total pixels/total cell area).
Cell adhesion assay
The adhesive strength of muscle cells attached to the titanium surfaces was evaluated by the percentage of cells remaining after mechanical detachment, as established previously.Citation29 Cells incubated on the titanium discs for 24 hours were rinsed once with phosphate-buffered saline to remove nonadherent cells, and then detached from the surfaces by vibrating a culture dish (amplitude, 10 μm; frequency, 30 Hz) at 37°C for five minutes. The detached and remaining cells were stained with calcein AM and their numbers were measured using an enzyme-linked immunosorbent assay reader.
Gene expression analysis
Gene expression was analyzed using a reverse transcriptase-polymerase chain reaction on culture days 5 and 10. Total RNA in these cultures was extracted using TRIzol (Invitrogen, Carlsbad, CA) and a purif ication column (RNeasy, Qiagen, Valencia, CA). Following DNAse I treatment, reverse transcription of 0.5 μg of total RNA was performed using MMLV reverse transcriptase (Clontech, Carlsbad, CA) in the presence of oligo(dT) primer (Clontech). Polymerase chain reaction was performed using Taq DNA polymerase (EX Taq; Takara Bio, Madison, WI) to detect collagen type I, collagen type III, myosin, and troponin T mRNA using the primer designs and polymerase chain reaction condition established previously.Citation24,Citation30 Polymerase chain reaction products were visualized on 1.5% agarose gel by ethidium bromide staining. Band intensity was detected and quantified under ultraviolet light and normalized with reference to GAPDH mRNA.
Collagen deposition and production
A Sirius red staining-based colorimetric assay was employed to quantify collagen production. On day 5, cultures were washed with prewarmed 1× phosphate-buffered saline at 37°C for one minute and fixed with Bouin’s fluid for one hour at room temperature. The cultures were washed with ddH2O and treated with 0.2% aqueous phosphomolybdic acid for one minute. The cultures were then washed again with ddH2O and stained with Sirius red dye (Pfaltz and Bauer, Stamford, CT) dissolved in saturated aqueous picric acid (pH 2.0) at a concentration of 100 mg/100 mL for 90 minutes with mild shaking. The cultures were washed with 0.01 N hydrochloric acid for two minutes to remove all nonbound dye. Afterwards, 600 μL of 0.1 N sodium hydroxide was added to dissolve the staining using a microplate shaker for 30 minutes at room temperature. The optical density of the solution was then measured using a spectrophotometer at 550 nm against 0.1 N sodium hydroxide as a blank. Additionally, to visualize collagen deposition, the above-mentioned protocol was employed. Instead of dissolving the stained dye, the cultures were dehydrated in a graded series of ethanol (50%–100%) followed by Xylene and examined using a confocal laser scanning microscope.
Statistical analyses
Three samples were used for all experiments (n = 3) except for those involving the assessment of surface roughness and chemistry assessment of titanium discs (n = 6) and cell morphometry (n = 6). One-way analysis of variance was used to examine the effects of TiO2 coating. If necessary, a post hoc Bonferroni test was used as a multiple-comparisons test. P < 0.05 was considered to be significant.
Results
Pico-to-nanometer thickness TiO2 coating
Low and high magnification scanning electron micrographic images of acid-etched titanium surfaces before and after TiO2 deposition are presented in . The acid-etched surfaces before TiO2 deposition showed a typical microroughened morphology, consisting of microscale pits, ranging 0.5–1.5 μm in peak-to-peak distance (approximately 1 μm on average). The acid-etched surfaces after five, 10, and 15 minutes of TiO2 deposition showed very similar surface morphologies and there were no recognizable changes before and after TiO2 deposition in roughness, uniformity, and appearance of the micropit features in low and high magnification images. However, the acid-etched surface with 30 minutes of TiO2 deposition showed apparent morphological changes (), in which the sharp peaks turned into dull and rounded ridges. Undefined structures were added along the inclines of the ridges, which made the slopes irregular and less steep.
Figure 1 TiO2 super-thin coating to pre-microroughened titanium surfaces. Acid-etched micropit titanium surfaces were coated with molten TiO2 nanoparticles using a slow-rate sputter deposition for various times, ie, five, 10, 15, and 30 minutes. (A) Scanning electron microscopy before and after TiO2 sputter deposition. (B) Atomic percentage of surface oxygen plotted against TiO2 deposition time. (C) Ti:O ratio plotted against TiO2 deposition time. (D) Contact angle of 10 μm ddH2O plotted against TiO2 deposition time.
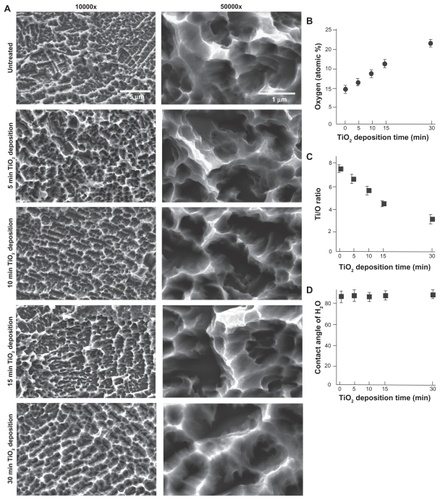
Energy dispersive x-ray spectroscopic analysis showed that TiO2 deposition increased the elemental oxygen on titanium surfaces in a deposition time-dependent manner (). Atomic oxygen, which was less than 10% before TiO2 deposition, increased to 17% after 15 minutes of deposition and eventually to 22% after 30 minutes of deposition. Accordingly, the Ti:O ratio decreased as the TiO2 deposition time increased (). A hydrophilicity test did not show any significant difference in the contact angle of ddH2O with and without TiO2 deposition, demonstrating that all surfaces tested were hydrophobic (defined as a contact angle >60°) regardless of the presence or absence of TiO2 deposition ().
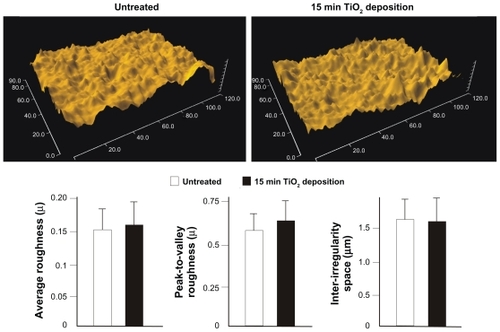
One of the objectives of this study was to modulate the surface chemistry of microroughened titanium surfaces without altering the morphology. As shown in , scanning electron micrographs indicated that TiO2 deposition for 15 minutes or less did not affect the original morphology of the acid-etched microroughened surface, at least in a visually recognizable manner at microscale. To confirm this, a further quantitative assessment was performed comparing untreated acid-etched surfaces and 15 minute TiO2 coated acid-etched surfaces. As shown in the representative optical images, the two surfaces were indistinguishable and similar in three-dimensional architecture (). No significant differences were observed between the microroughened surfaces with and without TiO2 coating in the average roughness, peak-to-valley roughness, and inter-irregularity space ().
Figure 2 Quantitative measurement of surface roughness of the microroughened titanium surfaces with and without TiO2 super-thin coating for 15 minutes.
Notes: Data are shown as the mean ± standard deviation (n = 6). Three-dimensional surface images are also presented. There was no statistically significant difference for any of the parameters tested between the two surfaces.
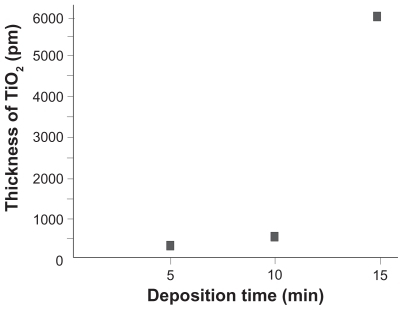
After confirming the controllable change in surface chemistry and absence of morphological changes on the titanium discs after application of 15 minutes or less deposition, we determined the thickness of the TiO2 coating. Based on the optically measured transmissivity data of different TiO2 coatings on a transparent acrylic plate, the thickness of TiO2 coatings for five, 10, and 15 minutes were calculated to be 300 pm, 600 pm, and 6.3 nm, respectively (). The TiO2 thickness after 30 minutes of deposition was not measurable because of 0% transmissivity. These surface characterization results indicate the establishment of a pico-to-nanometer thickness TiO2 coating that controllably and exclusively altered the surface chemistry of microroughened titanium surfaces, but did not alter their microscale morphology.
Muscle cell attachment, spread, and adhesion on super-thin TiO2 coating
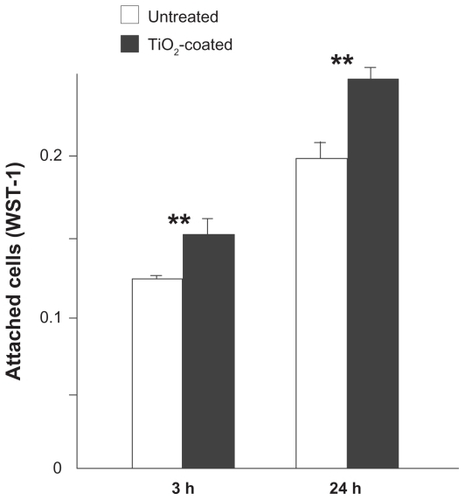
After establishing that 15 minutes of coating of TiO2 forming a thin layer of 6.3 nm thickness renders the most significant chemical alteration on the acid-etched titanium substrates while not altering the existing microscale morphology, titanium discs with 15 minutes of TiO2 deposition were compared with untreated acid-etched titanium discs for various biological properties. The number of muscle cells attached to the titanium discs was evaluated after three and 24 hours of incubation using a WST-1-based colorimetric assay (). After three hours of seeding, a significantly greater number of cells (20%) attached to the super-thin TiO2-coated surfaces when compared with untreated surfaces (P < 0.01). Even after 24 hours, the difference remained significant with the number of cells attached to super-thin TiO2-coated surfaces being greater, indicating that cell attachment is not just expedited but also enhanced on the super-thin TiO2-coated surfaces.
Figure 4 Cell attachment to microroughened titanium surfaces with and without 15 minutes of TiO2 coating evaluated by WST-1 colorimetric assay after three and 24 hours of incubation of muscle cells.
Notes: Data are shown as the mean ± standard deviation (n = 3). **P < 0.01, statistically significant difference between the two substrates.
Confocal microscopic images of cells with dual-staining of actin filaments and vinculin at three and 24 hours after seeding demonstrated that there were more cells on the TiO2-coated surfaces than on untreated surfaces, which confirmed the results of the WST-1 assay (). High magnification confocal images at three hours showed that the cells extended their cell processes more on TiO2-coated surfaces than on untreated surfaces (). At 24 hours, the cells were even larger on TiO2-coated surfaces, while the size of the cells on untreated surfaces remained similar between three and 24 hours.
Figure 5 Initial spread and cytoskeletal arrangement, and establishment of focal adhesion of muscle cells three and 24 hours after seeding onto the microroughened titanium surfaces with and without 15 minutes of TiO2 coating. Representative low (A) and high (B) magnification confocal microscopic images of cells stained with rhodamine phalloidin for actin filaments (red) and antivinculin (green). (C) Cytomorphometric evaluations for the cell area, perimeter, and Feret’s diameter of the cells. Densitometric analysis of vinculin (D) and actin expression per cell and cell area.
Notes: Data are the mean ± standard deviation (n = 6). *P < 0.05, **P < 0.01, statistically significant difference between the two substrates.
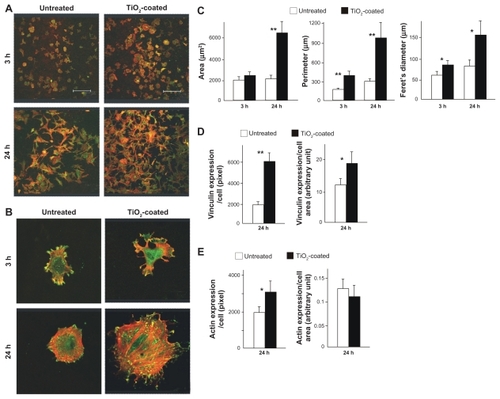
The cells on TiO2-coated surfaces showed more extensive staining of actin filaments within their cytoplasm (red color in images in ), indicating the expedited formation of a cytoskeleton. Moreover, vinculin immunopositivity was observed more extensively and intensively on TiO2-coated surfaces (green color in ), indicating expedited and advanced establishment of focal adhesion. Cytomorphometric evaluations of area, perimeter, and Feret’s diameter demonstrated considerable increases in all these parameters in cells seeded on TiO2-coated surfaces compared with those on untreated surfaces at both three and 24 hours, except for the cell area at three hours (), which supports the observations of cellular images. Both cell-based and cell area-based expression levels of vinculin were significantly increased on TiO2-coated surfaces (), whereas actin expression was significantly increased on TiO2-coated surfaces when standardized by cell number but not by cell area ().
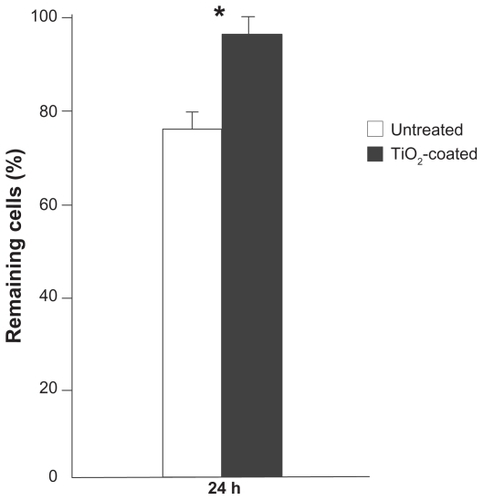
The strength of cell adhesion was assessed by evaluating the resistance against mechanical detachment (). Cells incubated for 24 hours were subjected to vibrating force as described in the Methods section. The number of cells remaining after detachment was 30% greater on TiO2-coated surfaces.
Cell proliferation and functional phenotypes on super-thin TiO2 coating
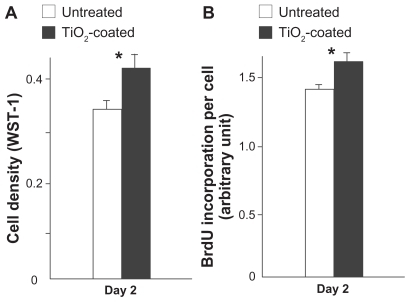
Cell density measured on day 2 was 25% higher on TiO2-coated surfaces than on untreated surfaces (). The result of BrdU incorporation per cell on day 2 confirmed the increase in proliferative activity of cells on TiO2-coated surfaces ().
Figure 7 Cell proliferative activity of muscle cells evaluated by cell density (A) and BrdU incorporation per cell (B) at day 2 of culture.
Notes: Data are the mean ± standard deviation (n = 3). *P < 0.05, statistically significant difference between the two substrates.
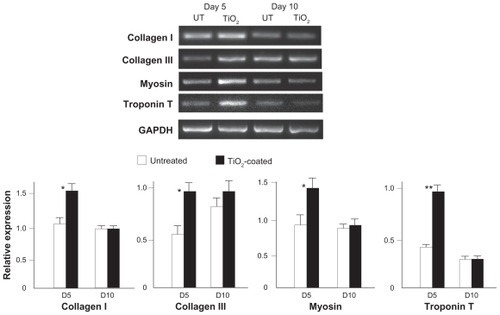
At day 5, the expression of all muscle cell-related genes tested was upregulated in cells cultured on TiO2-coated surfaces when compared with untreated surfaces (). The amount of upregulation was 60%–120%. At day 10, the expression levels of these genes were similar in surfaces with and without TiO2 coating.
Figure 8 Expression of muscle cell-related genes at days 5 and 10 of culture on the microroughened titanium surfaces with and without 15 minutes of TiO2 coating assessed using reverse transcriptase-polymerase chain reaction. Representative electrophoresis images are shown on top.
Notes: Data are the mean ± standard deviation (n = 3). *P < 0.05, statistically significant difference between the two substrates.
Abbreviation: UT, untreated surface.
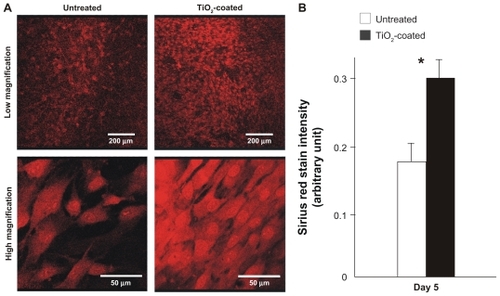
At day 5, there was more intensive and extensive detection of collagen molecules in the cells cultured on TiO2-coated surfaces (). The colorimetric measurement showed that cells on the TiO2-coated surface produced a 70% greater amount of collagen than those on untreated surfaces ().
Figure 9 Collagen production by muscle cells cultured on the microroughened titanium surfaces with and without 15 minutes of TiO2 coating at day 5. (A) Low and high magnification confocal microscopic images of cells after Sirius red staining. (B) The stained Sirius red was quantified colorimetrically.
Notes: Data are mean ± standard deviation (n = 3). *P < 0.05, statistically significant difference between the two substrates.
Discussion
This study tested the hypothesis that complex titanium surfaces, ie, morphologically enhanced surfaces in modern implant therapy, can be further enhanced biologically by modifying the surface chemistry without altering the surface morphology. In particular, this study focused on the behavior and response of skeletal muscle cells, for which little information is available in terms of their interaction with titanium. This hypothesis was supported by a comprehensive in vitro data set that revealed more favorable behaviors and increased levels of function of the cells on TiO2-coated microroughened surfaces.
We used TiO2 coating to modify the microroughened titanium surfaces chemically. A subtle difference in the superficial chemistry of titanium is known to affect its biological capability. There is a great variation in the thickness of the oxide layer and the amount of oxygen incorporated into titanium among the differently prepared titanium surfaces.Citation31–Citation35 Although titanium surfaces oxidize (primarily to TiO2) on exposure to the atmosphere, surface layers formed by titanium or TiO2 deposition may possess different biological properties. A recent study demonstrated that osteoblasts show greater affinity to deposited TiO2 surfaces than to the deposited Ti surfaces.Citation22 There seem to be specific biological events that can be enhanced by TiO2 in specific cell types. For instance, cell proliferation but not functional differentiation was particularly increased in osteoblasts cultured on TiO2 surfaces. Another rationale for using TiO2 coating was that TiO2 deposition chemically and structurally integrates well with the titanium surface.Citation36 Cross-sectional elemental mapping showed that the interface between the newly deposited TiO2 and base titanium is chemically seamless and the interfacial strength is sufficient to retain the deposited layer against exogenous detachment force equivalent to the one by a simulated load-bearing device. In this study, even five and 10 minutes of TiO2 deposition formed a 300 pm and 600 pm thick TiO2 coating, respectively. Deposition for different periods of time resulted in a linear correlation with the surface chemistry of titanium, ie, the oxygen percentage increased in a deposition time-dependent manner. The surface chemistry of the substrates was evaluated by energy dispersive x-ray spectroscopy in this study and the deposition time-dependent increase of surface oxygen was successfully monitored. However, energy dispersive x-ray spectroscopy is not capable of characterizing atomic components at the very superficial layer, and rather captures collectively elements within a layer of 2–5 μm depth. This may explain why the Ti/O ratio was higher than 4 even on 15 minute deposition surfaces, as opposed to an ideal 0.5 of a stoichiometric TiO2. It needs to be considered that the percentage of oxygen represents only a relative quantification and may be diluted in relation to the one of titanium element in this study. Evaluating TiO2-coated surfaces using x-ray photoelectron spectroscopy should be imminent in future studies for their precise characterization. Within the limitation of the present surface chemistry characterization, to ensure a higher probability of obtaining significant biological effects, the 15 minutes of deposition that produced the most significant increase in surface oxygen, while not altering the microscale morphology, was used for biological testings. To the best of our knowledge, a TiO2 coating of this thickness has rarely been created or tested for its biological potential and can be considered as a super-thin coating because of the technical advantage, that it preserves the existing microscale morphology.
We found no difference in hydrophilic nature between Ti substrates with and without TiO2 coating. The hydrophobic or hydrophilic nature of biomaterials significantly affects the behavior of various cells.Citation28,Citation37,Citation38 Various surface treatments, such as autoclaving, after processing titanium substrates could affect the level of hydrophilicity. Also, time after surface processing is known to decrease the level of hydrophilicity of titanium surfaces because of the progressive accumulation of surface contaminants, eg, hydrocarbons.Citation28,Citation39 In this study, to standardize the effect of these factors, titanium samples were autoclaved and stored for four weeks as described in the Materials and methods section. The hydrophilicity level of TiO2-coated substrates before autoclaving as well as that after different types of sterilization methods would be of great interest.
Another issue we considered was the deposition protocol. Recent studies have reported the phenomenon of nanostructure self-assembly during chemical deposition of metals onto specifically conditioned microtopographical surfaces.Citation29,Citation36,Citation40 For instance, when titanium or TiO2 chemical deposition of a thickness of 100 nm or higher is applied to titanium surfaces with acid-etched microtopography, unique nanonodular structures form within the existing micropits, creating a micro-nano hybrid topography.Citation29,Citation40 The depositioning protocol seems to have a key role in enabling nanostructure self-assembly. To create these nanostructures intentionally, deposition rates of 18.5–300 Å/min were used in those studies. To prevent accidental and unpredictable nanostructuring, this study employed a very slow deposition protocol. Based on the optical data for 10 minutes of deposition, the deposition rate in this study was as slow as 0.6 Å/min. Even at this rate, 30 minutes of deposition resulted in the formation of undefined structures along the peaks and flanks of ridges on the existing microarchitectures (). More importantly, even with the slow rate of deposition, a chemically controllable and biologically effective TiO2 coating was accomplished in as short as 15 minutes, which can be considered a significant technological advancement in improving current titanium-based materials.
The reason why the different deposition time did not lead to a linear correlation with TiO2 thickness may be due to the method used in this study to evaluate the thickness of TiO2. The TiO2 thickness was measured based on the transmissivity of light. A rapid decrease in light transmissivity was found between 10 and 15 minutes of deposition, implying that deposition of 10 minutes or less may have created voids or defects of TiO2 coating, and deposition of 15 minutes is likely to create a minimum thickness of coating that is even and uniform. In contrast, energy dispersive x-ray spectroscopic chemical analysis gave a stable and linear increase in oxygen percentage even for five and 10 minutes of deposition. Probably, this is because energy dispersive x-ray spectroscopic analysis captured a relatively larger area of the depositioned surfaces and was not significantly affected by such voids or defects of coating. These findings were also a rationale why 15 minutes of deposition was selected for biological capability testing. Future studies should pursue an even thinner TiO2 coating and an establishment of a linear deposition time-thickness curve by utilizing an even slower rate deposition protocol that enables more even and uniform coating. With this achievement, a next step in this line of research will be an optimization of TiO2 thickness from the technological and biological perspectives. Because titanium is a widely used implantable and ex vivo tissue engineering material, biological optimization will have to be carried out considering the behavior and response of different cell types, such as osteoblasts and fibroblasts.
An increased number of attached cells as well as increased levels of cellular spread were observed on the TiO2 coating not just at three hours but also at 24 hours, indicating that initial cell behavior was expedited and enhanced. The enhanced cell attachment was associated with enhanced expression of vinculin, providing molecular evidence explaining how attachment of muscle cells to TiO2-coated surfaces is enhanced. Not only cell-based but also cell area-based expression of vinculin was increased, indicating that the increased expression was not because of the enlargement of the cells but because of substantially upregulated expression of the protein. Vinculin is involved in the linkage between cell adhesion membranous molecules, integrins, and actin filaments, and serves a key role in initiating and establishing cell attachment, adhesion, formation of cell shape, and cytoskeletal development.Citation41–Citation44 In fact, the increased vinculin expression seems to have affected actin formation positively, as shown in the increased cell-based expression of actin. Moreover, the number of remnant cells after mechanical detachment was significantly greater on TiO2-coated surfaces. This indicates that cellular adhesion and retention are enhanced on the substrates, which is assumed to be because of the collective effects of increased expression of vinculin and actin molecules. The functional phenotypes of the cells, such as production of collagen, were also substantially increased on TiO2-coated surfaces. However, the question still remains as to whether this is because of the increased quantity of cells or advanced function in individual cells, or both. Gene expression analysis revealed that the expression of collagen types I and III was upregulated at the early culture stage of day 5, showing that not just the quantity of cells but also the function of the cells was advanced.
Collagens are the main constituents of the muscle extra-cellular matrix in the form of myofibrils. This study examined the gene expression of collagen type I and III, which are representative fibrillar collagens. These collagens are used as molecular markers during muscle cell differentiation.Citation45 Sirius red staining is also known to detect such fibrillar collagen molecules specifically. The presence of collagen fibers promotes the migration, proliferation, and differentiation of myoblasts, as well as myogenesis in stem cells, supposedly by creating a biologically, structurally, and biomechanically favorable environment.Citation46–Citation49 Myosins bind to actin filaments within the skeletal muscle cells and play a key role in muscle contraction. Troponin molecules play a key role in the relaxation and contraction of muscles by controlling the interaction between tropomyosin and calcium channels, wherein tropomyosin lies in the actin filaments. These genes were consistently upregulated on TiO2-coated surfaces at day 5, indicating that the function of the muscle cells, at least at the transcription level, may be uniformly enhanced.
Although TiO2 coating-induced upregulation was consistent for all of the genes tested, gene upregulation was found only at the early stage of day 5 but not at the later stage of day 10. This implies that the effect of TiO2 coating may primarily be on the initial and early stages of muscle cell culture and specifically to increase the attachment and proliferation of cells. The increased quantity of the cells may have then resulted in the increase in collagen production and gene expression because of the increased cellular interaction. These biological effects may be diluted at the later stage of culture as the cells reach the confluency even on noncoated titanium surfaces. In order to address this, as well as the physiological function of muscles cells which could potentially be affected by the surface chemistry of titanium, more systematic in vitro studies must be planned. Simultaneously, the results obtained from this study will warrant immediate planning of in vivo studies to explore the potential usefulness of super-thin TiO2 coating for implantable materials. The most significant advance made by the present study would be successful pilot application of a controllable and exclusive chemical modification on titanium, and may open a new avenue for surface modification of titanium-based biomaterials for better cell and tissue affinity and reaction.
Conclusion
This study tested and proved the hypothesis that micro-roughened titanium surfaces can be sufficiently chemically modified to enhance their biological properties without altering the existing microscale morphology. Pico-to-nanometer thickness molten TiO2 coating by slow-rate sputter deposition increased the surface oxygen on the titanium substrates in a controllable manner, but did not alter the existing microscale architecture and roughness of the substrates. Cells derived from rat skeletal muscles showed increased attachment, spread, adhesion strength, proliferation, gene expression, and collagen production at the initial and early stage of culture on 6.3 nm thick TiO2-coated microroughened titanium surfaces when compared with uncoated titanium surfaces.
Acknowledgment
This study was in part supported by Phiten Co Ltd, and was conducted in a facility constructed with grant support from the Research Facilities Improvement Program of the National Center for Research Resources, National Institutes of Health.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- SahlinHContrerasRGaskillDFBjurstenLMFrangosJAAnti- inflammatory properties of micropatterned titanium coatingsJ Biomed Mater Res A200677434916345099
- OvergaardLDanielsenNBjurstenLMAnti-inflammatory properties of titanium in the joint environment. An experimental study in ratsJ Bone Joint Surg Br1998808888939768904
- YangBGanLQuYYueCAnti-inflammatory properties of bioactive titanium metalsJ Biomed Mater Res A20109470070520205239
- SuzukiRMuycoJMcKittrickJFrangosJAReactive oxygen species inhibited by titanium oxide coatingsJ Biomed Mater Res A20036639640212889010
- ChiharaYFujimotoKKondoHAnti-tumor effects of liposomeencapsulated titanium dioxide in nude micePathobiology20077435335818087200
- PauneskuTRajhTWiederrechtGBiology of TiO2-oligonucleotide nanocompositesNat Mater2003234334612692534
- Carballo-VilaMMoreno-BurrielBChinarroEJuradoJRCasan-PastorNCollazos-CastroJETitanium oxide as substrate for neural cell growthJ Biomed Mater Res A2009909410518481786
- CehreliMCOnurMASahinSEffects of hydroxyapatite-coated and commercially pure titanium oral implant surfaces on compound nerve action potentialsClin Oral Implants Res20031426927212755776
- OnurMATasZGurpinarASahinSCehreliMCMorphologic, functional and behavioral effects of titanium dioxide exposure on nervesClin Oral Implants Res20041551351915355392
- KorteMInfluence of Aquatitan tape on nerve cells of the central nervous systemJ Clin Biochem Nutr2008431418648653
- AoiWTakanamiYKawaiYRelaxant effect of microtitan via regulation of autonomic nerve activity in miceLife Sci20098540841119632242
- WadsworthDPWalmsleyARowlandsDSAquatitan garments extend joint range of motion without effect on run performanceMed Sci Sports Exerc2010422273228120421834
- PennekampPHWimmerMAEschbachLBurianBKochPKraftCNMicrovasculatory reaction of skeletal muscle to Ti-15Mo in comparison to well-established titanium alloysJ Mater Sci Mater Med2007182053206017558479
- KraftCNHansisMArensSMengerMDVollmarBStriated muscle microvascular response to silver implants: A comparative in vivo study with titanium and stainless steelJ Biomed Mater Res20004919219910571905
- WildemannBKandzioraFKrummreyGLocal and controlled release of growth factors (combination of IGF-I and TGF-beta I, and BMP-2 alone) from a polylactide coating of titanium implants does not lead to ectopic bone formation in sheep muscleJ Control Release20049524925614980773
- RyhanenJKallioinenMTuukkanenJIn vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: muscle and perineural tissue responses and encapsule membrane thicknessJ Biomed Mater Res1998414814889659619
- McGeachieJSmithERobertsPGroundsMReaction of skeletal muscle to small implants of titanium or stainless steel: a quantitative histological and autoradiographic studyBiomaterials1992135625681633231
- ChoudharySBerheMHaberstrohKMWebsterTJIncreased endothelial and vascular smooth muscle cell adhesion on nanostructured titanium and CoCrMoInt J Nanomedicine20061414917722261
- Annual industry report. US markets for dental implants: Executive summaryImplant Dent200312108111
- SamarooHDLuJWebsterTJEnhanced endothelial cell density on NiTi surfaces with sub-micron to nanometer roughnessInt J Nanomedicine20083758218488418
- AttWYamadaMOgawaTEffect of titanium surface characteristics on the behavior and function of oral fibroblastsInt J Oral Maxillofac Implants20092441943119587863
- TsukimuraNKojimaNKuboKThe effect of superficial chemistry of titanium on osteoblastic functionJ Biomed Mater Res A20088410811617600332
- FengBWengJYangBCQuSXZhangXDCharacterization of surface oxide films on titanium and adhesion of osteoblastBiomaterials2003244663467012951009
- TakeuchiKSaruwatariLNakamuraHKYangJMOgawaTEnhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surfaceJ Biomed Mater Res A200572A29630515654712
- ButzFAitaHWangCJOgawaTHarder and stiffer bone osseointegrated to roughened titaniumJ Dent Res20068556056516723656
- OgawaTNishimuraIGenes differentially expressed in titanium implant healingJ Dent Res20068556657016723657
- CooperLFA role for surface topography in creating and maintaining bone at titanium endosseous implantsJ Prosthet Dent20008452253411105008
- AttWHoriNTakeuchiMTime-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materialsBiomaterials2009305352536319595450
- KuboKTsukimuraNIwasaFCellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy modelBiomaterials2009305319532919589591
- SaruwatariLAitaHButzFOsteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructureJ Bone Miner Res2005202002201616234974
- McAlarneyMEOshiroMAMcAlarneyCVEffects of titanium dioxide passive film crystal structure, thickness, and crystallinity on C3 adsorptionInt J Oral Maxillofac Implants19961173808820125
- WangGChengXRCharacterizations of three surface-modified titanium oxide filmsChin J Dent Res20003495211314519
- OjiMOWoodJVDownesSEffects of surface-treated cpTi and Ti6Al4V alloy on the initial attachment of human osteoblast cellsJ Mater Sci Mater Med19991086987215347967
- MacDonaldDERapuanoBEDeoNStranickMSomasundaranPBoskeyALThermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachmentBiomaterials2004253135314614980408
- DenholmEMCauchonEPoulinCSilverPJInhibition of human dermal fibroblast proliferation by removal of dermatan sulfateEur J Pharmacol200040014515310988328
- TsukimuraNYamadaMIwasaFSynergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titaniumBiomaterials2011324358436821421270
- AitaHAttWUenoTUltraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiationActa Biomater200953247325719427421
- MiyauchiTYamadaMYamamotoAThe enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfacesBiomaterials2010313827383920153521
- HoriNAttWUenoTAge-dependent degradation of the protein adsorption capacity of titaniumJ Dent Res20098866366719641155
- OgawaTSaruwatariLTakeuchiKAitaHOhnoNTi nano-nodular structuring for bone integration and regenerationJ Dent Res20088775175618650547
- HumphriesJDWangPStreuliCGeigerBHumphriesMJBallestremCVinculin controls focal adhesion formation by direct interactions with talin and actinJ Cell Biol20071791043105718056416
- BaillyMConnecting cell adhesion to the actin polymerization machinery: vinculin as the missing linkTrends Cell Biol20031316316512667752
- WenKKRubensteinPADemaliKAVinculin nucleates actin polymerization and modifies actin filament structureJ Biol Chem2009284304633047319736312
- GoldmannWHIngberDEIntact vinculin protein is required for control of cell shape, cell mechanics, and rac-dependent lamellipodia formationBiochem Biophys Res Commun200229074975511785963
- GerstenfeldLCCrawfordDRBoedtkerHDotyPExpression of type I and III collagen genes during differentiation of embryonic chicken myoblasts in cultureMol Cell Biol19844148314926548546
- ChaudhuriTRehfeldtFSweeneyHLDischerDEPreparation of collagen-coated gels that maximize in vitro myogenesis of stem cells by matching the lateral elasticity of in vivo muscleMethods Mol Biol201062118520220405368
- BeierJPKlumppDRudisileMCollagen matrices from sponge to nano: new perspectives for tissue engineering of skeletal muscleBMC Biotechnol200993419368709
- GoetschKPKallmeyerKNieslerCUDecorin modulates collagen I-stimulated, but not fibronectin-stimulated, migration of C2C12 myoblastsMatrix Biol20113010911721059388
- ArnesenSMoslerSLarsenNGadegaardNPurslowPLawsonMThe effects of collagen type I topography on myoblasts in vitroConnect Tissue Res20044523824715763933