Abstract
Aim
Our objective was to prepare a new nano-sized realgar particle and characterize its anti-tumor effect on tumor cells.
Methods
Nanoparticles were prepared by coprecipitation and were detected by transmission electron microscopy, scanning electron microscopy, energy dispersive spectrometry (EDS), and dynamic light scattering. An anti-proliferative effect of realgar nanoparticles on rat glioma (C6) cells was determined by the MTT assay. Cell cycle and apoptosis rates were observed by flow cytometry. Apoptosis-related gene expression was detected by immunofluorescence staining.
Results
Realgar nanoparticles were successfully prepared. The particles were spherical, with an average diameter of approximately 80 nm, and contained arsenic and sulfur elements. Realgar nanoparticles inhibited C6 cell proliferation and induced apoptosis in a dose- and time-dependent manner. Treatment of C6 cells with realgar nanoparticles significantly increased the proportions of cells in S and G2/M phases, decreased the proportion of cells in G0/G1 phase, downregulated Bcl-2 expression, and substantially upregulated Bax expression.
Conclusion
Realgar nanoparticles significantly inhibited C6 glioma cell proliferation and promoted cell apoptosis by inducing the upregulation of Bax and downregulation of Bcl-2 expression. Realgar nanoparticles are a promising in vitro anti-cancer strategy and may be applicable for human cancer therapy studies.
Introduction
Glioma is a highly chemoresistant and radioresistant cancer of the brain, with high morbidity, high mortality, and extremely poor prognosis. Despite recent advances in diagnosis and new treatment options, the median survival time of glioma patients is generally less than 2 years.Citation1 Novel and efficient therapeutic strategies are needed for this deadly disease.
Realgar has been used as a traditional Chinese medicine for thousands of years. In ancient China, realgar (called Xiong-Huang in Chinese) was applied for the treatment of carbuncles, scalds and burns, insect bites, abdominal pains, infantile convulsions, and psoriasis, among other things.Citation2 Recently, much attention has been given to its cytotoxic effect on a variety of cancer cells and its effect on many kinds of leukemia such as acute promyelocytic leukemia.Citation3 However, the application of realgar has been limited by its insolubility in water. Enhanced solubility and/or preparation as nanoparticles may improve the therapeutic potential of realgar. Although the methods of cryo-grinding and high-energy ball-milling for the production of realgar nanoparticles have been reported for many years,Citation3,Citation4 few studies have considered other methods of preparation, and none have evaluated the anti-proliferative effect of realgar nanoparticles on solid cancers. Here, we developed a novel method for preparing realgar nanoparticles for cancer treatment and evaluated their inhibitory effect on rat glioma cells.
Materials and methods
Materials
Realgar was obtained from Nanjing Medical Material Co (Jiangsu Province, China). High-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin, and fetal bovine serum were purchased from Gibco (Carlsbad, CA). Penicillin, streptomycin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and propidium iodide (PI) were purchased from Sigma (St Louis, MO). All other reagents were commercially available and of analytical grade. Rat glioma (C6) cells and mouse fibroblasts (L929) cells were provided by the Institute of Biochemistry and Cell Biology (Shanghai Institute of Biological Science, Chinese Academy of Sciences, China).
Preparation of realgar nanoparticles
Realgar powder was crushed in a mortar, added to 20 mL of 2 M HCl, and magnetically stirred at 40°C for 3 hours. The mixture was washed with distilled water several times until the suspension reached a neutral pH, and purified realgar was obtained after filtration. Purified realgar was added to a saturated Na2S solution, at a molar ratio of 1.4:1, with magnetic stirring at a warm temperature. After reaction for 2 hours under nitrogen protection, and filtered with distilled water several times, 0.2 M HCl was slowly added to the dissolved compound with constant stirring. The freshly prepared aqueous suspension was stored at 4°C in the dark.
Characterization of realgar nanoparticles
Transmission electron microscopy (TEM)
All observations were made using a transmission electron microscope (H7650; Hitachi High-Technologies Pte Ltd, Singapore).
Dynamic light scattering (DLS) analysis
The average size of realgar nanoparticles was measured by DLS at a wavelength of 633.0 nm with a detection angle of 90° using a Brookhaven BI9000AT system (Brookhaven Instruments Corp, Holtsville, NY) at 25°C.
Energy dispersive spectrometry (EDS)
The elemental composition of realgar nanoparticles and the proportions of each element were detected by an energy dispersive spectrometer (Thermo NORAN, Middleton, WI).
Inhibitory effects of realgar nanoparticles on C6 cells
MTT assay to measure cell viability and proliferation
Both C6 cells and L929 cells were seeded in 96-well plates at a density of 1 × 104 cells per well in 200 mL of high-glucose DMEM supplemented with 10% fetal bovine serum, and cultured at 37°C in an incubator with an atmosphere of 5% CO2. After 24 hours, the cells were treated with realgar nanoparticles, purified realgar, or traditional realgar at different concentrations (10, 20, 40, or 80 μg/mL) in triplicate. The cells were incubated for another 24, 48, or 72 hours. Untreated cells were used as controls, and wells with culture medium without cells were used as blank controls. For the cell proliferation assay, 100 μL of MTT (5 mg/mL in PBS) was added to each well and incubated at 37°C for 4 hours. The culture medium was removed, and 200 μL of dimethyl sulfoxide was added to each well for 5 minutes. The absorbance was measured at 570 nm using a universal microplate reader (Model EL x800G; BioTek Instruments, Winooski, VT). The half maximal inhibitory concentration (IC50) was calculated using SPSS software (v 18.0; SPSS Inc, Chicago, IL). Each experiment was performed at least three times. For both C6 and L929 cells, the percentage of cell survival according to the concentration of realgar was calculated using the following equation:Citation5 percentage (%) cell survival = [(At − Ab)/(Ac − Ab)] × 100, where At is the mean absorbance of the test well, Ab is the mean absorbance of the blank control, and Ac is the mean absorbance of the negative control.
Flow cytometric cell cycle analysis
To evaluate the presence of DNA fragments, cells were incubated with realgar nanoparticles, purified realgar, or traditional realgar at their respective IC50 values for 24 hours, harvested by trypsinization, flushed with complete culture medium, washed with PBS, and gently resuspended in 250 μL of hypotonic fluorochrome solution (50 μg of PI, 0.1% sodium citrate, and 0.1% Triton X-100 in PBS) with 100 U/mL RNase A to stain cell nuclei. The DNA content was determined by flow cytometry (FACSCalibur; Becton Dickinson, Mountain View, CA) and analyzed with CellQuest (Becton Dickenson) and ModFit LT (Verity Software House, Topsham, ME) software. For each sample, a total of 20,000 events were collected, and cell cycle distribution was determined based on DNA content.
Flow cytometric analysis of apoptosis
After treatment of C6 cells with realgar nanoparticles, purified realgar, or traditional realgar at their respective IC50 values for 24 hours, the cells were collected by centrifugation at 1500 rpm for 3 minutes, washed three times with cold PBS (0.15 M, pH 7.2), resuspended in 100 mL of binding buffer, and stained with 5 mL of Annexin V-FITC solution together with 5 mL of PI solution for 20 minutes at room temperature in the dark. To each sample tube, 400 μL of 1× binding buffer was added, and the samples were analyzed using a FACSCalibur and CellQuest software. All experiments were performed in triplicate.
Immunofluorescence staining
C6 cells were grown on coverslips in 24-well plates, serum-starved overnight, and treated with realgar nanoparticles, purified realgar, or traditional realgar at their respective IC50 values for 24 hours. The cells were fixed with 4% paraformaldehyde in PBS for 15 minutes, blocked with 1% bovine serum albumin in PBS for 1 hours, and incubated with specific primary antibody (anti-Bcl-2 or anti-Bax diluted 1:200 or 1:100, respectively, in blocking buffer) at 4°C overnight. The cells were then incubated with secondary antibody (AlexaFluor 532-conjugated anti-rabbit IgG, diluted 1:200 in blocking buffer) for 1 hour at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (0.5 mg/mL; DAPI) for 10 minutes. Images of DAPI and AlexaFluor 532 fluorescence were obtained using a fluorescence microscope (IX 71; Olympus, Tokyo, Japan) with a ×40 objective lens. Three independent experiments were performed with triplicate sample wells.
Statistical analysis
All experiments were repeated at least three times, and data for each sample were compiled from a minimum of triplicate wells. Data are expressed as means ± standard error. The results from treated and control cells were analyzed by ANOVA. Values of P < 0.05 were taken to indicate statistical significance.
Results and discussion
Characterization of realgar nanoparticles
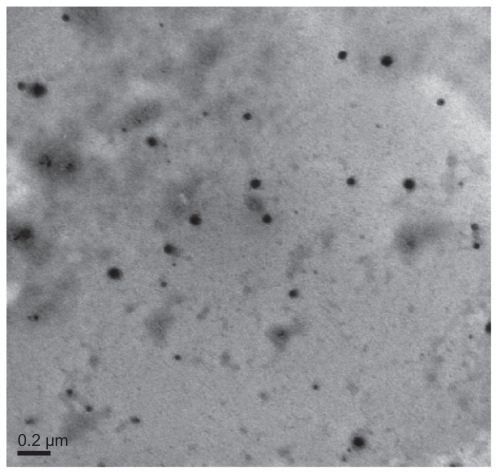
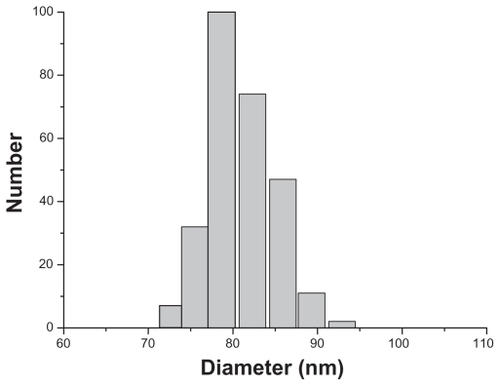
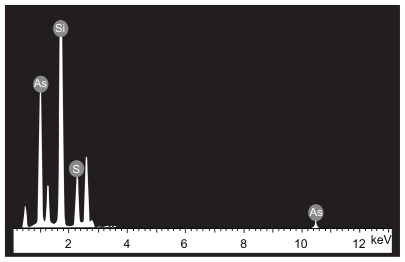
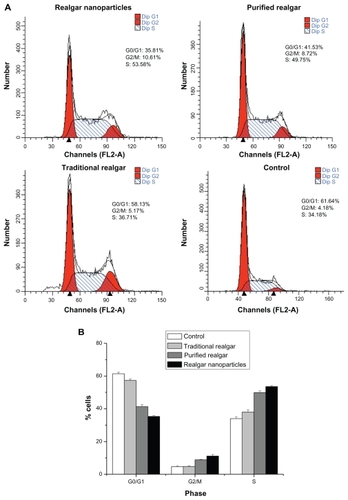
The morphological structure of the realgar nanoparticles was analyzed by TEM (). The nanoparticles appeared round or elliptical in shape, with a mean diameter of approximately 60–70 nm. No obvious particle aggregation was observed. DLS analyses revealed that the hydrodynamic diameter of realgar nanoparticles in water was approximately 80 nm (). Based on EDS ( and ), the realgar nanoparticle contained silicon (Si), arsenic (As), and sulfur (S). Arsenic and sulfur were present at mass percentages of 26.40% and 11.26%, respectively, and atomic percentages 25.68% and 26.12%, respectively, indicating that the atomic ratio was approximately 1:1.
Table 1 Elemental composition of realgar nanoparticles determined by energy dispersive spectrometric analysis
Inhibitory effects of realgar on C6 cells
Cell viability and proliferation
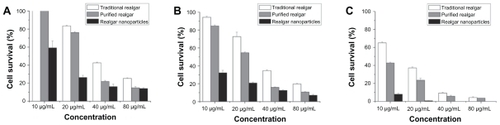
MTT assays demonstrated that realgar nanoparticles, purified realgar, and traditional realgar inhibited the growth of C6 cells in a dose- and time-dependent manner (). These findings are consistent with those of previous studies showing that realgar significantly suppressed the proliferation of tumor cells, including HaCaT, SiHa, and NB4-R1 cells, in a dose-dependent manner.Citation6–Citation8 Realgar nanoparticles showed the greatest anti-proliferative effect, followed by purified realgar and then traditional realgar (P < 0.05 for each). The IC50 values of realgar nanoparticles were 13 μg/mL at 24 hours and 3.2 μg/mL at 48 hours; no cells survived after realgar nanoparticle treatment for 72 hours. The IC50 values of purified realgar were 29.32 μg/mL at 24 hours, 22.25 μg/mL at 48 hours, and 5.88 μg/mL at 72 hours, and those of traditional realgar were 36.56 μg/mL at 24 hours, 31.79 μg/mL at 48 hours, and 15.41 μg/mL at 72 hours. We chose to use 13 μg/mL, the IC50 of realgar nanoparticles at 24 hours, for treatments in subsequent experiments. The effects of realgar nanoparticles, purified realgar, and traditional realgar on the growth of L929 cells showed no difference (data not shown).
Cell cycle analysis
The cell cycle distribution of C6 cells treated with realgar nanoparticles, purified realgar, traditional realgar, or PBS for 24 hours was analyzed by comparing the percentages of cells in G0/G1, S, and G2/M phases, without considering apoptotic cells (ie, cells in sub-G0/G1 phase). As shown in , after treatment with realgar nanoparticles, purified realgar, traditional realgar, or PBS for 24 hours, the percentage of cells in G1 was significantly decreased, and the percentages in G2/M and S phases were increased. These results indicate that realgar promoted the arrest of cells in the G2/M phase of the cell cycle, thereby inhibiting C6 cell proliferation. Similar findings have been reported in human ovarian and cervical cancer cells.Citation9
Figure 5 Realgar treatment altered the cell cycle distribution. C6 cells treated with realgar nanoparticles, purified realgar, traditional realgar, or PBS for 24 h were stained with PI and analyzed by flow cytometry (A). Graphical representation of realgar-treated cells in different phases of cellular cycle. Realgar treatment decreased the percentage of cells in G0/G1 and increased the percentages of cells in G2/M and S phases (B).
Notes: Results represent means ± SD of three independent experiments.
Apoptosis analysis
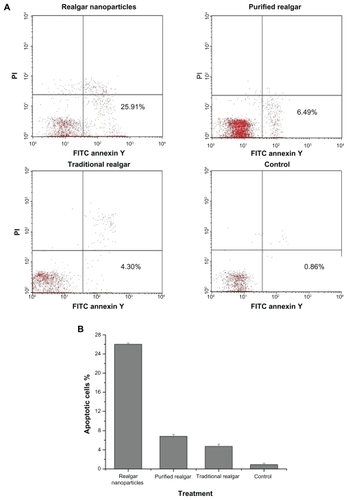
The anti-apoptotic effect of realgar nanoparticles on C6 cells was greater than that of purified or traditional realgar (P < 0.05; ). Purified realgar had a greater anti-apoptotic effect than traditional realgar (P < 0.05), which had a greater effect than control treatment (P < 0.05). The percentages of apoptotic cells after treatment with realgar nanoparticles, purified realgar, traditional realgar, and control were 26.02% ± 0.28%, 6.83% ± 0.39%, 4.73% ± 0.46%, and 0.91% ± 0.30%, respectively.
Figure 6 Realgar treatment promoted apoptosis. C6 cells were treated with realgar nanoparticles, purified realgar, traditional realgar, or PBS for 24 hours, stained with Annexin V-FITC/PI, and analyzed by flow cytometry (A). Realgar treatment increased the percentage of apoptotic cells (B).
Note: Results represent means ± SD of three independent experiments.
Immunofluorescence staining
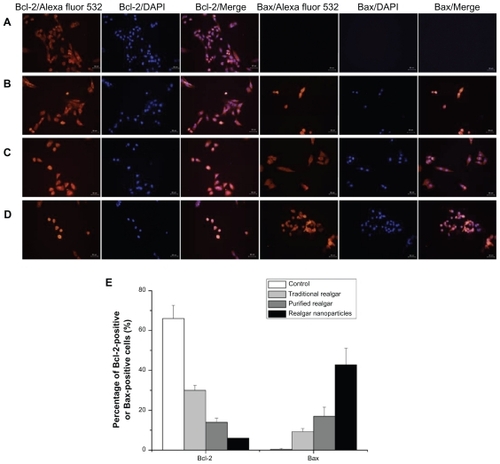
The apoptosis-related proteins Bcl-2 and Bax play crucial roles in apoptotic progression.Citation10 We determined the expression of Bcl-2 and Bax in realgar-treated C6 cells. Compared with the Bcl-2 immunofluorescence intensity in PBS-treated control cells, Bcl-2 immunofluorescence intensity was significantly reduced in realgar-treated cells (P < 0.05 for each; ). The greatest decrease was observed in cells treated with realgar nanoparticles, with smaller decreases in cells treated with purified realgar or traditional realgar. Bcl-2-positive cell frequencies after treatment with traditional realgar, purified realgar, and realgar nanoparticles were 30% ± 2.52%, 14% ± 2%, and 6% ± 4.16%, respectively (). These results indicate that realgar induced the downregulated expression of the anti-apoptotic Bcl-2 protein in C6 cells. In contrast, the expression of the pro-apoptotic Bax protein was upregulated by realgar treatment. Bax-positive cell frequencies after treatment with traditional realgar, purified realgar, and realgar nanoparticles were 9.3% ± 1.53%, 17% ± 4.5%, and 42.7% ± 8.33%, respectively (). These findings suggest that realgar nanoparticles can trigger apoptosis in C6 cells.
Figure 7 Immunofluorescence staining of Bcl-2 and Bax proteins in C6 cells treated with PBS (A), traditional realgar (B), purified realgar (C), or realgar nanoparticles (D). Semi-quantitative determination of the percentage of Bcl-2-positive or Bax-positive cells (E).
Notes: Magnification, ×400. Experiments were repeated in triplicate. For each sample, the percentage of positive cells was determined by counting four representative areas under the fluorescence microscope.
Conclusions
In this study, we successfully developed a new method for preparing realgar nanoparticles and showed that they significantly inhibited the growth of C6 glioma cells and led to cell apoptosis. Furthermore, we demonstrated realgar nanoparticles promoted apoptosis by inducing the upregulation of Bax and downregulation of Bcl-2 expression. Our study suggests that realgar nanoparticles are a promising in vitro anti-cancer strategy and may be applicable for human cancer therapy studies and clinical trials.
Acknowledgments
This work was supported by the Chinese National 863 Plan (no 2007 AA03Z356) and the Chinese National Natural Science Foundation (no 30770584 and 81171452).
Disclosure
The authors report no conflicts of interest in this work.
References
- JingYAokiHKakinumaKTherapeutic efficacy of targeting chemotherapy using local hyperthermia and thermosensitive liposome: evaluation of drug distribution in a rat glioma modelInt J Hyperthermia200420659560515370816
- ZhaoQHZhangYLiuYAnticancer effect of realgar nanoparticles on mouse melanoma skin cancer in vivo via transdermal drug deliveryMed Oncol201027220321219280372
- WangXBGaoHYHouBLHuangJXiRGWuLJNanoparticle realgar powders induce apoptosis in U937 cells through caspase MAPK and mitochondrial pathwaysArch Pharm Res200730565365817615687
- BalážPFabiánMPastorekMCholujováDSedlákJMechanochemical preparation and anticancer effect of realgar (As4S4) nanoparticleMater Lett2009631715421544
- PalizbanAASadeghi-AliabadiHAbdollahpourFEffect of cerium lanthanide on HeLa and MCF-7 cancer cell growth in the presence of transferringRes Pharm Sci20105211912521589800
- TseWPChengCHCheCTRealgar-mediated growth inhibition on HaCaT human keratinocytes is associated with induction of apoptosisInt J Mol Med200924218919619578792
- LiuRPuDLiuYInduction of SiHa cell apoptosis by nanometer realgar suspension and its mechanismJ Huazhong Univ Sci Technolog Med Sci200828331732118563332
- QiJHePChenWWangHWangXZhangMComparative proteome study of apoptosis induced by As4S4 in retinoid acid resistant human acute promyelocytic leukemia NB4-R1 cellsLeuk Res201034111506151620403635
- WuJZHoPCEvaluation of the in vitro activity and in vivo bioavailability of realgar nanoparticles prepared by cryo-grindingEur J Pharm Sci2006291354416824739
- KirschDGDoseffAChauBNCaspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome cJ Biol Chem199927430211552116110409669