Abstract
Engineered nanoparticles that can be injected into the human body hold tremendous potential to detect and treat complex diseases. Understanding of the endocytosis and exocytosis mechanisms of nanoparticles is essential for safe and efficient therapeutic application. In particular, exocytosis is of significance in the removal of nanoparticles with drugs and contrast agents from the body, while endocytosis is of great importance for the targeting of nanoparticles in disease sites. Here, we review the recent research on the endocytosis and exocytosis of functionalized nanoparticles based on various sizes, shapes, and surface chemistries. We believe that this review contributes to the design of safe nanoparticles that can efficiently enter and leave human cells and tissues.
Introduction
Nano-sized materials have been increasingly used in the medical field to improve the target efficiency of drugs.Citation1–Citation3 In order to successfully apply nanoparticles in drug delivery, their physical and chemical properties must first be understood, thereby assisting in controlling the biological responses to their use. Because drug delivery nanosystems transport pharmaceutical compounds in the body, it is important to understand their physiochemical properties to safely achieve a desired therapeutic effect.
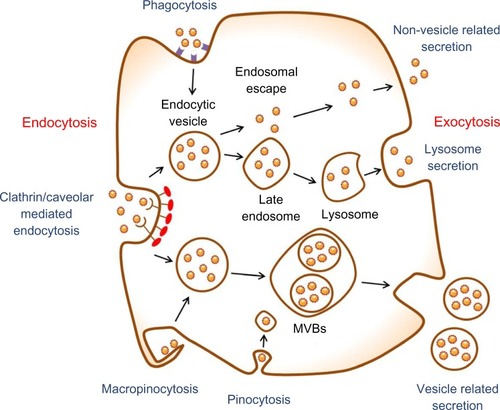
However, these drug delivery nanosystems have shown some limitations regarding the toxicity of the nanoscale materials in the body.Citation4,Citation5 In order to reduce their toxicity, it is crucial to study endocytosis, exocytosis, and clearance mechanisms for nanoparticles released from the nanoparticle–drug conjugates. Nanoparticles exposed to the bloodstream interact with opsonin proteins. When opsonin proteins attach to the surface of nanoparticles, they allow macrophages of the mononuclear phagocytic system (MPS) to easily recognize the nanoparticles and hence the nanoparticles eventually accumulate in the MPS organs, such as liver and spleen. These phenomena cause low targeting efficiency and severe systemic toxicity of the drug-delivery nanosystems. Therefore, this review focuses on endocytosis and exocytosis patterns of nanoparticles in mammalian cells with respect to their size, shape, and surface chemistry ().
Figure 1 Schematic of endocytosis and exocytosis patterns of nanoparticles. Nanoparticles enter the cell via four types of pathway: clathrin/caveolar-mediated endocytosis, phagocytosis, macropinocytosis, and pinocytosis. Nanoparticles exit the cell via three types of pathway: lysosome secretion, vesicle-related secretion, and non-vesicle-related secretion.
Abbreviation: MVBs, multivesicular bodies.
Nanoparticle stability
Nanoparticles have been widely used in the fields of drug delivery and bioimaging because their size, shape, and surface properties can be precisely engineered for specific diseases.Citation6,Citation7 The nanoparticle surface can be modified with various targeting molecules (eg, antibody, peptide, aptamer, etc) in order to achieve efficient targeting to disease sites.
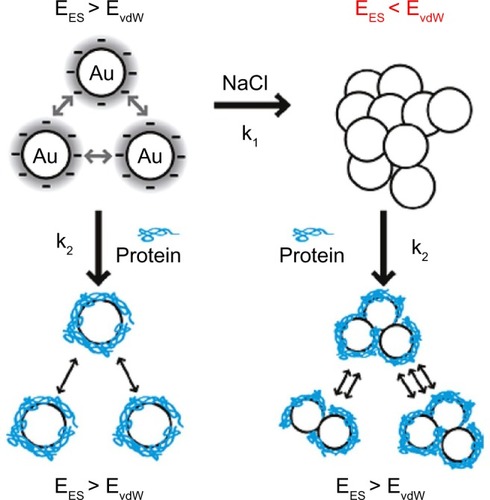
Recently, many scientists have begun to investigate the effects of different sizes, shapes, and surface chemistries on endocytosis, toxicity, and gene regulation.Citation8–Citation10 However, aggregates (strongly bonded between nanoparticles) and agglomerates (loosely bonded between nanoparticles or aggregates acting under weak forces, eg, van der Waals force) formed by forces between nanoparticles and components in biological media have not been fully considered to optimize their physicochemical properties for biological applications. It was recently suggested that the aggregates or agglomerates occur when the van der Waals attractive forces between nanoparticles are larger than the electrostatic repulsive forces ().Citation11 Aggregated or agglomerated forms of nanoparticles would behave differently within biological systems than would nanoparticles in their single form. Thus, size uniformity of nanoparticles should be considered when the effect of physical and chemical properties of nanoparticles on their interactions with biological systems is examined.
Figure 2 Scheme of aggregation or agglomeration mechanism. The stabilizing electrostatic forces (EES) on the surface of bare nanoparticles are neutralized by NaCl ions in the biological solution, causing the van der Waals forces (EvdW) to drive formation of aggregation or agglomeration. The protein coating of nanoparticles can reduce the aggregation or agglomeration.
Note: Reproduced with permission from Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478–5489.Citation11 Copyright © 2011 American Chemical Society.
In biological solutions, such as blood, saliva, and cell culture media, the surface chemistry of nanoparticles plays a crucial role in determining their behavior because they are directly related to types and compositions of biomolecules attached to the nanoparticle surface. The surface chemistries on the nanoparticle surface are dynamically changed because various biomolecules are attached and detached based on their binding affinity to the surface. Pre-coating of the nanoparticle surface with stabilizing molecules such as polyethylene glycol (PEG), deoxyribonucleic acid (DNA), and albumin has been utilized to reduce ionic strength and prevent nanoparticles from aggregation or agglomeration in the biological solutions.Citation12–Citation14
Additionally, individual nanoparticles can be naturally coated with various biomolecules, forming the nanoparticle–protein complex, when solubilized in biological solutions. The stability lifetimes of the nanoparticle–protein complexes range from hours to days in the biological solutions. The proteins covering the nanoparticle surface further prevent the individual nanoparticles from aggregation or agglomeration. Because the formation of nanoparticle–protein complexes is mainly determined by surface chemistries of the nanoparticles, it is important to investigate which surface chemistry is the most favorable to form the nanoparticle–protein complex. Therefore, natural nanoparticle–protein complexes formed in biological environments would allow us to study how individual nanoparticles interact with various types of cells.
When we study the endocytosis and exocytosis of nanoparticles, cells are treated with the nanoparticles in the culture medium containing various serum proteins. Most of the nanoparticles are first coated with the serum proteins and then met with the plasma membrane of cells. If the nanoparticles are already aggregated or agglomerated prior to binding to the membrane, their endocytosis patterns would differ from the endocytosis patterns of individual nanoparticles. The degree of aggregation or agglomeration of the nanoparticles can be determined by measuring time-dependent change of size and surface charge of the nanoparticles in the culture medium. The (hydrodynamic) size has been mainly analyzed using transmission electron microscopy (TEM) and dynamic light scattering (DLS) while the surface charge is determined by zeta potential measurements. In particular, the ultraviolet–visible (UV/Vis) spectrophotometry has also been used to monitor the size of gold nanoparticles because their localized surface plasmon resonance peaks can be shifted to a longer wavelength by increasing their size.Citation15 The DLS technique has been the most widely used to monitor size change because it directly measures hydrodynamic sizes of protein-coated nanoparticles in the biological solution with nanometer precision. Furthermore, the zeta potentials of protein-coated nanoparticles mostly appeared as a negative surface charge although the nanoparticles had different original surface chemistries. It suggests that most proteins attached on the nanoparticle surface seem to be negatively charged, regardless of their content and composition, although they may alter depending on the surface chemistries of the nanoparticles. Thus, the content and composition of proteins preferentially attached on the nanoparticle surfaces should be studied for the cellular uptake and immune response of nanoparticles.Citation16,Citation17
Nanoparticle endocytosis
Endocytosis mechanism
All types of cells in the body use the endocytosis process to communicate with the biological environments. This process is an energy-dependent process through which cells internalize ions and biomolecules.Citation18 In particular, the cells internalize nutrients and signaling molecules to obtain energy and interact with other cells, respectively. The endocytosis pathways are typically classified into clathrin- and caveolae-mediated endocytosis, phagocytosis, macropinocytosis, and pinocytosis (). Clathrin- and caveolae-mediated endocytosis indicates receptor-mediated endocytosis. Many types of cells use the clathrin- and caveolae-mediated endocytosis pathways to internalize nanoscale materials, including viruses and nanoparticles.Citation19–Citation21 These endocytosis pathways are the most important pathways for the internalization of nanoparticles into cells because the nanoparticles are directly coated with the plasma proteins when exposed to physiological solutions. The phagocytosis pathway is used when phagocytic cells internalize foreign materials with sizes larger than 0.5 μm.Citation16 The phagocytosis pathway is actin-dependent and restricted to professional phagocytes, such as macrophages, dendritic cells, and neutrophils. The macropinocytosis pathway is a non-specific process to internalize fluids and particles together into the cell, whereas the pinocytosis pathway absorbs biological fluids from the external environment of a cell.Citation22 These pathways are very important to translocate single nanoparticles with sizes below 10 nm into the cell.
Table 1 Classification of endocytosis pathways
When nanoparticles are systemically administered into the body, they are confronted with many types of cells. Since nanoparticles have emerged as effective drug carriers to treat complex diseases, it has become crucial to understand nanoparticle endocytosis mechanisms. It is believed that the endocytosis efficiency of nanoparticles is dependent on the physicochemical properties, such as size, shape, and surface chemistry, as well as cell type. Thus, interaction of nanoparticles with cells depending on their physicochemical properties is discussed in the following sections.
Factors affecting nanoparticle endocytosis
Nanoparticles circulating in the bloodstream happen to meet and internalize into many types of cells through the plasma membrane. The plasma membrane is a selectively permeable membrane that transfers materials that are essential for sustaining the cell’s life. Naturally, materials necessary for the cell’s life, such as ions and nano-sized proteins, can pass through the lipid bilayer using specialized membrane-transport protein channels.Citation23 Thus, the plasma membrane of cells would select the endocytosis pathways of nanoparticles depending on their size, shape, and surface chemistry ().
Table 2 Endocytosis of nanoparticles
Size
Size-dependent cellular uptake of nanoparticles has been extensively investigated in various cell lines because the nanoparticle size has been known to be a key determinant of the uptake pathways. Many critical in vivo functions of nanoparticles, such as circulation time, targeting, internalization, and clearance, depend on their size. Thus, the cellular uptake of nanoparticles with various sizes is reviewed in this subsection.
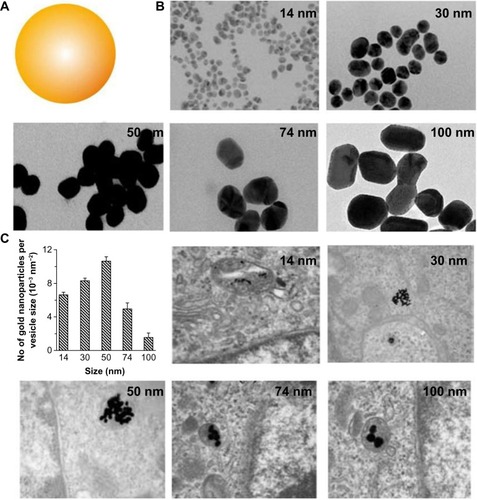
Much interest has focused on understanding size-dependent internalization of nanoparticles in cancer cells and fibroblasts. The cellular uptake of gold nanoparticles of various sizes was studied in human cervical cancer cells ().Citation8,Citation9,Citation24 Researchers at the University of Toronto, Canada, demonstrated that uptake mechanism and saturation concentration of nanoparticles was dependent on their size. The 50 nm gold nanoparticles showed the most efficient cellular uptake compared with other sizes. The cellular uptake of polystyrene (PS) nanoparticles of various sizes was also tested on a human colon adenocarcinoma cell line.Citation25 The PS nanoparticles with a size of 100 nm were taken up into the cells more efficiently than those with sizes of 50, 200, 500, and 1,000 nm. The internalization efficiency of the PS nanoparticles with a size of 50 nm was the lowest of all sizes. These two studies suggest that the patterns of cellular uptake could also vary according to nanoparticle material type. Although different cancer cell lines were used for each experiment, the stiffness of nanoparticles could affect their cellular uptake. Stiffer nanoparticles would interact tightly with the plasma membrane of cell, thereby causing rapid endocytosis.
Figure 3 (A) Schematic representation of gold nanoparticles. (B) Transmission electron microscope images of citrate-coated gold nanoparticles with various sizes. (C) Transmission electron microscope images of the gold nanoparticles entrapped in cellular vesicles. Graph showing the number of the gold nanoparticles per vesicle diameter.
Note: Reproduced with permission from Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668.Citation8 Copyright © 2007 American Chemical Society.
Single-walled carbon nanotubes (SWNTs) coated with DNA molecules were used to investigate length-dependent cellular uptake.Citation26 The results demonstrated that long (660 ± 40 nm) and short (130 ± 18 nm) SWNTs have lower uptake efficiencies in the fibroblasts than SWNTs with average lengths of 430 ± 35 nm and 320 ± 30 nm. The SWNTs with an average length of 320 ± 30 nm had the greatest uptake pattern.
In addition to endocytosis in non-phagocytic cells, much attention has recently been paid to understanding interactions between nanoparticles and phagocytic cells such as macrophages, because it could be relevant to the design of nanoparticles to avoid the immune system, thus increasing their target efficiency. Nanoparticles with sizes larger than 0.5 μm have been known to enter phagocytic cells via phagocytosis pathways. Polymeric microspheres with diameters of 2–3 μm exhibited the maximal phagocytosis rate.Citation27 Interestingly, this size range coincides with the general size of the bacteria that are the most common targets of the MPS.
On the other hand, there have been some efforts to examine size-dependent phagocytosis of nanoparticles smaller than 0.5 μm. Among many types of nanoparticles, lipid-based nanoparticles, including US Food and Drug Administration (FDA)-approved liposomes have been of particular interest in the drug-delivery field due to their drug-loading capability and biocompatibility. Lipid nanoparticles with sizes of 20, 50, and 100 nm were taken up into the macrophages by complement receptor-mediated phagocytosis.Citation28 In addition, liposomes with sizes ranging from 100 to 2,000 nm were also tested for their intracellular uptake in the macrophages.Citation29 The amount of liposomes taken up by the macrophages increased with size over the range 100–1,000 nm, but the uptake rate became constant with sizes over 1,000 nm. Size-dependent phagocytosis of gold nanoparticles was also studied in many research groups. It was reported that gold nanoparticles with sizes below 100 nm were phagocytosed via scavenger receptor-mediated phagocytosis.Citation30 Tsai et alCitation31 also demonstrated that gold nanoparticles with a size of 4 nm showed the highest uptake in the macrophages based on the number of nanoparticles taken up per cell, compared with those sized 11, 19, 35, and 45 nm. The 4 nm gold nanoparticles exhibited the highest potency in inhibiting tumor necrosis factor (TNF)-α production related to TNR9-mediated innate immune systems.
Although the experimental results introduced here showed size-dependent cellular uptake of nanoparticles in many types of cells, the physical size would not be fully reflected when they meet the plasma membrane of the cell. Most nanoparticles tend to aggregate in biological solutions, increasing their overall size. The results regarding the size effect would be influenced by nanoparticle aggregation before entering the cell. Thus, it should be tested whether nanoparticles prepared to study their size-dependent endocytosis retain their singularity in the biological media before they enter the cell.
Surface chemistry
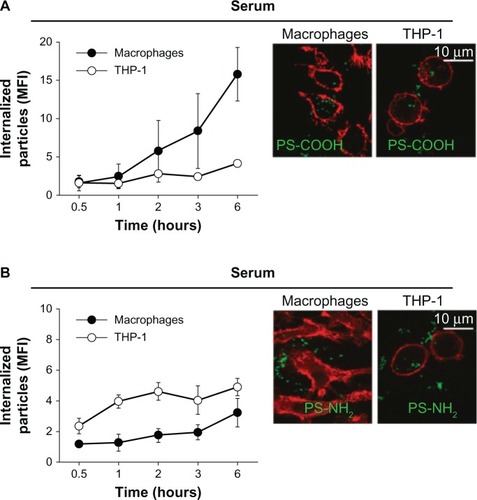
Surface chemistry (or surface charge) of nanoparticles can be determined by the chemical composition on the nanoparticle surface. The surface charge of nanoparticles can affect their efficiency and the pathway of cellular uptake, because biological systems consist of numerous biomolecules with various charges. Therefore, the charge of biomolecules covering the nanoparticle surface can influence the endocytosis patterns of nanoparticles. In this subsection, the effect of nanoparticle surface chemistries on the endocytosis pattern is discussed. For polymeric nanoparticles, carboxymethyl chitosan-grafted nanoparticles as negatively charged nanoparticles, and chitosan hydrochloride-grafted nanoparticles as positively charged nanoparticles, were used to test their cellular uptake efficiency.Citation32 The different surface charges significantly affected their uptake by macrophages. The positively charged nanoparticles exhibited a higher phagocytic uptake than did the negatively or neutrally charged nanoparticles. Moreover, when the uptake efficiency of the positively charged nanoparticles was compared with that of the negatively charged, neutrally charged, and PEGylated nanoparticles, the positively and negatively charged nanoparticles were internalized more rapidly than the neutrally and PEGylated charged nanoparticles. It was also demonstrated that negatively charged polymeric nanoparticles with diameters of around 100 nm were more efficiently phagocytized by macrophages than positively charged nanoparticles ().Citation33 Cellular uptake was also greater in the macrophages than in the monocytes.
Figure 4 Kinetics of cellular uptake of negatively (–COOH) (A) and positively (–NH2) (B) charged polymeric nanoparticles in macrophages and monocytes (THP-1). Confocal fluorescence images (right) were taken after 2 hours’ incubation with the indicated nanoparticles. Cell membrane was stained with red dyes and the nanoparticles were tagged with green dyes.
Note: Reproduced with permission from Lunov O, Syrovets T, Loos C, et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano. 2011;5:1657–1669.Citation33 Copyright © 2011 American Chemical Society.
Abbreviation: MFI, mean fluorescence index.
Furthermore, the surface functionalization with PEG, poloxamer, and poloxamine polymers prevented phagocytosis because these polymers protect the nanoparticles from ionic strength, promote particle dispersion, and reduce absorption of proteins in blood on their surface.Citation34 In addition, the uptake efficiency of PEGylated nanoparticles is closely related to PEG grafting density, which can determine the protein absorption.Citation17 That is, high PEG-grafting density inhibits protein adsorption on the nanoparticle surface in the biological solution. Interaction between nanoparticles and biological media can further lead to surface modification that eventually affects their phagocytosis through the attachment of complementary proteins and immunoglobulins.Citation35
Recently, there has been much effort to utilize biological nanomaterials in drug delivery applications, due to their biocompatibility and natural cell-binding ability. Apoferritin, a demineralized form of ferritin, was suggested as a drug carrier because it has been known to enter the cell via ferritin receptor-mediated endocytosis.Citation36,Citation37 Apoferritin can be further utilized for a switchable delivery system because the endocytosis can be reversibly inhibited in various ways. Additionally, the internal cavity of apoferritin can be used to load therapeutic molecules through channels on the protein shell. Other virus- and protein-based biomaterials also have great potential to serve as biocompatible nano-platforms in the drug-delivery system.Citation38
Shape
As mentioned in previous subsections, size and surface properties of nanoparticles play crucial roles in controlling the interaction between nanoparticles and biological systems. The nanoparticle shape might also be important in determining biological behaviors of nanoparticles. For example, it has been reported that bacteria with various shapes, such as rods, spirals, and ellipsoids, have implications for macrophage recognition.Citation16 These shapes can directly affect the endocytosis pattern of bacteria. Recent experiments have demonstrated a shape effect of the nanoparticle on cellular uptake.Citation8,Citation39 Nanoscale rods exhibit the highest uptake in human cervical cancer cells, followed by spheres, cylinders, and cubes, and the cellular uptake of cylindrical particles depends strongly on their aspect ratio. However, the receptor-mediated endocytosis of gold nanorods was vastly decreased with increases in their aspect ratio. The comparison of intracellular uptake efficiency between rod-shaped and spherical nanoparticles has also been investigated in many types of cells.Citation40 Interestingly, the results demonstrated that the uptake of rod-shaped nanoparticles by macrophages was more efficient than that of the spherical nanoparticles, while the spherical nanoparticles were taken up by cervical cancer cells and human lung epithelial cells more efficiently than were rod-shaped nanoparticles.
Nanoparticle exocytosis
An understanding of the cellular uptake and organ distribution of nanoparticles is important when examining their targeting and therapeutic efficiency in drug-delivery applications. Nanoparticles administered into the body are eventually cleared by organs in the MPS, such as the liver and spleen. Nanoparticles remain in these organs for a long time after being taken up by the macrophages, which increases the likelihood of unintended acute or chronic toxicity. Thus, it is also crucial to study exocytosis of the internalized nanoparticles from many types of cells, particularly macrophages, to evaluate their biosafety. However, compared with investigations of nanoparticle endocytosis, relatively little effort has been made to investigate the exocytosis of nanoparticles that may be responsible for their systemic elimination and toxicity. Thus, the exocytosis of functionalized nanoparticles is reviewed in this section ().
Table 3 Exocytosis of nanoparticles
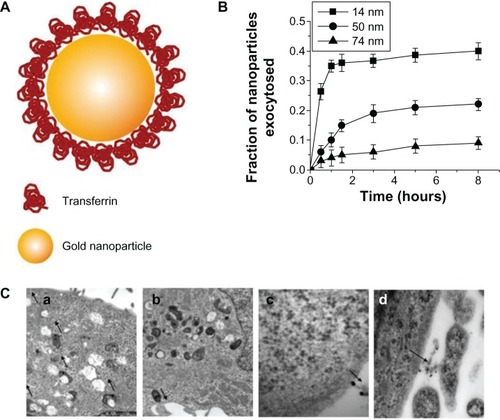
Many studies have compared the exocytosis of rod-shaped nanoparticles with that of spherical nanoparticles. Chithrani and ChanCitation9 examined differences in exocytosis phenomena between spherical and rod-shaped gold nanoparticles using various cell types (). The surface of gold nanoparticles was coated with transferrin proteins for their receptor-mediated endocytosis. Importantly, this work described that the cellular uptake could be considered as a result of competition between the thermodynamic driving force for wrapping and the receptor diffusion kinetics. In that respect, the 50 nm gold nanoparticles showed the fastest wrapping time, and therefore the receptor-ligand interaction could produce sufficient free energy to drive the nanoparticles into the cell. On the other hand, smaller nanoparticles with a slower wrapping time exhibited a faster rate of exocytosis. The exocytosis rate of the 14 nm nanoparticles was much faster than that of the 74 nm nanoparticles. In addition, the fraction of the rod-shaped nanoparticles released outside the cells was generally higher than that of the spherical nanoparticles. The exocytosis of peptide-coated gold nanoparticles was also investigated in endothelial cells.Citation41 Nanoparticles functionalized with KATWLPPR peptides have been known to bind to plasma membrane receptors on endothelial cells for endocytosis, while nanoparticles coated with KPRQPSLP peptides did not interact with the receptors for endocytosis. KATWLPPR peptide-coated nanoparticles taken up by cells were progressively exocytosed up until 6 hours. On the other hand, KPRQPSLP peptide-coated nanoparticles showed a more complex exocytosis profile. Interestingly, it was found that the exocytosed KPRQPSLP peptide-coated nanoparticles were re-taken up by the cells after 4 hours.
Figure 5 (A) Schematic depicting of transferrin-coated gold nanoparticles. (B) Kinetics of exocytosis patterns of the nanoparticles with different sizes. (C) Different stages of exocytosis patterns: (a) Movement of the vesicles containing nanoparticles toward the cell membrane; (b) Docking of one of the vesicles at the cell membrane; (c) Excretion of nanoparticles; (d) Cluster of nanoparticles after exocytosis.
Note: Reproduced with permission from Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550.Citation9 Copyright © 2007 American Chemical Society.
The exocytosis of poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles was also examined in vascular smooth muscle cells.Citation42 The size and zeta potential of PLGA nanoparticles coated with bovine serum albumin (BSA) were around 97 nm and –20 mV, respectively. The cellular uptake of nanoparticles increased with incubation time. The exocytosis of nanoparticles increased up to 65% of the internalized fraction within 30 minutes when nanoparticles in the culture media were removed. In addition, the exocytosis of nanoparticles was found to be energy-dependent because it was significantly inhibited with sodium azide and deoxyglucose. Furthermore, authors demonstrated that the exocytosis pattern of nanoparticles was dependent on the proteins in the medium, because the proteins were carried into the cells along with the nanoparticles and interacted with biological systems inside the cells.
Exocytosis of polysaccharide cationic nanoparticles was also studied in airway epithelium cells.Citation43 The cationic polymer hydroxycholine was used to coat nanoparticles. After the surface modification, the nanoparticles appeared to be an approximate size of 60 nm. For exocytosis experiments, human bronchial epithelial cells were treated with the nanoparticles for 30 minutes. The amount of exocytosed nanoparticles increased significantly after 1 hour. In addition, cholesterol depletion completely blocked the exocytosis of nanoparticles, indicating that their exocytosis is cholesterol-dependent.
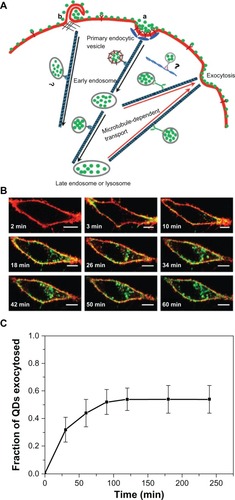
The exocytosis patterns of superparamagnetic iron oxide nanoparticles and quantum dots were also studied in many research groups. Serda et alCitation44 investigated the intracellular trafficking of iron oxide nanoparticles delivered into macrophage cells using porous silicon microcarriers (). In the exocytosis process, the amine-functionalized nanoparticles enriched in the multivesicular bodies were incorporated into the membrane vesicles in the intracellular region and efficiently secreted from the cells 6 days after intracellular delivery. The exocytosis rate of 15 nm nanoparticles was faster than that of 30 nm nanoparticles, thus indicating that smaller nanoparticles are more favorable for exocytosis. Exocytosis of zwitterionic quantum dots was also examined in human cervical cancer cells ().Citation45 Quantum dots were coated with D-penicillamine to improve colloidal stability in biological solutions. The size of D-penicillamine-coated quantum dots was around 4 nm, which is much smaller than conventional nanoparticles studied for exocytosis. In the endocytosis process, most of these smaller nanoparticles were observed on the plasma membrane prior to internalization. In addition, the quantum dots were found to enter the cells via a clathrin-mediated endocytosis pathway and macropinocytosis. In the exocytosis process, some of the quantum dots trapped in endosomes were actively transported to the cell periphery and exocytosed to the media within 21 minutes after internalization.
Figure 6 (A) A scanning electron microscopy image of a macrophage showing endocytosis of porous silicon particles incorporated with iron oxide nanoparticles, intracellular partitioning of the particles, endosomal escape of the particles, and exocytosis of the incorporated iron oxide nanoparticles. (B) Exocytosis of iron oxide nanoparticles. Upper column shows transmission electron microscopy images of iron oxide nanoparticles released from the porous silicon carrier in a macrophage. Middle column shows transmission electron microscopy images of the released iron oxide nanoparticles located in the intracellular region of the macrophage 6 days after uptake of the porous silicon particles. Bottom column shows that graph displayed time-dependent iron content in the supernatant and transmission electron microscopy images showed the internalized iron oxide nanoparticles were exocytosed by membrane vesicles.
Note: Reproduced with permission from Serda RE, Mack A, van de Ven AL, et al. Logic-embedded vectors for intracellular partitioning, endosomal escape, and exocytosis of nanoparticles. Small. 2010;6:2691–2700.Citation44 Copyright © 2010 John Wiley & Sons, Inc.
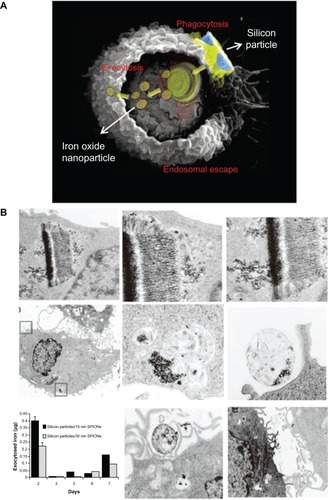
Figure 7 (A) Schematic diagram showing the endocytosis and exocytosis processes of D-penicillamine-coated quantum dots; a: Clathrin-mediated endocytosis; b: Macropinocytosis. (B) Interaction of D-penicillamine-coated quantum dots (green) with plasma membrane of a HeLa cell before internalization. The plasma membrane was stained with the red membrane dye. Scale bar: 10 μm. (C) Kinetics of exocytosis of D-penicillamine-coated quantum dots after removing the nanoparticles in the media.
Note: Reproduced with permission from Jiang X, Röcker C, Hafner M, Brandholt S, Dörlich RM, Nienhaus GU. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano. 2010;4:6787–6797.Citation45 Copyright © 2010 American Chemical Society.
Abbreviations: QDs, quantum dots; min, minutes.
Summary and future prospects
This review article summarizes the endocytosis and exocytosis of nanoparticles of different sizes, shapes, and surface chemistries in many types of cells. Generally, smaller nanoparticles seem to enter and exit the cell more efficiently. Spherical nanoparticles are more favorable to be internalized into the cell than cylindrical nanoparticles. Positively charged nanoparticles exhibited much higher rates of endocytosis than negatively or neutrally charged nanoparticles, while they seem to remain in the cell longer.
Although nanoparticle-based drug delivery has been actively developed to treat complex diseases, concerns regarding nanoparticle biosafety have still been raised, limiting their clinical translations. Understanding the endocytosis of nanoparticles enables us to develop more efficient intracellular drug-delivery nanosystems, while understating their exocytosis allow us to develop more clearable delivery nanosystems after drug delivery. However, the exocytosis patterns of nanoparticles remain not yet fully understood, although the cellular uptake of nanoparticles has been studied by many research groups. Thus, a considerable amount of effort to study the endocytosis and exocytosis of nanoparticles should be made in order to develop clinically translatable targeted nanoparticles in the drug-delivery field.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (grant no 2012M3A9C6050125), the National R&D Program for Cancer Control, Ministry for Health and Welfare (grant no 1220070), and the World Class University program funded by the Ministry of Education, Science and Technology, Republic of Korea (grant no R32-2008-000-10218-0).
References
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer20055316117115738981
- RuoslahtiEBhatiaSNSailorMJTargeting of drugs and nanoparticles to tumorsJ Cell Biol2010188675976820231381
- JainRKStylianopoulosTDelivering nanomedicine to solid tumorsNat Rev Clin Oncol201071165366420838415
- ChoiHSLiuWMisraPRenal clearance of quantum dotsNat Biotechnol200725101165117017891134
- ParkJHGuLvon MaltzahnGRuoslahtiEBhatiaSNSailorMJBiodegradable luminescent porous silicon nanoparticles for in vivo applicationsNat Mat200984331336
- NiikuraKIyoNMatsuoYMitomoHIjiroKSub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiationACS Appl Mater Interfaces201353900390723566248
- YuXSongSKChenJHigh-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agentMagn Reson Med20004486787211108623
- ChithraniBDGhazaniAAChanWCDetermining the size and shape dependence of gold nanoparticle uptake into mammalian cellsNano Lett2006666266816608261
- ChithraniBDChanWCElucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapesNano Lett200771542155017465586
- ChoECXieJWurmPAXiaYUnderstanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchantNano Lett200991080108419199477
- AlbaneseAChanWCEffect of gold nanoparticle aggregation on cell uptake and toxicityACS Nano201155478548921692495
- GillichTAcikgözCIsaLSchlüterADSpencerNDTextorMPEG-stabilized core-shell nanoparticles: impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregationACS Nano2013731632923214719
- EhrenbergMSFriedmanAEFinkelsteinJNOberdörsterGMcGrathJLThe influence of protein adsorption on nanoparticle association with cultured endothelial cellsBiomaterials20093060361019012960
- ParkHGOhJHLeeJSAssembly-based titration for the determination of monodisperse plasmonic nanoparticle concentrations using DNAAnal Chem2011834989499521615088
- NamJWonNJinHChungHKimSpH-Induced aggregation of gold nanoparticles for photothermal cancer therapyJ Am Chem Soc2009131136391364519772360
- YoungKDThe selective value of bacterial shapeMicrobiol Mol Biol Rev20067066070316959965
- WalkeyCDOlsenJBGuoHEmiliAChanWCNanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptakeJ Am Chem Soc20121342139214722191645
- IversenTGSkotlandTSandvigKEndocytosis and intracellular transport of nanoparticles: present knowledge and need for future studiesNano Today20116176185
- PelkmansLKartenbeckJHeleniusACaveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ERNat Cell Biol2001347348311331875
- WangZTiruppathiCMinshallRDMalikABSize and dynamics of caveolae studied using nanoparticles in living endothelial cellsACS Nano200934110411619919048
- TomatisMTurciFCeschinoRHigh aspect ratio materials: role of surface chemistry vs length in the historical “long and short amosite asbestos fibers”Inhal Toxicol20102298499820718637
- GeiserMUpdate on macrophage clearance of inhaled micro- and nanoparticlesJ Aerosol Med Pulm Drug Deliv20102320721720109124
- WoolfTBRouxBStructure, energetics, and dynamics of lipid-protein interactions: a molecular dynamics study of the gramicidin A channel in a DMPC bilayerProteins199624921148628736
- JiangWKimBYRutkaJTChanWCNanoparticle-mediated cellular response is size-dependentNat Nanotechnol2008314515018654486
- WinKYFengSSEffects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugsBiomaterials2005262713272215585275
- JinHHellerDASharmaRStranoMSSize-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticlesACS Nano2009314915819206261
- ChampionJAWalkerAMitragotriSRole of particle size in phagocytosis of polymeric microspheresPharm Res2008251815182118373181
- VonarbourgAPassiraniCSaulnierPSimardPLerouxJCBenoitJPEvaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptakeJ Biomed Mater Res A20067862062816779767
- ChonoSTaninoTSekiTMorimotoKUptake characteristics of liposomes by rat alveolar macrophages: influence of particle size and surface mannose modificationJ Pharm Pharmacol200759758017227623
- FrançaAAggarwalPBarsovEVKozlovSVDobrovolskaiaMAGonzález-FernándezÁMacrophage scavenger receptor A mediates the uptake of gold colloids by macrophages in vitroNanomedicine (Lond)201161175118821675859
- TsaiCYLuSLHuCWYehCSLeeGBLeiHYSize-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophagesJ Immunol2012188687622156340
- HeCHuYYinLTangCYinCEffects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticlesBiomaterials2010313657366620138662
- LunovOSyrovetsTLoosCDifferential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell lineACS Nano201151657166921344890
- MoghimiSMRe-establishing the long circulatory behaviour of poloxamine-coated particles after repeated intravenous administration: applications in cancer drug delivery and imagingBiochim Biophys Acta1999147239940310572962
- DobrovolskaiaMAMcNeilSEImmunological properties of engineered nanomaterialsNat Nanotechnol2007246947818654343
- ZhangLFischerWPippelEHauseGBrandschMKnezMReceptor-mediated cellular uptake of nanoparticles: a switchable delivery systemSmall201171538154121538872
- ZhangLLaugLMünchgesangWReducing stress on cells with apoferritin-encapsulated platinum nanoparticlesNano Lett20101021922320017497
- KaiserCRFlennikenMLGillitzerEBiodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivoInt J Nanomedicine2007271573318203438
- QiuYLuYWangLSurface chemistry and aspect ratio mediated cellular uptake of Au nanorodsBiomaterials2010317606761920656344
- BartneckMKeulHASinghSRapid uptake of gold nanorods by primary human blood phagocytes and immunomodulatory effects of surface chemistryACS Nano201043073308620507158
- BartczakDNittiSMillarTMKanarasAGExocytosis of peptide functionalized gold nanoparticles in endothelial cellsNanoscale201244470447222743818
- PanyamJLabhasetwarVDynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cellsPharm Res20032021222012636159
- DombuCYKroubiMZiboucheRMatranRBetbederDCharacterization of endocytosis and exocytosis of cationic nanoparticles in airway epithelium cellsNanotechnology20102135510220689164
- SerdaREMackAvan de VenALLogic-embedded vectors for intracellular partitioning, endosomal escape, and exocytosis of nanoparticlesSmall201062691270020957619
- JiangXRöckerCHafnerMBrandholtSDörlichRMNienhausGUEndo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cellsACS Nano201046787679721028844
- NativoPPriorIABrustMUptake and intracellular fate of surface-modified gold nanoparticlesACS Nano200821639164419206367
- VillanuevaACañeteMRocaAGThe influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cellsNanotechnology20092011510319420433
