Abstract
Background
Multidrug resistance (MDR) of cancers can be circumvented by inducing programmed cell death, which is known as apoptosis. Mitochondria play a crucial role in apoptosis. Mitochondria-specific therapy would provide an efficient strategy for treating resistant cancers.
Design and methods
A strategy was proposed here to overcome MDR by designing cancer mitochondria-specific drug-loaded liposomes, namely, antiresistant epirubicin mitosomes, aimed at treating resistant leukemia by targeting mitochondria. Evaluations were performed on human chronic leukemia K562, MDR K562/ADR cells, and female BALB/c nude mice xenografted with MDR K562/ADR cells. The liposomes were characterized through assays of cytotoxicity, mitochondrial targeting, caspase-9 and caspase-3, antitumor activities, and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) analysis.
Results
The average size of antiresistant epirubicin mitosomes was in the range of 105–115 nm. Antiresistant epirubicin mitosomes were effective in inhibiting proliferation of MDR K562/ADR cells in vitro and selectively accumulated into the mitochondria. Caspase-9 and caspase-3 activity was increased after applying antiresistant epirubicin mitosomes. In xenografted resistant MDR K562/ADR tumor in nude mice, antiresistant tumor effect of antiresistant epirubicin mitosomes was evidently observed. Apoptotic inducing effects by antiresistant epirubicin mitosomes were noticeably evidenced via mitochondrial pathway.
Conclusions
Antiresistant epirubicin mitosomes had significant inhibitory effect against resistant leukemia in vitro and in vivo, hence providing a promising strategy for improving therapeutic efficacy in resistant human leukemia.
Introduction
Multidrug resistance (MDR) is a major obstacle to successful cancer chemotherapy, and has been evidenced in a variety of cancers, including leukemia and breast, ovarian, lung, and lower gastrointestinal tract cancers.Citation1 The major mechanisms of MDR could be derived from acquired and/or intrinsic factors.Citation2 Acquired resistance develops during chemotherapy, resulting in the following outcomes: (1) less drug accumulation in cancer cells due to the efflux of drugs by overexpression of adenosine triphosphate (ATP)-binding cassette (ABC) transporters; (2) an enhanced drug-detoxifying function of cancer cells due to the enhanced deoxyribonucleic acid (DNA) repair ability and the increased activity of cytochrome P-450; and (3) decreased sensitivity of apoptosis due to the alteration of caspases.Citation3 In contrast, intrinsic resistance already exists at the beginning of chemotherapy. It may be derived from (1) host factors, (2) the genetic/epigenetic alternations such as activation of oncogenes and inactivation of tumor suppressor genes, or (3) the alternation of the apoptosis pathways.Citation4 Most likely, the MDR of leukemia is due to the intrinsic resistance.
Mitochondria are the powerhouses of cells, and the process of creating cell energy is known as cellular respiration. Most chemical reactions involved in cellular respiration happen in the mitochondria. The process by which mitochondria synthesize ATP by oxidative phosphorylation via the respiratory chain creates a transmembrane electrochemical gradient.Citation5 The membrane potential of mitochondria in vitro is estimated to be in the range of 180–200 mV,Citation6 while the membrane potential of mitochondria in living cells and organisms is lower (130–150 mV) due to metabolic processes such as ATP synthesis and ion transport.Citation7 This feature makes it possible to accumulate organic cations into mitochondria. Sufficient lipophilicity and delocalization of the positive charge are the prerequisites for mitochondrial accumulation of organic cations in response to the mitochondrial membrane potential.Citation8
Mitochondria are also known to play a role in programmed cell death via apoptosis.Citation9 Generally speaking, there are two semi-interdependent routes leading to apoptosis.Citation10 One route involves the ligand binding to death receptors at the cell surface,Citation11 while another involves mitochondria.Citation12 In the latter route, apoptotic inducer induces an increase in the permeability of the outer membrane, causing the release of cytochrome c,Citation13 and therefore triggering apoptosis. Cytochrome c may be released from mitochondria to the cytosol through Bax/Bak channels, permeability transition (PT) pores, or damaged mitochondrial membrane.Citation13 Meanwhile, the activated caspase family leads to a cascade of events in the cytosol,Citation14 eventually resulting in partial self-digestion of cells. There are two kinds of apoptotic gene-encoded proteins on the outer membrane of the mitochondria. One is pro-apoptotic gene-encoded proteins such as Bid and Bax, and the other one is apoptotic suppressing gene-encoded proteins such as Bcl-2 and Bcl-XL.Citation15–Citation18 These proteins play important roles in the process of apoptosis. As mitochondria play a major step-limiting role in apoptosis of cells,Citation12 it is necessary to deliver drugs to the mitochondria in order to activate pro-apoptotic genes or inhibit apoptotic suppressing genes.
Proposed here is a new strategy to circumvent the MDR of leukemia by designing cancer cell mitochondria-specific drug-loaded liposomes, ie, antiresistant epirubicin mitosomes. In the lipid-based mitosomes, epirubicin was encapsulated into the vesicle as an anticancer agent, amlodipine was simultaneously incorporated into the vesicle as an apoptotic inducer of leukemia, and dequalinium was inserted into the lipid bilayer membrane of the mitosome as a mitochondrial targeting molecule. Antiresistant epirubicin mitosomes were developed for the following purposes: (1) the resistant/nonresistant leukemia cells could be killed directly by antiresistant epirubicin mitosomes; (2) the mitochondrial genes could be regulated by apoptotic inducer amlodipine, which was selectively delivered to the mitochondria by the antiresistant epirubicin mitosomes.
Epirubicin is an anthracycline drug used for chemotherapy. It acts by intercalating with DNA strands and triggering DNA cleavage via topoisomerase II, resulting in death of cancer cells. In addition, epirubicin generates free radicals that cause cell and DNA damage.Citation19 Amlodipine is a dihydropyridine Ca2+ channel blocker used for treating hypertension. In the present study, amlodipine was used as a pro-apoptotic inducer of leukemia because it was previously found that amlodipine was able to potentiate the apoptosis of leukemia cells.Citation20 Dequalinium is a dicationic compound resembling “bola”-form electrolytes, and a symmetrical molecule with two charge centers separated at a relatively large distance. It is able to accumulate in the mitochondria of living cells in response to mitochondrial membrane potential.Citation21,Citation22 Dequalinium was thus modified on the mitosomes as a specific ligand for targeting mitochondria of leukemia cells.
In the present study, the building of antiresistant epirubicin mitosomes was based on the following hypotheses: antiresistant epirubicin mitosomes may enter into the cancer cells by phagocytosis and remain as two forms: namely, the integrated mitosomes and the ruptured mitosomes. The integrated mitosomes can target mitochondria due to mitochondrial affinity of dequalinium modified on the mitosomes.Citation23 Accordingly, epirubicin and amlodipine may activitate the Bax/Bak channel, induce the PT pore opening, damage the membrane of mitochondria, and thus result in the release of apoptogenic factors like cytochrome c and apoptosis-inducing factor (AIF). The degradation of nuclear DNA caused by the released AIF is not via the caspase pathway. On the contrary, released cytochrome c triggers the apoptosis pathway depending on the activating caspase family, including binding with apoptosis protease activating factor (Apaf)-1 and then stimulating caspase-9. The cleavage of caspase-9 initiates a cascade of caspase activation like caspase-3, leading to apoptosis of cancer cells.Citation24 On the other hand, the mitosomes may induce apoptosis by inhibiting Bcl-2 and Bcl-XL in mitochondria. Furthermore, epirubicin from the ruptured mitosomes may intercalate DNA strands in the nucleus and directly result in the death of cancer cells.
The objectives of the present study were to prepare the antiresistant epirubicin mitosomes and characterize their properties on human chronic myelogenous leukemia K562 and MDR K562/ADR cells in vitro and in vivo.
Design and methods
Preparation of antiresistant epirubicin mitosomes
Four types of liposomes were prepared: (1) antiresistant epirubicin mitosomes, which were used as the cancer cell mitochondria-specific liposomes; (2) antiresistant epirubicin liposomes; (3) epirubicin liposomes; and (4) amlodipine liposomes. The latter three types of liposomes were used as the controls.
Antiresistant epirubicin mitosomes
Egg phosphatidylcholine (EPC), 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol hydrochloride (DC-cholesterol HCl; Avanti Polar Lipids, Alabaster, AL), polyethylene glycol-distearoylphosphosphatidyl ethanolamine (PEG2000-DSPE; NOF Corporation, Tokyo, Japan), and dequalinium (Hangzhou Sanhe Chemicals Co, Ltd, Hangzhou, China) (in a 60:25:5:15 μmol ratio) were dissolved in methanol in a pear-shaped flask. The methanol was evaporated to dryness under vacuum with a rotary evaporator, and then the formed lipid film was hydrated with 250 mM ammonium sulfate by sonication in the water bath for 5 minutes and followed by sonication using a probe-type sonicator for 6.8 minutes. The suspensions after hydration were successively extruded through polycarbonate membranes (Millipore, Bedford, MA), with pore sizes of 400 and 200 nm, three times, respectively. The blank liposomes modified with dequalinium were then obtained and dialyzed (12,000–14,000 molecular mass cutoff) in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4) three times (12 hours each time). Epirubicin and amlodipine were loaded using an ammonium sulfate gradient loading method, as reported previously.Citation20 The blank liposomes modified with dequalinium were mixed with an appropriate amount of epirubicin hydrochloride (Nanjing Tianzun Zezhong Chemicals Ltd, Jinagsu, China), incubated at 60°C in a water bath, and intermittently shaken for 20 minutes. An appropriate amount of amlodipine besylate (Beijing Yimin Pharmaceuticals Co, Ltd, Beijing, China) was added to the above liposomes, further incubated for 10 minutes, producing the antiresistant epirubicin mitosomes.
Antiresistant epirubicin liposomes
The antiresistant epirubicin liposomes were prepared using the same procedure used to prepare the antiresistant epirubicin mitosomes, excluding the addition of dequalinium in the formed lipid membrane.
Epirubicin liposomes
The epirubicin liposomes were prepared using the same procedure used to prepare the antiresistant epirubicin liposomes, excluding the addition of amlodipine during drug loading.
Amlodipine liposomes
The amlodipine liposomes were prepared using the same procedure used to prepare the antiresistant epirubicin liposomes, excluding the addition of epirubicin during drug loading. The incubation duration for loading amlodipine was 30 minutes.
Characterization of the liposomes
Epirubicin, amlodipine, and dequalinium were measured on ODS column (Nucleodur 100-5 C18, 250 mm × 4.6 mm, 5 μm) by high performance liquid chromatography (HPLC) system with ultraviolet detector (Agilent Technologies Inc, Cotati, CA). The mobile phase consisted of acetonitrile, 0.02 M NaH2PO4 and triethylamine (34.0:66.0:0.3, v/v) and adjusted to pH 4.0 with phosphoric acid. The detection wavelength was set at 240 nm, the flow rate was 1.0 mL/min, and the injection volume was 20 μL.
All the liposomes were passed over a Sephadex G-50 column (Sigma-Aldrich Corporation agent, Beijing, China) to remove the unencapsulated epirubicin and amlodipine or unmodified dequalinium. The encapsulation efficiency of epirubicin or amlodipine was calculated with the formula: EE = Wencap/Wtotal × 100%, where EE is the encapsulation efficiency of epirubicin or amlodipine, Wencap is the measured amount of epirubicin or amlodipine in the liposome suspensions after passing over the column, and Wtotal is the measured amount of epirubicin or amlodipine in the equal volume of liposome suspensions before passing over the column. The modifying efficiency of dequalinium was defined as Wdeq per μmol lipids, where Wdeq is the measured amount of dequalinium on the liposome surface. The liposome sample was destroyed and measured by HPLC as above.
The particle sizes, polydispersity indexes (PDI), and zeta potential values of all liposomes were measured with Zetasizer 3000HSA (Malvern Instruments Ltd, Worcestershire, UK). The antiresistant epirubicin mitosomes were stained with 1% uranyl acetate and observed under transmission electron microscopy (TEM) (Tecnai G2 20ST, FEI Co, Tokyo, Japan).
In-vitro drug release assays of epirubicin and amlodipine from all liposomes were performed using the method reported previously.Citation25 A volume of 2 mL antiresistant epirubicin mitosomes, antiresistant epirubicin liposomes, epirubicin liposomes, or amlodipine liposomes was mixed with 2 mL of release medium (a mixture of PBS and 10% fetal bovine serum [FBS]), and the mixture was then placed into the dialysis tubing. The dialysis tubing was placed into 20.0 mL of the release medium, and oscillated with a shaker at a rate of 100 times per minute at 37°C. A volume of 0.2 mL release medium was sampled at 0, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 12.00, 24.00, and 48.00 hours, followed by immediately adding an equal volume of fresh release medium. The content of epirubicin or amlodipine was determined by HPLC as above, and the release rate was estimated with the formula: RR = (Wi/Wtotal) × 100%, where RR is the drug release rate (%), Wi is the measured drug amount of each sample, and Wtotal is the total drug amount in an equal volume of liposome suspension before performing the release experiment.
Cells and cell culture
Human chronic myelogenous leukemia (K562) and multidrug resistant human chronic myelogenous leukemia (MDR K562/ADR cells) (Institute of Hematology, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China) were used. The culture medium was prepared with RPMI (Roswell Park Memorial Institute) 1640 (Macgene Biotech Co, Ltd, Beijing, China) supplemented with 12% heat-inactivated FBS (Invitrogen Corporation, New York, NY), antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL), and 2 mM L-glutamine. The cell culture was performed in the incubator (37°C and 5% CO2).
Cytotoxicity assay
Microtiter tetrazolium (MTT) assay was performed according to a standard MTT-based colorimetric assay.Citation26 Briefly, K562, or MDR K562/ADR cells were seeded into a 96-well plate at 2 × 104 cells per well, and the cells were cultured in the incubator for 24 hours. Fresh medium containing serial concentrations of various drug formulations was then added into the plate well, including free epirubicin, free amlodipine, mixture of various concentrations of free epirubicin and amlodipine, epirubicin liposomes, amlodipine liposomes, antiresistant epirubicin liposomes, antiresistant epirubicin mitosomes, and PBS-loaded mitosomes. Cells incubated in medium without any drug were used as controls. After treatment, cells were incubated for 43 hours, and then 20 μL/well 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (5 mg/mL) was added. The plates were incubated for an additional 5 hours. The cells were then lyzed using 150 μL of sodium dodecyl sulfate (SDS) solution (10 g SDS, 5 mL iso-butyl alcohol, 10 mL 0.12 mol/L HCl, deionized water made up to 100 mL) and placed overnight in the incubator at 37°C. The absorbance values of the lyzed cells were read on a microplate reader (Model 680; BIO-RAD Laboratories, Tokyo, Japan) at a wavelength of 490 nm. The survival rates were calculated with the formula: survival rate (%) = (A490 nm treated cells/A490 nm control cells) × 100%, where A490 nm is the absorbance value at 490 nm.
Mitochondrial targeting
A confocal laser scanning fluorescent microscope with Leica confocal software (Leica, Heidelberg, Germany) was used to observe the mitochondrial targeting properties of antiresistant epirubicin mitosomes. Briefly, K562 or MDR K562/ADR cells were seeded into 6-well plates at 1 × 106 cells per well. After incubation for 24 hours, drugs were added, respectively, including free epirubicin (10 μM), epirubicin liposomes (10 μM), antiresistant epirubicin liposomes (10 μM for epirubicin or for amlodipine), and antiresistant epirubicin mitosomes (10 μM for epirubicin or for amlodipine). After treatment, cells were incubated for 1 hour, and then the cells were washed with PBS and stained with MitoTracker® Green FM (InvivoGen Corporation agent, Beijing, China). Composite images were made by overlapping the images of the individual channels.
Caspase-9 and -3 activity assays
The activity of caspase-9 was measured using caspase-9 colorimetric assay kit (Nanjing KeyGen Biotech Co, Ltd, Nanjing, China). Briefly, K562 or MDR K562/ADR cells were cultured for 24 hours. Drugs were added, respectively, including free epirubicin (10 μM), free amlodipine (10 μM), epirubicin liposomes (10 μM), antiresistant epirubicin liposomes (10 μM for epirubicin or for amlodipine) and antiresistant epirubicin mitosomes (10 μM for epirubicin or for amlodipine). The cells were further incubated for 12 hours, collected, and washed twice with PBS by centrifugation at 2000 revolutions per minute (rpm) for 5 minutes. Cold lysis buffer containing dithiothreitol was then added into the collected cells and mixed homogeneously. The cells were incubated on ice for 60 minutes and then vortexed three or four times for 10 seconds each time. The cell lysates were then centrifuged at 10,000 rpm for 1 minute at 4°C. Finally, the supernatants were sucked off and transferred to new tubes on ice. The assay for caspase-9 was based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate LEHD-pNA. The pNA light emission was quantified using a microplate reader at 405 nm. Caspase-9 activity was calculated with the formula: caspase-9 activity = (caspase-9 protein level of the treated cells)/(caspase-9 protein level of the control cells).
Caspase-3 activity was measured using the caspase-3 colorimetric assay kit (Nanjing KeyGen Biotech Co, Ltd, Nanjing, China), and the procedure was the same as for measuring caspase-9 activity. The assay is based on spectrophotometric detection of the chromophore pNA after cleavage from the labeled substrate DEVD-pNA. The pNA light emission was quantified using a microplate reader at 405 nm. Caspase-3 activity was calculated with the formula: caspase-3 activity = (caspase-3 protein level of the treated cells)/(caspase-3 protein level of the control cells).
In-vivo antitumor effects and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) analysis
All animal experiments were adhered to the principles of care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Peking University. Female BALB/c nude mice (initially weighing 14–16 g, Peking University Health Science Center) were divided into five groups (six in each). Cyclophosphamide was injected intraperitoneally into the mice at a dose of 2 mg per mouse per day for two consecutive days. Approximately 2.0 × 107 MDR K562/ADR cells re-suspended in 200 μL of serum-free cell culture medium were subcutaneously injected into the right flanks of the nude mice. On day 7, 9, 11, 13, 15, and 17 after inoculation, physiological saline, free epirubicin (3 mg/kg), epirubicin liposomes (3 mg/kg epirubicin), antiresistant epirubicin liposomes (3 mg/kg epirubicin plus 1.2 mg/kg amlodipine), and antiresistant epirubicin mitosomes (3 mg/kg epirubicin plus 1.2 mg/kg amlodipine) were injected into mice via the tail vein. The tumor sizes of the mice were monitored once daily, and tumor volumes were calculated as length × width2 × 0.52 (mm3).Citation27
Tumor-bearing mice were sacrificed on day 20 by cervical dislocation. Tumor masses were carefully isolated, and fixed for analysis using TUNEL. An ApopTag plus peroxidase in-situ apoptosis detection kit (Intergen Co, Ltd, Burlington, MA) was used to visualize the cells with DNA fragmentation, as reported previously.Citation28,Citation29 Nonnecrotic zone was selected in the tumor section, and images were randomly selected under the light microscope. In the presence of apoptosis, the apoptotic cells manifested as brownish staining in the nuclei. At least 1000 tumor cells were counted, and the percentage of TUNEL-positive cells was determined.
Statistics
Data are presented as means ± standard deviations. One- way analysis of variance was used to determine significance among groups, after which post-hoc tests with the Bonferroni correction were used for comparison between individual groups. A value of P < 0.05 was considered to be significant.
Results
Characterization of the mitosomes
For antiresistant epirubicin mitosomes, antiresistant epirubicin liposomes, epirubicin liposomes, and amlodipine liposomes, the encapsulation efficiencies of epirubicin or amlodipine were all ≥90%. For antiresistant epirubicin mitosomes, the modifying eff iciency of dequalinium was approximately 70 μg/μmol lipids. For antiresistant epirubicin mitosomes, antiresistant epirubicin liposomes, epirubicin liposomes, and amlodipine liposomes, the mean particle sizes were 109.2 ± 0.8, 107.8 ± 1.4, 113.7 ± 1.4, and 112.6 ± 5.7 nm, respectively. The particle size distributions (PDI) were 0.196 ± 0.002, 0.222 ± 0.006, 0.253 ± 0.007, and 0.251 ± 0.026, respectively. The zeta potential values were 1.564 ± 0.370, 1.129 ± 0.223, 0.900 ± 0.450, and 0.666 ± 0.169 mV, respectively.
For antiresistant epirubicin mitosomes, antiresistant epirubicin liposomes, and epirubicin liposomes in vitro, the release rates of epirubicin at 48 hours were 10.36% ± 0.19%, 30.42% ± 0.97%, and 21.38% ± 0.14%, respectively (). For antiresistant epirubicin mitosomes, antiresistant epirubicin liposomes, and amlodipine liposomes in vitro, the release rates of amlodipine at 48 hours were 24.41% ± 0.17%, 38.57% ± 0.63%, and 14.65% ± 0.22%, respectively ().
Figure 1 (A) In-vitro release rates (%) of epirubicin from epirubicin liposomes, amlodipine liposomes, anti-resistant epirubicin liposomes, and anti-resistant epirubicin mitosomes in PBS containing 10% FBS at 37°C. (B) In-vitro release rates (%) of amlodipine from epirubicin liposomes, amlodipine liposomes, anti-resistant epirubicin liposomes, and anti-resistant epirubicin mitosomes in PBS containing 10% FBS at 37°C. Data are presented as means ± standard deviations (n = 3). (C) Transmission electron microscopy image of anti-resistant epirubicin mitosomes.
Note: Scale bar = 100 nm.
Abbreviations: PBS, phosphate-buffered saline; FBS, fetal bovine serum.
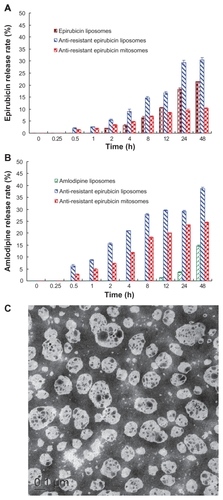
shows the TEM image of antiresistant epirubicin mitosomes. Results showed that antiresistant epirubicin mitosomes were monodispersed and spherical with a diameter between 100 and 110 nm.
Cytotoxicity
show the MTT assay results after epirubicin alone or epirubicin plus amlodipine were applied to K562 cells and MDR K562/ADR cells, respectively. Epirubicin alone (0.05–2.0 μM) was effective in inhibiting the proliferation of K562 cells, showing a dose-dependent manner. After the addition of amlodipine (10 or 20 μM), the inhibitory rates of epirubicin (0.05–2.0 μM) to K562 cells were significantly increased, displaying an evident synergistic effect. The inhibitory rates to MDR K562/ADR cells of epirubicin were <20% at a concentration ranging from 0.05 to 2.0 μM. After co-treating various concentrations of epirubicin (0.05–2.0 μM) with a fixed concentration of amlodipine (5.0, 10.0, or 20.0 μM), the survival rates of MDR K562/ADR cells were significantly reduced, compared with those treated with epirubicin alone at the same concentration. The inhibiting effect was increased with the rise of amlodipine concentration, displaying a dose-dependent manner.
Figure 2 (A) Effects of free epirubicin, free amlodipine, or free epirubicin co-treated with free amlodipine on the viable rates of K562 (i) and MDR K562/ADR cells (ii) measured by microtiter tetrazolium assay. (B) Effects of various formulations on the viable rates of K562 (i) and MDR K562/ADR cells (ii). (C) Effects of phosphate-buffered saline-loaded mitosomes on the viable rates of K562 and K562/ADR cells.
Notes: aP < 0.05, versus free epirubicin; bP < 0.05, versus free epirubicin of various concentrations plus amlodipine (5.0 μM); cP < 0.05, versus free epirubicin of various concentrations plus amlodipine (10.0 μM); dP < 0.05, versus free amlodipine; eP < 0.05, versus epirubicin liposomes; fP < 0.05, versus amlodipine liposomes; gP < 0.05, versus epirubicin plus amlodipine liposomes; *the membrane components were comparable to those of anti-resistant epirubicin mitosomes at the designated concentration point but not containing drugs in the empty mitosomes. Data are presented as the means ± standard deviations (n = 3).
Abbreviation: MDR, multidrug resistant.
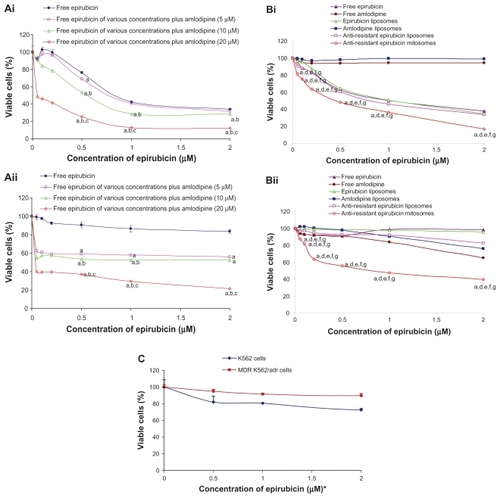
depict the effects on the proliferation of K562 and MDR K562/ADR cells after applying free epirubicin, free amlodipine, epirubicin liposomes, amlodipine liposomes, antiresistant epirubicin liposomes, and antiresistant epirubicin mitosomes. Results showed that antiresistant epirubicin mitosomes exhibited the strongest inhibitory effect to the proliferation among all the groups in both K562 cells and MDR K562/ADR cells. The inhibiting effect to the cells was increased with the rise of epirubicin or amlodipine concentration in antiresistant epirubicin mitosomes, displaying a dose-dependent manner.
shows the growth inhibition effect on K562 and MDR K562/ADR cells of PBS-loaded mitosomes. The survival rates of K562 and MDR K562/ADR cells after applying PBS-loaded mitosomes for 43 hours were 87.10% ± 5.10% and 68.65% ± 9.14%, respectively, where the membrane components were equivalent to antiresistant epirubicin mitosomes loaded with 10 μM epirubicin.
Mitochondria-targeting by confocal assay
represent the confocal laser scanning microscopic images of K562 and MDR K562/ADR cells after applying drugs to the cells for 1 hour at 37°C and stained with MitoTracker Green, respectively. represent the confocal laser scanning microscopic images after applying free epirubicin, epirubicin liposomes, antiresistant epirubicin liposomes, and antiresistant epirubicin mitosomes to K562 cells, respectively. Similarly, denote the results after applying the above drugs to MDR K562/ADR cells, respectively.
Figure 3 (A–F, M) Laser confocal fluorescence images of K562 cells incubated with various formulations for 1 hour at 37°C (magnification ×200 or ×600). (G–L, N) Laser confocal fluorescence images of MDR K562/ADR cells incubated with various formulations for 1 hour at 37°C (magnification ×200 or ×600). (A and G) Treated with PBS as a blank control. (B and H) Treated with PBS and stained with MitoTracker Green. (C and I) Treated with free epirubicin (10 μM) and stained with MitoTracker Green. (D and J) Treated with epirubicin liposomes (10 μM epirubicin) and stained with MitoTracker Green. (E and K) Treated with anti-resistant epirubicin liposomes (10 μM epirubicin plus 10 μM amlodipine) and stained with MitoTracker Green. (F, L, M and N) Treated with anti-resistant epirubicin mitosomes (10 μM epirubicin plus 10 μM amlodipine) and stained with MitoTracker Green.
Notes: (i) Green channel: MitoTracker Green® (InvivoGen Corporation agent, Beijing, China) stained mitochondria. (ii) Red channel: epirubicin. (iii) Composite images of (i) and (ii). The bright yellow fluorescence in Fiii, Liii, Miii, and Niii shows that anti-resistant epirubicin mitosomes are co-localized into the mitochondria of both K562 and MDR K562/ADR cells, demonstrating that the anti-resistant epirubicin mitosomes are selectively accumulated into the mitochondria of both leukemia cells.
Abbreviations: MDR, multidrug resistant; PBS, phosphate-buffered saline.
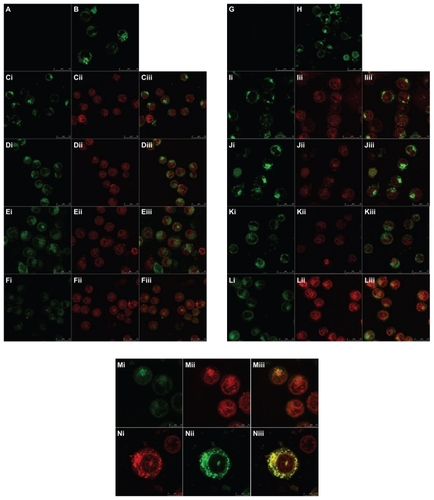
In the images, mitochondria were seen as the green fluorescent color stained by MitoTracker Green (; ), and epirubicin from free epirubicin, epirubicin liposomes, antiresistant epirubicin liposomes, or antiresistant epirubicin mitosomes was shown as a red fluorescent color (; ). A bright yellow fluorescent color represented a composition of red and green fluorescence, demonstrating that the liposomes containing epirubicin were co-localized into mitochondria (; ).
The results show that free epirubicin, epirubicin liposomes, and antiresistant epirubicin liposomes mostly distributed in the nucleus of the K562 cells and MDR K562/ADR cells (; ). On the contrary, antiresistant epirubicin mitosomes selectively accumulated into the mitochondria of both the K562 cells () and MDR K562/ADR cells ().
Caspase-9 and -3 activity
show the results of caspase-9 activity assays and caspase-3 activity assays in K562 cells and MDR K562/ADR cells, respectively. Results from caspase-9 assay showed that the activity of caspase-9, in both K562 and MDR K562/ADR cells, was significantly increased by antiresistant epirubicin mitosomes, compared with that of control. The results in K562 cells indicated that the caspase-9 activity of K562 cells was increased after adding free epirubicin, free amlodipine, epirubicin liposomes, antiresistant epirubicin liposomes, or antiresistant epirubicin mitosomes. The caspase-9 activity of K562 cells after treating with antiresistant epirubicin mitosomes was the highest among all the treatment groups (). The results in MDR K562/ADR cells indicated that the caspase-9 activity of MDR K562/ADR cells was increased after adding antiresistant epirubicin liposomes or antiresistant epirubicin mitosomes. The caspase-9 activity of MDR K562/ADR cells was slightly higher after treating with antiresistant epirubicin mitosomes compared with that after treating with antiresistant epirubicin liposomes ().
Figure 4 (A) Caspase-9 activity in K562 (Ai and Bi) and MDR K562/ADR cells (Aii and Bii) induced by various formulations. (B) Caspase-3 activity in K562 (Ai and Bi) and MDR K562/ADR cells (Aii and Bii) induced by various formulations.
Notes: Data are presented as means ± standard deviations (n = 3). aP < 0.05, versus the blank control; bP < 0.05, versus free epirubicin; cP < 0.05, versus free amlodipine; dP < 0.05, versus epirubicin liposomes; eP < 0.05, versus antiresistant epirubicin liposomes.
Abbreviation: MDR, multidrug resistant.
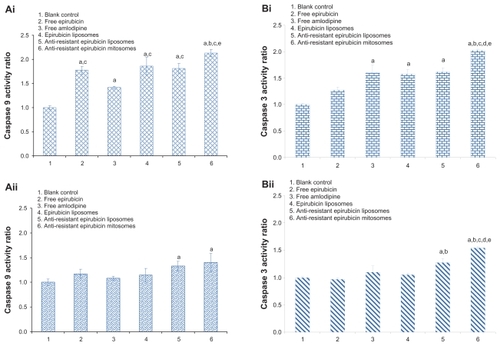
Results from caspase-3 assay in K562 and MDR K562/ADR cells showed a similar result to that of caspase-9 ().
In-vivo antitumor effects and TUNEL analysis
The cancer masses appeared at the 5th or 6th day after MDR K562/ADR cells were inoculated in the nude mice. Compared with the control group administered as physiological saline, the tumor growth was obviously inhibited in all treatment groups, but the antitumor effect varied. After giving antiresistant epirubicin mitosomes, the tumor volumes from the 8th to the 13th day were significantly smaller compared with those after administering free epirubicin alone, and at the 20th day were significantly smaller compared with those after injecting free epirubicin alone, epirubicin liposomes, or antiresistant epirubicin liposomes. In comparison among groups, the rank order for antitumor effect in vivo was as follows: antiresistant epirubicin mitosomes > antiresistant epirubicin liposomes > epirubicin liposomes > free epirubicin alone > physiological saline (the control) (). Results showed that the treatment of antiresistant epirubicin mitosomes resulted in a robust efficacy in reducing the tumor volume.
Figure 5 (A) Effect of anti-resistant epirubicin mitosomes on the MDR K562/ADR xenografts in female nude mice. At the seventh, ninth, eleventh, thirteenth, fifteenth, and seventeeth days after inoculation, physiological saline, free epirubicin (3 mg/kg), epirubicin liposomes (3 mg/kg epirubicin), anti-resistant epirubicin liposomes (3 mg/kg epirubicin plus 1.2 mg/kg amlodipine), and anti-resistant epirubicin mitosomes (3 mg/kg epirubicin plus 1.2 mg/kg amlodipine) were given to mice via tail vein, respectively. (B) Paraffin sections showing terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeled cells in the tumor tissues on the 20th day after inoculation of MDR K562/ADR cells in the female nude mice (magnification ×200). Apoptotic cells (shown by arrowhead) are characterized by a dense staining of nuclei. The MDR K562/ADR xenografted mice were given intravenously with physiological saline as a blank control (i), free epirubicin (ii), epirubicin liposomes (iii), anti-resistant epirubicin liposomes (iv), and anti-resistant epirubicin mitosomes (v).
Notes: Data are presented as the means ± standard deviations (n = 6). aP < 0.05, versus physiological saline; bP < 0.05, versus free epirubicin; cP < 0.05, versus epirubicin liposomes; dP < 0.05, versus anti-resistant epirubicin liposomes.
Abbreviation: MDR, multidrug resistant.
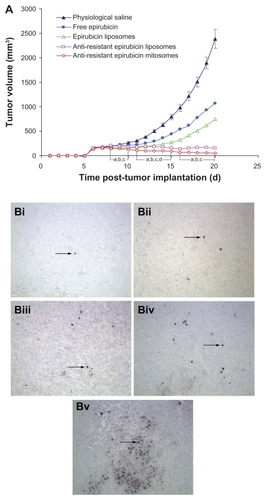
The TUNEL assay showed that the apoptotic percentages were 7.2% ± 0.8% for antiresistant epirubicin mitosomes (), 4.8% ± 1.0% for antiresistant epirubicin liposomes (), 3.0% ± 0.6% for epirubicin liposomes (), and 2.0% ± 0.9% for free epirubicin (), compared with 0.5% ± 0.5% for control ().
Discussion
In the present study, the mitochondrial targeting antiresistant epirubicin liposomes named as the antiresistant epirubicin mitosomes are constructed by modifying dequalinium on their surface as a mitochondrial targeting agent. Antiresistant epirubicin mitosomes show selective mitochondrial accumulation behavior and induce apoptotic effect on the resistant human chronic myelogenous leukemia. Resistant human myeloid leukemia cells are well known to be resistant to a number of pro-apoptotic agents like anticancer drugs because of the overexpression of anti-apoptosis factors, including Bcr-Abl tyrosine kinase, Bcl-2, Bcl-xL, P-glycoprotein, or multidrug resistance protein.Citation30–Citation32 There is also a set of pro- apoptosis genes expressed in leukemia cells which can be used as the target for chemotherapy. In the membrane of cancer mitochondria, two kinds of apoptotic genes, namely apoptotic suppressing genes and pro-apoptotic genes, are associated with the drug resistance of cancer cells.Citation33 Consequently, antiresistant epirubicin mitosomes were constructed for suppressing the anti-apoptosis genes and for activating the pro-apoptotic genes. The mitosomes were able to maintain the characteristics of the pegylated liposomes, including the long circulatory effect in the blood system.Citation34 Therefore, antiresistant epirubicin mitosomes may provide a thorough approach for eliminating resistant and nonresistant tumors.
In the present study, cytotoxicity assays were performed on human chronic myelogenous leukemia K562 and MDR K562/ADR cells to evaluate the additive antiproliferative effects of amlodipine and the antiproliferative effect of antiresistant epirubicin mitosomes, respectively. Results indicated that epirubicin alone is effective in inhibiting the growth of nonresistant K562 cells, but resistant to that of MDR K562/ADR cells. In the MDR K562/ADR cells, epirubicin in combination with amlodipine is effective in inhibiting the growth of MDR K562/ADR cells, indicating amlodipine was effective in reversing the resistance derived from the MDR K562/ADR cells. The reverse effect was increased with the rise in amlodipine concentration, exhibiting a dose-dependent manner. In K562 cells, the synergistic inhibitory effects are observed after co-treating with epirubicin plus amlodipine, suggesting that amlodipine was effective in enhancing the antitumor effect of epirubicin. Compared with other controls, antiresistant epirubicin mitosomes exhibited the strongest anticancer effect in K562 or MDR K562/ADR cells. After applying antiresistant epirubicin mitosomes, higher inhibition rates (60.5% in MDR K562/ADR cells) indicated that antiresistant epirubicin mitosomes were effective in inhibiting the proliferation of MDR K562/ADR cells in vitro. The reasons were as follows: (1) apoptotic inducer amlodipine could enhance anticancer efficacy of epirubicin and reverse the resistance of MDR K562/ADR cells when epirubicin and amlodipine were simultaneously incorporated into the mitosomes; (2) antiresistant epirubicin mitosomes could enhance concentrations of the drugs accumulated in mitochondria by selectively accumulating into the mitochondria, which may enhance apoptosis of K562 and MDR K562/ADR cells.
Dequalinium is a “single-chain bala-amphiphile”Citation35 with two charge centers separated at a relatively large distance. Such symmetric bola-like structures are well known from archaeal lipids, which usually consist of two glycerol backbones connected by two hydrophobic chains.Citation36 Dequalinium is able to self-associate into mitochondriotropic cationic “bola-lipid”-based vesicles, named DQAsomes.Citation23,Citation37 The DQAsomes have been developed for transport of drugs and DNA to mitochondria in living cells.Citation38,Citation39 In the present study, dequalinium is modified on the antiresistant epirubicin mitosomes with the same intention for targeting mitochondria. Mitochondria-targeting studies of antiresistant epirubicin mitosomes were performed on leukemia K562 and MDR K562/ADR cells using the confocal laser fluorescent microscopy technique. Results demonstrated that antiresistant epirubicin mitosomes were able to be colocalized into mitochondria of K562 and MDR K562/ADR cells, indicating that antiresistant epirubicin mitosomes were selectively accumulated into mitochondria of both leukemia cells, respectively.
After treating with drugs, cancer cells may be killed by injurious agents or by inducing programmed cell death (apoptosis), which may be generated by external signals (death receptor- mediated) or by internal signals (mitochondria-mediated). Activation of the effector cascade differs between extrinsic and intrinsic pathways. In the intrinsic pathways mediated by mitochondria, the apoptotic inducer increases the permeability of the outer membrane, causes cytochrome c to be released, then results in activation of caspase-9 and subsequent effector caspases, such as caspase-3, and finally leads to apoptosis of cells.Citation40,Citation41 In the present study, caspase-9 and caspase-3 activity were measured to discover whether these pathways were involved in the process of apoptosis initiated by antiresistant epirubicin mitosomes. Results showed that, in both K562 and MDR K562/ADR cells, caspase-9 and caspase-3 activity were most evidently increased after applying antiresistant epirubicin mitosomes. It was then postulated that antiresistant epirubicin mitosomes could enter into the cancer cells, target mitochondria to induce the PT pore opening, and thereby result in the swelling of mitochondria and then spill-age of cytochrome c. The released cytochrome c bound with Apaf-1 in the cytosol, and changed conformation of Apaf-1, leading to subsequent bindings with pro-caspase-9 and dATP. As a result, caspase-9 was cleaved, thus initiating a cascade of caspase activation like caspase-3 and leading to apoptosis of resistant or nonresistant leukemia cells.
Antitumor activities and apoptosis studies in vivo were performed on MDR K562/ADR cell xenografts in female BALB/c nude mice. Results showed that the antitumor effect of antiresistant epirubicin mitosomes was the most effective, compared with free epirubicin, epirubicin liposomes, or antiresistant epirubicin liposomes. TUNEL analysis demonstrated that antiresistant epirubicin mitosomes exerted favorable inducing apoptotic effect compared with other controls. The possible reasons may be as follows: (1) amlodipine as an apoptotic inducer could enhance anticancer efficacy and reverse the resistance in vivo; (2) the mitosomes as stealth liposomes with smaller particle size around 100 nm were able to extravasate through the “pores” of the capillary endothelium of tumor vasculature in a more efficient manner, resulting in an increased accumulation of those mitosomes in the interstitial spaces of tumor tissues;Citation42,Citation43 (3) the mitosomes as stealth liposomes exhibited prolonged half lives and sustained drug-release profiles in vivo, which could improve antitumor activity;Citation44 and (4) the mitochondrial genes could be regulated by apoptotic inducer amlodipine, which was selectively delivered to mitochondria by antiresistant epirubicin mitosomes.
In conclusion, a new kind of cancer mitochondria-specific drug-loaded liposome was designed, antiresistant epirubicin mitosomes, which could provide a complete solution to the intrinsic MDR of cancers. Antiresistant epirubicin mitosomes could selectively accumulate into mitochondria of cancer cells and initiate the intrinsic pathway mediated by mitochondria through inducing the PT pore opening, resulting in release of cytochrome c, followed by activating caspase-9, and subsequently the effector caspase-3. In vitro, antiresistant epirubicin mitosomes were effective in inhibiting the proliferation of both K562 and MDR K562/ADR cells, and in reversing drug resistance of MDR K562 cells. In vivo, antiresistant epirubicin mitosomes had the enhanced antitumor effects, and inducing apoptotic effects in the MDR K562/ADR xenografts. Therefore, antiresistant epirubicin mitosomes may provide a promising strategy for improving therapeutic efficacy in the resistant human leukemia.
Acknowledgments
This work was supported by the Key Grant of Beijing Natural Science Foundation (No. 7091005), the National Natural Science Foundation of China (No. 81172991), and the National Key Science Research Program of China (973 program, 2009CB930300).
Disclosure
The authors report no potential conflicts of interest in this work.
Authorship
WLL contributed to the conception and design of the study. YM performed the majority of experiments and drafted the article; XXW participated in some of the experiments and revised the article. WLL revised the article and obtained the necessary funding. Other authors contributed to some of the experiments.
References
- LautierDCanitrotYDeeleyRGColeSPMultidrug resistance mediated by the multidrug resistance protein (MRP) geneBiochem Pharmacol19965279679778831715
- JohnstoneRWRuefliAALoweSWApoptosis: a link between cancer genetics and chemotherapyCell2002108215316411832206
- GottesmanMMMechanisms of cancer drug resistanceAnnu Rev Med20025361562711818492
- KastrupIBWormJRalfkiaerEHoklandPGuldbergPGrønbaekKGenetic and epigenetic alterations of the reduced folate carrier in untreated diffuse large B-cell lymphomaEur J Haematol2008801616618028428
- MitchellPCoupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanismNature196119114414813771349
- MurphyMPSlip and leak in mitochondrial oxidative phosphorylationBiochim Biophys Acta198997721231412553111
- MurphyMPSmithRADrug delivery to mitochondria: the key to mitochondrial medicineAdv Drug Deliv Rev200041223525010699318
- WeissMJWongJRHaCSDequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulationProc Natl Acad Sci U S A19878415544454483474661
- BrownGCNichollsDGCooperCEMitochondria and Cell DeathPrinceton, NJPrinceton University Press1999viiviii
- HengartnerMOThe biochemistry of apoptosisNature2000407680577077611048727
- AshkenaziADixitVMApoptosis control by death and decoy receptorsCurr Opin Cell Biol199911225526010209153
- GreenDRReedJCMitochondria and apoptosisScience19982815381130913129721092
- MartinouJCDesagherSAntonssonBCytochrome c release from mitochondria: all or nothingNat Cell Biol200023E414310707095
- EarnshawWCMartinsLMKaufmannSHMammalian caspases: structure, activation, substrates, and functions during apoptosisAnnu Rev Biochem19996838342410872455
- GalluzziLLarochetteNZamzamiNKroemerGMitochondria as therapeutic targets for cancer chemotherapyOncogene200625344812483016892093
- MatsuyamaSReedJCMitochondria-dependent apoptosis and cellular pH regulationCell Death Differ20007121155116511175252
- GreenDRKroemerGThe pathophysiology of mitochondrial cell deathScience2004305568462662915286356
- AntignaniAYouleRJHow do Bax and Bak lead to permeabilization of the outer mitochondrial membrane?Curr Opin Cell Biol200618668568917046225
- PloskerGLFauldsDEpirubicin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapyDrugs19934557888567686469
- LiXRuanGRLuWLA novel stealth liposomal topotecan with amlodipine: apoptotic effect is associated with deletion of intracellular Ca2+ by amlodipine thus leading to an enhanced antitumor activity in leukemiaJ Control Release2006112218619816516327
- BernalSDLampidisTJMcIsaacRMChenLBAnticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dyeScience198322246201691726623064
- Modica-NapolitanoJSArilleJRDelocalized lipophilic cations selectively target the mitochondria of carcinoma cellsAdv Drug Deliv Rev2001491–2637011377803
- WeissigVTorchilinVPTowards mitochondrial gene therapy: DQA-somes as a strategyJ Drug Target20019111311378519
- YamadaYHarashimaHMitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseasesAdv Drug Deliv Rev20086013–141439146218655816
- YingXWenHLuWLDual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animalsJ Control Release2010141218319219799948
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- LiangZHWuPHLiLXueGZengYXHuangWLInhibition of tumor growth in xenografted nude mice with adenovirus-mediated endostatin gene comparison with recombinant endostatin proteinChin Med J2004117121809181415603709
- MoriMTeruiYTanakaMAntitumor effect of beta2-microglobulin in leukemic cell-bearing mice via apoptosis-inducing activity: activation of caspase-3 and nuclear factor-kappaBCancer Res200161114414441711389069
- SunPRenXDZhangHWSerum from rabbit orally administered cobra venom inhibits growth of implanted hepatocellular carcinoma cells in miceWorld J Gastroenterol20039112441244414606072
- PerkinsCKimCNFangGBhallaKNBhallaArsenic induces apoptosis of multidrug-resistant myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-xLBlood20009531014102210648417
- SchapiraAHMitochondrial diseaseLancet2006368708216815381
- WallaceDCThe mitochondrial genome in human adaptive radiation and disease: on the road to therapeutics and performance enhancementGene200535416918016024186
- SzewczykAWojtczakLMitochondria as a pharmacological targetPharmacol Rev20025410112711870261
- KaleAATorchilinVPEnvironment-responsive multifunctional liposomesMethods Mol Biol201060521324220072884
- WeissigVTorchilinVPCationic single-chain bolaamphiphiles as new materials for applications in medicine and biotechnological applicationsWorld J Microbiol Biotechnol199511115131
- De RosaMGambacortaAGlioziAStructure, biosynthesis, and physicochemical properties of archaebacterial lipidsMicrobiol Rev198650170803083222
- WeissigVLaschJErdosGMeyerHWRoweTCHughesJDQA-somes: a novel potential drug and gene delivery system made from dequaliniumPharm Res19981523343379523323
- D’SouzaGGRammohannRChengSMTorchilinVPWeissigVDQAsome-mediated delivery of plasmid DNA toward itochondria in living cellsJ Control Release2003921–218919714499196
- WeissigVChengSMD’SouzaGGMitochondrial pharmaceuticsMitochondrion20043422924416120357
- TwomeyCMcCarthyJVPathways of apoptosis and importance in developmentJ Cell Mol Med20059234535915963254
- LiPNijhawanDBudihardjoICytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascadeCell19979144794899390557
- DedharSHanniganGERakJKerbelRSThe extracellular environment and cancerTannockIFHillRPThe Basic Science of Oncology3rd edNew YorkMcGraw-Hill1998198218
- GabizonAASelective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomesCancer Res19925248918961737351
- SadzukaYSugiyamaITsurudaTSonobeTCharacterization and cytotoxicity of mixed polyethyleneglycol modified liposomes containing doxorubicinInt J Pharm20063121–2838916457972