Abstract
Research on liposome formulations has progressed from that on conventional vesicles to new generation liposomes, such as cationic liposomes, temperature sensitive liposomes, and virosomes, by modulating the formulation techniques and lipid composition. Many research papers focus on the correlation of blood circulation time and drug accumulation in target tissues with physicochemical properties of liposomal formulations, including particle size, membrane lamellarity, surface charge, permeability, encapsulation volume, shelf time, and release rate. This review is mainly to compare the therapeutic effect of current clinically approved liposome-based drugs with free drugs, and to also determine the clinical effect via liposomal variations in lipid composition. Furthermore, the major preclinical and clinical data related to the principal liposomal formulations are also summarized.
Introduction
The clinical utility of most conventional chemotherapeutics is limited either by the inability to deliver therapeutic drug concentrations to the target tissues or by severe and harmful toxic effects on normal organs and tissues. Liposomes are small, spherical, and enclosed compartments separating an aqueous medium from another by phospholipid bilayer. Many hundreds of drugs, including anticancer and antimicrobial agents, chelating agents, peptide hormones, enzymes, proteins, vaccines, and genetic materials, have been incorporated into the aqueous or lipid phases of liposomes, with various sizes, compositions, and other characteristics, to provide selective delivery to the target site for in vivo application. Several techniques, such as the Bangham, detergent-depletion, ether/ethanol injection, reverse phase evaporation, and emulsion methods, have been reported for preparing liposomes with high-entrapment efficiency, narrow particle size distribution, and long-term stability.Citation1–Citation7 Recently, some alternative methods including dense gas and supercritical fluid techniques have been introduced for liposome preparation without using any organic solvent.Citation1,Citation7–Citation9 Due to the differences in preparation methods and lipid compositions, liposomes can be classified according to their lamellarity (uni- and multilamellar vesicles), size (small [≤100 nm], intermediate [100–250 nm], or large [≥250 nm]), and surface charge (anionic, cationic, or neutral).Citation10–Citation12 In clinical studies, liposomes show improved pharmacokinetics and biodistribution of therapeutic agents and thus minimize toxicity by their accumulation at the target tissue.Citation13,Citation14
Liposomes were first discovered by Bangham in 1965 and the first liposomal pharmaceutical product, Doxil®, (Ben Venue Laboratories, Inc Bedford, OH) received US Food and Drug Administration (FDA) approval in 1995 for the treatment of chemotherapy refractory acquired immune deficiency syndrome (AIDS)-related Kaposi’s sarcoma.Citation13–Citation15 Currently, there are about twelve liposome-based drugs approved for clinical use and more are in various stages of clinical trials ( and ).Citation13–Citation62 Most liposomal drug formulations, such as Doxil and Myocet™ (GP-Pharm, Barcelona, Spain), are approved for intravenous application.Citation63 Other administration routes such as intramuscular delivery have been approved for delivery of surface antigens derived from the hepatitis A or influenza virus (Epaxal® [Berna Biotech Ltd, Berne Switzerland] and Inflexal® V [Berna Biotech España SA, Madrid, Spain]).Citation37,Citation38 Oral delivery has also been examined; however, this is more troublesome due to the potential for liposome breakdown following exposure to bile salts.Citation64
Table 1 Liposome-based drugs on market
Table 2 Liposome-based drugs in clinical trials
Storage of liposomes: lyophilization
Liposomes dispersed in aqueous solution generally face physical and chemical instabilities after long-term storage.Citation65 Hydrolysis and oxidation of phospholipids and liposome aggregation are the common cause of liposome instabilities. According to the literature, many methods have been investigated for the stabilization of liposomes, such as lyophilization, freezing, and spraying drying. In commercial liposome-based drugs (), AmBisome® (Gilead Sciences, Inc, San Dimas, CA), Amphotec® (Ben Venue Laboratories, Inc, Bedford, OH), Myocet, Visudyne® (Novartis Pharma AG, Basel, Switzerland), and LEP-ETU (liposome-entrapped paclitaxel easy-to-use; NeoPharm, Inc, Lake Bluff, IL) are all lyophilized products. In general, freeze-drying increases the shelf-life of liposomal formulations and preserves them in dried form as lyophilized cakes to be reconstituted with water for injection prior to administration.Citation66 Furthermore, cryoprotectants need to be added to maintain particle size distribution of liposomes after the freeze-drying-rehydration cycle. Various types and concentrations of sugars have been investigated for their ability to protect liposomes against fusion and leakage during lyophilization processes.Citation66 In commercial liposome lyophilized products, lactose has been used as a cryoprotectant in the formulations of Amphotec, Myocet, and Visudyne, and sucrose was added in the formulations of AmBisome and LEP-ETU to increase liposome stability during lyophilization. Interestingly, these commercial lyophilized products showed similar shelf-life in comparison with other liposome products (eg, suspension and emulsions) and hence lyophilization may not have the expected effect on liposome stability. In 1998, Clemons and Stevens compared the potency and therapeutic efficacy among the different lipid-based formulations of amphotericin B (Amphotec, AmBisome, and Abelcet® (Sigma-Tau PharmaSource, Inc, Indianapolis, IN)) for the treatment of systemic and meningeal cryptococcal disease.Citation67 Their work indicated that the therapeutic efficacy of Amphotec and AmBisome was superior to that of Abelcet, by up to ten-fold, in survival and in clearing infection from all organs. In these three commercially available lipid-based formulations of amphotericin B, Amphotec and AmBisome are both lyophilized products and Abelcet is formulated as a suspension. Therefore, lyophilization may not extend the shelf-life of products but may increase therapeutic efficacy in vivo. Similar results were also reported in our previous studies.Citation70 We investigated the stability of the siRNA-loaded liposomes in suspension and lyophilized powder form up to 1 month postmanufacture.Citation68 Following formulation, the siRNA-loaded liposomes were stored at either 4°C or room temperature. The particle size and zeta potential of siRNA-loaded liposomes remained unchanged in both storage conditions. However, siRNA entrapment efficiencies were observed to have decreased slightly after 1 month in storage for both suspension (90% → 83%) and lyophilized powder (94% → 84%) forms. Surprisingly, the gene-silencing efficiency of siRNA-loaded liposomes in aqueous solution showed 80% reduction following 1 month of storage at either 4°C or room temperature. This was in contrast to liposomes prepared in the lyophilized powder form where 100% of the gene-silencing efficiency was retained following storage at either 4°C or room temperature for 1 month. Although therapeutic efficiency of liposome-based drugs may vary depending on the choice of lipids, the preparation technique, physico-chemical characteristics of the bioactive materials, and overall charge of the liposome, lyophilization is useful for the long-term storage of liposome-based drugs.
Clinical studies of liposomal-based anticancer drugs: doxorubicin
Liposome delivery systems offer the potential to enhance the therapeutic index of anticancer drugs, either by increasing the drug concentration in tumor cells or by decreasing the exposure in normal host tissues. Doxorubicin is an anthracycline widely used to treat solid and hematological tumors, but its major drawback is its related cardiotoxicity. In cardiotoxicity, positively charged doxorubicin’s affinity for negatively charged cardiolipin, a lipid abundant in heart tissue, is thought to be involved in drug localization in the heart tissue.Citation69 Therefore, doxorubicin-loaded liposomes were developed to combat aggressive tumors, like breast and ovary metastatic cancers and Kaposi’s sarcoma. Myocet and Doxil were the first-approved liposome-based drugs for cancer treatment. Both products contain doxorubicin but are different, particularly in the presence of polyethylene glycol (PEG) coating (). In pharmacokinetic studies of doxorubicin-loaded liposomes, free doxorubicin had an elimination half-life of 0.2 hours and an area under the plasma concentration–time curve (AU) of 4 μg h mL−1 in patients as compared with 2.5 hours and 45 μg h mL−1 for Myocet and with 55 hours and 900 μg h mL−1 for Doxil, respectively.Citation25 The particle size of Myocet is about 190 nm and Doxil is about 100 nm. Both liposome products have longer circulating half-life in blood as compared with the free drug, but Doxil has a much longer circulation time in blood than Myocet. Generally, the blood circulation time of liposomes (T1/2) increases with decreasing size, negative charge density, and fluidity in the bilayer or PEG surface coating. In a Phase III head-to-head comparison of free doxorubicin vs Myocet in patients with metastatic breast cancer, similar results were presented in first-year survival rate (64% vs 69%) and progression-free survival (3.8 vs 4.3 months), but Myocet had low incidence of cardiac events (13% vs 29%), mucositis/stomatitis (8.6% vs 11.9%), and nausea/vomiting (12.3% vs 20.3%).Citation70,Citation71 Therefore, Myocet tends to reduce drug-related toxicity (eg, cardiotoxicity) rather than to enhance antitumor efficacy. Similar to Myocet, Doxil had a better safety profile, including reduction of cardiotoxicity (3.9% vs 18.8%), neutropenia (4% vs 10%), vomiting (19% vs 31%), and alopecia (20% vs 66%) in a Phase III trial of metastatic breast cancer, whereas its progression-free survival times (6.9 vs 7.8 months) and overall survival times (21 vs 22 months) demonstrated equivalent efficacy to conventional doxorubicin.Citation72 However, palmar-plantar erythrodysesthesia (48% vs 2%), stomatitis (22% vs 15%), and mucositis (23% vs 13%) were found to be more often associated with Doxil than free doxorubicin.
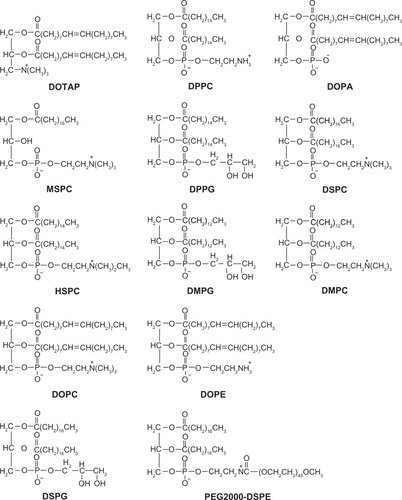
Figure 1 Chemical structures of lipids in liposome formulations.
Abbreviations: DOTAP, 1,2-dioleoyl-3-trimethylammonium propane; DPPC, dipalmitoylphosphatidylcholine; DOPA, 1,2-Dioleoyl-sn-Glycero-3-Phosphate; MSPC, monostearoylphosphatidylcholine; DPPG, dipalmitoylphosphatidylglycerol; DSPC, distearoylphosphatidylcholine; HSPC, hydrogenated soy PC; DMPG, l-α-dimyristoylphosphatidylglycerol; DMPC, 1-α-dimyristoylphosphatidylcholine; DOPC, 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DOPE, dioleoyl phosphatidylethanolamine; DSPG, distearoylphosphatidylglycerol; PEG2000-DSPE, polyethylene glycol 2000-distearoylphosphatidylethanolamine.
Lipo-dox® (TTY Biopharm Company Ltd, Taipei Taiwan) is the second generation of PEGylated liposomal doxorubicin, composed of distearoylphosphatidylcholine (DSPC) and cholesterol with a surface coating of PEG.Citation27 DSPC, which has two completely saturated fatty acids (both stearic acids), has high phase-transition temperature (Tm), 55°C, and good compatibility with cholesterol. Normally, lipid bilayer has two thermodynamic phases: gel or liquid-crystal phase. At temperature < Tm, the lipid membrane is in the gel phase, which is relatively rigid and tight because the lipid molecules have lower energy of random motion and the hydrocarbon chains are fully extended and closely packed. Liposomes composed of phospholipids like DSPC have higher stability compared with others containing unsaturated fatty acid (egg phosphatidycholine [PC]) or fatty acids of shorter or not uniform carbon chains like hydrogenated soy PC (HSPC). In a Phase I clinical study, Lipo-dox achieved the most prolonged circulation half-life (65 hours).Citation73 However, Tseng et al demonstrated that there were no differences in survival between free doxorubicin only (median survival time of 23 days) and Lipo-dox (medium survival time of 23.5 days) in a murine B-cell lymphoma model.Citation74 In patients with metastatic breast cancer, the median time to disease progression of 163 days represented the result of Lipo-dox treatment and the median duration of response in responding patients (286 days) are comparable with those of Doxil treatment.Citation75 Neutropenia, stomatitis, and skin toxicity were reported in many cases of Lipo-dox administration. Moreover, stomatitis became the new dose-limiting toxicity of PEGylated liposomal doxorubicin. For Lipo-dox, stomatitis appeared at doses of 30 mg/m2 and reached dose limit at 50 mg/m2.Citation27 In contrast, Doxil reached dose limit at 80 mg/m2 and hence Lipo-dox had higher incidence of severe stomatitis than Doxil. In comparison with Myocet (the non-PEGylated form of liposomal doxorubicin), Doxil and Lipo-dox (both PEGylated forms of liposomal doxorubicin) both showed significant incidence of stomatitis and this is mainly due to the long circulation properties of PEGylated liposomes.Citation27,Citation71,Citation72
The new generation of doxorubicin-loaded liposomes are thermosensitive liposomes (TSLs), which release their encapsulated drugs in regions where local tissue temperatures are elevated.Citation76 Compared with non-TSLs that remain stable and do not release drug in the physiologic temperature range, TSLs undergo a gel-to-liquid crystalline phase change when heated that renders the liposomes more permeable, releasing their encapsulated drugs. ThermoDox® (Celsion Corporation, Lawrenceville, NJ), a proprietary TSL encapsulation of doxorubicin, has recently begun Phase III clinical trials for the treatment of hepatocellular carcinoma.Citation77 ThermoDox is composed of dipalmitoylphosphatidylcholine (DPPC), monostearoylphosphatidylcholine (MSPC), and polyethylene glycol 2000-distearoylphosphatidylethanolamine (PEG 2000-DSPE) in 90:10:4 molar ratio.Citation49,Citation50 In the design of TSLs, it is necessary to choose a phospholipid that has a gel-to-liquid crystalline phase transition temperature (Tc) in the temperature range of clinically attainable local hyperthermia (41°C– 42°C). The mechanism behind TSLs is the temperature-induced membrane instability at the Tc of the used lipids. DPPC with a Tc = 41.5°C is an ideal lipid according to temperature-triggered technology.Citation78,Citation79 For liposomes composed of DPPC alone, the rate of release and the amount released are relatively small. By incorporating a small amount of lysolipids, such as MSPC or monopalmitoylphosphatidylcholine, into DPPC liposomes, Tc is shifted down slightly and membrane instability and drug release rate is significantly enhanced at Tc. In vitro release studies, monopalmitoylphosphatidylcholine-containing TSLs released about 45% of encapsulated doxorubicin in bovine serum at 42°C in a few seconds (20 seconds), while pure DPPC liposomes released only 20% over 1 hour.Citation79 Banno et al demonstrated that the presence of MSPC, rather than PEG 2000-DSPE, in DPPC liposomes would give rise to the rapid drug-release profile in vitro, suggesting that lysolipid is the more important component in determining the rate of TSL content release.Citation80 Indeed, Banno’s in vivo data showed that the presence of 9.6 mol% MSPC in TSL could result in more rapid elimination of the encapsulated doxorubicin (T1/2 = 1.29 h), compared with the formulation without lysolipid (T1/2 = 2.91 h). In 2007, Dromi et al compared the accumulation of doxorubicin in mice tumors among free doxorubicin, Doxil, and ThermoDox.Citation50 Results showed that over time, doxorubicin gradually increased in tumors when both Doxil and ThermoDox were used but not with free doxorubicin. At 24 hours after administration, doxorubicin concentrations in tumors were found to be significantly higher with Doxil than ThermoDox. ThermoDox is currently under evaluation in clinical trials and hence the therapeutic efficacy of ThermoDox is still unknown.
Clinical studies of liposomal-based anticancer drugs: daunorubicin
Daunorubicin is classified as an anthracycline anticancer drug in the treatment of leukemia and a wide variety of solid tumors, but its major drawbacks are myelosuppression and cardiotoxicity.Citation81 Daunorubicin has also been incorporated into liposomes for the formulation of liposomal anticancer chemotherapy drugs. DaunoXome® (Gilead Sciences, Inc) is a commercial liposomal formulation of daunorubicin in which the drug is entrapped into small unilamellar vesicles (45 nm) composed of DSPC and cholesterol in 2:1 molar ratio. In animal studies with tumor models in mice, DaunoXome increased tumor uptake of daunorubicin ten-fold when measured against free drug (2470.5 vs 245.1 μg · hr/mL for 0–48 hours).Citation82 Furthermore, clinical pharmacokinetic studies have demonstrated that DaunoXome was 36-fold higher in AUC (375.3 vs 10.33 μg · hr/mL) in comparison with conventional daunorubicin.Citation83 In a Phase III trial of DaunoX-ome versus a conventional combination of doxorubicin, bleomycin, and vincristine (ABV) in AIDS-related Kaposi’s sarcoma, the efficacy of DaunoXome was comparable to that of vincristine. Response rates (25% vs 28%), time to treatment failure (115 vs 99 days), and overall survival (369 vs 342 days) were similar on both treatment arms.Citation84 Moreover, patients treated with DaunoXome experienced less alopecia (8% vs 36%) and neuropathy (13% vs 41%) and their cardiac function remained stable. Therefore, DaunoXome may provide another comparable but safer chemotherapy.
Clinical studies of liposomal-based anticancer drugs: paclitaxel
Taxol® (paclitaxel) is a marketed product for the treatment of ovarian, breast, non-small cell lung cancer, and AIDS-related Kaposi’s sarcoma.Citation40 However, paclitaxel is only sparingly soluble in water and, therefore, intravenous administration depends on the use of the non-ionic surfactant Cremophor EL® (polyethoxylated castor oil) to achieve a clinically relevant concentrated solution. Unfortunately, Cremophor EL increases toxicity and leads to hypersensitivity reactions in certain patients.Citation85 The LEP-ETU formulation of paclitaxel is being developed to potentially reduce toxicities associated with Taxol by eliminating the drug formulation component polyoxyethylated castor oil. LEP-ETU formulations composed of 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, and cardiolipin in 90:5:5 molar ratio were prepared by the modified thin-film hydration method. DOPC, a zwitterionic natural phospholipid, is chosen as one of the lipid components in the LEP-ETU formulation because of a low Tc (−22°C) and hence DOPC can form more flexible liposomes to entrap highly hydrophobic molecules. Moreover, cholesterol is included in LEP-ETU formulations to increase the liposome stability. Liposomes containing cardiolipin reportedly reduced cardiotoxicity associated with doxorubicin by altering the pharmacokinetics and tissue distribution of the drug and hence cardiolipin may also exert similar results in LEP-ETU.Citation86 Fetterly et al evaluated the maximum tolerated dose, dose-limiting toxicities, and pharmacokinetics of liposome-encapsulated paclitaxel (LEP-ETU) in comparison with Taxol.Citation87,Citation88 The maximum tolerated dose of LEP-ETU was 325 mg/m2 in a Phase I study of patients with locally advanced or metastatic carcinoma.Citation88,Citation89 This dose is higher than that achieved with Taxol, which is typically delivered at a dose range of 135 to 200 mg/m2. The major toxicity to administration of paclitaxel is neuropathy. In the Phase I study, neurotoxicity occurred in 5 of 12 patients (42%) treated with LEP-ETU at ≥325 mg/m2. Although a direct comparison with Taxol is not possible, neutropenia was seen in 53% of metastatic breast cancer patients treated with 250 mg/m2 Taxol as demonstrated by Winer et al.Citation89 Therefore, the neuropathy caused by LEP-ETU appears to be no worse than that reported for Taxol within 3 weeks of treatment. Following LEP-ETU administration, the AUC of paclitaxel in patients with advanced or metastatic carcinoma was improved (8.2 to 6.16 μg · hr/mL) with increasing dose (135 to 375 mg/m2), which is similar to Taxol. Although similarities exist between the plasma pharmacokinetics of the two formulations, the clinical evidence obtained from the Phase I study shows LEP-ETU can be administered safely at higher doses than Taxol.Citation88,Citation89
Another liposome formation of paclitaxel is EndoTAG-1.Citation42–Citation44 The formulation of EndoTAG-1 is prepared by 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), DOPC, and paclitaxel in 50:47:3 molar ratio. DOTAP is a cationic synthetic lipid, which comprises one positive charge at the head group. The use of cationic lipids to enhance gene delivery has been studied extensively, but their application in clinic is relatively unexplored. Recently, there has been great interest in cationic liposomes, mainly due to their inherent ability to selectively target tumor vasculature. This selective affinity of cationic liposomes to tumor vasculature provides an opportunity for the development of many anti-angiogenic and anticancer formulations based on cationic liposomes.Citation42 EndoTAG-1 is the first formulation of cationic liposomes carrying paclitaxel in clinical trial. For commercial storage, EndoTAG-1 formulations are lyophilized, and they are reconstituted with water for injection directly prior use. In preclinical programs, EndoTAG1-1 inhibited tumor growth also in Taxol-resistant animal tumor models such as B16 melanoma and Sk-Mel 28 melanoma. EndoTAG-1 demonstrated a strong antivascular effect on the preexisting tumor vasculature and affected several tumor microcirculatory parameters. In a Phase II trial of patients with pancreatic adenocarcinoma who were not candidates for surgery, EndoTAG-1 in combination with gemcitabine substantially extended overall survival compared with gem-citabine alone.Citation90 Median survival in patients who received gemcitabine alone was 7.2 months, whereas it was up to 9.4 months in those who received combination treatment of EndoTAG-1 plus gemcitabine. After 6 and 12 months of treatment, survival rate was superior for all EndoTAG-1 doses plus gemcitabine compared with gemcitabine alone. The 12-month survival rates in patients given the two higher doses of EndoTAG-1 (22 and 44 mg/m2 plus gemcitabine) were 36% and 33%, respectively, compared with 17.5% in those given gemcitabine alone. Combination treatment with EndoTAG-1 plus gemcitabine was well tolerated and led to substantially more prolonged survival rates compared with standard therapy in this Phase II trial. Further clinical studies are warranted to demonstrate a statistically significant survival benefit associated with EndoTAG-1 plus gemcitabine in advanced pancreatic cancer.
Liposome application in vaccine formulation: Epaxal and Inflexal V
The incorporation of viral membrane proteins or peptide antigens into liposomes has been shown to potentiate cell-mediated and humoral immune response, and generate solid and durable immunity against the pathogen. Virosomes are reconstituted virus liposomes, constructed without the genetic information of the virus making them unable to replicate or cause infection.Citation91,Citation92 The lipid layers of virosomes, composed of dioleoyl phosphatidylethanolamine (DOPE) and DOPC, are employed to mimic viral membrane for vaccine delivery. Epaxal and Inflexal V are both vaccine products using the virosome-based antigen delivery system for commercial use (). For the production of Inflexal V, the influenza viruses, grown in hens’ eggs, are first inactivated with beta-propiolactone. The influenza surface antigens, hemagglutinin and neuraminidase, are then purified and mixed with the phospholipid lecithin to form virosomes. Due to the virosomal technology, hepatitis A virus (HAV) vaccine Epaxal, and influenza vaccine Inflexal V are highly efficacious by mimicking natural viral infection. The use of virosomes to deliver hepatitis A or influenza antigens stimulates a strong immune response of immunocompetent cells. In contrast to other commercially available HAV vaccines, Epaxal is an aluminium-free vaccine based on formalin-inactivated hepatitis A (strain RG-SB) antigen-incorporated virosomes. In a clinical study by Usonis et al, seroprotection rates were 100% in all infants and children at 1 and 12 months after primary vaccination with Epaxal.Citation35 In contrast, the seroprotection rate after vaccination with the aluminium-containing vaccine Havrix™ (GlaxoSmithKline Biologicals Rixensart, Belgium) was 67.7% in infants with pre-existing maternal anti-HAV antibodies, and a booster vaccination was required for complete seroprotection. Moreover, Epaxal was generally well tolerated by infants and children, with no serious systemic or local events reported after either primary or booster vaccination.
For Inflexal V, most studies have shown inferior efficacy or ineffectiveness on clinical parameters for these vaccines compared with the nonadjuvanted, split-virus, or subunit seasonal vaccines.Citation93 Kanra et al, compared the immunogenicity and safety of Inflexal V in children with a split influenza vaccine, Fluarix (GlaxoSmithKline Biologicals, Dresden, Germany).Citation94 Both vaccines were well tolerated and could induce effective immune responses in children. Interestingly, the virosome-adjuvanted influenza vaccine showed greater immunogenicity (88.8% seroconversion rates for H3 N2) over the split influenza vaccine (77.5% seroconversion rates for H3 N2) in unprimed children. In essence, virosomal techniques may not be able to give superior protective immunity in clinic but play an important role in preventing morbidity and lethality associated with vaccine.
Liposomal formulations in ophthalmology: Visudyne®
Verteporfin is a hydrophobic chlorin-like photosensitizer, which has been shown to be highly effective for photodynamic therapy in vivo. However, verteporfin also has a tendency to undergo self-aggregation in aqueous media, which can severely limit drug bioavailability to biological systems. It is important to introduce verteporfin into the bloodstream in its monomeric form and hence verteporfin was encapsulated in liposomes (Visudyne) for intravenous drug delivery.Citation29–Citation31 The lipid layers of Visudyne are composed of unsaturated egg phosphatidylglycerol and dimyristoyl phosphatidyl choline in 3:5 molar ratio. Visudyne was the only drug approved by the FDA for the photodynamic treatment of age-related macular degeneration. Visudyne treatment prevents the growth of the destructive blood vessels without hurting the surrounding tissues. Phase I and II clinical trials were conducted for 609 patients with age-related macular degeneration.Citation95,Citation96 After 12 months of treatment, the group treated with Visudyne (6 mg/m2 body surface area) had statistically better visual acuity, contrast sensitivity, and fluorescein angiographic outcomes than those who had placebo treatment (5% dextrose in water). At the examination 12-months posttreatment, 246 (61%) of 402 eyes assigned to verteporfin compared with 96 (46%) of 207 eyes assigned to placebo had lost fewer than 15 letters of visual acuity from baseline. In subgroup analyses, the visual acuity benefit of verteporfin therapy was clearly demonstrated (67% vs 39%) when the area of choroidal neovascularization, caused by age-related macular degeneration, occupied 50% or more of the area of the entire lesion. However, Chowdhary et al reported that Visudyne was readily destabilized in the presence of relatively low concentrations of plasma.Citation29 Therefore, the aim of future investigation of liposomal formulations in ophthalmology is to develop stable liposome structures for extending the plasma circulation time following intravenous injection.
Future approaches
Since the first liposomal pharmaceutical product, Doxil, received FDA approval in 1995, liposomes have been widely applied as drug carriers in clinic. Until now, several important types of liposomes, such as PEGylated liposomes (Doxil and Lipo-dox), temperature sensitive liposomes (ThermoDox), cationic liposomes (EndoTAG1-1), and virosomes (Expal and Inflexal V) have been investigated for clinic use. In contrast with liposomal-based drugs on the market (), liposome-based drugs in clinical trials () focused more on the types of delivered drugs (eg, Cisplatin, BLP25 lipopeptide, Grb2 antisense oligodeoxynucleotide, Bacteriophage T4 endonuclease 5, etc) and therapeutic applications (from topical delivery systems to portable aerosol delivery systems). New liposomal formulations, such as PEGylated liposomes, may extend blood circulation time, vary drug distribution in the body, and hence reduce the possible side effects related to the drugs (eg, cardiotoxicity). However, PEGylated liposomes (Doxil and Lipo-dox) displayed significant incidence of stomatitis in clinical trials, which may be related to PEGylation. Moreover, some of the new generation liposomes showed only comparable or even poor therapeutic efficiency compared with free drug or conventional vesicles in clinical trials. In comparison with Doxil, ThermoDox displayed significantly weaker doxorubicin accumulation in mice tumors at 24 hours after administration.Citation50 EndoTAG-1 plus gemcitabine and EndoTAG-1 plus paclitaxel achieved excellent therapeutic effect in two Phase II clinical trials in pancreatic cancer and triple receptor-negative breast cancer, but EndoTAG-1 therapy alone in triple receptor-negative breast cancer resulted in poor survival rate (34%) and median progression-free survival time (3.4 months) in comparison with paclitaxel (48% and 3.7 months).Citation97 SPI-077, the first liposomal formulation of cisplatin, had limited clinical efficacy in a Phase II clinical trial of advanced non-small cell lung cancer, even though SPI-077 demonstrated enhanced cisplatin tumor accumulation in preclinical models.Citation98 Similar to SPI-077, a Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer had no detectable antitumor activity.Citation99 Although new liposomal-based drugs have been well studied in preclinical animal models, these liposomal pharmaceutical products may be unable to provide promising therapeutic effects in clinical trials. For future development of liposomal-based drugs, the comparison of drug circulation time in blood, drug accumulation in tissues, and possible toxicity between conventional vesicles and new generations of liposomes should be investigated in preclinical animal models. Furthermore, there should also be focus on the clinical therapeutic effects and toxic side effects of liposomal lipid composition.
Acknowledgements
This work was supported by the National Science Council (Taiwan) (grant numbers: NSC 98-2320-B-415-001-MY3 and NSC 98-2320-B-016-003-MY3).
Disclosure
The authors report no conflicts of interest in this work.
References
- OtakeKImuraTSakaiHAbeMDevelopment of a new preparation method of liposomes using supercritical carbon dioxideLangmuir2001171338983901
- UhumwanghoMUOkorRSCurrent trends in the production and biomedical applications of liposomes: a reviewPak J Pharm Sci200541921
- JiskootWTeerlinkTBeuveryECCrommelinDJPreparation of liposomes via detergent removal from mixed micelles by dilution. The effect of bilayer composition and process parameters on liposome characteristicsPharm Weekbl Sci1986852592653786108
- DeamerQWPreparation and properties of ether-injection liposomesAnn N Y Acad Sci1978308250258279292
- SzokaFJrPapahadjopoulosDProcedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporationProc Natl Acad Sci U S A197875941944198279908
- KimSMartinGMPreparation of cell-size unilamellar liposomes with high captured volume and defined size distributionBiochim Biophys Acta19816461197272296
- MeureLAFosterNRDehghaniFConventional and dense gas techniques for the production of liposomes: a reviewAAPS Pharm Sci Tech200893798809
- LesoinLBoutinOCramponCBadensECO2/water/surfactant ternary systems and liposome formation using supercritical CO2: a reviewColloid Surface Physicochem Eng Aspect20113771–3114
- CastorTPPhospholipid nanosomesCurr Drug Deliv20052432934016305436
- JesorkaAOrwarOLiposomes: technologies and analytical applicationsAnnu Rev Anal Chem (Palo Alto Calif)2008180183220636098
- MakinoKShibataAChapter 2: Surface properties of liposomes depending on their compositionLeitmannova LiuAAdvances in Planar Lipid Bilayers and LiposomesNetherlandsElsvier Science200644977
- IracheJMEsparzaIGamazoCAgüerosMEspuelasSNanomedicine: novel approaches in human and veterinary therapeuticsVet Parasitol20011801–2477121680101
- ImmordinoMLDosioFCattelLStealth liposomes: review of the basic science, rationale, and clinical applications, existing and potentialInt J Nanomedicine20061329731517717971
- ParkJWLiposome-based drug delivery in breast cancer treatmentBreast Cancer Res200243959912052251
- BanghamADDiffusion of univalent ions across unilamellar of swollen phospholipidsJ Mol Biol1965132382525859039
- AllenTMMoaseEHTherapeutic opportunities for targeted liposomal drug deliveryAdv Drug Deliv Rev1996212117133
- Astellas Pharma US, Inc [homepage on the Internet]AmBisome® (Amphotericin B) liposome for injection Available from: http://www.ambisome.com/. Accessed November 11, 2011.
- MeunierFPrenticeHGRingdénOLiposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trialJ Antimicrob Chemother199128Suppl B83911778895
- Enzon Pharmaceuticals, Inc [homepage on the Internet]ABELCET® (amphotericin B lipid complex) Injection Available from: http://enzon.com/. Accessed November 11, 2011.
- WasanKMLopez-BeresteinGCharacteristics of lipid-based formulations that influence their biological behavior in the plasma of patientsClin Infect Dis1996235112611388922813
- DenningDWLeeJYHostetlerJSNIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosisAm J Med1994971351448059779
- Three Rivers Pharmaceuticals, LLC. [homepage on the Internet]AMPHOTEC® Amphotericin B Cholesteryl Sulfate Complex for Injection Available from: http://www.3riverspharma.com/. Accessed November 11, 2011.
- Gilead Sciences, Inc [homepage on the Internet]DaunoXome® (daunorubicin citrate liposome injection) Available from: http://www.gilead.com/. Accessed November 11, 2011.
- RiveraELiposomal anthracyclines in metastatic breast cancer: clinical updateOncologist20038Suppl 23913679590
- TomkinsonBBendeleRGilesFJOSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALLLeuk Res200327111039105012859997
- HoarauDDelmasPDavidSRouxELerouxJCNovel long-circulating lipid nanocapsulesPharm Res200421101783178915553223
- HongRLLiposomal anti-cancer drug researches the myth of long circulationJ Chinese Oncol Soc20042021021
- GardikisKTsimplouliCDimasKMicha-ScrettasMDemetzosCNew chimeric advanced Drug Delivery nano Systems (chi-aDDnSs) as doxorubicin carriersInt J Pharm20104021–223123720934501
- ChowdharyRKShariffIDolphinDDrug release characteristics of lipid based benzoporphyrin derivativeJ Pharm Pharmaceut Sci2003611319
- FahrAvan HoogevestPMaySBergstrandNS LeighMLTransfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug deliveryEur J Pharm Sci2005263–425126516112849
- Novartis Ophthalmics, Inc [homepage on the Internet]Visudyne® (verteporfin for injection) Available from: http://www.visudyne.com/. Accessed November 11, 2011.
- Drugs.com [homepage on the Internet]DepoCyt® (cytarabine liposome injection) Prescribing Information Available from: http://www.drugs.com/pro/depocyt.html. Revised Sep 2011. Accessed November 11, 2011.
- Drugs.com [homepage on the Internet]DepoDur (morphine sulfate extended-release liposome injection) Prescribing Information Available from: http://www.drugs.com/pro/depodur.html. Revised Jan 2008. Accessed November 11, 2011.
- PatilSDBurgessDJLiposomes, design and manufacturingBurgessDJInjectable Dispersed Systems: Formulation, Processing and Performance (Drugs and The Pharmaceutical Sciences Series)New YorkMarcel Dekker2005249303
- UsonisVBakasénasVValentelisRKatilieneGVidzenieneDHerzogCAntibody titres after primary and booster vaccination of infants and young children with a virosomal hepatitis A vaccine (Epaxal)Vaccine200321314588459214575771
- D’AcremontVHerzogCGentonBImmunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderlyJ Travel Med2006132788316553593
- The electronic Medicines Compendium (eMC) [homepage on the Internet]Epaxal Prescribing Information Available from: http://www.medicines.org.uk/EMC/medicine/12742/SPC/Epaxal/. Revised January 4, 2011. Accessed November 11, 2011.
- The electronic Medicines Compendium (eMC) [homepage on the Internet]INFLEXAL V Prescribing Information Available from: http://www.medicines.org.uk/emc/medicine/24941/SPC/inflexalv/. Revised Oct 2010. Accessed November 11, 2011.
- HerzogCHartmannKKünziVEleven years of Inflexal V-a virosomal adjuvanted influenza vaccineVaccine200927334381438719450630
- ZhangJAAnyarambhatlaGMaLDevelopment and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEP-ETU) formulationEur J Pharm Biopharm200559117718715567316
- CattaneoAGGornatiRSabbioniENanotechnology and human health: risks and benef itsJ Appl Toxicol201030873074421117037
- MichaelisUHaasHTargeting of cationic liposomes to endothelial tissueGregoriadisGLiposome Technology, Volume III: Interactions of Liposomes with the Biological MilieuLondon, UKInforma Healthcare2007151170
- EichhornMEBeckerSStriethSPaclitaxel encapsulated in cationic lipid complexes (MBT-0206) impairs functional tumor vascular properties as detected by dynamic contrast enhanced magnetic resonance imagingCancer Biol Ther200651899616357513
- RobertBCampbellRBYingBKuestersGMHemphillRFighting cancer: from the bench to bedside using second generation cationic liposomal therapeuticsJ Pharm Sci200998241142918563780
- LiZZhangYWurtzWCharacterization of nebulized liposomal amikacin (Arikace) as a function of droplet sizeJ Aerosol Med Pulm Drug Deliv200821324525418759656
- MossalamMDixonASLimCSControlling subcellular delivery to optimize therapeutic effectTher Deliv20101116919321113240
- RodriguezMAPytlikRKozakTVincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 studyCancer2009115153475348219536896
- KrishnaRWebbMSSt OngeGMayerLDLiposomal and non-liposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution propertiesJ Pharmacol Exp Ther200129831206121211504822
- YarmolenkoPSZhaoYLandonCComparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumoursInt J Hyperthermia201026548549820597627
- DromiSFrenkelVLukAPulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effectClin Cancer Res20071392722272717473205
- ZahidSBrownellIRepairing DNA damage in xeroderma pigmentosum: T4N5 lotion and gene therapyJ Drugs Dermatol20087440540818459526
- TariAMGutiérrez-PuenteYMonacoGLiposome-incorporated Grb2 antisense oligodeoxynucleotide increases the survival of mice bearing bcr-abl-positive leukemia xenograftsInt J Oncol20073151243125017912453
- PalAKhanSWangYFPreclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulationAnticancer Res2005251A33134115816556
- DragovichTMendelsonDKurtinSRichardsonKVon HoffDHoosAA Phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancerCancer Chemother Pharmacol200658675976416847673
- ClinicalTrials.gov [homepage on the Internet]Prostaglandin E1 (Liprostin) treatment with lower limb angioplasty for peripheral arterial occlusive disease Available from: http://clinicaltrials.gov/ct2/show/NCT00053716. Accessed November 11, 2011.
- PowellEChowLQBLP-25 liposomal vaccine: a promising potential therapy in non-small-cell lung cancerExpert Rev Respir Med200821374520477220
- OhyanagiFHoraiTSekineISafety of BLP25 liposome vaccine (L-BLP25) in Japanese patients with unresectable stage III NSCLC after primary chemoradiotherapy: preliminary results from a Phase I/II studyJpn J Clin Oncol201141571872221393255
- StathopoulosGPBoulikasTLipoplatin formulation review articleJ Drug Deliv2012201258136321904682
- YuNYConwayCPenaRLChenJYSTEALTH liposomal CKD-602, a topoisomerase I iInhibitor, improves the therapeutic index in human tumor xenograft modelsAnticancer Res2007274B2541254517695551
- HwangJHLimMCSeoSSParkSYKangSPhase II study of belotecan (CKD 602) as a single agent in patients with recurrent or progressive carcinoma of uterine cervixJpn J Clin Oncol201141562462921355002
- DuffaudFBornerMCholletPPhase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC New Drug Development Group studyEur J Cancer200440182748275215571957
- SempleSCLeoneRWangJOptimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activityJ Pharm Sci20059451024103815793796
- MrossKNiemannBMassingUPharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose studyCancer Chemother Pharmacol200454651452415322827
- ShajiJPatoleVProtein and Peptide drug delivery: oral approachesIndian J Pharm Sci200870326927720046732
- ChenCHanDCaiCTangXAn overview of liposome lyophilization and its future potentialJ Control Release2010142329931119874861
- EL-NeserOHYahiyaSAEL-GazayerlyONEffect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomesSaudi Pharm J201018217224
- ClemonsKVStevensDAComparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosisAntimicrob Agents Chemother19984248999029559804
- WuSYPutralLNLiangMDevelopment of a novel method for formulating stable siRNA-loaded lipid particles for in vivo usePharm Res200926351252219023647
- RahmanAMYusufSWEwerMSAnthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulationInt J Nanomedicine20072456758318203425
- LammersTHenninkWEStormGTumour-targeted nanomedicines: principles and practiceBr J Cancer200899339239718648371
- HarrisLBatistGBeltRTLC D-99 Study GroupLiposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinomaCancer2002941253611815957
- O’BrienMEWiglerNInbarMCAELYX Breast Cancer Study GroupReduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancerAnn Oncol200415344044914998846
- HongRLTsengYLPhase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in patients with solid tumorCancer2001911826183311335910
- TsengYLHongRLTaoMHChangFHSterically stabilized anti-idiotype immunoliposomes improve the therapeutic efficacy of doxorubicin in a murine B-cell lymphoma modelInt J Cancer199980572373010048974
- ChaoTCWangWSYenCCChiouTJLiuJHChenPMA dose-escalating pilot study of sterically stabilized, pegylated liposomal doxorubicin (Lipo-Dox) in patients with metastatic breast cancerCancer Invest200321683784714735687
- HossannMWangTWiggenhornMSize of thermosensitive liposomes influences content releaseJ Control Release2010147343644320727921
- ClinicalTrials.gov [homepage on the Internet]Phase 3 study of ThermoDox with Radiofrequency Ablation (RFA) in Treatment of Hepatocellular Carcinoma (HCC) Available from: http://clinicaltrials.gov/ct2/show/NCT00617981. Accessed November 11, 2011.
- MillsJKNeedhamDLysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transitionBiochim Biophys Acta200517162779616216216
- NeedhamDAnyarambhatlaGKongGDewhirstMWA new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft modelCancer Res2000601197120110728674
- BannoBIckensteinLMChiuGNThe functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivoJ Pharm Sci20109952295230819902527
- O’ByrneKJThomasALSharmaRAA phase I dose-escalating study of DaunoXome, liposomal daunorubicin, in metastatic breast cancerBr J Cancer2002871152012085249
- ForssenEACoulterDMProffittRTSelective in vivo localisation of daunorubicin small unilamellar vesicles in solid tumorsCancer Res19925212325532611596882
- ForssenEARossMEDaunoxome® treatment of solid tumors: preclinical and linical investigationsJ Liposome Res199441481512
- GillPSWernzJScaddenDTRandomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcomaJ Clin Oncol1996148235323648708728
- SzebeniJComplement activation-related pseudoallergy: a new class of drug-induced acute immune toxicityToxicology200521610612116140450
- MehtaRRBurkeTGMembrane biophysical parameters influencing anthracycline actionPriebeWAnthracycline AntibioticsNew YorkAmerican Chemical Society Publication2004222240
- FetterlyGJStraubingerRMPharmacokinetics of paclitaxel-containing liposomes in ratsAAPS Pharm Sci200354E32
- FetterlyGJGraselaTHShermanJWPharmacokinetic/pharmacodynamic modeling and simulation of neutropenia during phase I development of liposome-entrapped paclitaxelClin Cancer Res200814185856586318794097
- WinerEPBerryDAWoolfSFailure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B Trial 9342J Clin Oncol200422112061206815169793
- BayraktarSBayraktarUDRocha-LimaCMRecent developments in palliative chemotherapy for locally advanced and metastatic pancreas cancerWorld J Gastroenterol201016667368220135714
- StegmannTMorseltHWBooyFPFunctional reconstitution of influenza virus envelopesEMBO J198769265126593678202
- GlückRMischlerRBrantschenSJustMAlthausBCryzSJJrImmunopotentiating reconstituted influenza virus virosome vaccine delivery system for immunization against hepatitis AJ Clin Invest1992906249124951334977
- NicholsonKGThompsonCIKlapJMSafety and immunogenicity of whole-virus, alum-adjuvanted whole-virus, virosomal, and whole-virus intradermal influenza A/H9N2 vaccine formulationsVaccine200928117117819799843
- KanraGMarchisioPFeiterna-SperlingCComparison of immunogenicity and tolerability of a virosome-adjuvanted and a split influenza vaccine in childrenPediatr Infect Dis J200423430030615071282
- BresslerNMBresslerSBPhotodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciencesInvest Ophthalmol Vis Sci200041362462810711673
- Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP report. Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study GroupArch Ophthalmol19991171329134510532441
- Drugs.com [homepage on the Internet]MediGene reports additional phase II results of EndoTAG-1 for the treatment of triple receptor-negative breast cancer Prescribing Information Available from: http://www.drugs.com/clinical_trials/medigene-reports-additional-phase-ii-results-endotag-1-triple-receptor-negative-breast-cancer-9689.html. Posted Jun 2010. Accessed November 15, 2011.
- WhiteSCLoriganPMargisonGPPhase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancerBr J Cancer200695782282816969346
- BooserDJEstevaFJRiveraEPhase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancerCancer Chemother Pharmacol20025016812111105