Abstract
Many studies in recent years have focused on surface engineering of implant materials in order to improve their biocompatibility and other performance. Porous tantalum implants have increasingly been used in implant surgeries, due to their biocompatibility, physical stability, and good mechanical strength. In this study we functionalized the porous tantalum implant for sustained drug delivery capability via electrostatic self-assembly of polyelectrolytes of hyaluronic acid, methylated collagen, and terpolymer on the surface of a porous tantalum implant. The anticancer drug doxorubicin was encapsulated into the multilayer copolymer membranes on the porous tantalum implants. Results showed the sustained released of doxorubicin from the functionalized porous tantalum implants for up to 1 month. The drug release solutions in 1 month all had inhibitory effects on the proliferation of chondrosarcoma cell line SW1353. These results suggest that this functionalized implant could be used in reconstructive surgery for the treatment of bone tumor as a local, sustained drug delivery system.
Introduction
Bone tumors (either primary or secondary) are one of the most severe diseases in orthopedic clinical practice. The common approach for bone tumors is a combination of surgery, chemotherapy, and radiology. Reconstructive surgery for bone cancer patients requires precise removal of the affected tissue and replacement with a bone implant followed by postoperational chemotherapy.Citation1,Citation2
Metallic implants using titanium and tantalum have been widely used due to their superior mechanical strength, physical stability, and biocompatibility.Citation3–Citation7 Basic scientific and clinical studies have shown that osseointegration is a well-established property of porous tantalum implant,Citation8–Citation10 which is promising for reconstructive surgeries.Citation4,Citation11
On the other hand, systemic chemotherapy is the most commonly applied strategy of postoperational chemotherapy. The effectiveness of systemic chemotherapy has been improved; however, there are still limitations in clinical practice, including a number of side effects, such as renal damage, liver damage, cardiomyopathy, and myelosuppression, which may necessitate the suspension of treatment. Furthermore, systemic chemotherapy is not efficient, because the concentration of drug is diluted at the targeted site. Therefore, local drug delivery systems have received increasing interest in recent years. In 1997, Fröschle et alCitation12 reported that daunorubicin-polymethylmethacrylate in the resection cavity delayed or reduced recurrences in bone metastasis in animals. Recently, El-Ghannam et al tested a ceramic-based anticancer drug, 5-fluorouracil, to treat breast cancer in a murine model.Citation13 To the best of our knowledge, there is still not an implantable drug delivery system available on the market for bone tumor patients. Researchers are still trying to design a multifunctionalized implant to not only support the skeletal structure but also provide local, controllable release of an anticancer drug in order to prevent cancer recurrence.Citation14,Citation15
In the current study we intended to functionalize the surface of the porous tantalum implant so as to have a sustained drug delivery capability via electrostatic self-assembly of polyelectrolytes of hyaluronic acid, methylated collagen, and terpolymer of hydroxylethyl methacrylate-methyl methacrylate-methylacrylic acid (HEMA-MMA-MAA). The anticancer drug doxorubicin (DOX) was encapsulated into the multilayer copolymer membranes on the porous tantalum implants. Previously, we developed this electrostatic self-assembly technique for the improvement of osteogenic differentiation of mesenchymal stem cells and calcium deposition, which are important parameters of bone tissue engineering.Citation16 Here, we focused on the drug release aspect and the bioactivity test of the loaded drug DOX. Firstly, we optimized the self-assembly membranes to obtain the optimal drug release rate by the use of an orthogonal design table. Then, we monitored the drug release rate from the implants for 1 month. Finally, we tested the bioactivity of DOX, which was released from functionized porous tantalum implants, by the inhibition of chondrosarcoma cell line SW1353. We aimed at adding a drug delivery capability to the reconstructive porous tantalum implant.
Materials and methods
The porous tantalum implants, Trabecular Metal™ (Ta), were supplied from Zimmer Inc. (Minneapolis, MN). They are made by chemical vapor deposition of tantalum precursors on a vitreous carbon skeleton. The average pore size of the tantalum implant is 430 microns, and the porosity is 75%– 80%. Hyaluronic acid (780 kDa, Lot P9805-9A) was purchased from Lifecore Biomedical Inc. (Chaska, MN) HEMA, MMA, and MAA were purchased from Sigma-Aldrich, Shanghai, China. DOX standard was purchased from Wanle, Shenzhen, China. The collagen (mainly bovine collagen type I) was courtesy of Professor Yinjun Wang, Materials and Engineer College, South China University of Technology, China.
Materials preparation
Collagen methylation
Type I bovine collagen was modified to be cationic via esterification, as described previously.Citation17 The degree of methylation was controlled by adjusting the time and temperature of the reaction.Citation18 The precipitated collagen type I was dissolved in 0.1 M HCl containing methanol for 6 days at 4°C. At the end of the reaction, the solution was dialyzed against deionized water at 4°C using dialysis tubing with molecular weight cut-off (MWCO) of 8000–15,000 until the pH of the external reservoir reached 6.4, followed by freeze-drying. The modified collagen was stored at −20°C for use.
Terpolymer preparation
Terpolymer of HEMA, MMA, and MAA was synthesized by solution polymerization at 78°C in 2-propanol using 2,2′-azobisisobutyronitrile (AIBN) as an initiator, as described previously.Citation17 The themolar feed ratio of HEMA, MMA, and MAA was 25:50:25. Terpolymer 3% and 10% in phosphate-buffered saline solution was used in this study.
Surface modification
Pretreatment of porous tantalum implants
The porous tantalum implants were placed in the 0.1 M of NaOH solution for 24 hours at 60°C. Then, the implants were rinsed with distilled water five times and dried in an electric oven at 80°C. After this pretreatment, the implants were used for surface modification and drug loading.
Assembly of membranes
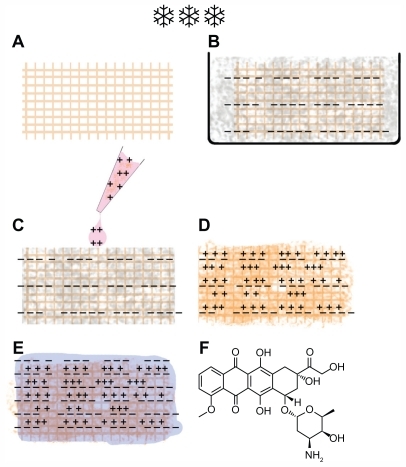
Porous tantalum implants were immersed in the hyaluronic acid solution for 30 minutes and then freeze-dried. A mixed solution of 100 μL of methylated collagen with DOX was added into the hyaluronic acid precoated porous tantalum implant and dried in the desiccator in a vacuum for 30 minutes. The final coating layer was done by pipetting 100 μL of copolymer solution into the precoated porous tantalum implants, which were left at room temperature for 1 hour and then dried in the desiccator under vacuum. The implant functional procedure is shown in .
Figure 1 Procedure of implant functionalization and doxorubicin (DOX) loading. (A) A macroporous tantalum implant. (B) Implant embedded with hyaluronic acid followed with lyophilization. (C) DOX mixed with methylated collagen was dropped on to the embedded implant. (D) A hyaluronic acid-embedded implant was loaded with DOX and methylated collagen. (E) Terpolymer of hydroxylethyl methacrylate-methyl methacrylate-methylacrylic acid was added into the previous functional implant (D) and an implant with the copolymeric multilayer membranes loaded with DOX was made. “−− ” represents negative charge, and “+++” represents positive charge. (F) The chemical structure of DOX.
Scanning electron microscopy (SEM, Nova NanoSEM 600, FEI Company, Eindhoven, The Netherland) was used to visualize the morphology and distribution of the coating on the tantalum implant.
The orthogonal experimental design for optimization of sustained drug release
We optimized the self-assembly membranes to obtain the optimal drug release rate by the use of an orthogonal design table. Three parameters, including the concentrations of hyaluronic acid (A), methylated collagen (B), and terpolymer (C), were defined as main factors. Nine formulations were designed for the test according to the standard L9 (33) orthogonal experimental design, in order to screen the optimal formulations of prescription. Cumulative DOX release rates (Q) from porous tantalum implants at 2 hours and 30 days were selected as indexes to determine the optimal factors. Experiments have been orthogonally designed for arranging the three factors with three levels (for A and B) and two levels (for C), as shown in .
Table 1 Levels of each of the three factors for the sustained drug release (mg/mL)
In vitro drug release test
The determination of DOX by chromatography
The high-performance liquid chromatography system consisted of the 1200 Series lsocratic pump (Agilent Corporation, CA), a manual injector (20 mL loop), an Agilent 1200 Series-fluorescent detector, and Agilent Technologies chemstation. The separation was carried out on a 4.6 × 150 mm (5 μm) reversed-phase C18 Luna column. The mobile phase consisted of (5% phosphoric acid) water:methyl cyanides:methanol:isopropyl alcohol (65:15:10:10, v:v:v). The flow rate was maintained at 1 mL/ minute. The effluents were monitored at λEx = 505 nm and λEm = 550 nm. All chromatographic analyses were performed at room temperature.
Standard solutions
DOX (0.05–10 μg/mL) was prepared in the mobile phase. DOX (0.0010 g) was dissolved in a 5 mL mobile phase in a volumetric flask. Various standard solutions were then prepared from this stock solution after adequate dilution with a mobile phase.
In vitro DOX release from the implants
The release profile of DOX from porous tantalum implants was determined by incubating an implant in 2.0 mL stimulated body fluid in a shaking water bath at 37°C for 1 month. At the series time points of 0.33 hours, 0.67 hours, 1 hour, 2 hours, 6 hours, 12 hours, 24 hours, 48 hours, 96 hours, 144 hours, 15 days, and 30 days, 2 mL of solution was collected and replaced with 2 mL of fresh stimulated body fluid. The drug concentrations of samples were measured by high-performance liquid chromatography, and the cumulative release rates were calculated afterwards.
In vitro cytotoxicity test of DOX released from porous tantalum implant
Chondrosarcoma cell line SW1353 was seeded in 96-well plates at a density of 2000 cells/well in each well with 200 μL Dulbecco’s Modified Eagle Medium containing 10% fetal calf serum. After 2 days’ culture, 10 μL of sterile-filtered DOX-released solutions from series time points of 2 hours, 24 hours, 15 days, and 30 days were added into the culture medium. Cells that were cultured in stimulated body fluid were used as control. Each treatment had six replicates. After 3 days, SW1353 cells were collected for viability testing determined by MTT assay. Briefly, to each well was added 10 μL of MTT stock solution (5 g/L), which was cultured at 37°C for 4 hours. Media were removed and the converted dye was dissolved in 100 μL of DMSO solution. Absorbance of converted dye was measured at a wavelength of 490 nm. Cell inhibitory rate was calculated as % IC = (1 − ODtesting group/ODcontrol) × 100%.
Results and discussion
Characterization of surface modification by SEM
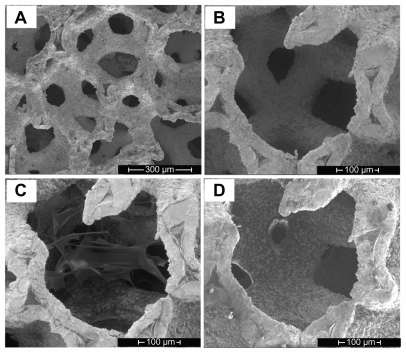
From the SEM micrographs () we could observe that the porous tantalum implant has a trabecular bone-like structure with highly interconnected pores, ranging from 200 μm to 400 μm (). We chose a representative position of the porous tantalum implant () to characterize the modification procedure. After the initial layer coating of hyaluronic acid, nanofibres of hyaluronic acid were observed on the surface of the trabacula as well as the pores (). After adding methylated collagen and terpolymer, we found that a hyaluronic acid-methylated collagen-terpolymer complex was formed within the porous tantalum implant. The copolymer membranes were uniform, covering the surface of porous tantalum trabecula, and did not block the porous structures ().
Figure 2 Scanning electron microscopy micrographs of the porous tantalum implant. (A) The bare porous tantalum implant with highly interconnected pores. (B) A representative position before surface modification. (C) The representative position after hyaluronic acid modification. (D) The representative position after hyaluronic acid, methylated collagen, and terpolymer modification.
The copolymer membranes formed on the surfaces of the porous tantalum implant occurred by electrostatic self- assembly of oppositely charged polyelectrolytes. When negative and positive polymers are mixed, charge–charge interactions result in the formation of a polyelectrolyte complex. This technique has been widely applied in drug delivery systemsCitation19 and tissue engineering devices.Citation17,Citation20 Quek et alCitation21 used methylated collagen and terpolymer of HEMA-MMA-MAA to form a two-layered membrane for encapsulation of hepatocytes. In our previous study we used hyaluronic acid, methylated collagen, and terpolymer of HEMA-MMA-MAA to form a composite matrix for functionalization of bone tissue engineering scaffolds.Citation16
In this study, porous tantalum implants were pretreated with NaOH to obtain an amorphous sodium tantalate layer on the surface to increase the bioactivity of the implant.Citation22 Due to the viscosity of hyaluronic acid solution, there were networks of hyaluronic acid nanofibres covering the trabecular implant after the freeze-dryer procedure. The porous tantalum implant was embedded in the hyaluronic acid and the surface area of the implant was increased, so the implant was finally embedded in the membranes after the electrostatic assembly among hyaluronic acid, methylated collagen, and terpolymer of HEMA-MMA-MAA. This was different from the study carried out by Toh et al,Citation23 which used methylated collagen and terpolymer to macroencapsulate a whole implant.
For the drug loading study, DOX was mixed in the methylated collagen solution, which was because DOX dispersed better in the collagen solution compared with in the hyaluronic acid solution, and also avoided direct contact of DOX with the porous tantalum metal surface. Because the chemical structure of DOX () contains an amino group with a pKa of 8.6, it is positively charged at pH 7.4. DOX can interact with the polyanions, either with hyaluronic acid or with the terpolymer of HEMA-MMA-MAA, and form polymer-drug complexes due to electrostatic interaction between oppositely charged polyions. In this study, DOX served as both a coating component and a functional drug.
Polyelectrolytes and polyelectrolyte complexes have been extensively studied in the development of drug delivery as drug carriers or substances. The polyelectrolyte complexes can be polymer-polymer, polymer-drug, and polymer- drug-polymer. Drug-carrying polyelectrolyte microparticles have been shown to prolong drug release time.Citation24 Polyelectrolyte multilayer nanofilms have been studied for delivery of charged dyes, metal and inorganic nanoparticles, DNA, proteins, and viruses.Citation19
Determination of the optimal formulations of prescription
The formulation factors affecting cumulative DOX release rates were studied in an orthogonal experimental design. The results are listed in (DOX release rate at 2 hours) and (DOX release rate at 30 days).
Table 2 Results of the test of orthogonal design of cumulative doxorubicin release rates (Q1) from the functionalized porous tantalum implants at 2 hours
Table 3 Results of the test of orthogonal design of cumulative doxorubicin release rates (Q2) from the functionalized porous tantalum implants at 30 days
The effects of each factor were analyzed by comparing the K (average value of Q of each factor at same level) value and the range R (Kmax–Kmin). The range reflected the extent of each factor effect on index. With a bigger range, the extent affected was greater.
Effects of each factor on the cumulative DOX release rate from the functionalized porous tantalum implants at 2 hours
According to the R values, three factors in the present experiment were ranged as B > A > C (). To avoid a burst release at the early time point, a lower Q1 is better for the optimal formulation. The optimized level for each factor was analyzed: A: 1 > 2 > 3; B: 1 > 2 > 3; C: 1 > 2. The best combination for the formulation was chosen by combining the optimal levels of each factor. The optimal combination was A1B1C1, which is hyaluronic acid (1 mg/mL), methylated collagen (1.5 mg/mL), and terpolymer (30 mg/mL).
A burst release effect is that the cumulative drug release rate at the first 2 hours exceeds 30%–40% of the total amount of drug, so B3 was excluded for the formulation of prescription.
Effects of each factor on the DOX release from the porous tantalum implants at 30 days
According to the R values, three factors in the present experiment were ranged as B > A > C (). For more drug release from the functionalized implants, a higher Q2 is better for the optimal formulation. The optimized level for each factor was analyzed: A: 1 > 2 > 3; B: 2 > 1; C: 2 > 1. The best combination for the formulation was chosen by combining the optimal levels of each factor. The optimal combination was A1B2C2, which is hyaluronic acid (1 mg/mL), methylated collagen (3 mg/mL), and terpolymer (100 mg/mL).
Taking the results from and together, three factors in the present experiment were ranged as B > A > C, which means that Factor B has the greatest impact on the cumulative drug release and Factor C has the least impact. For Factor A, it is the same level A1 as the optimal level for both indexes Q1 and Q2. For Factor B, there is no big difference between B1 and B2 for Q1. However, Q2 is too low at the B1 level. So the optimal formulation of prescription for the composite membrane is A1B2C2, which is hyaluronic acid (1 mg/mL), methylated collagen (3 mg/mL), and terpolymer (100 mg/mL).
Through prescription optimization we can see that the DOX release rates were greatly affected by the concentrations of the three polyions. The concentration of methylated collagen has the highest effect on the drug release rate. Both DOX and methylated collagen are polycations. In this study we fixed the DOX loading amount at 100 μg, so concentration of methylated collagen has a significant effect and reacts with the other two negative charges: hyaluronic acid and terpolymer of HEMA-MMA-MAA.
Polyelectrolyte multilayer nanofilms have been widely used for surface modification of biomedical devices and drug delivery systems. The properties of the polyelectrolyte multilayer are controllable by selecting either different polyelectrolytes or different processing procedures. The choice of the assembly components is very flexible because many biological molecules are polyelectrolytes, (eg, nucleic acid, protein, glycoprotein, proteoglycan, glycosaminoglycans, alginates, and chitosan). The polyelectrolyte multilayer nano-films are usually prepared by a layer-by-layer self-assembly technique to coat a device or substrate. By controlling the number of film layers, the concentration of the polyelectrolytes, and the drug incubation time, the drug loading and release from the polyelectrolyte multilayers are tunable.
With this versatile technique it is possible to build a multi-drug release system to have a multifunction device. Different therapeutic agents can be loaded into different film layers. Taking an orthopedic implant as an example, prevention of implant infection and promoting tissue regeneration are the main tasks for implant fixation. Polyelectrolyte multilayer nanofilms with antibiotics, cytokines, or growth factors have been used to modify the biomedical devices for different aims. The study has shown that interleukin 12 multilayer polypeptide nanoscale coatings at the implant/tissue interface substantially decreased infections in vivo.Citation25 Recombinant human bone morphogenetic protein 2 (rhBMP-2) was delivered by cross-linked poly(L-lysine)/hyaluronan layer-by-layer films to induce myoblast differentiation to osteoblasts.Citation26 Multifunctional implants could be manufactured by incorporating multiple drugs on the surface of implants via the processing of polyelectrolyte multilayer nanofilms. Such devices can use rhBMP-2 as the initial layer to induce osteogenic differentiation of mesenchymal stem cells, and use antibiotics such as gentamicin for the upper layer to prevent infection.
Based on our present study, we can also load rhBMP-2 as an initial layer, and anti-cancer drug DOX as the outside layer. This multifunctional implant could firstly release anticancer drug to prevent cancer recurrence and then promote bone tissue engineering.
In vitro study of DOX release from functionalized porous tantalum implants
DOX was loaded into the porous tantalum implants with the optimization of prescription. The DOX concentrations released from the functionalized porous tantalum implants at series time points and the cumulative release curve are depicted in .
Figure 3 (A) Doxorubicin concentration released from functionalized porous tantalum implants at series time points. (B) Cumulative release rate of doxorubicin from functionalized porous tantalum implants in 30 days.
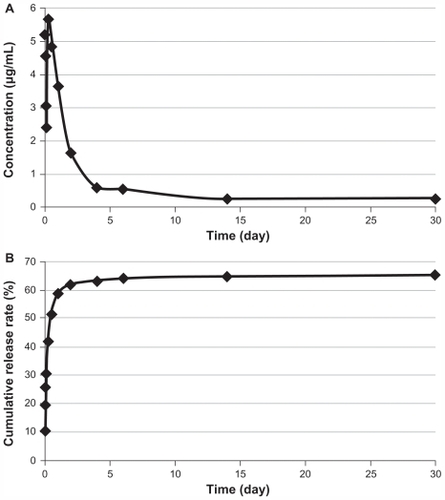
DOX released rapidly from the implants at the first 24 hours and then slowly sustained release for 30 days. The cumulative release curve was closed to zero-order rate from Day 2. We loaded 100 μg of DOX in each functionalized porous tantalum implant, and the drug release concentrations ranged from 0.25 μg/mL to 5.64 μg/mL for 30 days. The highest concentration of the release solutions was 5.64 μg/mL at 6 hours. A release concentration greater than 0.25 μg/mL was maintained.
Drug release rates from polyelectrolyte multilayers depend on the polyelectrolytes applied in the drug delivery system. Drug release mechanisms are different. They can be induced by charge, temperature, degradation of polyelectrolyte multilayer, hydrolysis, or redox.Citation27 Many studies have been trying to control drug release rates by controlling the release environment pH,Citation28 the temperature,Citation29 or the construction of the polyelectrolyte multilayer.Citation30
In the present study, there are two phases of drug release rates: an initial burst release and a sustained release. The initial burst release on the first day is mainly caused by the absorption of the free DOX, which is not encapsulated into the copolymeric membranes but physically absorbed on the implant. The second phase of sustained release is mainly due to the degradation of hyaluronic acid, which induces the change of net charges of polyelectrolytes and releases the DOX. DOX release from the implant may also involve a diffusion mechanism, where DOX diffuses from the higher concentration of the implant to the release buffer.
In clinical practice, DOX is given with a dose of 60 mg/m2 every 3 weeks. It is reported that DOX reaches peak plasma concentration at 2.7 μg/mL.Citation31 The implantable drug delivery system in the present study shows the high efficiency of the loaded drug, with a satisfactory sustained-release capability.
A study by Itokazu et alCitation32 showed that a local drug delivery system has the advantage of minimizing systemic side effects and has long-term localized delivery of anticancer drug. They showed that the release of DOX was sustained for 66 days in vitro and 4 weeks in vivo. DOX concentrations in plasma, liver, and kidney ranged from 0.25% to 10% of that at the implanted site. However, in their system, the hydroxyapatite blocks did not have adequate mechanical support for the bone-bearing part.
Cytotoxicity of DOX release solutions from the functionalized porous tantalum implants
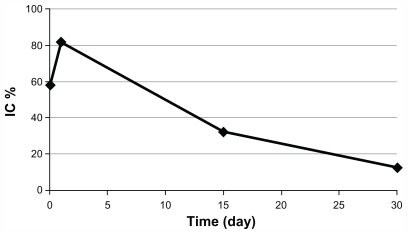
shows that DOX release solutions from the porous tantalum implants in 30 days all had an inhibition effect on the growth of chondrosarcoma cells. The inhibitory ratios of DOX release solutions from 2 hours, 24 hours, 15 days, and 30 days were 57.87%, 81.42%, 31.78%, and 12.19%, respectively.
Figure 4 Inhibitory effects of doxorubicin release solutions on the growth of chondrosarcoma cell line SW1353. Data represent the mean ±SD (n = 6).
These results proved that the drug loading process did not change the bioactivity of DOX. When the implant is used as a drug delivery vehicle, it is very important to consider the interaction between the material and the loaded drug. The implant should not have any effects on the loaded drug and also be able to protect the stability and bioactivity of the loaded drug.
Conclusion
This study investigated a modification procedure to incorporate an anticancer drug into a widely clinically used porous tantalum implant in order to have an implantable drug delivery system. Polyelectrolytes of hyaluronic acid, methylated collagen, and terpolymer of HEMA-MMA-MAA were electrostatically self-assembled on the surface of the porous tantalum implants. We optimized three factors of the self-assembly membranes by orthogonal experimental design and obtained the optimal drug release of the loaded drug DOX. Our results showed that the functionalized porous tantalum implants loaded with DOX had a stable and sustained drug release for 30 days and that the drug release solutions all had inhibitory effects on the growth of chondrosarcoma cell SW1353.
Base on the aforementioned results, it is predicted that this drug-loaded porous tantalum implant could be placed into the surgical defect created by bone tumor resection for immediate load-bearing support and local anticancer treatment to prevent recurrence. The functionalized technique in the present study can also apply to different types of implants to have different local drug delivery systems for various therapeutic aims. Thus, further in vivo experiments should be proposed in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30571892, u0732001) and the Danish Research Council (Jr.nr. 09-063120). Type I bovine collagen was supplied courtesy of Professor Yingjun Wang, the Key Lab for Special Functional Materials of the Ministry of Education, South China University of Technology, China.
Disclosure
The authors disclose no conflicts of interest in this work.
References
- DuboussetJStrategy for the surgical treatment of primary bone tumors of the spine in childrenChir Organi Mov1990751 Suppl89902249567
- DraganSWallAKrawczykALocal experience in the surgical treatment of primary malignant and metastatic bone tumors of the proximal femurOrtop Traumatol Rehabil20057660461017611422
- NiinomiMMetallic biomaterialsJ Artif Organs200811310511018836869
- LevineBRSporerSPoggieRAExperimental and clinical performance of porous tantalum in orthopedic surgeryBiomaterials200627274671468116737737
- BobynJDTohKKHackingSATissue response to porous tantalum acetabular cups: a canine modelJ Arthroplasty199914334735410220190
- ZouXLiHBüngerMBone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigsSpine J2004419910514749198
- LongMRackHJTitanium alloys in total joint replacement: a materials science perspectiveBiomaterials19981918162116399839998
- BobynJDStackpoolGJHackingSACharacteristics of bone in growth and interface mechanics of a new porous tantalum biomaterialJ Bone Joint Surg Br199981590791410530861
- AlberiusPBone reactions to tantalum markers A scanning electron microscopic studyActa Anat (Basel)198311543103186845968
- AronsonASHanssonLIEffect of tantalum markers of longitudinal bone growthActa Orthop Scand1976475515519998187
- PatilNLeeKGoodmanSBPorous tantalum in hip and knee reconstructive surgeryJ Biomed Mater Res Part B Appl Biomater200989124225118837451
- FröschleGWMählitzJLangendorffHURelease of daunorubicin from polymethylmethacrylate for the improvement of the local growth control of bone metastasis animal experimentsAnticancer Res1997172A99510029137440
- El-GhannamARicciKMalkawiAA ceramic-based anticancer drug delivery system to treat breast cancerJ Mater Sci Mater Med20102192701271020644983
- PorterJRuckhTPopatKCBone tissue engineering: a review in bone biomimetics and drug delivery strategiesBiotechnol Progress200925615391560
- DefailAJEdingtonHDMatthewsSControlled release of bioactive doxorubicin from microspheres embedded within gelatin scaffoldsJ Biomed Mater Res2006794954962
- ChenMLeDBaatrupASelf-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineeringActa Biomaterialia201111320619368
- ChiaSMLeongKWLiJHepatocyte encapsulation for enhanced cellular functionsTissue Eng20006548149511074935
- ZhangJWeiHPQuekCHQuantitative measurement of collagen methylation by capillary electrophoresisElectrophoresis200425203416342115490447
- LankalapalliSKolapalliVRPolyelectrolyte complexes: a review of their applicability in drug delivery technologyIndian J Pharm Sci200971548148720502564
- SunTChanMLZhouYUse of ultrathin shell microcapsules of hepatocytes in bioartificial liver-assist deviceTissue Eng20039Suppl 1S657514511471
- QuekCHLiJSunTPhoto-crosslinkable microcapsules formed by polyelectrolyte copolymer and modified collagen for rat hepatocyte encapsulationBiomaterials200425173531354015020127
- MiyazakiTKimHMMiyajiFBioactive tantalum metal prepared by NaOH treatmentJ Biomed Mater Res2000501354210644961
- TohYCHoSTZhouYApplication of a polyelectrolyte complex coacervation method to improve seeding efficiency of bone marrow stromal cells in a 3D culture systemBiomaterials200526194149416015664642
- LiaoICWanACYimEKLeongKWControlled release from fibers of polyelectrolyte complexesJ Control Release2005104234735815907585
- LiBJiangBBoyceBMLindseyBAMultilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infectionsBiomaterials200930132552255819215980
- CrouzierTRenKNicolasCLayer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: controlled differentiation of myoblasts to osteoblastsSmall20095559860819219837
- JiangBBarnettJBLiBAdvances in polyelectrolyte multilayer nanofilms as tunable drug delivery systemsNanotechnol Sci Appl200922127
- JiangBLiBTunable drug loading and release from polypeptide multilayer nanofilmsInt J Nanomedicine20094375319421369
- QuinnJFCarusoFThermoresponsive nanoassemblies: layer-by-layer assembly of hydrophilic-hydrophobic alternating copolymersMacromolecules20053834143419
- BergMCZhaiLCohenRERubnerMFControlled drug release from porous polyelectrolyte multilayersBiomacromolecules20067135736416398536
- MüllerIJennerABrucheltGEffect of concentration on the cytotoxic mechanism of doxorubicin – apoptosis and oxidative DNA damageBiochem Biophys Res Commun199723022542579016760
- ItokazuMKumazawaSWadaEWenyiYSustained release of adriamycin from implanted hydroxyapatite blocks for the treatment of experimental osteogenic sarcoma in miceCancer Lett1996107111188913261