Abstract
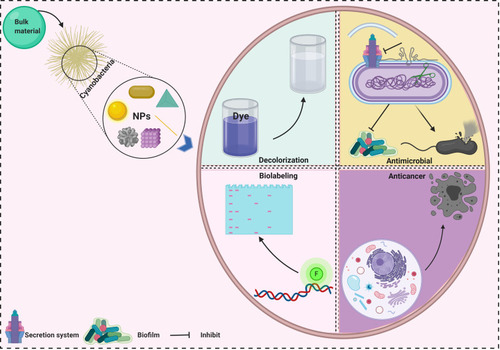
Green synthesis of nanoparticles (NPs) is a global ecofriendly method to develop and produce nanomaterials with unique biological, physical, and chemical properties. Recently, attention has shifted toward biological synthesis, owing to the disadvantages of physical and chemical synthesis, which include toxic yields, time and energy consumption, and high cost. Many natural sources are used in green fabrication processes, including yeasts, plants, fungi, actinomycetes, algae, and cyanobacteria. Cyanobacteria are among the most beneficial natural candidates used in the biosynthesis of NPs, due to their ability to accumulate heavy metals from their environment. They also contain a variety of bioactive compounds, such as pigments and enzymes, that may act as reducing and stabilizing agents. Cyanobacteria-mediated NPs have potential antibacterial, antifungal, antialgal, anticancer, and photocatalytic activities. The present review paper highlights the characteristics and applications in various fields of NPs produced by cyanobacteria-mediated synthesis.
Introduction
Nanotechnology is an emerging field that includes synthesis, characterization, and development of various nanomaterials,Citation1,Citation2 that have significant roles in daily life, providing valuable products that improve industrial production, agriculture, communication, and medicine.Citation3 Currently, around 1000 commercial nanoproducts are available in world markets.Citation4,Citation5 The term “nano” denotes any particles or materials with at least one nanosized (1–100 nm) dimension. Nanoparticles (NPs) differ significantly from their bulk materials in terms of physical, chemical, and biological properties.Citation6 These differences are mainly due to their high surface area to volume ratio, which results in considerable differences in catalytic and thermal activities, melting point, conductivity, mechanical properties, and optical absorption. These properties make NPs applicable in almost all fields.Citation7
NPs have significant roles in bio-diagnostic and optical biosensing, nanophotonics, and imaging and treatment of many diseases affecting human health.Citation8 Silver NPs (Ag-NPs) can interact effectively with microbe surfaces due to their small size and large surface area and are thus used as antimicrobial agents.Citation9 Ag-NPs synthesized using Fusarium Keratoplasticum and embedded in cotton fiber showed significant antibacterial activity against pathogenic bacteria.Citation10 Carbon nanotubes have a key role in drug delivery, because of their capacity to carry drugs and control their release into target cells.Citation11 Quantum dot NPs have been used to detect the location of malignant cells inside the body.Citation12 Iron oxide NPs are used in resonance imaging and diagnosis of tumors.Citation13 Gold NPs (Au-NPs) are in high demand for various applications in multiple fields owing to their low toxicity.Citation14 In addition, Au-NPs have high photothermal and photoacoustic activity, making them suitable for use in photothermal therapy for cancer. Other biogenic NPs such as copper oxide NPs,Citation15 zinc oxide NPs,Citation16 selenium NPs,Citation17 acted as potent anticancer agents. The variation in NP shapes, including spherical, cubic, needles, triangular, rod, etc., enables them to be used in diverse areas such as device manufacture, electronics, optics, and biofuel cells ().Citation18
The physicochemical routes used to produce NPs are often unwieldy, expensive, and result in liberation of toxic byproducts that threaten ecological systems.Citation19 To avoid these drawbacks, green synthesis of NPs using biogenic agents has become an alternative to chemical and physical synthesis.Citation20 Diatoms, mushrooms, algae, plants, fungi, bacteria, actinomycetes, lichen, cyanobacteria, and microalgae have been shown to successfully reduce metal precursors to their corresponding NPs.Citation21 Intra- and extracellular green synthesis techniques have been developed to reduce bulk materials to nanoforms using biological extracts.Citation22 These nanomaterials can be synthesized in the presence of biocompounds such as flavanones, amides, enzymes, proteins, pigments, polysaccharides, phenolics, terpenoids, or alkaloids, to aid the reduction and stabilization of NPs.Citation21 A high surface area to volume ratio is the target physical feature of NPs, as it confers their versatile applicability and ability to withstand harsh conditions.Citation23 The shape and size of NPs synthesize by microorganisms, can be controlled by various abiotic and biotic factors, including pH, temperature, the nature of the microorganisms, their biochemical activity, and interactions with heavy metals.Citation24–Citation26
In the past few years, the synthesis of NPs using cyanobacteria has become an active research field.Citation24,Citation27 Cyanobacteria are a diverse group of photoautotrophic prokaryotes that exist in a wide range of ecosystems.Citation28 They are distinguished by their ability to fix atmospheric nitrogen (N2) by reducing nitrogen gas to ammonia using nitrogen reductase enzymes. This is a significant advantage in their role in biotransformation of metals to NPs,Citation19 as they possess the ability to eliminate heavy metal ions from their surrounding environment. Furthermore, they contain various biomolecules including secondary metabolites, proteins, enzymes, and pigments that confer important biological properties such as antimicrobial and anticancer activity.Citation27,Citation29,Citation30 In addition to these features, cyanobacteria have a high growth rate that facilitates high biomass production. Thus, they represent important nanotechnology-mediated microorganisms that can act as nanobiofactories for NPs.Citation31,Citation32 Although there have been several detailed reviews of biological synthesis using microorganisms, few studies have focused on synthesis of NPs using cyanobacteria.
The current paper comprehensively reviews work on cyanobacteria-mediated fabrication of NPs, their abiotic and biotic conditions used during biosynthesis process including illumination, pH, temperature, type of synthesis process (extracellular and intracellular), followed with the factors influence on the synthesis process of NPs, the corresponding mechanisms of the biological synthesis, applications of NPs in various areas as well as the toxicity strategies of NPs against living cells.
Classification of NPs
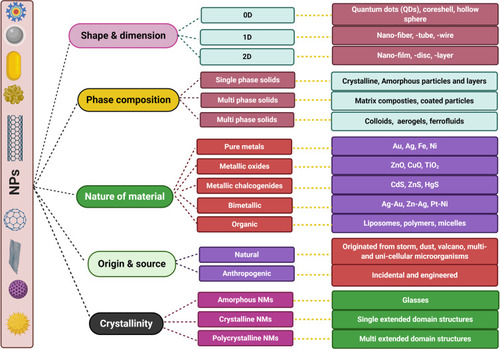
NPs can be classified according to various factors, such as shape and dimension, phase compositions, nature, and origin ().Citation33 For instance, natural NPs include those that exist in the biosphere as a result of fabrication from heavy metals by living organisms, as well as those generated incidentally by forest fires, volcanic eruptions, weathering of rocks, explosion of clay minerals, soil erosions, and sandstorms.Citation34 By contrast, engineered NPs are produced using chemical and physical routes. NPs can also be classified according to their chemical nature. Inorganic NPs include metal and metal oxide NPs such as silver, gold, platinum, titanium oxide, iron oxide, copper oxide, and zinc oxide NPs. Organic NPs include carbon NPs, N-halamine compounds, and chitosan NPs.Citation21
Figure 2 Nanoparticles classifications.
Note: ©2019. Dove Medical Press. Adapted from Ahmad S, Munir S, Zeb N, et al. Green nanotechnology: a review on green synthesis of silver nanoparticles–an ecofriendly approach. Int J Nanomedicine. 2019;14:5087Citation33 and adapted from J Microbiol Methods, 163, Khanna P, Kaur A, Goyal D. Algae-based metallic nanoparticles: synthesis, characterization and applications, page number:105656, Copyright (2019), with permission from Elsevier.Citation19
Synthesis and Characterization of NPs
There are several methodologies for NP synthesis, including conventional methods such as chemical and physical approaches as well as modern methods such as biological synthesis using natural living organisms,Citation25 enzymes,Citation35 vitamins,Citation36 etc.Citation6 The chemical routes are a type of bottom-up fabrication method, in which the atom assembled to nuclei and then grown to NPs.Citation37 In chemical methods, the main components are the precursor metals and the stabilizing and reducing agents. Reducing agents include sodium citrate, ascorbate, and sodium borohydride;Citation38 stabilizing agents include polyvinyl pyrrolidone, starch, and sodium carboxyl methylcellulose.Citation39 Physical synthesis strategies belong to top-down route, in which, the metals are converted to their nanoforms using physical approaches such as laser ablation,Citation40 mechanical milling,Citation41 and sputtering.Citation37,Citation42
The drawbacks of the physicochemical approaches include power consumption, high cost, and a slow production rate, and, most importantly, these processes are not more eco-friendly due to their toxic yields that threaten ecological systems.Citation6,Citation18 Thus, there are global efforts towards development and usage of eco-friendly methods to synthesize NPs.
Green chemistry represents an advanced version of bottom-up nanotechnology approaches used to fabricate NPs.Citation19 But still now, biological synthesis process fights many obstacles to compete physicochemical synthesis such as obtaining uniform shape and size of NPs.Citation43 The aim of the biological synthesis methodology is to utilize natural sources to convert bulk material into nanoscaled particles with unique properties.Citation32 The natural sources used in biofabrication of NPs vary from unicellular to multicellular organisms, moreover; this process can be carried out in the presence of single proteins, enzymes, pigments, etc.Citation21 The unicellular organisms include bacteria,Citation44 cyanobacteria,Citation31 and diatoms,Citation45 while multicellular organisms include algae and plant.Citation46,Citation47 The variety of potential natural sources and biomolecules facilitate the production of NPs with different unique properties. NPs are also subjected to various characterization analyses to assess their physical and chemical properties, including size, distribution, dispersion, stability, charge, and surface morphology.Citation48,Citation49 These analyses use spectroscopic techniques such as ultraviolet (UV)-visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), zeta potential, dynamic light scattering, and nuclear magnetic resonance spectroscopy to determine wavelength ranges,Citation27 and the chemical composition of biocoats surrounding biogenic NPs,Citation31 and to confirm the formation of NPs, as well as characterizing their charge and hydrodynamic diameter.Citation50 X-ray based analyses such as X-ray diffraction (XRD),Citation49 X-ray photo-electron spectroscopy (XPS),Citation23 and energy dispersive spectroscopy (EDAX or EDS) are employed to evaluate composition, structure and crystal phase, structure and crystal phase.Citation49 Microscopic techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution TEM, and atomic force microscopy are used to determine the size and morphological appearance of NPs.Citation43,Citation51 Also, there are several techniques perform to estimate the magnetic properties of NPs such as ferromagnetic resonance (FMR), vibrating sample magnetometer (VSM), etc. ().Citation52,Citation53
Table 1 Techniques Performed to Characterize Nanoparticles
Potential Mechanisms of Green Synthesis Methods
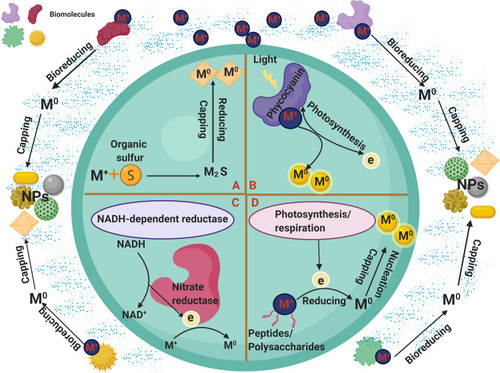
The method of biofabrication of NPs differs from organism to another.Citation54 Moreover, differences are found among different strains of the same species.Citation55,Citation56 Naturally, NPs are synthesized when microorganisms are exposed to toxic substances, in response to which they secrete extracellular substances to capture the material, or mediated through electrostatic interactions.Citation54,Citation57 Mukherjee et al showed that attraction electrostatic forces were among the factors aiding the reduction process of NPs in fungal cells.Citation57 The biotransformation of NPs follows a general pattern wherein metal ions are trapped in the microbial cells or on the microbial surface in the presence of enzymes, and then reduced to form NPs.Citation54 Furthermore, the reduction of NPs by microorganisms can be classified into intracellular and extracellular synthesis ().
Figure 3 Potential mechanisms of green synthesis methods. (A-D) represented different intracellular synthesis methods. (A) Organic sulfur-mediated synthesis of NPs. (B) pigment-mediated synthesis of NPs. (C) Enzyme-mediated synthesis of NPs. (D) Biomolecules-mediated synthesis of NPs.
Abbreviations: M+, metal; M0, reduced NPs; e, electron; S, sulfur, M2S, metal sulfide.
Intracellular Synthesis of NPs
Intracellular formation of NPs refers to cases where the reduction of bulk material into NPs is performed inside host cells under the control of various biological factors.Citation20 Methods for in vivo synthesis of NPs in the laboratory involve three general steps: i) culturing the target microorganisms; ii) the interaction between the precursor solution and living cells; and iii) the separation and purification of NPs from cells for characterization using physicochemical techniques.Citation24 In the latter step, the mixture of living microorganisms and precipitated NPs is centrifuged at 12,000 g. The pellets are then washed multiple times with distilled water for direct examination, and/or the cells are lysed using methods such as sonication to extract NPs for analysis ().Citation58
Figure 4 Potential protocols of intracellular synthesis methods.
Notes: (A) microorganisms are individually cultured along with the bulk material solution and kept under culture conditions. (B) synthesis NPs utilizing algal biomass in the logarithmic phase.
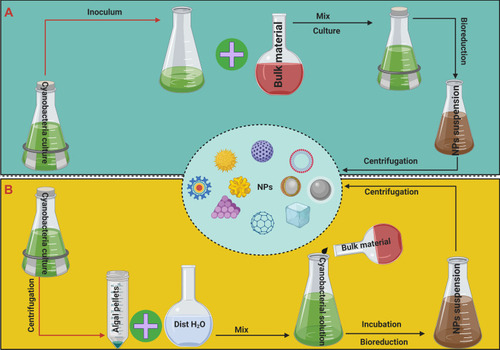
Intracellular interactions between bulk materials and microorganisms can be achieved by two different protocols. The first involves collecting cellular biomass of target microorganisms in the logarithmic phase by centrifugation, then washing this biomass to remove any traces of salts from the medium.Citation59 The cleaned biomass is dissolved in water and mixed with a suitable concentration of precursor material solution. In the second method, microorganisms are individually cultured along with the bulk material solution and kept under culture conditions for 2 weeks.Citation60
In cyanobacteria, ions are intracellularly reduced by electrons produced via the electron transport system, including energy-generating reactions in photosynthesis and, to some extent, in the respiratory electron transport system as a result of the existence of enzymes such as NADH-dependent reductases and redox reactions occur in the cytoplasm, thylakoid membranes, and cell membranes.Citation22,Citation61,Citation62
Lengke et al showed that after reaction between Plectonema boryanum UTEX 485 system and silver nitrate, spherical, octahedral, and anhedral Ag-NPs were precipitated inside cells and in the culture solution.Citation24 They suggested that the silver ions were reduced by an intracellular electron donor or exported by a membrane transporter system. This indicates that the metabolic status (eg, respiration or photosynthesis) and the growth phase of an organism determine its ability to synthesize NPs. Remarkably, P. boryanum was shown to reduce gold(III)-chloride solutions to Au-NPs intracellularly.Citation63 The authors attributed the biorecovery mechanism of P. boryanum strains to either the gold(III)-chloride solution or the acidic medium, which killed cyanobacteria and liberated more organic sulfur, leading to further precipitation of gold particles. The interactions of cyanobacteria or organic material released through the cyanobacterial membrane with gold(III)-chloride enhanced the growth of metallic Au-NPs.
Kalishwaralal et al suggested that nitrate reductase enzymes may help to induce the enzyme responsible for synthesis of Ag-NPs in Bacillus licheniformis.Citation64 Another study showed that nitrogenases (nitrogen-fixing enzymes) may be potent reducing agents for fabrication of gold ions into Au-NPs.Citation65 Shabnam and Pardha-Saradhi reported that thylakoids extracted from Potamogeton nodosus (an aquatic plant) and Spinacia oleracea (a terrestrial plant) may have been responsible for the fabrication of gold ions into Au-NPs during photochemical reactions.Citation66 Dahoumane et al suggested that polysaccharides and other macromolecules may be responsible for internal reduction and stabilization of NPs.Citation67 Other reports have suggested that cyanobacterial pigments act as potent bioreductant molecules that intracellularly reduce bulk material into NPs.Citation68,Citation69
Extracellular Synthesis of NPs
Extracellular synthesis takes place outside the cell, with exuded biological molecules such as pigments, ions, proteins, enzymes, hormones, and antioxidants having a significant role in the reduction process of NPs.Citation70,Citation71 These biomolecules act as reducing and capping agents during the biofabrication process. Various methods exist for extracellular synthesis of NPs, including use of cell-free culture media, cell biomass extracts, and biomolecules ().
Figure 5 Potential protocols of extracellular synthesis methods.
Notes: (A–D) represent different protocols of extracellular synthesis of NPs. (A) cell biomass mediated synthesis of NPs (B) biomolecules mediated synthesis of NPs. (C) cell-free media mediated synthesis of NPs. (D) cell filtrate mediated synthesis of NPs.
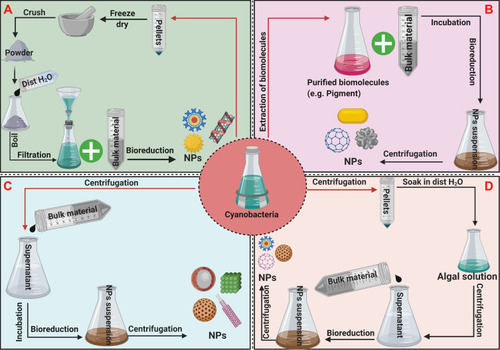
Cell-Free Media
NPs can be synthesized using the culture media of microorganisms after removal of biomass by centrifugation. The filtrated extracellular culture media are mixed with metal precursor solutions under certain conditions to synthesis NPs. Keskin et al tested five cyanobacterial strains and showed that cell-free culture media of Synechococcus sp. were able to synthesis Ag-NPs under light conditions.Citation72 Ag-NPs were also extracellularly synthesized using the supernatant of Pseudomonas sp. THG-LS1.4 strain.Citation73 Many studies have demonstrated the vital role of biocontents such as enzymes, antioxidants, and phenolic and alkaloid compounds present in the cell-free culture in controlling the bioreduction process of NPs. Shivaji et al suggested that biomolecules such as NADH-reductases and sulfur-containing proteins in the cell-free supernatant are involved in biofabrication of metallic NPs.Citation74
Cell Biomass Filtrate
Several reports describe the cell biomass filtrate synthesis approach, in which microorganism cultures are centrifuged and the pellets washed at least three times with distilled water to eliminate any excess metal from the medium.Citation31,Citation32,Citation75 Then, the cleaned pellets are freeze-dried using a lyophilizer or dried in an oven almost at 40 °C, then crushed using a mortar and pestle. The resulting fine powder is mixed with distilled water then filtrated using Whatman filter paper. Finally, the filtrate is mixed with a metal precursor solution and incubated under suitable conditions. In this method, active biomoieties such as proteins and enzymes in the cells filtrate have significant roles in the biofabrication and stabilization of NPs.Citation31 Other route mentioned by Hassan et al in which cell biomass collected at mid-exponential phase and wash several times to exclude any trace elements then soaked in distilled water for specific time. Afterward, the mixture is centrifuged, and the supernatant is used for NPs synthesis.Citation15
Biomolecule-Based NP Synthesis
Pigments
Cyanobacteria, microalgae, actinomycete, algae, etc. are distinguished by the predominance of pigments in their cells, such as carotenoid,Citation76 C-phycocyanin,Citation69 and R-phycoerythrin,Citation77 that are able to synthesis NPs. C-phycocyanin is a blue photosynthetic accessory pigment that forms part of phycobilisomes, structures attached to thylakoids involved in light harvesting and transferring electrons toward photosystem II reaction centers.Citation78,Citation79 It has been shown that C-phycocyanin controls electron transfer and has the ability to bind to heavy metals.Citation80,Citation81 Recent studies have demonstrated the role of C-phycocyanin in fabrication of NPs; Pate et al showed that utilizing C-phycocyanin extracted from two different cyanobacteria species (Spirulina and Limnothrix species) resulted in production of Ag-NPs with different shapes and sizes.Citation55 The authors attributed these differences in morphological features to the fact that the two C-phycocyanin preparations differed in purity and molecular weight. Also, they showed that there was a relationship between the ability of C-phycocyanin to synthesis NPs and the presence of light, as the pigment failed to reduce silver nitrate into Ag-NPs under dark conditions. Wei et al demonstrated the importance of C-phycocyanin extracted from Spirulina as a protective (capping) agent during the reduction process of silver nitrate into Ag-NPs.Citation82 El Naggar et al reported that a proteinaceous pigment phycocyanin isolated from Nostoc linckia reduced silver nitrate into Ag-NPs under direct illumination (2400–2600 Lux) for 24 h at room temperature.Citation69 In another study, phycoerythrin was extracted from Nostoc carneum and used as a reducing agent to synthesize Ag-NPs.Citation83 Mubarak Ali et al successfully synthesized cadmium sulfide (CdS) NPs using C-phycoerythrin extracted from cyanobacteria species Phormidium tenue NTDM05.Citation27 The mechanism by which C-phycocyanin mediates Ag-NPs synthesis is not clear; however, Patel et al suggest that it is similar to the mechanism of the red pigment R-phycoerythrin extracted from red algae, which can synthesize Ag-NPs without the addition of any reductant materials.Citation55
Proteins
Many studies have considered the role of proteins in stabilization and reduction of NPs.Citation20,Citation31,Citation69 Bharde et al showed that cationic proteins secreted by Verticillium sp. and Fusarium oxysporum had hydrolytic activity, which enabled them to reduce and/or cap magnetite NPs.Citation84 Reddy et al found that a bacterial protein of molecular weight between 25 and 66 kDa contributed to the reduction process of Au-NPs, whereas a protein of molecular weight between 66 and 116 kDa was responsible for SNP fabrication.Citation85 Khalifa et al suggested that amino acids and proteins acted as stabilizing and reducing agents in the biofabrication process of metal nanoparticles (MNPs).Citation86 Mubarak Ali et al showed that the presence of amino, carboxyl, sulfate, etc. groups in the amino acid sequence of cyanobacterial proteins facilitates the bioreduction process of extremely small NPs with uniform particle size distribution.Citation87 Govindaraju et al performed extracellular synthesis of silver-gold core shell NPs, in addition to Ag-NPs and Au-NPs, using a single cell protein Spirulina plantensis.Citation8 They identified the FTIR bands at 1653, 1541, and 1242 cm−1 as amide I–III bands of polypeptides/proteins, and suggested that algal proteins played a crucial part in the stabilization process of these NPs. Rahman et al reported that under metal stress, new or modified proteins were expressed to perform dual functions, fabricating precursor materials into CuO NPs and acting as stabilizing agents.Citation88 They showed that a 22 kDa protein of Phormidium was responsible for the reduction of copper sulfate into copper oxide NPs. Rosken et al first detected Au-NPs in the extracellular polymeric material of vegetative cells and the heterocyst polysaccharide layer of Anabaena cylindrica.Citation89
Enzymes
Many studies have investigated the roles of various enzymes, including nitrate reductases and NADH-dependent enzymes in the bioreduction process of NPs.Citation65 Furthermore, Yin et al reported that superoxide dismutase has a vital role in the extracellular production of Ag-NPs using F. oxysporum.Citation54 Mostly, assimilatory type of nitrate reductases is metalloproteins having molybdenum ions as cofactor and catalyzes several reactions in nitrogen, carbon and sulfur cycle. Many studies have demonstrated the importance of these enzymes as a potent reducing agent in the biofabrication process of MNPs.Citation90
Oza et al suggested that nitrate reductase aided the NADH-dependent extracellular synthesis of Au-NPs by Chlorella pyrenoidosa.Citation65 Brayner et al synthesized Au-NPs, Ag-NPs, Pt NPs, and Pd NPs in the presence of cyanobacteria using nitrogenases as reducing agents.Citation91 They chose three strains, Calothrix pulvinate, Anabaena flos-aquae, and Leptolyngbya foveolarum, to form the NPs. C. pulvinate synthesized Pd and Pt NPs after 30 min and 15 days, respectively, Au-NPs after a few hours, and Ag-NPs after 24 h. By contrast, when the same precursor materials and strains were incubated with nitrogenases, Pd and Pt, Au, and Ag NPs were formed after 2 h, 72 h, and 10 min, respectively.
Polysaccharides
Morsy et al used extracellular polysaccharides (EPSs) from Nostoc commune to reduce silver nitrate to Ag-NPs.Citation92 Briefly, they mixed 10 g of dried cyanobacteria with water, suspended the mixture in 100 mL of potassium phosphate buffer (pH 7), and homogenized it in a blender at medium velocity for 10 s, followed by stirring overnight at ambient temperature. The suspension was then homogenized again at least three times for 10 s and left to stand at ambient temperature for 10 min. The upper layer (polysaccharides) was removed using a spatula, and the lower aqueous layer was centrifuged at 6000 x g for 10 min at 20 °C. The pellets were discarded, and the supernatant containing the water-soluble fraction of the EPSs was dialyzed overnight against distilled water and freeze-dried using a lyophilizer. Then, 50 mL of the aqueous EPS solution (10 mg/mL) was added to 30 mM of silver nitrate solution. The reaction was autoclaved at 15 psi and 121 °C for 5 min. To precipitate biogenic Ag-NPs the mixture was centrifuged at 15,000 x g for 30 min at 20 °C. They reported that the EPSs functioned as potent reducing and capping agents, and showed that coating Ag-NPs with EPSs prevented the particles from coming into contacting with oxygen and increased their stability.
Factors Influencing Biofabrication of NPs Using Microorganisms
Many factors should be considered during biological synthesis, including illumination, time of exposure, pH, temperature, and concentration. Control of these factors can facilitate the production of NPs with suitable physicochemical properties.Citation22,Citation67
Illumination
The presence or absence of light has an important role in the biosynthesis of NPs. Some researchers report that light is needed at least to accelerate the bioreduction process for synthesis of NPs.Citation93 Patel et al suggested that illumination conditions are significant factors controlling the intracellular and extracellular synthesis of Ag-NPs using phototrophic cyanobacteria.Citation55 They found that some cyanobacteria could not produce NPs under dark conditions. However, under the same conditions but after exposure to light, the same strains were able to fabricate Ag-NPs from silver nitrate. On the other hand, other strains including Anabaena sp., Limnothrix sp., and Synechocystis sp. were able to produce NPs under both light and dark conditions. This can be explained by the fact that different strains produce different compounds capable of NPs synthesis, only some of which require light activation.Citation55 Another important point related to illumination is the light intensity. Mankad et al reported complete extracellular reduction of silver ions to Ag-NPs after exposure of a mixture of Azadirachta indica leaf extract and silver nitrate solution to sunlight for only 5 min.Citation93
Time of Exposure
The time of reaction varies among different microorganisms, with some able to synthesize NPs within minutes whereas others require weeks. Lengke et al reported that P. boryanum UTEX 485 took 28 days to synthesize Ag-NPs, whereas Zada et al showed that Leptolyngbya sp. JSC-1 fabricated Ag-NPs extracellularly from silver nitrate after 20 min.Citation94,Citation95 A recent study showed that the dispersivity and stability of silver-NPs synthesized using Oscillatoria limnetica changed with increasing time. After 48 h of synthesis, the UV spectrum wavelength value was constant, and after 9 months of incubation, the optical intensity had shifted from 0.71 to 0.599 nm, indicating about the stability of Ag-NPs with low agglomeration.Citation26 The stability of Anabaena-mediated Ag-NPs was evaluated after 6 months of storage by Singh et al.Citation96 The results showed that the Ag-NPs were stable with no change in spectral absorbance, at least in the first 3 months.
pH
El Naggar et al reported that pH significantly affected the size and distribution of Ag-NPs.Citation83 They reported that silver-NPs extracellular synthesis using phycoerythrin was inhibited in acidic media (pH), whereas the reaction was enhanced under alkaline conditions (pH 10). In addition, smaller and more highly dispersed NPs were observed at pH 10, whereas, larger and more aggregated NPs were observed at pH 12.
Temperature
Lengke et al reported the first successful alternative to abiotic chemical approaches for fabricating NPs.Citation97 They screened the ability of P. boryanum UTEX 485 to form extra-, intracellular platinum NPs. The results demonstrated the effects of a variable range of temperatures on NP formation, showing that crystallization and recrystallization of NPs depend on temperature. Decreasing temperature resulted in amorphous distribution, while a crystalline structure was dominant at higher temperatures.
Concentration of Precursors and Natural Reducing Agents
The concentration of bioreductant materials has an important influence on the features of NPs, including shape and size.Citation26 The synthesis of NPs is dose dependent and is also related to the type of algae used.Citation65,Citation67 Hamouda et al showed that increasing the concentration of cyanobacteria Oscillatoria limnetica increased the absorbance of the resulting peaks.Citation26 Moreover, they reported that peaks shifted in position from 420 to 430 nm, accompanied by broadening of peaks, indicating an increase in the size of particles. They also found that the extracellular biofabrication reaction of Ag-NPs was dependent on the dose of silver nitrate. Overall, increasing the concentration of precursor materials resulted in an increased optical intensity recorded by UV spectrophotometry.
Nature of Microorganisms
The nature of microorganisms is related to their biomolecular contents and the types of these molecules, such as polysaccharides, peptides, and pigments.Citation21,Citation87 Thus, different microorganisms vary in their ability to form NPs. Brayner et al screened the reduction activity of three cyanobacteria cultures and observed that the size and shape of NPs varied according to the genus of cyanobacteria used.Citation91 Moreover, different strains from the same species were found to produce NPs with different physicochemical and biological characteristics. Recent studies used different strains from the same Nostoc species, including Nostoc sp. Bahar M,Citation32 Nostoc HKAR-2,Citation98 and Nostoc muscorum NCCU-442,Citation56 resulting in different nanosize ranges (8.5–26.44, 51–100, and 42 nm, respectively) of the resulting Ag-NPs.
Other factors may control NP features, including the microorganisms’ habitats and their ability to eliminate metals from their environment.Citation21 Blue-green algae are distinguished by their ability to accumulate heavy metals from the surrounding environment and degrade these metals into NPs.Citation20 Husain et al showed that 30 cyanobacteria strains isolated from a marine habitat had significant potential to extracellularly reduce silver nitrate to Ag-NPs.Citation56 By contrast, other Eubacteria known to be resistant to heavy metals such as Aphanizomenon sp. could not fabricate Ag-NPs from silver metal under laboratory conditions.Citation55
Cyanobacteria as Biomachinery for NPs Synthesis
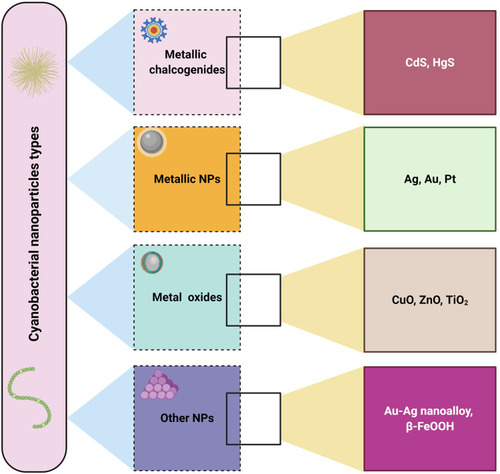
The blue-green algae (cyanobacteria) are the only known oxygen-producing prokaryotes. All cyanobacteria are microscopic and widespread in aquatic environments, with some terrestrial species. The aquatic forms mostly occur in fresh water and few are marine.Citation99 Cyanobacteria contain non-membrane-bound organelles including chloroplasts, unstacked thylakoids, phycobiliprotein pigments, and cyanophycean starch and peptidoglycan matrices or walls. In addition, they are a rich source of biomolecules such as proteins, enzymes, antioxidants, vitamins, and polysaccharides.Citation27 Although there have been many reports of biological synthesis with different micro- or macro-organisms, using plants, algae, bacteria, and fungi to obtain NPs of various sizes and shapes, few studies have used cyanobacteria as the biological machinery for NP fabrication.Citation12 Cyanobacteria are considered to be important biofactories that can be used to synthesize NPs by either extracellular or intracellular methods, owing to their ease of culture at ambient temperature and under atmospheric pressure, their high growth rate, the variable biocompounds inside their cells, their capacity to take up heavy metal ions, and the environmental safety of their products.Citation20 Different studies have demonstrated the capacity of different cyanobacterial strains to form different types of NPs, including metallic NPs such as Ag-NPs and Au-NPs, and metal oxide NPs such as ZnO NPs and CuO NPs, as well as other nanomaterials such as Ag-Au nanoalloys and semiconductor NPs such as CdS NPs ().Citation2,Citation63,Citation99,Citation100
Figure 6 Type of metallic NPs synthesized by cyanobacteria.
Abbreviations: Cds, cadmium sulfide; HgS, mercury sulfide; Au, gold; Ag, silver; Pt, platinum; CuO, copper oxide; ZnO, zinc oxide; β-FeOOH, akaganéite.
Silver Nanoparticles (Ag-NPs)
Silver-NPs are the most commonly used MNPs in various fields and can be found in many industrial and medical products including dressings, surgical instruments and masks, detergents, and care products.Citation101,Citation102 Conventionally, Ag-NPs have been fabricated by chemical and physical approaches. Due to the drawbacks of these routes include their high cost, high energy requirements, and toxic byproducts, green synthesis methods have been developed to produce clean NPs and avoid the harmful effects of physicochemical techniques.Citation6,Citation12 For instances, Kim et al mentioned that the injection rate and reaction temperature are crucial factors to synthesize Ag-NPs using polyol process and a modified precursor injection approach.Citation103 They generated spherical Ag-NPs with nanosize of 17 nm at 100 °C. In contrast, many studies using neutral organisms could synthesis Ag-NPs with smallest size ranged from 10, 10–15, 14.9 nm at room temperature.Citation32,Citation91,Citation104 Also, Jin et al synthesized Ag-NPs utilizing photoinduced route.Citation105 They found that Ag-NPs have triangular shape with size range between 30 and 120 nm. The scholars used chemically stabilizing agents such as citrate and poly (styrene sulfonate) to obtain stable Ag-NPs. While Arthrospira maxima SAE and Arthrospira platensis were able to generate triangular Ag-NPs (61 and 46 nm, respectively) at 30 °C for 24 h without needing to any stabilizing agents.Citation56
Ecofriendly synthesis research has expanded rapidly to discover new microbes with hydrolytic capacity to synthesize NPs. Among these biological entities, blue-green algae provide a straightforward route for producing the desired Ag-NPs. Desertifilum IPPAS B-1220 was explored for the fabrication of Ag-NPs for the first time by Hamida et al,Citation31 who extracellularly synthesized Ag-NPs using a cell biomass extract under direct illumination for 24 h at room temperature. The formation of D-SNPs confirmed by UV-absorption peak at 421 nm. XRD diffraction pattern exhibited five 2 θ degree at 29.7, 38.4, 44.6, 64.7and 77.7 indicates the nanocrystallinity of D-SNPs. FTIR data demonstrated different IR peaks at 601.81 cm−1, 1042.35 cm−1, 1626.05 cm−1, 2353.23 cm−1, and 3453.72 cm−1 for D-SNPs. According to dominant FTIR spectra, the scholars reported the main biomolecules responsible for the bioreduction and stabilization processes were proteins and polysaccharides. Also, they found that Desertifilum IPPAS B-1220 sp. could produce spherical silver-NPs with sizes ranging from 4.5 to 26 nm.
Lengke et al used P. boryanum UTEX 485 for the first time for both intracellular and extracellular synthesis of Ag-NPs.Citation106 They found that Ag-NPs have octahedral and spherical shapes with nanosize ranged from 1 to 200 and 10 nm, respectively. EDS and XPS indicated the association of trace of iron, phosphorus, and sulfur with silver nanoparticles. They reported that the biofabrication process of silver nitrate could be linked with metabolic process using nitrate by reducing nitrate to nitrite and ammonium which is fixed as glutamine before cyanobacterial death. However, liberating of organic materials after bacterial death resulted in precipitation of Ag-NPs in solution. A recent study investigated the hydrolytic activity of a new strain of cyanobacteria, Nostoc sp. Bahar M, to extracellularly fabricate silver-NPs from silver nitrate.Citation32 The authors mentioned that the plasmon resonance of Nostoc-mediated silver-NPs was at 403 nm, indicating about the smaller size of these NPs. Also, XRD pattern confirmed the face-centered cubic crystalline structure of silver-NPs. TEM and SEM showed that silver-NP produced by Nostoc sp. Bahar M was uniformly distributed and spherical in shape, with average nanosize of 14.9 nm. Also, FTIR data exhibited that protein molecules play important role in the biofabrication process of Ag-NPs. Recently, an aqueous extract of Oscillatoria limnetica biomass was also used to synthesize Ag-NPs at room temperature for 18 h.Citation26 The Ag-NPs were spherical in shape and have diverse size ranged between 3.30 and 17.97 nm. Also, the authors studied variable abiotic factors such as pH, time, and concentration of natural reducing materials and silver nitrate to determine the optimal conditions for silver-NP synthesis. They found that the most suitable conditions to produce Ag-NPs from Oscillatoria limnetica are at pH of 6.7 and for 18 h as well as the synthesis process is dose-dependent manner for both silver nitrate and cyanobacterial extract concentrations.
Thirty cyanobacterial species were screened for extracellular synthesis of Ag-NPs.Citation56 The results showed that all 30 cyanobacteria were able to form Ag-NPs from their precursor materials, with variable size ranges. Cylindrospermum stagnale performed the best, producing the smallest Ag-NPs with nanosizes of 38–40 nm. Similarly, Sudha et al screened the Ag-NP fabrication ability of several cyanobacteria species isolated from Muthupet mangroves, including Aphanothece, Oscillatoria, Microcoleus, Aphanocapsa, Phormidium, Lyngbya, Gloeocapsa, Spirulina, and Synechococcus.Citation68 Of all the tested microorganisms, only Microcoleus sp. could reduce silver nitrate and form spherical Ag-NPs, with an average diameter of 55 nm.
Patel et al performed both extracellular and intracellular synthesis of Ag-NPs with light and dark incubation using different cyanobacterial isolates, including Anabaena sp., Aphanizomenon sp., Cylindrospermospsis sp. 121–1, Cylindrospermopsis sp. USC CRB3, Lyngbya sp., Limnothrix sp., Synechocystis sp., and Synechococcus sp. With the exception of Aphanizomenon sp., all tested cyanobacteria reduced silver nitrate into Ag-NPs under direct light exposure using either cell-free culture liquids or cell biomass extracts. In addition, only the cell biomass extracts of Anabaena sp., Limnothrix sp., and Synechocystis sp. were able to synthesis Ag-NPs under dark incubation. Only Aphanizomenon sp. failed to tailor the bulk material into its nanoform ().Citation55
Table 2 Cyanobacteria-Mediated Synthesis of Silver Nanoparticles (Ag-NPs)
Gold Nanoparticles
Au-NPs are known to be good heat conductors and low toxicity NPs against normal cells that are suitable for use in photothermal therapy to treat cancer.Citation107 As well as physiochemical methods, biological synthesis can be used to synthesize Au-NPs in an ecofriendly manner. The first investigation of the ability of Anabaena laxa to catalyze the conversion of hydrogen tetrachloroaurate (III) into Au-NPs was performed by Lenartowicz et al.Citation108 They reported that A. laxa were able to intracellularly fabricate Au-NPs with diverse morphologies and sizes using three different concentrations of auric chloride (HAuCl4; 0.1, 0.5, and 1 mM). The authors reported that the maximum UV spectra peak was formed at 545 and 560 nm for 0.5 and 1 mM of HAuCl4. Three 2θ degree (38.2°, 44.3° and 64.6°) were detected by X-ray diffraction analysis indicating the crystallinity of Au-NPs. Also, TEM micrographs showed that the Au-NPs appearing at 0.5 mM were spherical, however, few NPs with other shapes (triangular, hexagonal, and irregular) were observed. The size of these NPs ranged from 0 to 30 nm; however, the largest particles have size range from 30 to 100 nm. At higher concentration (1 mM of HAuCl4), triangular, hexagonal, and irregular shapes of Au-NPs appeared with nanosize range from 20 to 50 nm. In addition, living cyanobacteria cultures were able to form Au-NPs more efficiently than dead ones, indicating a role of metabolic activity in the bioreduction process of Au-NPs.
Similarly, Dahoumane et al and Brayner et al demonstrated the bioreduction ability of A. flos-aquae to intracellularly synthesize Au-NPs.Citation22,Citation91 Rosken et al showed that A. cylindrica and other Anabaena sp. reduced gold ions to Au-NPs without releasing toxic anatoxin-a.Citation89,Citation109 They mentioned that A. cylindrica were able to intracellularly produce Au-NPs after 4 h under direct illumination incubation. XRD pattern of Au-NPs showed that these NPs have ellipsoidal shape with an aspect ratio of 1.15. The biosynthesized Au-NPs were spherical, and their average size was 10 nm. Moreover, TEM micrographs showed that the Au-NPs mainly distributed in vegetative cells (50%) in comparing with heterocysts and found to be located along the thylakoid membranes. The scholars suggested that heterocysts are not as important for NPs fabrication, due to their results showed that more than 10 vegetative cells contained Au-NPs while only one heterocysts contained NPs. The bioreduction activities of three species of cyanobacteria, Phormidium valderianum, P. tenue, and Microcoleus chthonoplastes, were screened against HAuCl4 solution at different pH values by Parial et al.Citation14 They reported that all three tested cyanobacterial strains were able to synthesis Au-NPs; however, only P. valderianum was able to produce Au-NPs of different shapes and sizes, including nanospheres (15 nm) and triangular (24 nm) and hexagonal (25 nm) NPs, in response to variations in pH. Gloeocapasa sp. were exploited for intracellular synthesis of Au-NPs using cleaned whole cells;Citation110 90 mL of 10−3 HAuCl4 solution was mixed with 10 mL of Gloeocapasa culture and incubated with stirring at ambient temperature. After a few hours, Au-NPs had formed, and their UV absorbance was 547 nm. Moreover, FTIR results showed that algal proteins were responsible for the bioreduction process that formed these NPs, as strong peaks were observed at 3424.96 cm−1, corresponding to N-H stretching, and at 1640.16 cm−1, corresponding to amide I. In addition, SEM and TEM micrographs showed that the Au-NPs had spherical shape and diameters of less than 100 nm ().
Table 3 Cyanobacteria-Mediated Synthesis of Gold Nanoparticles (Au-NPs)
Other Nanomaterials
The ability of cyanobacteria to reduce metallic NPs is not limited to Ag-NPs and Au-NPs, but extends to other metals including platinum, palladium, and cadmium, as well as metal oxides including copper oxide and zinc oxide. Lengke et al screened the ability of P. boryanum UTEX 485 to form platinum and palladium NPs under variable temperatures.Citation96,Citation111 The scholars mentioned that Pd NPs formed after incubation with P. boryanum for 28 days at 25–100 °C. TEM micrographs of Pd NPs showed that these particles have spherical and elongated shapes, but at 100 °C only spherical palladium hydride appeared. XRD analysis of Pd NPs synthesized at 100°C and 60°C showed different peaks at a Bragg angle (2θ) of 46.9, 54.5, and 81.2 indicates the nanocrystalline of Pd NPs, however, at 25 °C there no diffraction peaks detected. Similarly, different physicochemical analysis of Pt NPs synthesized by P. boryanum UTEX 485 at 25–100 °C for 28 days and at 180 °C for 1 day were performed by Lengke et al XPS results showed the association of sulfur, phosphorus and nitrogen with platinum(II), and EDX analysis indicated the present of sulfur with platinum. So, the authors speculated that these organic elements play significant role in the reduction process of platinum ions into Pt NPs. Brayner et al intracellularly synthesized Au-NPs, Ag-NPs, Pt NPs, and Pd NPs utilizing the biomass of C. pulvinata, A. flos-aquae, and L. foveolarum.Citation91 They suggested that polysaccharides were responsible for NP stabilization.
CdS NPs are well-known and widely studied semiconductors. They have been synthesized using various microorganisms, including yeasts, algae, and fungi.Citation112–Citation114 C-phycoerythrin (CPE) pigment extracted from P. tenue was also used to synthesis 5 nm CdS NPs from their precursors, using an aqueous mixture of CdCl2 and Na2S ().Citation27 The CdS NPs were formed after incubation with C-phycoerythrin pigment for 24 h at 37 °C. The formation of CdS NPs was confirmed by the absorption peak at 470 nm. FTIR analysis demonstrated new bands at 1543, 1364, 1223 cm−1 appeared only in CPE-coated CdS NPs indicates a new vibration due to C\S band. Other bands seen at 1364 and 1034 cm–1 was related to the C-N stretching vibrations of the aromatic amino amines. Furthermore, EDAX analysis confirmed the existence of both Cd and S in biosynthesized CdS NPs.
Table 4 Cyanobacteria-Mediated Synthesis of Other Nanomaterials (NMs)
Metal Oxides and Akaganéite NPs
Nostoc sp.Citation99 and Anabaena sp.Citation95 have been used to generate ZnO NPs, whereas copper oxide (CuO) NPs were produced by Phormidium cyanobacterium and S. plantensis respectively ().Citation88,Citation100 Brayner et al synthesized beta-iron oxyhydroxide (β-FeOOH) NPs using A. flos-aquae, C. pulvinata, and Klebsormidium sp.Citation115 They found that there were two basic mechanisms by which iron was acquired higher plants, denoted strategy I and strategy II. Strategy I involves soil acidification and formation of lateral roots and specific transfer cells in the rhizodermis, as well as induction of an Fe3+-chelate reductase and of Fe2+ transporters. This strategy was used in all plants except grasses. Grasses followed strategy II, in which iron is taken up as a Fe3+-siderophore complex. Based on these observations, the authors proposed that the mechanism of iron oxide fabrication by cyanobacteria followed strategy II (siderophore-based iron acquisition). Owing to limited availability of iron, the cells released organic Fe3+ metal-chelating molecules (siderophores) that could solubilize and scavenge iron ions from the environment and internalize them into the cells. Similarly, Dahmoune et al biologically generated akaganeite NPs using A. flos-aquae.Citation116 They showed that XRD analysis of freeze-dried cyanobacteria after reaction with iron salts exhibited the existence of tetragonal akaganeite NPs with some amorphous phase related to the cyanobacteria biomass. TEM ultrathin sections of treated cyanobacteria with iron precursors showed distribution of dark β-FeOOH akaganeite nanorods inside the bacterial cells. However, HR-TEM provided more information about the distribution pattern of akaganeite NPs inside the cells, in which the nanorods appeared as a complex arrangement of pores forming a spongelike structure.
Bimetallic NPs
Bimetallic NPs include a combination of two different metals, the ratio of which can be varied to provide novel physicochemical features derived from the constituting metals (). Citation8,Citation12 These NPs are attractive for various applications due to the extra degree of freedom they afford. Govindaraju et al used extracellular synthesis to produce silver-gold core shell NPs, in addition to Ag-NPs and Au-NPs, using single-cell protein S. plantensis.Citation8 The spectral wavelength of silver-gold bimetallic NPs was 509 nm and their size was 17–25 nm. Roychoudhury et al exposed Lyngbya majuscula to an equimolar solution of gold and silver (1 mM, pH 4) for 72 h to produce an Au-Ag nanoalloy.Citation117 They reported that Lyngbya sp. acted as environmentally friendly factories for Au-Ag nanoalloy fabrication. The UV absorbance of the nanoalloy was 481 nm, with a nanosize ranging from 5 to 25 nm.
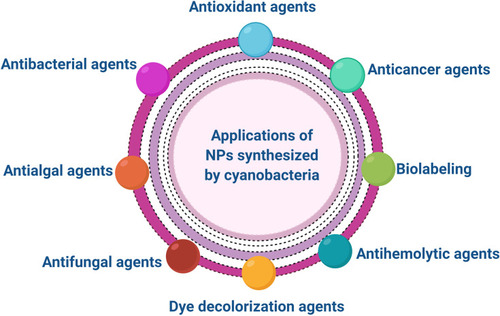
Applications of NPs Synthesized by Cyanobacteria
Many studies demonstrated the bioactivity of NPs synthesized by different strains of cyanobacteria. These bioactivities are including anticancer, antimicrobial, antioxidant activities ().
Antitumor Activity
Cancer is among the leading causes of death in humans.Citation118 The incidence of cancer is increasing because of various factors, including aging, an increasing population, and lifestyles that predispose to individuals to the disease.Citation118,Citation119 Advances in nanoscience have led to new methods to prevent tumor progression and metastasis.Citation31,Citation32 For example, magnetic iron oxide NPs have improved cellular imaging,Citation13 increasing the quality and speed of diagnostic process. Intravenous injection of Au-NPs can enhance radiotherapy (X-rays), with increased survival of test mice (86%) compared with those treated with X-rays alone (20%).Citation120 There have been many reports of the biological synthesis of NPs using cyanobacteria and their activities against different cancer cell lines (–). Green Ag-NPs synthesized by novel Desertifilum sp. induced a drastic loss of viability in HepG2 liver, MCF-7 breast, and Caco-2 colon cancer cell lines, with half-maximal inhibitory concentration (IC50) values of 32, 58, and 90 µg/mL, respectively,Citation31 suggested that they may have applications as potent anticancer agents. Bin-Meferij et al reported that Nostoc-mediated silver-NPs (N-SNPs) reduced Caco-2 cell viability by 50%, with an IC50 of 150 µg/mL.Citation32 The authors mentioned that N-SNPs resulted in morphological changes including cell shrinkage, clustering, cell detachment. They speculated that the cytotoxic effect of N-SNPs toward Caco-2 cells resulted from interaction of N-SNPs with different organelles such as mitochondria and endoplasmic reticulum as well as biomolecules such as DNA and proteins. Similarly, a recent study assessed the anticancer activity and biocompatibility of silver-NPs formed by Nostoc sp. against normal and tumor cells.Citation83 Ag-NPs suppressed the proliferation of MCF-7 cancer cells seven-fold compared with normal human lung fibroblast (WI38) and human amnion (WISH) cells. By contrast, the same concentration (13.07 ± 1.1 µg/mL) of a standard antitumor drug, 5-fluorouracil, had a greater cytotoxic effect against WI38 and WISH cells than against malignant cells. Thus, green Ag-NPs appear to be safer to normal cells than 5-fluorouracil. Moreover, the scholars exhibited that after injection of mice bearing Ehrlich ascites carcinoma with 5 mg of Ag-NPs lead to significant reduction in the tumor volume, tumor cells, WBCs count and body weight. Roychoudhury et al found that biosynthesized, ecofriendly, highly stable Ag-NPs showed appreciable antiproliferative effects against leukemic cells, especially the lymphoblastic leukemia (REH) cell line.Citation121 The Ag-NPs (100–1000 µg/mL) showed no effect on peripheral blood mononuclear cells. However, at the same concentrations of Ag-NPs, REH cell line was the most sensitive toward Ag-NPs than other leukemia cell lines including myeloblastic leukemia and lymphoblastic leukemia with IC50 of 620 ± 3.73, 1000 ± 2.79 and 1000 ± 4.38 µg/mL, respectively. Also, the scholars studied the nuclear morphological changes of REH treated with Ag-NPs using DAPI staining assay and observed that Ag-NPs resulted in nuclear damage, shrinkage, chromatin condensation and chromatin margin. A recent publication reported that Anabaena sp. could produce Ag-NPs with strong antitumor activities against Dalton’s lymphoma and colo205 cancer cells.Citation122 They found that low concentration of SNP was able to reduce the proliferation of both cell types via inducting the production of reactive oxygen species (ROS) and DNA damage led to cell death. The antitumor properties of Ag-NPs biosynthesized by Nostoc sp. HKAR-2 were screened against the MCF-7 cell line.Citation97 The Ag-NPs killed tumor cells effectively as their concentrations increased, suggesting potential applications in the treatment of cancerous cells. Ebadi et al found that a 50 µg/mL concentration of ZnO NPs eliminated 50% of cancerous A549 lung cancer cell line, while the same concentration killed less than 50% of normal (MRC-5) cells.Citation99
Antioxidative Activity
Singh et al tested the toxicity of biogenic ZnO NPs fabricated by cell extracts from Anabaena strain L31 and biogenic ZnO NP–shinorine conjugates in vivo.Citation95 They monitored the formation of ROS using DCFH-DA as a probe. Under a fluorescence microscope, they observed that ROS reached maximum activity 12 h after incubation with ZnO NPs, in contrast to control cells incubated with DCFH-DA alone. By contrast, ROS production decreased in cells spiked with the ZnO NP–shinorine conjugate. The authors suggested that biogenic ZnO NP–shinorine conjugates have antioxidative properties and could be used as non-toxic sunscreen agents. MubarakAli et al showed that Au-NPs synthesized by Phormidium sp. exhibited stronger antioxidant properties than citric acid.Citation2 They also found that increasing the concentration of Au-NPs increase their scavenging activity ().
Antimicrobial Activity
Antibacterial Activity
NPs are attractive for medical applications due to their high surface area to volume ratio.Citation12 Smaller NPs have a large surface area that increases the interaction between NPs and target bacterial cells.Citation123 Keskin et al reported that Ag-NPs synthesized by Synechococcus sp. have antibacterial properties against various bacterial strains, including Bacillus subtilis, Staphylococcus aureus, and Escherichia coli, of which B. subtilis was the most sensitive to biogenic Ag-NPs.Citation72
Hamida et al found that 1.54 mg/mL of silver-NPs synthesized from Desertifilum sp. (D-SNPs) had significant antibacterial activity against five strains of Gram-negative and Gram-positive bacteria: Pseudomonas aeruginosa, Bacillus cereus, B. subtilis, Shigella flexneri, and Salmonella enterica (clinical isolates).Citation31 S. flexneri showed the greatest response to D-SNPs, with an inhibition zone (IZ) diameter of 22.7 ± 0.3. In another recent study, E. coli, Salmonella typhimurium, drug-resistant Klebsiella pneumoniae, Streptococcus mutans, and methicillin-resistant S. aureus (MRSA) (clinical isolates) were treated with 1.5 mg/mL of an aqueous solution of silver-NPs synthesized by Desertifilum sp.Citation123 The D-SNPs were more effectual antibacterial agents than silver nitrate against all tested bacteria; MRSA showed the greatest response to D-SNPs, with an IZ of 23.7 ± 0.08. Treating MRSA with D-SNPs also resulted in ultrastructural changes, including bacterial membrane disruption, cytoplasmic lashing, and atrophy of cells leading to bacterial cell death. Also, the authors suggested that the potential mechanism of D-SNPs against MRSA via direct interaction between NPs and biomolecules such as proteins, enzymes, antioxidants and nucleic acids or/and indirect interaction through enhancing the generation of ROS leading to bacterial death. Ag-NPs (5mM) synthesized by phycocyanin extracted from N. linckia showed significant inhibitory activity against various pathogenic bacterial strains, including S. aureus, P. aeruginosa, E. coli, and K. pneumoniae.Citation69 The highest IZ diameter (10 mm) was observed in both P. aeruginosa and E. coli, while the IZ diameter of S. aureus and K. pneumoniae was (9 mm). The authors reported that Ag-NPs produced by N. linckia has ability to suppress the Gram-positive and Gram-negative bacteria.
In a recent study, the inhibitory activity of silver-NPs synthesized utilizing Nostoc sp. (N-SNPs) was assessed against drug-resistant K. pneumoniae.Citation124 The results demonstrated for the first time the relationship between biogenic silver-NPs and genes related to a bacterial secretion system. N-SNPs resulted in downregulation of the hns, hcp-1, VgrG-1, and VgrG-3 genes responsible for inducing the secretion system. Interactions of Au-NPs with Gram-positive organisms (B. subtilis and S. aureus) were investigated using TEM. The TEM micrographs of B. subtilis showed that the Au-NPs damaged the bacterial cell wall, resulting in pits at the exterior of the cell and deformation of the cell membrane, thereby disrupting bacterial functions such as respiration and permeability.Citation125
The antibacterial activity of Ag-NPs produced by Oscillatoria sp. was observed against pathogens E. coli, Klebsiella sp., Salmonella sp., and Pseudomonas sp. using the disc diffusion method. The maximum antibacterial effects were observed against Pseudomonas sp. (15 mm), and a minor antibacterial effect was recorded against E. coli (10 mm), indicating resistance to Ag-NPs.Citation126 Ag-NPs synthesized from Anabaena sp. showed strong antibacterial activity,Citation122 which was tested against three multidrug-resistant bacteria, K. pneumoniae, E. coli, and S. aureus, isolated from foot ulcers of diabetic patients. Sonker et al investigated the inhibitory activities of silver-NPs synthesized using a cyanobacterial extract of Nostoc sp. against two plant-pathogenic bacteria, Ralstonia solanacearum and Xanthomonas campestris.Citation97 The result showed that Ag-NPs (5, 10, 15 µg/mL) lead to suppress the growth of two bacterial strains with IZ ranges of 15 ± 4.5, 25 ± 4, 25 ± 5 and 18 ± 1.5, 23 ± 4, 23 ± 10, respectively.
Patel et al found that Ag-NPs synthesized from different species of cyanobacteria had bactericidal activity against Bacillus megaterium, E. coli, B. subtilis, S. aureus, P. aeruginosa, and Micrococcus luteus.Citation55 They also reported that all tested bacteria showed resistance to Ag-NPs fabricated by Limnothrix sp., while SNP synthesized by Anabaena sp. was the most significant one, with an IZ of 22 mm. Ahmed et al reported that Ag-NPs synthesized from Spirulina sp. and Nostoc sp. suppressed bacterial growth of the tested bacteria (including S. aureus, Staphylococcus epididymis, K. pneumoniae, E. coli and P. aeruginosa) more strongly than silver nitrate,Citation127 and that P. aeruginosa resisted Ag-NPs synthesized by two isolates.
Roychoudhury et al reported that the synthesized nontoxic, highly stable Ag-NPs synthesized by Lyngbya majuscula exhibited significant biocidal activity against P. aeruginosa.Citation121 The authors subjected the P. aeruginosa with 50 µL of different concentration of Ag-NPs including 1, 0.5, and 0.1 mg/mL for 24 h at 37 °C. The maximum IZ (8 mm) was resulted from treating P. aeruginosa with 0.5 mg/mL of Ag-NPs. Another study found that Ag-NPs synthesized by Nostoc sp., Scytonema sp. and Phormidium sp. were potent antibacterial agents against various pathogens including S. aureus, MRSA, P. aeruginosa, E. coli.Citation75 Seventy microliters of the Ag-NPs synthesized by Nostoc sp., Scytonema sp. and Phormidium sp. inhibited the bacterial growth of all previous tested microbes with IZ diameter of (10, 2, 9, 9 mm), (7, 12, 9, 9.5 mm) and (9, 11.5, 8, 10 mm) respectively. Nostoc-mediated ZnO NPs showed antibiofilm activity against P. aeruginosa, and S. aureus. Atomic force microscopic analysis showed that ZnO NPs destroyed the biofilms of the tested bacteria such that they protruded outward or were sunken inwards (–).Citation99
Antialgal Activity
El-Sheekh et al studied the inhibitory activity of Ag-NPs synthesized by S. platensis, Chlorella vulgaris, and Scenedesmus obliquus against Microcystis aeruginosa.129 M. aeruginosa are well known to be harmful microalgal, which secrete toxins that induce liver cancer. The results of this work encouraged the use of Ag-NPs as antialgal agents to reduce the harmful effect of M. aeruginosa. As the concentration of Ag-NPs increased, the viability of Microcystis cells decreased; at 10 mg/L, Ag-NPs caused a 92% reduction of viable cells, compared with a 30% reduction at 1 mg/L ().
Antifungal Activity
A recent study showed that Ag-NPs produced by Nostoc sp. HKAR-2 resulted in inhibition of Aspergillus niger and Trichoderma harzianum fungal activity with IZ ranges of 3–5 and 4–5, respectively.Citation97 Morsy et al studied the antifungal activity of Ag-NPs synthesized by EPSs of N. commune against different fungal strains infecting seeds of sorghum, maize, soybean, and sesame.Citation92 They sterilized the grain surface with Ag-NPs then grew the seeds in Dichloran chloramphenicol peptone agar at 25 °C by the direct plate method. Complete inhibition of Fusarium nygamai was observed on sorghum grains and soybean seeds with Ag-NP sterilization, compared with 17.24% and 14.63% of total Fusarium and 7.5% and 8.7% of total fungi with NaOCl sterilization, respectively ().
Photocatalytic Activity
Keskin et al reported that Ag-NPs produced from Synechococcus sp. had photocatalytic activity.Citation72 These Ag-NPs caused photodegradation of methylene blue; after 4 h, the decolorization of the organic pigment reached 18 ± 0.3% for 20 mg L−1 of aqueous dye solution. Similarly, Ag-NPs fabricated by Microchaete sp. NCCU-342 degraded methyl red dye (84.60%) within 2 h more effectively than cyanobacterial extract (49.80%) ().Citation128
Other Applications
Bakir et al and Younis et al reported that Au-NPs synthesized by L. majuscule have anti-myocardial infarction properties.Citation129,Citation130 They showed that Au-NPs alone or with cyanobacterial extract inhibit the isoproterenol-induced changes in serum cardiac injury markers, ECG, arterial pressure indices, and antioxidant capabilities of the heart. Also, Ag-NPs synthesized by phycobiliproteins extracted from Nostoc sp. showed antihemolytic activity.Citation83 The authors used 2, 2ʹ-azobis (2-amidinopropane) dihydrochloride (AAPH) to induce complete erythrocyte hemolysis (100%) of blood extracted from a rat. They mixed the mixture with Ag-NPs and observed that Ag-NPs inhibit hemolytic activity with inhibition of 96.4% (erythrocyte hemolysis was decreased to be 3.6%) which was similar to those caused by vitamin C (96%). Also, the scholars mentioned that the possible mechanism of antihemolytic activity of Ag-NPs is due to Nostoc material (such as amino compounds (-NH2), phenols (Ar-OH)) capped Ag-NPs which have the antihemolytic and antioxidants activity.
MubarakAli et al showed that C-phycoerythrin-CdS NPs could be used as fluorescence probes in biolabeling for ultrasensitive detection of DNA.Citation27 Similarly, Au-NP synthesized by Phormidium willei and Coelastrella sp. was proven to be used as a biolabeling by MubarakAli ( and ).Citation2 The scholars showed that at higher concentration, the green Au-NPs synthesized by Coelastrella sp only were actively binding to DNA.
Killing Strategies of NPs Against Living Cells
The exact killing mechanism of NPs against living cells such as microbial and cancer cells is still under investigation. However, several studies attributed the toxic effect of NPs against cells to two general strategies: (i) indirect influence in which NPs enhancing the production of ROS results in intensive oxidative stress leading to DNA damage, protein degradation, imbalance in enzyme activities resulting in metabolic toxicity and cellular dysfunctions that finally cause cell death;Citation131,Citation132 (ii) direct influence in which NPs directly interact with cellular structures (such as cellular borders and organelles) as well as cellular components (such as proteins, enzymes, antioxidants, nucleic acids, etc.) led to activate cell death pathways ().Citation123,Citation133
Figure 8 The killing strategies of NPs against living cells. (A) illustrating the direct influence of NPs, in which NPs interact directly with cellular structures and biomolecules led to cell death. (B) illustrating the indirect influence of NPs, in which NPs inducing the generation of reactive oxygen species (ROS) led to cell death.
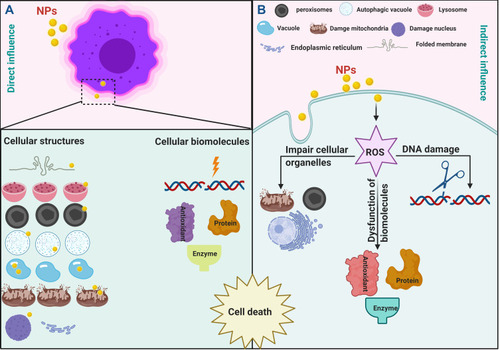
Oxidative stress is the dominant key of NP-induced cellular injury. Different factors control the oxidative stress-mediated by NPs including acellular factors (such as particle size, concentration and the chemistry of their surface) and cellular responses (such as mitochondrial respiration, NP-cellular interaction and immune cell stimulation) are responsible for ROS-mediated damage. The induction of oxidative stress by NPs is torch bearers for further pathophysiological influences such as genotoxicity, inflammation, proteins denaturation, enzyme and antioxidants dysfunction demonstrated by stimulation of associated cell signaling pathways causing cell death.Citation132
Many scholars reported that NPs stimulate the production of ROS such as superoxide anion (O2∙−), hydrogen peroxide (H2O2), hydroxyl radicals (OH∙), etc.Citation132,Citation134 This abundance of reactive oxygen species have the potentiality to impair the cellular functions causing oxidative stress. It results from the imbalance between ROS production and the capacity of cells to detoxify or repair the damage caused by free radical intermediates.Citation135 For illustration, the decrease in GSH level after exposure the cells to NPs lead to accumulation of ROS causing oxidative stress.Citation124 Similarly, the imbalance in antioxidant enzymes such as GPx,Citation123 superoxide dismutase, catalase, etc., results in oxidative stress causing cellular dysfunction and led to cell death.Citation136 On the other side, other studies suggested that the direct interaction between NPs and cellular targets (such as mitochondrial respiration, metabolism, inflammation, etc.) results in direct induction of oxidative stress.Citation124,Citation132,Citation137
In prokaryotic and eukaryotic cells, cellular membranes are the protective barriers against the external stress and controlling the transport of nutrients in/out the cells.Citation37 When NPs injected to the cells, it was observed that these particles attracted to cellular membranes may be due to charge of NPs and their surface chemistry.Citation123,Citation138 This direct interact between NPs and cellular membranes results in irregular, folded, pored membranes and sometimes causes completely lysed membranes affecting the membrane integrity and permeability, causing cell death.Citation138,Citation139 TEM micrographs taken by Hamida et al showed that N-SNPs attached to plasma membranes of MRSA, K. pneumoniae.Citation123,Citation124
After NPs well ingested into cells, result in cytoplasmic dissolution and nuclear damage including nucleoagglomeration, chromatin condensation and DNA damage.Citation133 On the other hand, NPs impaired with biomolecules including enzymes (such as adenosine triphosphatase (ATPase) causing metabolic toxicity),Citation123 antioxidants and antioxidant enzymes (such as GSH, GPx result in induction of oxidative stress),Citation124 proteins (such as membrane proteins causing protein denaturation led to influence biological activities),Citation123 nucleic acids (such as DNA causing DNA damage),Citation140,Citation141 enhancing cellular death pathways.
A recent study demonstrated the direct interaction between NPs and cellular organelles such as LDs, endosomes, mitochondria, etc.Citation142 Barkhade et al showed that cells treated with titanium dioxide NPs exhibited swallowing mitochondria and influence the mitochondrial membrane permeability.Citation143 Also, it was found that Au-NPs increase number of mitochondria and decrease the number of lipid droplets indicating that cells produce more energy in trial to remove the stress caused by NPs.Citation142 Furthermore, the high incidence of, autophagic vacuoles, endosomes, cytoplasmic vacuoles were observed after treatment cancer cells with Ag-NPs.Citation142,Citation144 Another study showed that Ag-NPs and copper NPs resulted in increasing lipid droplets that may be return to nanotoxicity that impaired the lipid catabolic activity. The authors reported also that high incidence of peroxisomes as well as lysosomes and low distribution of endoplasmic reticulum indicating necrosis.Citation144
Future Remarks
Green nanotechnology is considered a promising domain of modern science. This technology facilitates the synthesis of NPs by ecofriendly and inexpensive processes, resulting in high yields. Various natural resources are used to biosynthesize NPs, including plants, fungi, bacteria, cyanobacteria, and algae. However, some aspects still require more detailed investigation; for instance, many cyanobacterial strains remain unexplored. Further studies are needed to discover the biocatalytic activities of these cyanobacteria and their abilities to biotransform precursor metals into nanoforms.
Although many hypotheses have been proposed, the exact mechanism of NP synthesis using living organisms has not yet been elucidated. Thus, it will be necessary to further explore how microorganisms synthesize NPs and determine why different strains from the same species result in morphologically diverse NPs. Furthermore, studying the effects of biotic and abiotic conditions such as electrostatic force, metabolic status, growth conditions, and biomolecules (enzymes, pigments, proteins, etc.) on the green fabrication process will enable control of the physicochemical and biological properties of NPs. The emergence of serious diseases that threaten human health will motivate research to screen the activity of green NPs in various medical domains, including developing new antimicrobial and anticancer agents. In this respect, this review recommends that future studies focus on the cyanobacteria-nanotechnology field.
Conclusions
The use of cyanobacteria as bio-fabrication machinery to synthesize NPs has tremendous value, as it enables safe eco-friendly synthesis, as well as being inexpensive and saving energy, and produces novel NPs with varied shape and size. Cyanobacteria-mediated NPs have various biological, physical, and chemical features that provide versatile applicability. Many studies have shown that these NPs can serve as antimicrobial, anticancer, photocatalytic agents, etc. Cyanobacterial strains such as Desertifilum sp. and Nostoc sp. have been screened and used to synthesize Ag-NPs with significant anticancer activity against various cancer cell lines. Furthermore, Ag-NPs and Au-NPs fabricated using Spirulina sp. and Anabaena sp. show broad-spectrum inhibitory activity against Gram-positive and Gram-negative bacteria. Development and improvement of biosynthetic processes using cyanobacteria may lead to the discovery of new biogenic NPs with unique properties to serve various needs. Their incorporation into medical treatments could increase efficacy and diminish side effects ().
Highlights
The use of cyanobacteria as bio-fabrication machinery to synthesize NPs has tremendous value, as it enables safe eco-friendly synthesis, as well as being inexpensive and saving energy, and produces novel NPs with varied shape and size.
Cyanobacteria-mediated NPs have various biological, physical, and chemical features that provide versatile applicability.
Development and improvement of biosynthetic processes using cyanobacteria may lead to the discovery of new biogenic NPs with unique properties to serve various needs.
Abbreviations
NPs, nanoparticles; SNPs, silver nanoparticles; AgNO3, silver nitrate; nm, nanometer; min, minute; µL, microliter; mg, milligram; mL, milliliter; µg, microgram; mM, millimolar; h, hour; cm−1, inverse centimeter; rpm, revolutions per minute, SEM, scanning electron microscope; TEM, transmission electron microscope; DLS, dynamic light scattering; FTIR, Fourier-transform infrared spectroscopy; XRD, X-ray diffraction; kV, kilovolt; kb, kilobase; SPR, surface plasmon resonance; UV-vis, ultraviolet–visible spectroscopy.
Data Sharing Statement
The data supporting this article are available in – and –. The data sets analyzed in the present study are available from the corresponding authors on reasonable request.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Disclosure
The authors report no conflicts of interest in this work.
References
- Dubchak S, Ogar A, Mietelski J, Turnau K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Spanish J Agri Res. 2010;8(1):103–108. doi:10.5424/sjar/201008S1-1228
- MubarakAli D, Arunkumar J, Nag KH, et al. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms—Comparative studies on synthesis and its application on biolabelling. Colloids Surf B Biointerfaces. 2013;103:166–173. doi:10.1016/j.colsurfb.2012.10.01423201734
- He X, Deng H, Hwang H-M. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2018.
- Nanotechnology AOo. National Nanotechnology Strategy Annual Report 2008:2007-2008.
- Roco MC. The Long View of Nanotechnology Development: The National Nanotechnology Initiative at 10 Years. Springer; 2011.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262. doi:10.1016/j.nano.2009.07.00219616126
- Schmid G. Nanoparticles: From Theory to Application. John Wiley & Sons; 2011.
- Govindaraju K, Basha SK, Kumar VG, Singaravelu G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J Mater Sci. 2008;43(15):5115–5122. doi:10.1007/s10853-008-2745-4
- Durán N, Durán M, De Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12(3):789–799. doi:10.1016/j.nano.2015.11.01626724539
- Mohamed A, Fouda A, Elgamal M, et al. Enhancing of cotton fabric antibacterial properties by silver nanoparticles synthesized by new Egyptian strain Fusarium keratoplasticum A1-3. Egypt J Chem. 2017;60(ConferenceIssue (The 8th International Conference of The Textile Research Division (ICTRD 2017), National Research Centre, Cairo 12622, Egypt.)):63–71. doi:10.21608/ejchem.2017.1626.1137
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9(6):674–679. doi:10.1016/j.cbpa.2005.10.00516233988
- Pathak J, Ahmed H, Singh DK, Pandey A, Singh SP, Sinha RP. Recent developments in green synthesis of metal nanoparticles utilizing cyanobacterial cell factories In: Durgesh KT, editor. Nanomaterials in Plants, Algae and Microorganisms. Elsevier; 2019:237–265.
- Park JH, von Maltzahn G, Zhang L, et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv Mater. 2008;20(9):1630–1635. doi:10.1002/adma.20080000421687830
- Parial D, Patra HK, Dasgupta AK, Pal R. Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol. 2012;47(1):22–29. doi:10.1080/09670262.2011.653406
- Hassan SE-D, Fouda A, Radwan AA, et al. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J Biol Inorganic Chem. 2019;24(3):377–393. doi:10.1007/s00775-019-01654-5
- Fouda A, Saad E, Salem SS, Shaheen TI. In-vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb Pathog. 2018;125:252–261. doi:10.1016/j.micpath.2018.09.03030240818
- Salem SS, Fouda MM, Fouda A, et al. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J Cluster Sci. 2020;1–11.
- Gour A, Jain NK. Advances in green synthesis of nanoparticles. Artif Cells Nanomed Biotechnol. 2019;47(1):844–851. doi:10.1080/21691401.2019.157787830879351
- Khanna P, Kaur A, Goyal D. Algae-based metallic nanoparticles: synthesis, characterization and applications. J Microbiol Methods. 2019;163:105656. doi:10.1016/j.mimet.2019.10565631220512
- Sharma A, Sharma S, Sharma K, et al. Algae as crucial organisms in advancing nanotechnology: a systematic review. J Appl Phycol. 2016;28(3):1759–1774. doi:10.1007/s10811-015-0715-1
- Asmathunisha N, Kathiresan K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf B Biointerfaces. 2013;103:283–287. doi:10.1016/j.colsurfb.2012.10.03023202242
- Dahoumane SA, Djediat C, Yéprémian C, et al. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J Nanoparticle Res. 2012;14(6):883. doi:10.1007/s11051-012-0883-8
- Dahoumane SA, Wujcik EK, Jeffryes C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzyme Microb Technol. 2016;95:13–27. doi:10.1016/j.enzmictec.2016.06.00827866608
- Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir. 2007;23(5):2694–2699.17309217
- Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from Gold(I)−Thiosulfate and Gold(III)−Chloride complexes. Langmuir. 2006;22(6):2780–2787. doi:10.1021/la052652c16519482
- Hamouda RA, Hussein MH, Abo-Elmagd RA, Bawazir SS. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep. 2019;9(1):13071. doi:10.1038/s41598-019-49444-y31506473
- MubarakAli D, Gopinath V, Rameshbabu N, Thajuddin N. Synthesis and characterization of CdS nanoparticles using C-phycoerythrin from the marine cyanobacteria. Mater Lett. 2012;74:8–11. doi:10.1016/j.matlet.2012.01.026
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111(1):1–61. doi:10.1099/00221287-111-1-1
- Tyagi R, Kaushik B, Kumar J. Antimicrobial activity of some cyanobacteria In: Kharwar RN, editor. Microbial Diversity and Biotechnology in Food Security. Springer; 2014:463–470.
- Mukund S, Sivasubramanian V. Anticancer activity of oscillatoria terebrieormis cyanobacteria in human lung cancer cell line a549. Int J Appl Biol Pharm Technol. 2014;5(2):34–45.
- Hamida RS, Abdelmeguid NE, Ali MA, Bin-Meferij MM, Khalil MI. Synthesis of silver nanoparticles using a novel cyanobacteria desertifilum sp. extract: their antibacterial and cytotoxicity effects. Int J Nanomedicine. 2020;15:49–63. doi:10.2147/IJN.S23857532021164
- Bin-Meferij MM, Hamida RS. Biofabrication and antitumor activity of silver nanoparticles utilizing novel nostoc sp. Bahar M. Int J Nanomedicine. 2019;14:9019–9029. doi:10.2147/IJN.S23045731819416
- Ahmad S, Munir S, Zeb N, et al. Green nanotechnology: a review on green synthesis of silver nanoparticles—an ecofriendly approach. Int J Nanomedicine. 2019;14:5087. doi:10.2147/IJN.S20025431371949
- Baker S, Rakshith D, Kavitha KS, et al. Marine microbes: invisible nanofactories. Bioimpacts. 2013;3(3):111–117. doi:10.5681/bi.2013.01224163802
- Smuleac V, Varma R, Baruwati B, Sikdar S, Bhattacharyya D. Nanostructured membranes for enzyme catalysis and green synthesis of nanoparticles. ChemSusChem. 2011;4(12):1773–1777. doi:10.1002/cssc.20110021122086852
- Nadagouda MN, Varma RS. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: density-assisted self-assembly of nanospheres, wires and rods. Green Chem. 2006;8(6):516–518. doi:10.1039/b601271j
- Salem SS, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res. 2020.
- Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9).
- Patel K, Bharatiya B, Mukherjee T, Soni T, Shukla A, Suhagia B. Role of stabilizing agents in the formation of stable silver nanoparticles in aqueous solution: characterization and stability study. J Dispers Sci Technol. 2017;38(5):626–631. doi:10.1080/01932691.2016.1185374
- Amendola V, Meneghetti M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys Chem Chem Phys. 2009;11(20):3805–3821. doi:10.1039/b900654k19440607
- Arbain R, Othman M, Palaniandy S. Preparation of iron oxide nanoparticles by mechanical milling. Minerals Eng. 2011;24(1):1–9. doi:10.1016/j.mineng.2010.08.025
- Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63(3):152–160. doi:10.1016/j.addr.2010.09.00120840859
- Quester K, Avalos-Borja M, Castro-Longoria E. Biosynthesis and microscopic study of metallic nanoparticles. Micron. 2013;54:1–27. doi:10.1016/j.micron.2013.07.00323928107
- Saravanan C, Rajesh R, Kaviarasan T, Muthukumar K, Kavitake D, Shetty PH. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol Rep. 2017;15:33–40. doi:10.1016/j.btre.2017.02.006
- Chetia L, Kalita D, Ahmed GA. Synthesis of Ag nanoparticles using diatom cells for ammonia sensing. Sens Bio-Sensing Res. 2017;16:55–61. doi:10.1016/j.sbsr.2017.11.004
- Rajeshkumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Metals. 2014;2014.
- Aritonang HF, Koleangan H, Wuntu AD. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int J Microbiol. 2019;2019:8642303. doi:10.1155/2019/864230331354833
- Menon S, Rajeshkumar S, Kumar V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resource Eff Technol. 2017;3(4):516–527. doi:10.1016/j.reffit.2017.08.002
- Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. Green synthesis of metallic nanoparticles via biological entities. Materials (Basel). 2015;8(11):7278–7308. doi:10.3390/ma811537728793638
- Poinern GEJ. A Laboratory Course in Nanoscience and Nanotechnology. CRC Press; 2014.
- Khan I, Saeed K, Nanoparticles: KI. Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/j.arabjc.2017.05.011
- Upadhyay S, Parekh K, Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J Alloys Compd. 2016;678:478–485. doi:10.1016/j.jallcom.2016.03.279
- El-Din TS, Elzatahry AA, Aldhayan DM, Al-Enizi AM, Al-Deyab SS. Synthesis and characterization of magnetite zeolite nano composite. Int J Electrochem Sci. 2011;6:6177–6183.
- Yin Y, Yang X, Hu L, et al. Superoxide-mediated extracellular biosynthesis of silver nanoparticles by the fungus Fusarium oxysporum. Environ Sci Technol Lett. 2016;3(4):160–165. doi:10.1021/acs.estlett.6b00066
- Patel V, Berthold D, Puranik P, Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep (Amst). 2015;5:112–119. doi:10.1016/j.btre.2014.12.00128626689
- Husain S, Sardar M, Fatma T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J Microbiol Biotechnol. 2015;31(8):1279–1283. doi:10.1007/s11274-015-1869-325971548
- Mukherjee P, Senapati S, Mandal D, et al. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chembiochem. 2002;3(5):461–463. doi:10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X12007181
- Kalabegishvili T, Kirkesali E, Rcheulishvili A. Synthesis of Gold Nanoparticles by Blue-Green Algae Spirulina Platensis. Frank Lab. of Neutron Physics; 2012.
- Jena J, Pradhan N, Dash BP, Sukla LB, Panda PK. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int J Nanomater Biostruct. 2013;3(1):1–8.
- Merin DD, Prakash S, Bhimba BV. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac J Trop Med. 2010;3(10):797–799. doi:10.1016/S1995-7645(10)60191-5
- Focsan M, Ardelean II, Craciun C, Astilean S. Interplay between gold nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803. Nanotechnology. 2011;22(48):485101. doi:10.1088/0957-4484/22/48/48510122072064
- Jeffryes C, Agathos SN, Rorrer G. Biogenic nanomaterials from photosynthetic microorganisms. Curr Opin Biotechnol. 2015;33:23–31. doi:10.1016/j.copbio.2014.10.00525445544
- Lengke MF, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold(III)-chloride complex. Environ Sci Technol. 2006;40(20):6304–6309. doi:10.1021/es061040r17120557
- Kalishwaralal K, Deepak V, Ram Kumar Pandian S, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B Biointerfaces. 2010;77(2):257–262. doi:10.1016/j.colsurfb.2010.02.00720197229
- Oza G, Pandey S, Mewada A, et al. Facile biosynthesis of gold nanoparticles exploiting optimum pH and temperature of fresh water algae Chlorella pyrenoidusa. Adv Appl Sci Res. 2012;3(3):1405–1412.
- Shabnam N, Pardha-Saradhi P. Photosynthetic electron transport system promotes synthesis of Au-nanoparticles. PLoS One. 2013;8(8):e71123. doi:10.1371/journal.pone.007112323976990
- Dahoumane SA, Yéprémian C, Djédiat C, et al. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J Nanoparticle Res. 2014;16(10):2607. doi:10.1007/s11051-014-2607-8
- Sudha SS, Rajamanickam K, Rengaramanujam J. Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J Exp Biol. 2013;51(5):393–399.23821828
- El-Naggar NE, Hussein MH, El-Sawah AA. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci Rep. 2017;7(1):10844. doi:10.1038/s41598-017-11121-328883419
- Mata YN, Torres E, Blazquez ML, Ballester A, Gonzalez F, Munoz JA. Gold(III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J Hazard Mater. 2009;166(2–3):612–618. doi:10.1016/j.jhazmat.2008.11.06419124199
- Vijayan SR, Santhiyagu P, Singamuthu M, Kumari Ahila N, Jayaraman R, Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. ScientificWorldJournal. 2014;2014:938272. doi:10.1155/2014/93827224672397
- Keskin S, Oya N, Koçberber Kılıç N, Dönmez G, Tekinay T Green synthesis of silver nanoparticles using cyanobacteria and evaluation of their photocatalytic and antimicrobial activity. Paper presented at: Journal of Nano Research; 2016.
- Singh H, Du J, Singh P, Yi TH. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J Pharm Anal. 2018;8(4):258–264. doi:10.1016/j.jpha.2018.04.00430140490
- Ashmore DA, Chaudhari A, Barlow B, et al. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Revista do Instituto de Medicina Tropical de São Paulo. 2018;60:1–11. doi:10.1590/s1678-9946201860018
- Al Rashed S, Al Shehri S, Moubayed NM. Extracellular Biosynthesis of Silver Nanoparticles from Cyanobacteria. 2018.
- Sowani H, Mohite P, Damale S, Kulkarni M, Zinjarde S. Carotenoid stabilized gold and silver nanoparticles derived from the Actinomycete Gordonia amicalis HS-11 as effective free radical scavengers. Enzyme Microb Technol. 2016;95:164–173. doi:10.1016/j.enzmictec.2016.09.01627866612
- Bekasova O, Brekhovskikh A, Revina A, Dubinchuk V. Preparation and optical properties of silver nanoparticles in R-phycoerythrin, a protein matrix. Inorganic Mater. 2008;44(8):835. doi:10.1134/S0020168508080098
- Glazer AN. Phycobiliproteins—a family of valuable, widely used fluorophores. J Appl Phycol. 1994;6(2):105–112. doi:10.1007/BF02186064
- MacColl R. Cyanobacterial phycobilisomes. J Struct Biol. 1998;124(2–3):311–334. doi:10.1006/jsbi.1998.406210049814
- Gelagutashvili E. Binding of heavy metals with C-phycocyanin: a comparison between equilibrium dialysis, fluorescence and absorption titration. Am J Biomed Life Sci. 2013;1:12–16. doi:10.11648/j.ajbls.20130101.13
- Chen SS, Berns DS. Effect of plastocyanin and phycocyanin on the photosensitivity of chlorophyll-containing bilayer membranes. J Membr Biol. 1979;47(2):113–127. doi:10.1007/BF01876112490619
- Wei N, Hou Y, Lu Z, Yu H, Wang Q Synthesis of silver nanoparticles stabilized with C-phycocyanin and for fluorimetric detection of copper ions. Paper presented at: IOP Conference Series: Earth and Environmental Science; 2018.
- El-Naggar NE, Hussein MH, El-Sawah AA. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci Rep. 2018;8(1):8925. doi:10.1038/s41598-018-27276-629895869
- Bharde A, Rautaray D, Bansal V, et al. Extracellular biosynthesis of magnetite using fungi. Small. 2006;2(1):135–141. doi:10.1002/smll.20050018017193569
- Reddy AS, Chen CY, Chen CC, et al. Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J Nanosci Nanotechnol. 2010;10(10):6567–6574. doi:10.1166/jnn.2010.251921137763
- Khalifa K, Hamouda R, Hanafy D, Hamza A. In vitro antitumor activity of silver nanoparticles biosynthesized by marine algae. Digest J Nanomater Biostruct. 2016;11(1):213–221.
- Ali DM, Sasikala M, Gunasekaran M, Thajuddin N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig J Nanomater Biostruct. 2011;6(2):385–390.
- Rahman A, Ismail A, Jumbianti D, Magdalena S, Sudrajat H. Synthesis of copper oxide nano particles by using Phormidium cyanobacterium. Indonesian J Chemi. 2009;9(3):355–360. doi:10.22146/ijc.21498
- Rosken LM, Cappel F, Korsten S, et al. Time-dependent growth of crystalline Au(0)-nanoparticles in cyanobacteria as self-reproducing bioreactors: 2. Anabaena cylindrica. Beilstein J Nanotechnol. 2016;7:312–327. doi:10.3762/bjnano.7.3027335727
- Ali J, Ali N, Wang L, Waseem H, Pan G. Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J Microbiol Methods. 2019;159:18–25. doi:10.1016/j.mimet.2019.02.01030797020
- Brayner R, Barberousse H, Hemadi M, et al. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J Nanosci Nanotechnol. 2007;7(8):2696–2708. doi:10.1166/jnn.2007.60017685286
- Morsy FM, Nafady NA, Abd-Alla MH, Elhady DA. Green synthesis of silver nanoparticles by water soluble fraction of the extracellular polysaccharides/matrix of the cyanobacterium Nostoc commune and its application as a potent fungal surface sterilizing agent of seed crops. Univ J Microbiol Res. 2014;2(2):36–43.
- Mankad M, Patil G, Patel D, Patel P, Patel A. Comparative studies of sunlight mediated green synthesis of silver nanoparaticles from Azadirachta indica leaf extract and its antibacterial effect on Xanthomonas oryzae pv. oryzae. Arab J Chem. 2018.
- Zada S, Ahmad A, Khan S, et al. Biogenic synthesis of silver nanoparticles using extracts of Leptolyngbya JSC-1 that induce apoptosis in HeLa cell line and exterminate pathogenic bacteria. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S471–S480. doi:10.1080/21691401.2018.149966330198334
- Singh G, Babele PK, Kumar A, Srivastava A, Sinha RP, Tyagi MB. Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. J Photochem Photobiol B. 2014;138:55–62. doi:10.1016/j.jphotobiol.2014.04.03024911272
- Lengke MF, Fleet ME, Southam G. Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum(IV)-chloride complex. Langmuir. 2006;22(17):7318–7323. doi:10.1021/la060873s16893232
- Sonker AS, Pathak J, Kannaujiya VK, Sinha RP. Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can J Biotechno. 2017;1(1):26. doi:10.24870/cjb.2017-000103
- Vashishta B. Botany for Degree Student. S. Chand; 1978.
- Ebadi M, Zolfaghari MR, Aghaei SS, et al. A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv. 2019;9(41):23508–23525. doi:10.1039/C9RA03962G
- Saran T, Sharma G, Kumar M, Ali M. Biosynthesis of copper oxide nanoparticles using cyanobacteria spirulina platensis and its antibacterial activity. Int J Pharm Sci Res. 2017;8:3887.
- Vijayaraghavan K, Nalini SK. Biotemplates in the green synthesis of silver nanoparticles. Biotechnol J. 2010;5(10):1098–1110. doi:10.1002/biot.20100016720669257
- Manivasagan P, Nam SY, Oh J. Marine microorganisms as potential biofactories for synthesis of metallic nanoparticles. Crit Rev Microbiol. 2016;42(6):1007–1019. doi:10.3109/1040841X.2015.113786026920850
- Kim D, Jeong S, Moon J. Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology. 2006;17(16):4019. doi:10.1088/0957-4484/17/16/00421727531
- Kratošová G, Konvičková Z, Vávra I, Zapomělová E, Schröfel A. Noble metal nanoparticles synthesis mediated by the genus Dolichospermum: perspective of green approach in the nanoparticles preparation. Adv Sci Lett. 2016;22(3):637–641. doi:10.1166/asl.2016.6993
- Jin R, Cao YC, Hao E, Métraux GS, Schatz GC, Mirkin CA. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature. 2003;425(6957):487–490. doi:10.1038/nature0202014523440
- Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir. 2007;23(5):2694–2699.17309217
- Vines JB, Yoon JH, Ryu NE, Lim DJ, Park H. Gold Nanoparticles for Photothermal Cancer Therapy. Front Chem. 2019;7:167. doi:10.3389/fchem.2019.0016731024882
- Lenartowicz M, Marek PH, Madura ID, Lipok J. Formation of variously shaped gold nanoparticles by Anabaena laxa. J Cluster Sci. 2017;28(5):3035–3055. doi:10.1007/s10876-017-1275-0
- Rösken LM, Körsten S, Fischer CB, et al. Time-dependent growth of crystalline Au 0-nanoparticles in cyanobacteria as self-reproducing bioreactors: 1. Anabaena sp. J Nanoparticle Res. 2014;16(4):2370. doi:10.1007/s11051-014-2370-x
- Geetha S, Sathakkathul Z, Aarthi R, Heizline B. Green synthesis of gold nanoparticle using marine cyanobacteria Gloeocapsa sp and the antitumor potential. J Chem Pharm Sci. 2014;4:172–174.
- Lengke MF, Fleet ME, Southam G. Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium(II) chloride complex. Langmuir. 2007;23(17):8982–8987. doi:10.1021/la701244617658865
- Ahmad A, Mukherjee P, Mandal D, et al. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc. 2002;124(41):12108–12109. doi:10.1021/ja027296o12371846
- Dameron C, Reese R, Mehra R, et al. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature. 1989;338(6216):596–597. doi:10.1038/338596a0
- Prasad K, Jha AK. Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J Colloid Interface Sci. 2010;342(1):68–72. doi:10.1016/j.jcis.2009.10.00319880131
- Brayner R, Yéprémian C, Djediat C, et al. Photosynthetic microorganism-mediated synthesis of akaganeite (β-FeOOH) nanorods. Langmuir. 2009;25(17):10062–10067. doi:10.1021/la901034519572505
- Dahoumane SA, Djediat C, Yéprémian C, Couté A, Fiévet F, Brayner R. Design of magnetic akaganeite-cyanobacteria hybrid biofilms. Thin Solid Films. 2010;518(19):5432–5436. doi:10.1016/j.tsf.2010.04.001
- Roychoudhury P, Ghosh S, Pal R. Cyanobacteria mediated green synthesis of gold-silver nanoalloy. J Plant Biochem Biotechnol. 2016;25(1):73–78. doi:10.1007/s13562-015-0311-0
- Varthamanan J. Anti cancer activity of silver nano particles bio-synthesized using stingless bee propolis (Tetragonula iridipennis) of Tamilnadu. Asian J Biomed Pharm Sci. 2015;5(40):30.
- Kathiravan V, Ravi S, Ashokkumar S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta a Mol Biomol Spectrosc. 2014;130:116–121. doi:10.1016/j.saa.2014.03.10724769382
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309. doi:10.1088/0031-9155/49/18/N0315509078
- Roychoudhury P, Gopal PK, Paul S, Pal R. Cyanobacteria assisted biosynthesis of silver nanoparticles—a potential antileukemic agent. J Appl Phycol. 2016;28(6):3387–3394. doi:10.1007/s10811-016-0852-1
- Singh G, Babele PK, Shahi SK, Sinha RP, Tyagi MB, Kumar A. Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J Microbiol Biotechnol. 2014;24(10):1354–1367. doi:10.4014/jmb.1405.0500324986675
- Hamida RS, Ali MA, Goda DA, Khalil MI, Al-Zaban MI. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of methicillin-resistant staphylococcus aureus (MRSA) strain. Front Bioeng Biotechnol. 2020;8:433. doi:10.3389/fbioe.2020.0043332548095
- Hamida RS, Ali MA, Goda DA, Khalil MI, Redhwan A. Cytotoxic effect of green silver nanoparticles against ampicillin-resistant Klebsiella pneumoniae. RSC Adv. 2020;10(36):21136–21146. doi:10.1039/D0RA03580G
- Suganya KS, Govindaraju K, Kumar VG, et al. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater Sci Eng C Mater Biol Appl. 2015;47:351–356. doi:10.1016/j.msec.2014.11.04325492207
- Pawar Sunil BA, Parvin M, Panchratna P, Swarali S. Screening of silver nanoparticles producing cyanobacteria and its characterization. Int Res J of Science & Engineering. 2017;2017(A1):44–54.
- Ahmed E, Hafez A, Ismail F, Elsonbaty M, Abbas H, Eldin R. Biosynthesis of silver nanoparticles by Spirulina platensis and Nostoc sp. Glo Adv Res J Microbiol. 2015;4(4):36–49.
- Husain S, Afreen S, Yasin D, Afzal B, Fatma T. Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J Microbiol Methods. 2019;162:77–82. doi:10.1016/j.mimet.2019.05.01131132377
- Bakir EM, Younis NS, Mohamed ME, El Semary NA. Cyanobacteria as nanogold factories: chemical and anti-myocardial infarction properties of gold nanoparticles synthesized by Lyngbya majuscula. Mar Drugs. 2018;16(6):217. doi:10.3390/md16060217
- Younis NS, Bakir EM, Mohamed ME, El Semary NA. Cyanobacteria as nanogold factories II: chemical reactivity and anti-myocardial infraction properties of customized gold nanoparticles biosynthesized by cyanothece sp. Mar Drugs. 2019;17(7):402. doi:10.3390/md17070402
- Krishnamoorthy K, Moon JY, Hyun HB, Cho SK, Kim S-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J Mater Chem. 2012;22(47):24610–24617. doi:10.1039/c2jm35087d
- Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013.
- Liu C-G, Han Y-H, Kankala RK, Wang S-B, Chen A-Z. Subcellular performance of nanoparticles in cancer therapy. Int J Nanomedicine. 2020;15:675. doi:10.2147/IJN.S22618632103936
- Flores‐López LZ, Espinoza‐Gómez H, Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J Appl Toxicol. 2019;39(1):16–26. doi:10.1002/jat.365429943411
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291–295. doi:10.1113/expphysiol.1997.sp0040249129943
- Jiang HS, Qiu XN, Li GB, Li W, Yin LY. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem. 2014;33(6):1398–1405. doi:10.1002/etc.257724619507
- Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhal Toxicol. 2002;14(1):5–27. doi:10.1080/08958370175333861312122558
- Romero-Urbina DG, Lara HH, Velázquez-Salazar JJ, et al. Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein J Nanotechnol. 2015;6(1):2396–2405. doi:10.3762/bjnano.6.24626734530
- Abalkhil TA, Alharbi SA, Salmen SH, Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol Biotechnol Equipment. 2017;31(2):411–417. doi:10.1080/13102818.2016.1267594
- Feng QL, Wu J, Chen G, Cui F, Kim T, Kim J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668. doi:10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-311033548
- Satapathy SR, Mohapatra P, Preet R, et al. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine. 2013;8(8):1307–1322. doi:10.2217/nnm.12.17623514434
- Kepsutlu B, Wycisk V, Achazi K, et al. Cells undergo major changes in the quantity of cytoplasmic organelles after uptake of gold nanoparticles with biologically relevant surface coatings. ACS Nano. 2020;14(2):2248–2264. doi:10.1021/acsnano.9b0926431951375
- Barkhade T, Mahapatra SK, Banerjee I. Study of mitochondrial swelling, membrane fluidity and ROS production induced by nano-TiO2 and prevented by Fe incorporation. Toxicol Res (Camb). 2019;8(5):711–722. doi:10.1039/c9tx00143c31588348
- Ostaszewska T, Śliwiński J, Kamaszewski M, Sysa P, Chojnacki M. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environ Sci Poll Res. 2018;25(1):908–915. doi:10.1007/s11356-017-0494-0
- Nikolić V, Ilić-Stojanović S, Petrović S, Tačić A, Nikolić L. Administration routes for nano drugs and characterization of nano drug loading In: Shyam SM, editor. Characterization and Biology of Nanomaterials for Drug Delivery. Elsevier; 2019:587–625.
- Dejous C, Hallil H, Raimbault V, Rukkumani R, Yakhmi JV. Using microsensors to promote the development of innovative therapeutic nanostructures In: Nanostructures for Novel Therapy. Elsevier; 2017:539–566.
- Russo R, Esposito E, Granata C, et al. Magnetic nanoparticle characterization using nano-SQUID based on niobium Dayem bridges. Phys Procedia. 2012;36:293–299. doi:10.1016/j.phpro.2012.06.162
- Cicci A, Sed G, Tirillò J, Stoller M, Bravi M. Production and characterization of silver nanoparticles in cultures of the cyanobacterium A. platensis (Spirulina). Chem Eng Trans. 2017;57:1405–1410.
- Gulin A, Koksharova O, Popova A, Astaf’ev A, Shakhov A, Nadtochenko V. Visualization of the spatial distribution of Ag ions in cyanobacteria Anabaena sp. PCC 7120 by time-of-flight secondary ion mass spectrometry and two-photon luminescence microscopy. Nanotechnol Russia. 2016;11(5–6):361–363. doi:10.1134/S1995078016030083
- Mira A-K, Yousef A-S, Abdullah A-N. Biosynthesis of Silver Nanoparticles by Cyanobacterium Gloeocapsa sp.
- Asmathunisha NAR, Kathiresan K. Synthesis of silver and gold nanoparticles by mangrove-derived cyanobacteria. J Advan Nanomater. 2018;3(3):1–7.
- Roychoudhury P, Pal R. Synthesis and characterization of nanosilver—a blue green approach. Indian J Appl Res. 2014;4:1.
- Hamida RS, Albasher G, Bin-Meferij, MM. Oxidative Stress and Apoptotic Responses Elicited by Nostoc-Synthesized Silver Nanoparticles against Different Cancer Cell Lines. Cancers. 2020;12:2099.
- El-Sheekh MM, El-Kassas HY. Application of biosynthesized silver nanoparticles against a cancer promoter cyanobacterium, Microcystis aeruginosa. Asian Pac J Cancer Prev. 2014;15(16):6773–6779. doi:10.7314/APJCP.2014.15.16.677325169524
- Sharma G, Jasuja ND, Kumar M, Ali MI. Biological synthesis of silver nanoparticles by cell-free extract of Spirulina platensis. J Nanotechnol. 2015;2015.
- Rejeeth C. Biosynthesis of silver nanoscale particles using Spirulina platensis induce growth-inhibitory effect on human breast cancer cell line MCF-7. Med Aromat Plants. 2014;3(03):2167–0412.1000163. doi:10.4172/2167-0412.1000163
- Kaliamurthi S, Selvaraj G, Çakmak ZE, Çakmak T. Production and characterization of spherical thermostable silver nanoparticles from Spirulina platensis (Cyanophyceae). Phycologia. 2016;55(5):568–576. doi:10.2216/15-98.1
- Tsibakhashvili NY, Kirkesali EI, Pataraya DT, et al. Microbial synthesis of silver nanoparticles by Streptomyces glaucus and Spirulina platensis. Adv Sci Lett. 2011;4(11–12):3408–3417. doi:10.1166/asl.2011.1915
- Sugandha V, Babita K, Shrivastava J. Green synthesis of silver nanoparticles using single cell protein of Spirulina platensis. Int J Pharm Bio Sci. 2014;5:2.
- Mahdieh M, Zolanvari A, Azimee A. Green biosynthesis of silver nanoparticles by Spirulina platensis. Scientia Iranica. 2012;19(3):926–929. doi:10.1016/j.scient.2012.01.010
- Lakshmi P, Priyanka D, Annamalai A. Reduction of silver ions by cell free extracts of westiellopsis sp. Int J Biomater. 2015;2015.
- Radtsig M, Koksharova O, Nadtochenko V. Production of gold nanoparticles by biogenesis using bacteria. Microbiology. 2016;85(1):63–70. doi:10.1134/S0026261716010094
- Roychoudhury P, Bhattacharya A, Dasgupta A, Pal R. Biogenic synthesis of gold nanoparticle using fractioned cellular components from eukaryotic algae and cyanobacteria. Phycol Res. 2016;64(3):133–140. doi:10.1111/pre.12127
- Parial D, Gopal PK, Paul S, Gold PR. (III) bioreduction by cyanobacteria with special reference to in vitro biosafety assay of gold nanoparticles. J Appl Phycol. 2016;28(6):3395–3406. doi:10.1007/s10811-016-0880-x
- Parial D, Pal R. Green synthesis of gold nanoparticles using cyanobacteria and their characterization. Indian J Appl Res. 2014;4:69–72. doi:10.15373/2249555X/JAN2014/22
- Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation–a novel phenomenon. J Appl Phycol. 2009;21(1):145. doi:10.1007/s10811-008-9343-3
- Parial D, Patra HK, Roychoudhury P, Dasgupta AK, Pal R. Gold nanorod production by cyanobacteria—a green chemistry approach. J Appl Phycol. 2012;24(1):55–60. doi:10.1007/s10811-010-9645-0