?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Among several attempts to integrate tissue engineering concepts into strategies to repair different parts of the human body, neuronal repair stands as a challenging area due to the complexity of the structure and function of the nervous system and the low efficiency of conventional repair approaches. Herein, electrospun polyvinyl alcohol (PVA)/chitosan nano-fibrous scaffolds have been synthesized with large pore sizes as potential matrices for nervous tissue engineering and repair. PVA fibers were modified through blending with chitosan and porosity of scaffolds was measured at various levels of their depth through an image analysis method. In addition, the structural, physicochemical, biodegradability, and swelling of the chitosan nanofibrous scaffolds were evaluated. The chitosan-containing scaffolds were used for in vitro cell culture in contact with PC12 nerve cells, and they were found to exhibit the most balanced properties to meet the basic required specifications for nerve cells. It could be concluded that addition of chitosan to the PVA scaffolds enhances viability and proliferation of nerve cells, which increases the biocompatibility of the scaffolds. In fact, addition of a small percentage of chitosan to the PVA scaffolds proved to be a promising approach for synthesis of a neural-friendly polymeric blend.
Introduction
Generally speaking, the chemical composition and structure of tissue engineering scaffolds should be optimized for supporting the reparative process in a particular tissue and for attachment and proliferation of particular cells.Citation1 Among different available scaffolds, those recently developed with nanofibrous structures seem to be promising substrates for tissue engineering applications due to their high structural similarity to native extracellular matrix.Citation2–Citation5
Fiber-based porous scaffolds, which structurally mimic the extracellular matrix (ECM), have been synthesized from numerous natural or synthetic biopolymers. These scaffolds have been specifically engineered by electrospinning platform technology, and were successfully used for nerve tissue engineering applications. Electrospun scaffolds could be optimized to closely mimic the chemical, physical, and biological properties of the extracellular matrix of a particular tissue by fine-tuning its fabrication method and modifying the components of the composites. For most tissues, the biological properties of the scaffolds, including promotion of cell adhesion, proliferation, and differentiation, are more important than its microstructure. However, for neural tissues, the microstructure of the scaffold, especially its three-dimensionality, is equally as important as its biological properties.Citation6–Citation9
Electrospinning is a unique technology which can produce non-woven fibrous structures with fiber diameters ranging from nanometers to microns. This range of fiber size is difficult to achieve by other fabrication methods.Citation10,Citation11 The scaffolds fabricated by these nanofibers possess an extremely high ratio of surface to volume, have adjustable porosity, and could easily be customized over a wide range of sizes, shapes, and mechanical properties, which makes them very suitable candidates for neural tissue engineering.Citation12,Citation13 In electrospinning, polymer blending is one of the most effective methods for preparation of composites with specific properties.Citation14 The key issue in this method is adjustment of the ratio of the components, which influences the diameter and morphology of the structure of the fibers and scaffolds and the biological properties of the scaffolds.Citation15 Fine-tuning of nanostructured topographical cues such as grooves, ridges, pores, and nodes is also important as they influence cell adhesion, migration, proliferation, and differentiation.Citation5
So far, neural scaffolds have been synthesized from several natural and synthetic materials using different fabrication techniques, including electrospinning. The electrospun biodegradable polymers were successfully tested for their efficacy to stimulate axonal regeneration and neural stem cell differentiation, taking into account their different structural properties such as the diameter and alignment of the nanofibers.Citation16–Citation22 The biodegradable polymers used for this purpose mostly include polylactic-co-glycolic acid (PLGA), polyvinyl alcohol (PVA), collagen, and chitosan.Citation23,Citation24 Of these, PVA is a non-toxic, hydrophilic, and biocompatible material which has also been used for other tissue engineering applications.Citation25,Citation26 Chitosan has been widely used in this field as well.Citation27,Citation28 However, most of the previous studies focus on a single polymer for fabrication of electrospun nanofibrous scaffolds.
The high biocompatibility of chitosan has led several groups to use different techniques for scaffold fabrication, such as different three-dimensional shapes including tubular conduits, using chitosan as the base material.Citation29,Citation30 However, the mechanical properties of these scaffolds are still not optimal for application in nervous tissue. It has been shown that chitosan has quite positive effects on nerve tissue regeneration.Citation31–Citation33 Here, we tried to modify the physicochemical and biological properties of PVA polymer by blending it with chitosan, and used electrospinning for fabrication of a neurocompatible scaffold.
Materials and methods
Materials
PVA (98% hydrolyzed, average molecular weight of 72000 gmol−1), acetic acid (AA), and glutaraldehyde (GA) (25% aqueous solution) were purchased from Merck (Darmstadt, Germany). Chitosan (medium molecular weight of 190,000–310,000) was purchased from Orbital Pharma Co (Hebei, China).
Electrospinning procedures
The electrospinning setup utilized in this study consisted of an adjustable high DC voltage power supply, two syringe pumps (SP-500; JMS, Tokyo, Japan) and a ground electrode (a stainless steel drum with an external diameter of 50 mm, length of 10 cm, and variable rotating speed). Polymer solution was placed into metal capillaries (an internal diameter of 0.8 mm and length of 20 mm) with a constant mass flow rate of 0.6 mL/hour. PVA was dissolved in distilled water (DW) at a concentration of 10 wt% and chitosan was dissolved in acetic acid-water (AA-water) solution (2 wt%) at a concentration of 2 wt%. The PVA-DW solution 10 wt% was mixed with the chitosan-AA solution 2 wt% at a weight ratio of (PVA/chitosan) 90/10. Then, the mixed solution and PVA-DW solution (10 wt%) were subjected to the electro-spinning experiment separately. The distance between the tips and the ground electrode was 15 cm, while the positive voltage applied to the polymer solutions was 20 kV. The drum was continuously rotating at 250 rpm throughout the course of electrospinning.
Finally, samples were soaked in a cross-linking bath with GA vapor for 24 hours to cross-link the polymeric chains, reduce degradation, and enhance the biomechanical properties of the scaffolds for tissue repair. After soaking in the bath, the samples were carefully washed with 2% glycine aqueous solution several times to remove the remaining amount of GA.
Characterization
Viscosity measurement
Before the electrospinning process, the viscosity of solutions was measured by Brookfield Model DV-III viscometer (Brookfield Engineering Laboratories Inc, Stoughton, MA).
Morphology and microstructure analysis
The morphology and microstructure of the synthesized samples were evaluated using SEM. The electrospun fiber samples were coated with a thin layer of gold (Au) by sputtering (Emitech K450X, Ashford, UK) and their morphologies were observed under a scanning electron microscope (AIS2100; Seron Technology, Uiwang-si, Gyeonggi-do, South Korea) that operated at the acceleration voltage of 15 kV.
Image analysis program ImageJ (US National Institute of Health, Bethesda, MD), which uses grayscale level processing based on image structure,Citation34 was used to characterize the SEM micrographs in the original magnification of 30×. The average diameters of fibers and the porosity of various layers were calculated by this software package. Using different thresholds, the SEM micrographs were converted to binary images, and porosity of scaffolds were calculated in various layers by a recently developed method described by Ghasemi-Mobarakeh et al.Citation35
FTIR analysis
The samples were examined by Fourier transform infrared (FTIR) analysis with a Nicolet 17DSX FT-IR spectrometer (Thermo Scientific, Waltham, MA). For IR analysis, 1 mg of the scraped samples was carefully mixed with 300 mg of KBr (infrared grade) and pelletized under vacuum. Then, pellets between 500 and 4000 cm−1 were analyzed with 120 scans averaging 4 cm−1 resolution. The FTIR analysis was used to characterize the presence of specific chemical groups of PVA and chitosan.
Swelling test
The prepared electrospun nanofibrous scaffolds were placed in a 24-well plate. Each well contained 1 mL of a phosphate buffered solution (PBS; pH 7.4). The scaffolds were incubated in vitro at 37°C for different periods of time (1, 3, 7, and 10 days).Citation36 After immersion of the scaffolds in PBS solution for these different periods, the amount of fluid uptake was determined by careful removal of samples from the medium after wiping off excess fluid with filter paper. The swelling value (S) was calculated using Equationequation 1(1) :
For this test the samples were weighed for determination of the wet weight (Ww) as a function of immersion time. Wd is the dried weight of the samples.
Degradation test
The degradation study of the scaffolds was carried out in vitro by incubating the samples in PBS at pH 7.4, 37°C for different periods of time. After each degradation period, the samples were washed and subsequently dried in a vacuum oven at room temperature for 24 hours. In order to find out the degradation index (Di), the weight of the samples (Wt) and the degradation index was calculated based on the mass loss using Equationequation 2(2) :
In vitro study in contact with PC12 nerve cell line
The in vitro cytotoxicity of the prepared scaffolds was tested using the PC12 nerve cell line. The line was kept in continuous culture in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and streptomycin/penicillin 100 U/mL (1%). The cells were detached with trypsin/EDTA before seeding on samples. For seeding, the cells were trypsinized, centrifuged, and resuspended in complete culture medium. In the 24-well plate, 90,000 cells were seeded in each well. Then, 6 mg/mL of synthesized scaffolds were added to each well. After 72 hours of incubation, MTT solution (5 mg/mL) (Sigma, Munich, Germany) was added into each well and incubated for 90–120 minutes.
Then, all the media was discarded and 600 μL DMSO was added to each well. The OD (optical density) values were measured after 30 minutes by an ELISA reader at 590 nm with a reference filter of 620 nm.
For scanning electron microscopy (SEM), the cells were harvested after 3 days of culture. The samples were fixed with 3% GA for 2 hours. Specimens were rinsed in water and dehydrated with graded concentrations (50%, 70%, 90%, 100% v/v) of ethanol. Subsequently, the samples were treated with hexamethyldisilazane (HMDS) and kept in a fume hood for air drying. Finally, the samples were coated with gold to observe cell morphology.
Statistical analysis
All experiments were performed in fifth replicate. The results were given as mean ± standard error (SE). Statistical analysis was performed by one-way ANOVA and Tukey’s test, with significance reported when P < 0.05. The Kolmogorov–Smirnov test was used for assessment of distribution of data.
Results and discussion
Viscosity behavior
Before electrospinning, the viscosities of the solutions were measured by Brookfield Model DV-III viscometer. The viscosity of PVA solution was 557 centipoise and that of PVA/chitosan solution (with the weight ratio of 90/10) was 1726 centipoise. This is in agreement with the data published by Paipitak et alCitation37 who reported a linear increase in viscosity of PVA solution after blending with increasing amounts of chitosan. The high viscosity increases the interaction of two polymers, mainly through hydrogen bonding, and decreases the effects of surface tension. This will result in formation of fibers with uniform morphology after electrospinning.Citation3
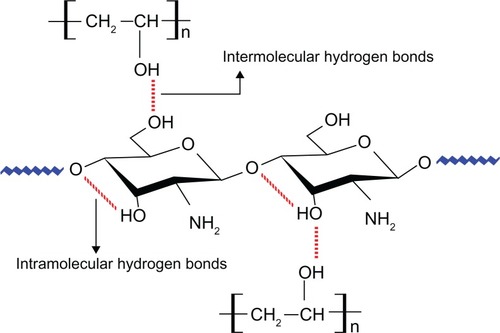
In recent years, polymer blending has become a method for providing polymeric materials with desirable properties for practical applications. In particular, chitosan blended with PVA has been reported to have good mechanical and chemical properties and, as a topic of great interest, has been extensively studied in the biomedical field.Citation38–Citation40 The enhanced property has been attributed to the interactions between chitosan and PVA in the blend through hydrophobic side-chain aggregation and intermolecular and intra-molecular hydrogen bondsCitation41,Citation42 as shown in .
Morphology and porosity of nanofibrous scaffolds
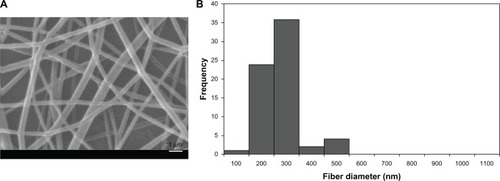
Electrospun PVA and PVA/chitosan scaffolds were observed by SEM in 3000 magnification. and show the SEM micrographs of the electrospun PVA and PVA/chitosan scaffolds, respectively. As can be seen in , in PVA/chitosan blend (weight ratio 90/10), the average fiber diameter was found to be 221 nm with a range of 94–410 nm; while in PVA alone, the average fiber diameter was 744 nm with a range of 395–1105 nm (). Similar observations have been made by Lin et alCitation43 and Ignatova et alCitation44 who investigated a series of PVA/chitosan blend nanofibrous membranes at different weight ratios and found a decrease in the average diameter of the nanofibers with increasing the chitosan content.
Figure 2 (A) SEM micrograph of electrospun PVA fibers; (B) fiber diameter distribution of PVA fibers.
Abbreviations: SEM, scanning electron micrograph; PVA, polyvinyl alcohol.
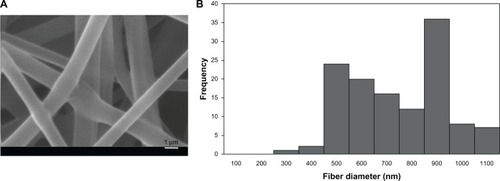
Figure 3 (A) SEM micrograph of electrospun PVA/chitosan nanofibers; (B) fiber diameter distribution of PVA/chitosan nanofibers.
Abbreviations: SEM, scanning electron micrograph; PVA, polyvinyl alcohol.
It should be noted that in electrospinning, the fiber diameter is dependent on the viscosity and charge of the solution. Typically, when the viscosity is increased, the diameter is increased proportionally. Chitosan affects not only the viscosity but also the charge density at the surface of the ejected jet through its cationic polyelectrolytic property. It increases the charge density at the surface of the jet which in turn increases the elongation force and decreases the diameter of the fiber.Citation45
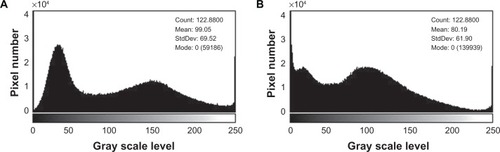
When the grayscale image was converted to binary form by ImageJ software, various layers of nanofibers could be seen by applying different thresholds. shows the configuration of histograms of PVA and PVA/chitosan nanofibrous scaffolds. As previously described by Ghasemi-Mobarakeh et al,Citation34 the results are not dependent upon the magnification or histogram of images. Various layers in most image magnifications and histograms allow calculation of porosity.
Figure 4 (A) Image histogram of PVA fibers; (B) image histogram of PVA/chitosan nanofibers.
Abbreviation: PVA, polyvinyl alcohol.
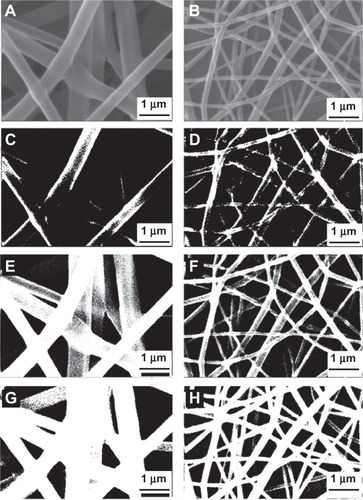
Analysis was performed on various layers of nanofibers by application of thresholds (see ). Threshold 1 eliminated the upper layer, and so, the surface layers were captured, a representative sum of surface and middle layers was captured using threshold 2, and threshold 3 was used for capture of all of the visible layers.
Figure 5 Various binary images with different thresholds for PVA and PVA/chitosan samples. (A) and (B) original images; (C) and (D) binary images with threshold 1; (E) and (F) binary images with threshold 2; (G) and (H) binary images with threshold 3.
After converting the original image to various binary images, the porosity of each binary image was calculated using the mean intensity of micrographsCitation34 using Equationequation 3(3) :
Table 1 Porosity measurement of binary images of PVA and PVA/chitosan with various thresholds
Interestingly, it was shown that the porosity of the same layers in the scaffolds fabricated with PVA and PVA/chitosan blend did not differ significantly. However, the pore morphologies were different. As mentioned above, PVA solution yielded fibers with relatively large diameters, which formed pores larger than those formed by small-diameter PVA/chitosan fibers. The numbers of pores were also different. PVA/chitosan scaffolds had a higher number of pores.
It should be noted that scaffold porosity and pore morphology are important for many tissue engineering applications as they allow migration of the cells and growth of blood vessels across the scaffold and ensure effective exchange of nutrients and waste products between the cells and their microenvironment. Here, we proposed and used an effective method for measurement of porosity at different layers of a scaffold.
Chemical bonding
FTIR spectroscopy was used to assess the chemical groups of the polymers. shows the FTIR spectra of PVA, chitosan, and PVA/chitosan blend. In , for the chitosan sample, the major characteristic peaks around 844 and 1151 cm−1 related to the saccharide structure (as the repeating unit of chitosan) are clearly observable.Citation46,Citation47 In addition, the strong absorption peaks at 1740, 1480, and 1346 cm−1 are shown, which are characteristic of chitosan and have been reported as amide I, II, and III peaks, respectively. The sharp peaks at 1382 and 1417 cm−1 could be assigned to the CH3 symmetrical deformation mode. Also, the broad peaks at 1081 and 1122 cm−1 indicate the C–O stretching vibration in chitosan, and another broad peak at 3447 cm−1 is caused by amine N–H symmetrical vibration. The peak observed at around 2947 cm−1 is due to the typical C–H stretch vibrations.Citation48 In , all major peaks related to hydroxyl and acetate groups are shown in the FTIR spectrum of PVA. More specifically, the broad band observed between 3550 and 3200 cm−1 is associated with the O–H stretch from the intermolecular and intramolecular hydrogen bonds. The vibrational band observed between 2840 and 3000 cm−1 is the result of the C–H stretch from alkyl groups and the peaks between 1730 and 1680 cm−1 are due to the C═O and C–O stretches from the remaining acetate groups in PVA (saponification reaction of polyvinyl acetate).Citation49–Citation51
Figure 6 FTIR analysis of (A) PVA; (B) chitosan; (C) PVA/chitosan samples.
Abbreviations: FTIR, Fourier transform infrared; PVA, polyvinyl alcohol.
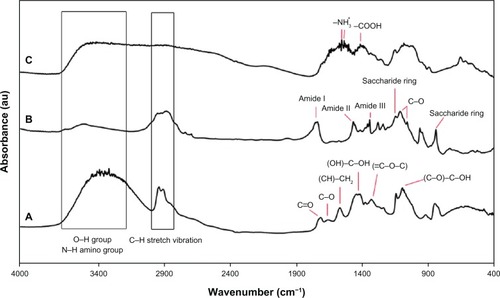
Moreover, the FTIR spectra of the PVA/chitosan fibers () are typical of the characteristic peaks of PVA and chitosan except those related to the ionization of the primary amino groups of chitosan. These peaks are shown at 1408 and 1548–1560 cm−1. Formation of the 1552–1558 cm−1 peak is due to the symmetric deformation of –NH3+ groups and the peak at 1408 cm−1 is the result of carboxylic acid. The peaks shown at 1700–1725 cm−1 are characteristic of carboxylic acid dimers.Citation10
Swelling behavior
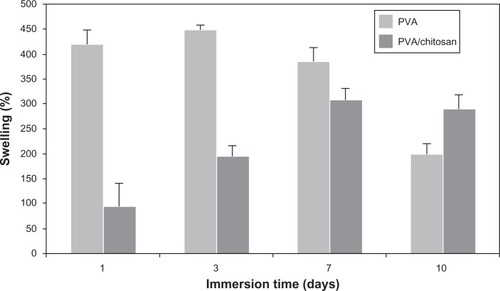
Fluid uptake is an important parameter, which influences the chemical and physical characteristics of the scaffolds after and prior to cell seeding. Herein, swelling experiments were performed after cross-linking of PVA and PVA/chitosan with GA vapor. A representative fluid uptake behavior is shown in for PVA and PVA/chitosan cross-linked scaffolds. The results revealed that chitosan strongly influences the swelling volume of the scaffold and decreases it from 450% (for PVA scaffold) to 300% (for PVA/chitosan scaffold). The reduced swelling volume could be attributed to a more rigid network formed by the inter- and intra-polymer reactions and also to the reduction of hydrophilic groups in PVA/chitosan blend. The latter is due to chitosan amine groups being more reactive to GA than to hydroxyls of PVA. This observation is in agreement with previous studies which reported that chitosan decreases the swelling rate when blended with PVA; the degree of reduction depends on factors such as weight ratio of the components, pH, temperature, and so on.Citation36,Citation48–Citation51
Degradation behavior
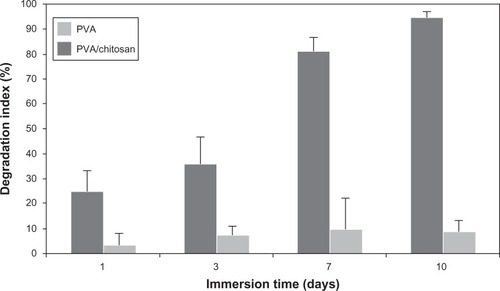
Degradation is the process through which useful physicochemical properties of the polymers are lost. This can include loss of polymer mass through mechanisms such as solvation and depolymerization. Degradation behavior of PVA and PVA/chitosan nanofibrous scaffolds using the PBS immersion method is shown in . It was clearly observed that the degradation rate of PVA/chitosan scaffolds was much slower than PVA samples. This could be due to higher density of chemical cross-linking between GA and amine groups of chitosan and leads to slower depolymerization.Citation52,Citation53
Viability of the nerve cells
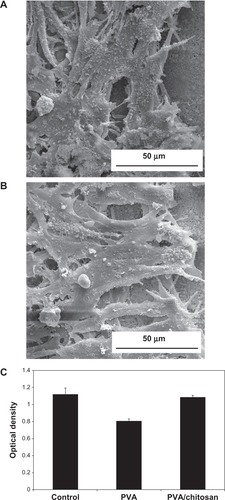
In this study, MTT assay was performed to evaluate the cell proliferation rate of PC12 nerve cells on the PVA and PVA/chitosan nanofibrous scaffolds. As shown in , the proliferation of cells on PVA/chitosan scaffolds was higher than on PVA scaffolds, indicating that the chitosan might have accelerated the proliferation rate of PC12 nerve cells. This could be attributed to the higher surface amine and the lower water contents of the swollen PVA/chitosan scaffold. In addition, it has been shown that fiber diameter can influence cell adhesion, proliferation, and migration. For example, it was shown by Christopherson et al that smaller fiber diameters favor better neural cell attachment and proliferation of cells cultured on non-woven electrospun fiber meshes.Citation54
Conclusion
In this study, polyvinyl alcohol (PVA) fibers were modified through blending with chitosan and electrospun to fabricate a nanofibrous scaffold. The PVA/chitosan scaffolds have been found to exhibit physicochemical and biological properties which, compared with PVA scaffolds, better meet the requirements of nerve cells.
Acknowledgements
The authors thank the many colleagues and collaborators who have made a vast contribution to this area of research.
Disclosure
The authors declare no conflict of interest in relation to this work.
References
- SamadikuchaksaraeiAScientific and industrial status of tissue engineeringAfr Biotechnol200762528972909
- ChewSYMiRHokeALeongKWThe effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturationBiomaterials200829665366117983651
- BhattaraiNEdmondsonDVeisehOMatsenFAZhangMElectrospun chitosan-based nanofibers and their cellular compatibilityBiomaterials200526316176618415885770
- CaoHLiuTChewSYThe application of nanofibrous scaffolds in neural tissue engineeringAdv Drug Deliv Rev200961121055106419643156
- YimEKReanoRMPangSWYeeAFChenCSLeongKWNanopattern-induced changes in morphology and motility of smooth muscle cellsBiomaterials200526265405541315814139
- LeeJCuddihyMJKotovNAThree-dimensional cell culture matrices: state of the artTissue Eng Part B Rev2008141618618454635
- YuLLeipzigNShoichetMPromoting neuron adhesion and growthMater Today20081153643
- MaPXZhangRSynthetic nano-scale fibrous extracellular matrixJ Biomed Mater Res1999461607210357136
- YangFXuCYKotakiMWangSRamakrishnaSCharacterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffoldJ Biomater Sci Polym Ed200415121483149715696794
- LiDXiaYElectrospinning of nanofibers: reinventing the wheel?Adv Mater2004161411511170
- Reneker DarrellHChunINanometre diameter fibres of polymer, produced by electrospinningNanotechnology199673216223
- JainKKRole of nanotechnology in developing new therapies for diseases of the nervous systemNanomedicine2006191217716203
- LiaoSLiBMaZWeiHChanCRamakrishnaSBiomimetic electrospun nanofibers for tissue regenerationBiomed Mater200613R45R5318458387
- ChengMDengJYangFGongYZhaoNZhangXStudy on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutionsBiomaterials200324172871288012742725
- SandersEHKloefkornRBowlinGLSimpsonDGWnekGETwo-phase electrospinning from a single electrified jet: microencapsulation of aqueous reservoirs in poly(ethylene-co-vinylacetate) fibersMacromolecules2003361138033805
- AhmedILiuHYMamiyaPCThree-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitroJ Biomed Mater Res A200676485186016345089
- BellamkondaRVPeripheral nerve regeneration: an opinion on channels, scaffolds and anisotropyBiomaterials200627193515351816533522
- BiniTBGaoSWangSRamakrishnaSPoly(l-lactide-co-glycolide) biodegradable microfibers and electrospun nanofibers for nerve tissue engineering: an in vitro studyJ Mater Sci2006411964536459
- ItoSWangWTsuneoOEfficacy of chitosan nanofiber mesh as a scaffold for regenerating nerve tissueKichin Kitosan Kenkyu200612194195
- PatelSKurpinskiKQuigleyRBioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidanceNano Lett2007772122212817567179
- SchnellEKlinkhammerKBalzerSGuidance of glial cell migration and axonal growth on electrospun nanofibers of poly-[epsilon]-caprolactone and a collagen/poly-[epsilon]-caprolactone blendBiomaterials200728193012302517408736
- CoreyJMLinDYMycekKBAligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growthJ Biomed Mater Res A200783A363664517508416
- ChenJPChangGYChenJKElectrospun collagen/chitosan nanofibrous membrane as wound dressingColloids Surf A: Physicochem Eng Asp2008313–314183188
- SahooSOuyangHGohJCHTayTETohSLCharacterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineeringTissue Eng2006121919916499446
- Zheng-QiuGJiu-MeiXXiang-HongZThe development of artificial articular cartilage – PVA-hydrogelBiomed Mater Eng19988275819830990
- MiyashitaHShimmuraSKobayashiHCollagen-immobilized poly (vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epitheliumJ Biomed Mater Res B Appl Biomater2006761566316044431
- JafariJEmamiSHSamadikuchaksaraeiABaharMAGorjipourFElectrospun chitosan-gelatin nanofiberous scaffold: fabrication and in vitro evaluationBioMed Mater Eng20112129911221654066
- EmamiSHAbadAMABonakdarSSamadikuchaksaraeiABaharMAPreparation and evaluation of chitosan-gelatin composite scaffolds modified with chondroitin-6-sulphateInt J Mater Res20101011012811285
- ZhangXFCaoWLGongYDA method for the preparation of porous chitosan tubeChina Patent2002 ZL 02149086. [Chinese].
- WangAAoQCaoWFiber-based chitosan tubular scaffolds for soft tissue engineering: fabrication and in vitro evaluationTsinghua Sci Technol2005104449453
- DillonGPYuXSridharanARanieriJPBellamkondaRVThe influence of physical structure and charge on neurite extension in a 3D hydrogel scaffoldJ Biomater Sci Polym Ed1998910104910699806445
- WangXHuWCaoYYaoJGuXDog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graftBrain200512881897191015872018
- CiardelliGChionoVMaterials for peripheral nerve regenerationMacromol Biosci200661132616374766
- SemnaniDLattifiMEffect of yarn appearance on apparent quality of weft knitted fabricJ Text I2005965295301
- Ghasemi-MobarakehLSemnaniDMorshedMA novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applicationsJ Appl Polym Sci2007106425362542
- de Souza Costa-JúniorEPereiraMMMansurHSProperties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinkedJ Mater Sci Mater Med200920255356118987949
- PaipitakKPornpraTMongkontalangPTechitdheerWPecharapaWCharacterization of PVA-chitosan nanofibers prepared by electrospinningProcedia Eng20118101105
- ZhengHDuYYuJHuangRZhangLPreparation and characterization of chitosan/poly(vinyl-alcohol) blend fibersJ Appl Polym Sci2001801325582565
- MiyaMIwamotoRMimaSFT-IR study of intermolecular interactions in polymer blendsJ Polym Sci Polym Phys Ed198422611491151
- NakatsukaSAndradyALPermeability of vitamin B-12 in chitosan membranes. Effect of crosslinking and blending with poly(vinyl alcohol) on permeabilityJ Appl Polym Sci19924411728
- ChoYWHanSSKoSWPVA containing chito-oligosaccharide side chainPolym200041620332039
- JinLBaiRMechanisms of lead absorption on chitosan/PVA hydrogel beadsLangmuir20021897659770
- LinTFangJWangHChengTWangXUsing chitosan as a thickener for electrospinning dilute PVA solutions to improve fibre uniformityNanotechnology200617153718
- IgnatovaMStarbovaKMarkovaNManolovaNRashkovIElectrospun nanofibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol)Carbohydr Res2006341122098210716750180
- SajeevUSAnandKAMenonDNairSControl of nanostructures in PVA, PVA/chitosan blends and PCL through electrospinningBull Mater Sci2008313343351
- MansurHSCostaHSNanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applicationsChem Eng J200813717283
- CostaEDSJrMansurHSPreparation and characterization of chitosan/poly(vinylalcohol)blend chemically crosslinked by glutaraldehyde for tissue engineering applicationQuímica Nova20083114601466
- DonTMKingCFChiuWYPengCAPreparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibilityCarb Polym2006633331339
- WangTTurhanMGunasekaranSSelected properties of pH-sensitive, biodegradable chitosan-poly(vinyl alcohol) hydrogelPolym Int2004537911918
- ShigemasaYMatsuuraHSashiwaHSaimotoHEvaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitinInt J Bio Macromol19961832372428729036
- MansurHSSadahiraCMSouzaANMansurAAPFTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehydeMater Sci Eng C2008284539548
- SuhJKMatthewHWApplication of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a reviewBiomaterials200021242589259811071608
- BergerJReistMMayerJMFeltOPeppasNAGurnyRStructure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applicationsEur J Pharm Biopharm2004571193414729078
- ChristophersonGTSongHMaoHQThe influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferationBiomaterials200930455656418977025