 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of the study was to determine the effect of carbon nanoparticles produced by different methods on the growth of brain tumor and the development of blood vessels. Glioblastoma multiforme cells were cultured on the chorioallantoic membrane of chicken embryo and after 7 days of incubation, were treated with carbon nanoparticles administered in ovo to the tumor. Both types of nanoparticles significantly decreased tumor mass and volume, and vessel area. Quantitative real-time polymerase chain reaction analysis showed downregulated fibroblast growth factor-2 and vascular endothelial growth factor expression at the messenger ribonucleic acid level. The present results demonstrate antiangiogenic activity of carbon nanoparticles, making them potential factors for anticancer therapy.
Introduction
Glioblastoma multiforme (GBM) is one of the most common tumors of the central nervous system. GBM grows in the brain, where it develops extensive tumors with specific infiltration along nerve fibers, somas, pia mater, and blood vessels. This prevents the total resection of the tumor. Glioblastoma is characterized by high proliferation of cancer cells, increased cellularity, necrosis, and a high potential for new blood vessels to develop from the vessels that already exist (angiogenesis).Citation1–Citation3 Angiogenesis is the main reason for the transformation of the small local lesions into extensive metastatic tumors.Citation4 The intensity of new blood vessel formation depends on the balance between proangiogenic factors, such as basic fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), and antiangiogenic factors, such as angiostatin, angiopoietin 2, and endostatin. Shifting of this equilibrium due to the increased expression of antiangiogenic or reduction of proangiogenic factors inhibits angiogenesis.Citation5–Citation8 Inhibition of tumor angiogenesis suppresses tumor growth and prevents metastasis, and is a key target in cancer therapy.Citation9 There have been several trials of angiogenic therapy applied to different places of cancer formation (breast, prostate, colon, liver, or kidney),Citation10–Citation14 as well as in the brain.Citation15,Citation16 Most utilize VEGF (VEGF-A and VEGF-B) or VEGF receptor (VEGFR1-3, PDGFRβ, and c-Kit) inhibition,Citation17–Citation20 while some also block pathways not dependent on VEGF (CD36 receptor, FGF pathway, cyclooxygenase-2, and hypoxia-inducible factors 1α)Citation21–Citation23 or inhibit endothelial cell migration (integrins αvβ1, αvβ3, αvβ5).Citation24 However, up to now, only bevacizumab, containing antiVEGF antibodies, has entered phase III clinical trials for treating GBM.Citation25
Recently, new biologically active substances have appeared that can be useful in antiangiogenic therapy: nanoparticles of carbon allotropes. They are not only biocompatible but also bioactive, inhibiting lipid peroxidation and regulating the expression of genes associated with cellular, genotoxic, and oxidative stress.Citation26,Citation27 Anticancer properties are characteristic for water-soluble C60 fullerenes. They inhibit growth of Lewis lung carcinoma tumors in mice, probably by inhibiting specific receptors (eg, endothelial growth factor receptor).Citation28 In anticancer therapy, allotropic forms of carbon are also applied as a drug delivery system. Conjugate water-soluble single-walled nanotube-palitaxelCitation29 and single-walled nanotube- doxorubicinCitation30 enhance permeability and retention in xenograft tumors, while not changing the effect of the medical treatment. Murugesan et alCitation31 proved the antiangiogenic properties of graphite nanoparticles, multi-walled carbon nanotubes, and C60 fullerenes on the blood vessels of chicken chorioallantoic membrane. They resulted from the binding of the proangiogenic factors FGF-2 or VEGF. Results of experiments on embryonic chicken heart have also showed that diamond and graphite nanoparticles downregulate FGF-2 expression.Citation32 There are no data concerning the influence of carbon nanoparticles on the development of blood vessels in GBM tumors.
It was hypothesized that the investigated carbon nanoparticles can downregulate the expression of factors associated with the formation of new blood vessels in GBM tumors. The objective of the study was to determine the effect of carbon nanoparticles manufactured by different methods on the growth of brain tumor and the development of its blood vessels.
Materials and methods
Carbon nanoparticles
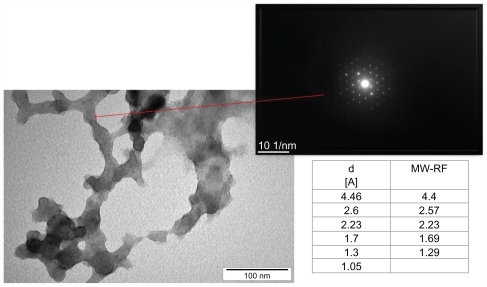
Nanoparticles of carbon allotropes: ultradispersed detonation diamond (UDD) and microwave-radiofrequency (MW-RF) carbon allotrope, were obtained from the Technical University of Lodz (Lodz, Poland). UDD were produced by the detonation method described by Danilenko.Citation33 MW-RF was synthesized by a plasma-assisted chemical vapor deposition process using the dual frequency methodCitation34 in MW (2.45 GHz) and RF (13.56 MHz) plasma in methane under a pressure of 100 Pa. The structure of nanoparticles and electron diffraction patterns were visualized by JEM-2000EX transmission electron microscope at 200 kV (JEOL Ltd, Tokyo, Japan). Transmission electron microscope images of UDD and MW-RF nanoparticles and electron diffraction patterns are presented in and , showing differences in the phase content and size of the particles. The size of the nanoparticles ranged from 2–10 nm for UDD and 30–100 nm for MW-RF.
Figure 1 Transmission electron microscope images and electron diffraction pattern of ultradispersed detonation diamond (UDD) nanoparticles.
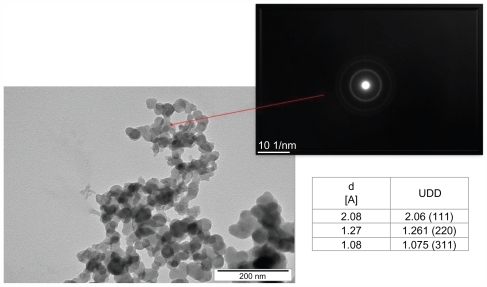
Figure 2 Transmission electron microscope images and electron diffraction pattern of microwave-radiofrequency (MW-RF) carbon nanoparticles.
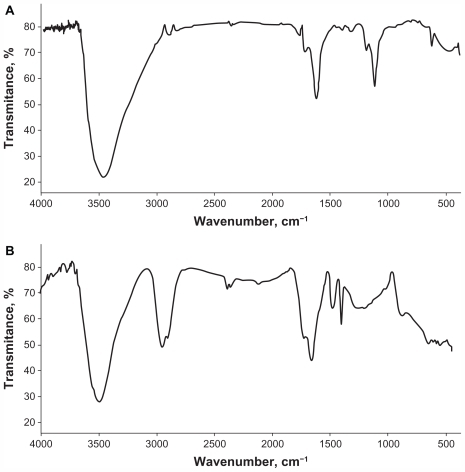
Infrared spectroscopy was used to detect the vibrational modes of the nanoparticles and, in particular, to reveal their surface termination. Fine potassium bromide powder and sample mixture at the ratio of 300:1 mg were used to prepare a pellet in the hydraulic press under a 10-ton pressure. Fourier transform infrared spectra were registered in the classic mid-infrared range (4000–400 cm−1) with a resolution of 4 cm−1. A pellet of pure potassium bromide was used as the background. Twenty-five scans were collected to obtain a spectrum for each sample using a PerkinElmer System 2000 spectrometer (Applied Biosystems, Inc, Foster City, CA), operated by Grams 2000 software (PerkinElmer, Inc, Waltham, MA). The Fourier transform infrared spectra of UDD and MW-RF nanoparticles are shown in . The measurements confirmed the chemical structures of the nanoparticles and indicated that the UDD nanoparticle had a surface termination at the carbonyl and carbon–oxygen polar groups, while the MW-RF nanoparticle had surface terminations at the carbon–hydrogen group.
Figure 3 Fourier transform infrared spectra of ultradispersed detonation diamond (A) and microwave-radiofrequency carbon (B) nanoparticles.
Zeta potential of hydrocolloids of nanoparticles was measured by light scattering method, using Zetasizer Nano ZS (ZEN3500; Malvern Instruments, Malvern, Worcestershire, United Kingdom). Each sample was measured after 120 seconds of stabilization at 25°C (20 replicates). Zeta potential of UDD nanoparticles hydrosol was +15 and MW-RF nanoparticles hydrosol was −19.3.
In , there is a summary of the physical and chemical characteristics of UDD and MW-RF nanoparticles.
Table 1 Physical and chemical characteristics of ultradispersed detonation diamond (UDD) and microwave-radiofrequency (MW-RF) carbon nanoparticles
Cells and embryos
Human U87 glioblastoma cells (HTB-14; American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s modified Eagle medium (Sigma-Aldrich Corporation, St Louis, MO) with 10% fetal bovine serum (Sigma-Aldrich). The fertilized eggs (Gallus gallus) (n = 60) were supplied by a commercial hatchery (Debowka, Poland).
Culture of GBM on chorioallantoic membrane
After 6 days of egg incubation, the silicone ring with the deposited 3–4 × 106 U87 cells suspended in 30 μL of culture medium was placed on the chorioallantoic membrane. The eggs were incubated for 7 days and then 36 eggs with visible tumor development were chosen. Eggs were divided into three groups of twelve: the control group, UDD group (injected with 200 μL of 500 μg/mL solution of UDD), and MW-RF group (injected with 200 μL of 500 μg/mL solution of MW-RF). The solutions were added directly into the tumors. After 2 days, the tumors were resected for further analysis.
Calculation of volume of tumor and blood vessel area
Digital photos of tumors were taken by a stereo microscope (SZX10, CellD software version 3.1; Olympus Corporation, Tokyo, Japan). The measurements were taken with cellSens Dimension Desktop version 1.3 (Olympus). The tumor volumes were calculated with the equation:
Measurement of the blood vessel area was performed in accordance with Seidlitz et al.Citation35 On each of the analyzed images of the tumor, five different areas of a fixed size of 400 μm2 were marked. The fragments with large blood vessels were not chosen. All the image pixels in shades of red, after strengthening of the picture contrast, were converted into the black pixels and counted. The result is a percentage count, in relation to all pixels in the analyzed field.
Histology analysis
After resection, the tumors were placed into 10% buffered formaldehyde (Sigma-Aldrich). Samples were embedded in paraffin (Paraplast®; Sigma-Aldrich) and cut into 5-μm sections. After staining with Harris hematoxylin (POCH S.A, Gliwice, Poland) and eosin (BDH Laboratory Supplies, Poole, Dorset, United Kingdom), the samples were analyzed by light microscopy (DM750; Leica Microsystems GmbH, Wetzlar, Germany) using LAS EZ version 2.0 software (Leica) ().
Quantitative real-time polymerase chain reaction analysis
Total ribonucleic acid (RNA) from tumor tissue was obtained using SV Total RNA Isolation System (Promega Corporation, Madison, WI). Total RNA (2 μg) was reverse-transcribed using reverse transcriptase (Promega), oligodeoxythymidylic acid, and random primers (TAG Copenhagen A/S, Copenhagen, Denmark). Relative messenger RNA levels of FGF-2 (locus NC_006091), VEGF (locus NC_006090), and the housekeeping gene EEF1A2 (locus NC_006107) were determined by real-time polymerase chain reaction using a LightCycler® 480 SYBR Green I Master and Light-Cycler® 480 Real-Time Polymerase Chain Reaction System (Roche Diagnostics GmbH, Mannheim, Germany), which was programmed for an initial step of 5 minutes at 95°C followed by 45 cycles of 10 seconds at 95°C, 10 seconds at 60°C, and 9 seconds at 72°C. The oligonucleotides used as specific primers were: 5′GGCACTGAAATGTGCAACAG3′ and 3′TCCAGGTCCAGTTTTTGGTC5′ for FGF-2; 5′TGAGGGCCTAGAATGTGTCC3′ and 3′TCTTTTGACCCTTCCCCTTT5′ for VEGF; and 5′AGCAGACTTTGTGACCTTGCC3′ and 3′TGACATGAGACAGACGGTTGC5′ for EEF1A2. All the reactions were performed in triplicate.
Statistical analysis
Results were expressed as mean and standard error. The data were analyzed using monofactorial analysis of variance and the differences between groups were tested by Duncan’s multiple range tests, using STATGRAPHICS® Plus 4.1 (StatPoint Technologies, Inc, Warrenton, VA). Differences with P < 0.05 were considered significant.
Results
Analysis of the antiangiogenic features of carbon nanoparticles (UDD and MW-RF) in terms of the growth of brain GBM tumors and their blood vessels was performed in ovo on the chicken embryo experimental model.
Tumor weight, volume, blood vessel area, and FGF-2 and VEGF expression levels were assessed. A decrease in tumor growth in terms of its weight and volume was observed in both treated groups (). In the UDD group, the weight was reduced by 73% and the volume by 61%, and in the MW-RF group, the weight decreased by 69% and the volume by 68% compared to the control group (P < 0.05).
Table 2 Weight, volume, and area of blood vessels of glioblastoma tumors
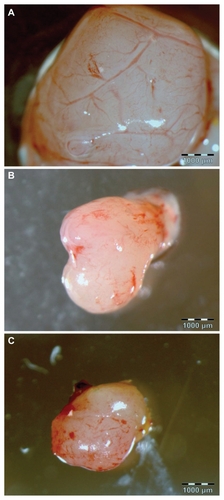
A decrease in blood vessel area was detected in the UDD and MW-RF groups versus the control group (). In the control group, 58% area of vessels was detected on average, but 19% and 25% in the UDD and MW-RF groups was detected, respectively. Except for the decreased blood vessel density after UDD and MW-RF treatment, characteristic changes in the macroscopic images of the observed blood vessels were found (). In the control group, distinctive blood vessel branching was observed; however, in the UDD and MW-RF groups, only the fragments of blood vessels and hemorrhagic processes were seen.
Figure 4 Images of a glioblastoma multiforme tumor cultured on chorioallantoic membrane: (A) control group, (B) ultradispersed detonation diamond group, and (C) microwave-radiofrequency group.
Note: Scale bar: 1000 μm.
In the histology of GBM between the control (), UDD (), and MW-RF () groups, strong differences were noticed. Chicken chorioallantoic membrane surrounding the tumor in the control group was thick with strong vascularity, while in the UDD and MW-RF groups, it was thin without any blood vessels. In the central part of the tumor in all the groups, fewer blood vessels were found than in the lateral part in contact with the host.
Figure 5 Histology of glioblastoma multiforme tumor cultured on chorioallantoic membrane: (A, B) control group, (C, D) ultradispersed detonation diamond group, and (E, F) microwave-radiofrequency group.
Notes: Scale bar: 100 μm. The images in (A), (C), and (E) were taken from the central part of the tumor, while those in (B), (D), and (F) from near its surface. Arrows point to blood vessels.
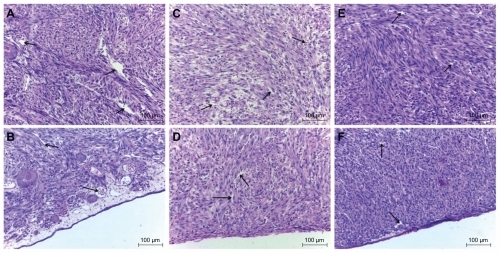
In the control group, the diameter of blood vessels varied between 2–30 μm, with a wide, transparent, and well-defined lumen. In the UDD and MW-RF groups, the blood vessels had similar dimensions as the capillary vessels (2–7 μm), while the lumen was irregular, narrow, and chink-like shaped. Endothelial cells of blood vessels in the control group had a typical shape with a flattened nucleus. In the UDD and MW-RF groups, endothelial cells had tube-like shapes and their nuclei were round. In these groups, there were also a lower number of blood vessels compared with the control group, with erythrocytes being present between the parenchymal cells of the tumor and necrotic area.
shows the results of the transcription of messenger RNA encoding FGF-2 and VEGF. There was a 69% decrease in FGF-2 expression in the UDD group compared to the control group (P < 0.05). A decrease of 33% was observed in the MW-RF group, but this was not significant. The amount of VEGF gene transcripts was also reduced in the UDD (by 72%) and MW-RF (by 48%) groups compared to the control (P < 0.05).
Table 3 Fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) gene expression profile in glioblastoma tumors following ultradispersed detonation diamond (UDD) and microwave-radiofrequency (MW-RF) treatment
Discussion
UDD and MW-RF nanoparticles reduced tumor mass and volume and inhibited new blood vessel development in GBM tumors cultured in ovo. It was observed that UDD nanoparticles significantly decreased FGF-2 and VEGF expression, while MW-RF nanoparticles only reduced VEGF expression, although there was a tendency of reduced FGF-2 expression too. VEGF is one of the most important factors influencing angiogenesis. The VEGF family (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor) is activated at different stages of the angiogenic cascade, including the induction of endothelial cell formation and proliferation, as well as stimulation of endothelial nitric oxide synthase.Citation36 The increased protein level of VEGF is linked to the higher permeability of the blood vessels and their untypical structure, which is characteristic of tumors.Citation37 Lowering of the VEGF level contributes to the normalization of blood vessels, facilitating the infiltration of other factors (eg, drugs) into the tumor,Citation38 while inhibiting VEGF expression limits the transport of nutrients into cancer cells.Citation39 In the present study, erythrocytes were observed to flow out to the parenchyma and necrotic area in tumors from the UDD and MW-RF groups. The occurrence of this process, in association with decreased levels of VEGF and FGF, indicates a breakdown and/or disappearance of blood vessels.
There are many possible mechanisms of action of carbon nanoparticles (UDD and MW-RF). The most probable seems to be the one described by Murugesan et alCitation31 who showed that multi-walled carbon nanotubes, C60 fullerenes, and graphite nanoparticles inhibited angiogenesis when VEGF and FGF-2 levels were elevated. Such a case takes place in the tumor microenvironment, where the level of proangiogenic factors is higher than that in healthy tissues. Carbon nanoparticles can bind to VEGF and FGF-2 or their receptors and in this way influence signal transduction between cells. The possibility of connecting the factors influencing angiogenesis is the property of carbon nanoparticles, but this has also been observed in experiments with nanosilverCitation40–Citation42 and nanogold.Citation43–Citation46 Nanosilver inhibits the phosphorylation of phosphatidylinositol-3-kinase/Akt at serine 473, leading to the inhibition of VEGF-induced angiogenesis.Citation41 Nanogold, by binding VEGF165 (a heparin-binding growth factor), inhibits its action and lowers the level of proliferation.Citation43 Moreover, mixing nanogold and nanosilver with heparan sulfate enhances heparan binding to FGF-2 and VEGF because silver and gold nanoparticles can both bind to amines and thiol groups.Citation47
The presence of different functional groups on the surface of UDD and MW-RF nanoparticles is probably the reason that although the effect of UDD and MW-RF nanoparticles is comparable (decrease of the weight and volume of the tumor as well as the number and area of the vessels), the mode of action is not identical (UDD nanoparticles inhibit VEGF and FGF-2, while MW-RF nanoparticles inhibit only VEGF []). This may be the result of different physical and chemical properties of the nanoparticles (). They differ not only in the way they are manufactured but also in size, zeta potential, atomic configuration, and surface termination. Due to the different functional groups on the surface, nanoparticles can bind different domains of proangiogenic factors or their receptors. It is interesting to note that despite different physicochemical features, both carbon nanoparticles exhibited antiangiogenic properties, but their intensity of action differed.
Regardless of the unclear mechanism of carbon nanoparticle activity, their biocompatibility together with the present results demonstrating their antiangiogenic properties make them potential factors for anticancer therapy.
Acknowledgments
This work was supported by the following grants: “New, multifunctional nanopowder of carbon” 357/ERA-NET 2008–2011 and Danish Agency for Science Technology and Innovation #2106-08-0025. The report is part of Marta Grodzik’s doctoral thesis.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ricci-VitianiLPalliniRBiffoniMTumour vascularization via endothelial differentiation of glioblastoma stem-like cellsNature2010468732582482821102434
- SalaczMEWatsonKRSchomasDAGlioblastoma: part I. Current state of affairsMo Med2011108318719421736079
- ChenJKesariSRooneyCInhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheresGenes Cancer20101882283521127729
- CarmelietPAngiogenesis in life, disease and medicineNature2005438707093293616355210
- HillenFGriffioenAWTumour vascularization: sprouting angiogenesis and beyondCancer Metastasis Rev2007263–448950217717633
- ReijneveldJCVoestEETaphoornMJAngiogenesis in malignant primary and metastatic brain tumorsJ Neurol2000247859760811041327
- BrekkenRAThorpePEVascular endothelial growth factor and vascular targeting of solid tumorsAnticancer Res2001216B4221422911908675
- FolkmanJAngiogenesis in cancer, vascular, rheumatoid and other diseaseNat Med19951127317584949
- PuduvalliVKSawayaRAntiangiogenesis – therapeutic strategies and clinical implications for brain tumorsJ Neurooncol2000501–218920011245279
- ImSAKimJSGomez-ManzanoCInhibition of breast cancer growth in vivo by antiangiogenesis gene therapy with adenovirus-mediated antisense-VEGFBr J Cancer20018491252125711336478
- Hartley-AspBVukanovicJJosephIBStrandgårdenKPolacekJIsaacsJTAnti-angiogenic treatment with linomide as adjuvant to surgical castration in experimental prostate cancerJ Urol19971583 Pt 19029079258116
- MurataKMoriyamaMIsoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesisCancer Res20076773263326817409434
- ZhangYJiangXQinXRKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinomaOncogene201029395404541520603618
- JieSLiHTianYBerberine inhibits angiogenic potential of Hep G2 cell line through VEGF down-regulation in vitroJ Gastroenterol Hepatol201126117918521175812
- BremSGrossmanSACarsonKAPhase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastomaNeuro Oncol20057324625316053699
- ZhouYXHuangYLAntiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo studyChin Med J (Eng)20091221416661673
- DrevsJHofmannIHugenschmidtHEffects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma modelCancer Res200060174819482410987292
- LuJZhangKNamSAndersonRAJoveRWenWNovel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signalingCarcinogenesis201031348148819969552
- WenWLuJZhangKChenSGrape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signaling pathwayCancer Prev Res (Phila)20081755456119139005
- ChamberlainMCBevacizumab for the treatment of recurrent glioblastomaClin Med Insights Oncol2011511712921603247
- BanuNADalyRSBudaAMoorghenMBakerJPignatelliMReduced tumour progression and angiogenesis in 1,2-dimethylhydrazine mice treated with NS-398 is associated with down-regulation of cyclooxygenase-2 and decreased beta-catenin nuclear localisationCell Commun Adhes2011181–21821679035
- RoncaRBenzoniPLealiDAntiangiogenic activity of a neutralizing human single-chain antibody fragment against fibroblast growth factor receptor 1Mol Cancer Ther20109123244325320940322
- KimTHHurEGKangSJNRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1αCancer Res20117162260227521278237
- SkuliNMonferranSDelmasCAlphavbeta3/alphavbeta5 integrins- FAK-RhoB: a novel pathway for hypoxia regulation in glioblastomaCancer Res20096983308331619351861
- WickWWellerMvan den BentMStuppRBevacizumab and recurrent malignant gliomas: a European perspectiveJ Clin Oncol20102812e188e18920159801
- Bakowicz-MituraKBartoszGMituraSInfluence of diamond powder particles on human gene expressionSurf Coat Technol20072011361316135
- LiuKKChengCLChangCCChaoJIBiocompatible and detectable carboxylated nanodiamond on human cellNanotechnology200718325102
- PrylutskaSVBurlakaAPPrylutskyyYIRitterUScharffPPristine C(60) fullerenes inhibit the rate of tumor growth and metastasisExp Oncol201133316216421956470
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res200868166652666018701489
- ChaudhuriPHarfoucheRSoniSHentschelDMSenguptaSShape effect of carbon nanovectors on angiogenesisACS Nano20104157458220043662
- MurugesanSMousaSAO’ConnorLJLincolnDW2ndLinhardtRJCarbon inhibits vascular endothelial growth factor-and fibroblast growth factor-promoted angiogenesisFEBS Lett200758161157116017331505
- MituraKChwalibogASawoszENanoparticles of diamond do not stimulate angiogenesis in chicken embryo modelPaper presented at: 2nd International Workshop on Science and Applications of Nanoscale Diamond Materials2010 June 28–July 2Zakopane, Poland.
- DanilenkoVVSynthesizing and Sintering of Diamond by ExplosionMoscowEnergoatomizdat2003 Russian
- KaczorowskiWNiedzielskiPMorphology and growth process of carbon films prepared by microwave/radio frequency plasma assisted CVDAdv Eng Mater2008107651656
- SeidlitzEKorbieDMarienLRichardsonMSinghGQuantification of anti-angiogenesis using the capillaries of the chick chorioallantoic membrane demonstrates that the effect of human angiostatin is age-dependentMicrovasc Res200467210511615020201
- NagyJAVasileEFengDVascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesisJ Exp Med2002196111497150612461084
- McDonaldDMBalukPSignificance of blood vessel leakiness in cancerCancer Res200262185381538512235011
- TongRTBoucherYKozinSVWinklerFHicklinDJJainRKVascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumorsCancer Res200464113731373615172975
- AbdollahiALipsonKESckellACombined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effectsCancer Res200363248890889814695206
- KalishwaralalKBanumathiERamkumarpandianSSilver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cellsColloids Surf B Biointerfaces2009731515719481908
- GurunathanSLeeKJKalishwaralalKSheikpranbabuSVaidyanathanREomSHAntiangiogenic properties of silver nanoparticlesBiomaterials200930316341635019698986
- SheikpranbabuSKalishwaralalKVenkataramanDEomSHParkJGurunathanSSilver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cellsJ Nanobiotechnology20097819878566
- BhattacharyaRMukherjeePXiongZAtalaASokerSMukhopadhyayDGold nanoparticles inhibit VEGF165-induced proliferation of HUVEC cellsNano Lett200441224792481
- KarthikeyanBKalishwaralalKSheikpranbabuSDeepakVHaribalaganeshRGurunathanSGold nanoparticles downregulate VEGF-and IL-1β-induced cell proliferation through Src kinase in retinal pigment epithelial cellsExp Eye Res201091576977820833166
- KalishwaralalKSheikpranbabuSBarathManiKanthSHaribalaganeshRRamkumarpandianSGurunathanSGold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via Src dependent pathway in retinal endothelial cellsAngiogenesis2011141294521061058
- ArvizoRRRanaSMirandaORBhattacharyaRRotelloVMMukherjeePMechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface chargeNanomedicine20117558058721333757
- KempMMKumarAMousaSGold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis propertiesNanotechnology2009204545510419822927