Abstract
Background
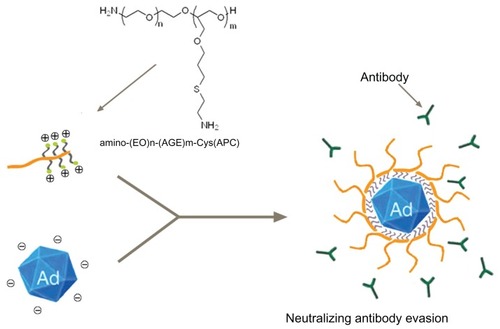
The generation of anti-adenovirus neutralizing antibody (NAb) in humans severely restricts the utilization of recombinant adenovirus serotype 5 (Ad5) vectors in gene therapy for a wide range of clinical trials. To overcome this limitation, we ionically complexed Ad5 with a newly synthesized copolymer, which we called APC, making an adenovirus shielded from NAb.
Methods
APC, a cationic polyethylene glycol derivative, was synthesized via two steps of ring-opening copolymerization of ethylene oxide and allyl glycidyl ether, followed by the addition of 2-mercaptoethylamine. The copolymer or the control PEI-2k was ionically complexed to anionic Ad5 in 5% glucose, and in vitro transduction assays were carried out in coxsackievirus and adenovirus receptor-positive cells (A549) and coxsackievirus and adenovirus receptor-negative cells (B16 and SKOV3). The physical properties and morphology of adenovirus alone or the complexes were investigated respectively by zeta potential, size distribution, and transmission electron microscopy image. Then cytotoxicity of APC was examined using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide assays. Finally, the ability of APC to protect adenovirus from NAb was evaluated by transfection assays after a neutralizing effect.
Results
APC was successfully synthesized and showed a low cytotoxicity. Positively charged Ad5/APC exhibited slightly increased diameter (130.2 ± 0.60 nm) than naked Ad5 (115.6 ± 5.46 nm) while Ad5/PEI-2k showed severe aggregation (1382 ± 79.9 nm). Ad5/APC achieved a gene transfection level as high as Ad5/PEI-2k in A549 or B16 cells, and significantly higher than Ad5/PEI-2k in SKOV3 cells. Most importantly, after the exposure to the neutralizing antibody, naked Ad5 and Ad5/PEI-2k exhibited poor gene expression while Ad5/APC still showed significantly efficient gene expression.
Conclusion
Our results demonstrated that Ad5/APC complex offered good protection for Ad5 against NAb in vitro and suggested a potential strategy of resistance to NAb in vivo.
Introduction
Recombinant adenovirus vectors (Adv) have been under extensive exploration as gene delivery systems for gene therapy or vaccines.Citation1–Citation3 As reported in 2009, viral vectors for gene therapy accounted for 66% of all those in clinical trials, approximately 25% of which were Adv-based vectors.Citation4 In particular, adenovirus-mediated p53 gene therapy has been proven effective in clinical trials by intratumoral administration for cancer treatment.Citation5,Citation6 Recently, Adv-based research has entered the new fields of nanomedicine and nanodiagnostics.Citation7 The broad application based on Adv originated from their special advantages over other viral and nonviral vectors, primarily containing their high efficiency in transduction and transgenic expression and low pathogenicity.
Unfortunately, capsid proteins of Adv are immunogenic; in animal and human studies anti-adenovirus neutralizing antibodies (NAbs) were elicited within one week after initial exposure to Adv.Citation8,Citation9 The titer of NAb increased to a peak within 4 weeks.Citation10 When readministrated, NAb recognized and neutralized Adv before internalizing into targeted cells, leading to an inefficient transduction. Moreover, pre-existing anti-Adv neutralizing antibodies in a large population of people worldwide, especially anti-Ad5 antibodies, is a barrier for Adv-mediated clinical applications.Citation11–Citation13 In addition, Adv are limited to local administration due to substantial liver tropism and subsequent severe hepatotoxicity after systemic administration.Citation14 Therefore, none of the Adv-mediated gene therapy drugs has been FDA-approved so far.
Over the past decade, the most frequently used strategies to solve this problem have been hybrid vectors that combine viral and nonviral vectors. Nonviral vectors are nonimmunogenic, thus a liposome envelope or a polymer coating shields Adv and thereby reduces the immunogenicity or prevents recognition by the immune system or NAb. Adv can be divided into two groups: those that are covalently chemically conjugated and those that are physically self-assembled, each of which has their drawbacks. A typical example of chemical conjugation is pegylation, which has been confirmed as an efficient technique in several studies.Citation15–Citation19 However, studies have shown that chemical conjugation reduced infection activity of Adv, associated with steric hindrance from flexible polyethylene glycol (PEG) chains, resulting in a decreased gene transduction and transgenic expression.Citation16,Citation20,Citation21 This can be partially compensated for by a targeted ligand covalently conjugated to the PEG (ligand-PEG-Adv).Citation22,Citation23 Moreover, chemical conjugation showed protection of Adv in a titer-related way, but still failed in complete avoidance of the immune response or a negligible influence by NAb. The techniques of pegylation need to be further optimized, including the covalent site on Adv, PEG size and density, and reaction conditions.Citation15,Citation21,Citation24,Citation25 Alternatively, Adv can be modified by physical self-assembly, which is easier to handle and avoids potential inactivity of Adv during chemical synthesis. Cationic polymers (polyethylenimine [PEI], lipofectamine, and chitosan) have been widely investigated in enhancing gene transduction abilities, but most of the complexes showed weak evasion from NAb.Citation26–Citation30 Moreover, aggregation of Adv induced by cationic polymers suggested unstable complexationCitation29 and simultaneous toxicity associated with cationic polymers also limited clinical applications.Citation31 For the efficient delivery of plasmid DNA, it has been reported that PEG covalently conjugated to cationic polymers such as PEG-poly-L-lysine (PLL),Citation32 PEG-PEI,Citation33 and PEG-chitosanCitation34 could form stable and low toxic nonviral vectors. Therefore, we hypothesized that a PEG grafted cationic polymer can improve the stability of Adv-cationic polymer complexes and that linear PEG extending outside can protect Ad5 from NAb as well as achieve effective transgenic expression.
In our study, cationic PEG derivatives (APC), a linear PEG with one side blocked by amine pendants, was synthesized to immobilize onto negatively charged capsid proteins of Ad5 for evading NAb (). We hypothesized that amines play a bridge-like effect, linking a linear PEG and Ad5. Simultaneously, the linear PEG extends outside, protecting Ad5 from interaction with NAb or cells in blood. Then replicate-incompetent Ad5 expressing β-gal (Ad5- LacZ) were complexed with APC or the control PEI-2k in a serial ratio. Next, in vitro transfection assays were carried out in coxsackievirus and adenovirus receptor (CAR)- positive cells (A549Citation35) and CAR-negative cells (B16,Citation36 SKOV3Citation37). Finally, the ability of APC to protect Ad5 from evading NAb was evaluated by in vitro neutralizing assays in A549 cells.
Materials and methods
Chemical reagents and biomaterials
Branched PEI (2 kd), allyl glycidyl ether (AGE), and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co, (St Louis, MO). 2-mercaptoethylamine was obtained from Aladdin Chemistry Co, Ltd, (Shanghai, China). Ethylene oxide (EO) was obtained from Xinhua Institute of Active Material (Changzhou, Jiangsu, China). O-nitrohenyl-β-D-galactopyranoside and cesium chloride (molecular biology grade) were obtained from Amersco (Solon, OH).
Cell lines
E1-transformed human embryonic kidney cell line HEK 293 was purchased from ATCC (CRL1573) and cultured in Dulbecco’s modified Eagle’s medium (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin, incubated at 37°C in a 95% humidified atmosphere containing 5% CO2. Non-small cell lung carcinoma cell line A549, murine melanoma cell line B16, and human ovarian cancer cell line SKOV3 were obtained from Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 (GIBCO-BRL, Grand Island, NY) complemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin, cultured in the same conditions as above.
Adenovirus preparation
E1/E3-deleted recombinant type 5 adenovirus vectors encoding LacZ reporter gene and expressing β-galactosidase (Ad5-LacZ) were purchased from the Vector Gene Technology Company Limited (Beijing, China). Replication-deficient Ad5 vectors were amplified in HEK 293 cells and purified from the cells’ lysate through two steps of CsCl gradients ultracentrifugation. Next, the underlayer of viral band was desalted by dialysis at 4°C for 4 hours against dialysis buffer (10 mM Tris pH 8.0, 2 mM MgCl2, 5% sucrose) with a cellulose ester membrane of molecular weight (MW) cut off 25 kd before being stored in aliquots at −80°C until use. Finally, the titer of virus stock was evaluated as 2.5 × 1011 plaque-forming units per mL (pfu/mL) by established plaque-forming assay or 2.0 × 1012 viral particles per mL (vps/mL) by measurements of ultraviolet absorption at 260 nm (OD260). Multiplicity of infection (MOI) value is defined as the ratio of total number of pfu per number of cells. In our in vitro study, MOI was approximately equivalent to 40.
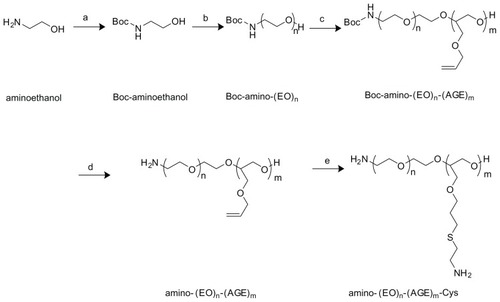
Synthesis of the copolymer (APC)
Protection of 2-aminoethanol with tertbutoxycarbonyl (Boc) group
Boc group was conjugated to 2-amino ethanol to prevent amine from oxidation in the following process. Typically, a solution of sodium hydroxide (10.03 g) in 50 mL distilled water was added to a solution of β-aminoethanol (7.37 g) in 25 mL methanol. While stirring in an ice bath, (Boc)2O (32.72 g) in 25 mL methanol was added dropwise to the mixture. After another 24 hours stirring at room temperature, the reaction mixture was filtrated and the filtrate was extracted three times by acetoacetate, and then washed with distilled water until it became neutral, followed by wash again with saturated salt solution. Next, the mixture was dried by waterless sodium sulfate, and the solvent was removed by rotary evaporation under reduced pressure. Finally a viscous product was obtained, namely Boc-aminoethanol.
Boc-(EO)n/(AGE)m copolymerization
Boc-(EO)n/(AGE)m was copolymerized by two steps of ring-opening of EO firstly and AGE. Typically, Boc-aminoethanol (0.88 g) as the initiator, after dehydration for 1 hour at 90°C through oil pump reduced pressure, was dissolved in nonaqueous dioxane. While stirring at 60°C, sodium cyanide (60%, 0.92 g) was added, and nonaqueous EO (100 mL) purified by reforming was added dropwise to the mixture by a constant pressure funnel connected with a condensing tube (4°C−8°C recirculation water) and a drying tube filled with waterless calcium chloride to maintain an anhydrous reaction system. After EO recirculation came to an end, AGE (30.1 g) was added to the reaction mixture and stirred for another 2 days at 60°C. Afterwards, water was added to stop the reaction, and 1 M hydrochloric acid was used to adjust pH to neutral. The solvent was then removed by rotary evaporation under reduced pressure, and the residue was again dissolved in dichloromethane, dried by waterless sodium sulfate, filtrated, centrifuged, and the supernatant was condensed by rotary evaporation under reduced pressure. Finally, after further purification by repeated precipitation of diethyl ether three times, a yellowish product resulted, namely Boc-(EO)n/(AGE) m, where n or m is represented as the number of monomers.
Deprotection of Boc-(EO)n/(AGE)m
Boc-(EO)n/(AGE)m was deprotected by trifluoroacetic acid. Briefly, Boc-(EO)n/(AGE)m (2.0 g) copolymer was dissolved in 10 mL dichloromethane containing 40% trifluoroacetic acid (v/v), which was stirred at RT for 2 hours. After removal of the solvent by rotary evaporation under reduced pressure, the mixture was redissolved in methanol and dialyzed for 2 days against distilled water through cellulose (3.5 kd). Finally, lyophilization of the solution gave a product, namely amino-(EO)n/(AGE)m.
Addition of 2-aminoethanethiol to amino-(EO)n/(AGE)m-CYS (APC)
Addition of 2-aminoethanethiol to the double bond of amino- (EO)n/(AGE)m was performed according to the protocol reported by Koyama et al.Citation38 Briefly, amino-(EO)n/(AGE)m (1.03 g) was dissolved in methanol (4 mL), and was added dropwise to the solution of 2-aminoethanethiol hydrochloride (3.01 g) in methanol (8 mL). After stirring at room temperature for 2 days, the reaction mixture was evaporated to remove the methanol. The residual syrup was dialyzed against distilled water for 3 days though cellulose membrane (MW cut off 3.5 kd). Lyophilization of the solution gave a yellowish product, namely amino-(EO)n/(AGE)m- Cys (APC).
Characterization of APC
To confirm synthesis of the APC polymer, the 1H-NMR spectras of intermediates and the final product APC were recorded on liquid samples (CD3CL or D2O; Sigma-Aldrich) in a Varian UNITY INOVA400 NMR Spectrometer (Palo Alto, CA) at 400 MHz.
The MW of APC was estimated by gel permeation chromatography (GPC), calibrated with PEG using Shodex KB-803 column (Shoko Co, Tokyo, Japan), Waters 515 pump, and Waters 2410 Refractive Index Detector (Waters Corporation, Milford, MA). Briefly, APC was dissolved in ultrapure water at a concentration of 10 mg/mL. Acetate buffer 0.5 M (acetate-sodium acetate) was used as eluent with a flow rate of 0.5 mL/min.
To determine the content of amines per molar of APC or indirectly charge APC bearing, analysis of nitrogen, carbon, and hydrogen was performed using an elementary analysis instrument (CARLO ERBA 1106; Carlo, Milan, Italy) and the sulfur atom was measured using the oxygen flask combustion method. The sample was in a completely dry form.
Preparation of Ad5 complexed cationic polymer
Complexes of Ad5 and APC or PEI-2k were formed as follows. Initially, stock solutions of APC (10 mg/mL), PEI-2k (10 mg/mL) and 5% glucose (w/v) in ultrapure water were respectively filtered through 0.22 μm pore sized filters. Then a serial concentration of dilutions of APC and PEI-2k in 5% glucose were respectively added dropwise to the isovolumic Ad5-LacZ dilution in 5% glucose with fixed particles of Ad5. After gently pipetting several times, the samples were incubated at room temperature for 25~30 min to form complexes of Ad5/APC or Ad5/PEI-2k. Complexes were freshly prepared before use every time.
In vitro transfection assays
To determine the best ratio of naked Ad5 and polymers (APC or PEI-2k), in vitro transfection assays were carried out in CAR over-expressing A549 cells. Briefly, A549 cells were seeded in 24 wells tissue culture plate at a density of 1 × 105 cells per well for 24 hours. When the cells reached 80%~90% confluence, Ad5/LacZ (1.25 × 107 pfu per well, 1 × 108 vps per well, MOI 40) were complexed with a serial dose of APC and PEI-2k (50, 150, 300, 450, 600, 750, 1000 ng) in 5% glucose. After staying at room temperature for 25~30 min, 50 μL per well of complexes were applied to the cells in 200 μL RPMI-1640 medium without serum. After incubation at 37°C for 4 hours, the medium was replaced by fresh RPMI-1640 complete medium. After 24 hours incubation, cells were washed by PBS and lyzed by cell lysis buffer. Next, β-galactosidae and total protein concentration were respectively quantified by colorimetry using o-nitrohenyl-β-D-galactopyranoside as the substrate and a BCA kit (Pierce, Rockford, IL). The transfection efficiency was represented as unit of β-galactosidase per milligram of total protein (u/mg protein).
After the optimal ratio of Ad5 and polymers were determined, in vitro transfection assays of naked Ad5 or complexes at the optimal ratio were also carried out in CAR negative cells (SKOV3 and B16) with the same processes as described above.
Cytotoxicity
For cytotoxicity assays, A549, SKOV3, and B16 cells were seeded in 96 wells tissue culture plate (1 × 104 cells/well) in RPMI-1640 complete medium. The background wells had no cells but RPMI-1640 complete medium. When cells achieved 80%~90% confluence, 10 μL per well of freshly prepared complexes of Ad5 (2 × 107 vps/well, 2.5 × 106 pfu/well), coated with a serial concentration of APC or PEI-2k dilutions respectively in 5% glucose were treated to the cells in 90 μL RPMI-1640 medium without serum, with the final concentrations of copolymers as follows: 1, 5, 10, 20 μg/mL. The control and background wells were added to 10 μL of 5% glucose in 90 μL RPMI-1640 medium without serum. Each sample had five repeated wells. After maintaining for 4 hours, the medium were replaced by 200 μL of fresh RPMI-1640 medium containing 10% FBS for a further 24 hours incubation, 20 μL per well of MTT solution (5 mg/mL) was added to the plate and further maintained for 4 hours at 37°C. Subsequently, the medium and MTT were carefully removed and replaced by 150 μL dimethyl sulfoxide. After shaking at 37°C for 30 min, the absorbance of each plate was read at 490 nm using varioskan@Flash instrument (Thermo Scientific, Waltham, MA). Relative cell viability was calculated by the formula as (Asample−Ablank)/(Acontrol−A blank) × 100%.
Size and zeta potential measurement of naked adenovirus and complexes
The average diameter and zeta potential of naked Ad5 and complexes of Ad5/APC and Ad5/PEI-2k at the optimal ratio were respectively measured by dynamic light scattering and electrophoretic light scattering, using a photon correlation spectroscopy (Malvern Zetasizer Nano ZS90, Malvern Instruments Ltd, Worcestershire, UK) with a 50 mV laser. Before measurement, 200 μL of naked Ad5 or freshly prepared complexed samples of Ad5/PEI-2k, Ad5/APC containing 2 × 1010 vps of Ad5-LacZ were diluted to 1 mL with ultrapure water. Then the measurement was performed at 25°C and a fixed angle of 90 with 2 min of equilibrium time and automatic cycles of measurement determined by the instrument. Each measurement was carried out in triplicate.
Morphological investigation by transmission electron microscopy (TEM)
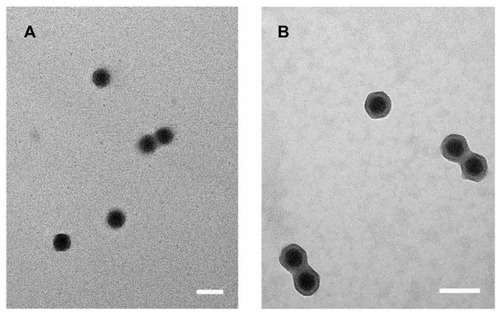
The morphology of Ad5 alone or complexes of Ad5/APC were observed with a transmission electron microscope (tecnaiG2F-20, FEI, Eindhoven, Holland) at an accelerating voltage of 20 kV, using a negative stain technique. Briefly, 20 μL of naked Ad5 or freshly prepared complex (Ad5/APC) at optimal ratio containing 1 × 109 vps of Ad5 were respectively added on a copper grid and left for 10 min, enabling a sufficient adsorption. Then the solution was removed with filter paper and replaced with 1% aqueous uranyl acetate, staining for 1 min. After removal of the dye with filter paper and dryness at 37°C, the samples were imaged by transmission electron microscopy.
Evaluation of APC protecting Ad5 from NAb
Primarily for preparation of antiserum from mice, C57BL/6 N mice (4 to 5 week old, male) were obtained from the Laboratory Animal Center of Sichuan University, and were housed under specific pathogen-free conditions for one week to acclimate. The animal experiments were approved by the Institutional Animal Care and Ethic Committee of Sichuan University. On day −28, the mice were randomly divided into two groups – one group of mice was immunized by intramuscular injection in the right hind limb with 5 × 1010 vps of recombinant adenovirus (Ad5-LacZ) in 50 μL of 5% glucose per mouse, and the other group was intramuscularly administered 5% glucose. On day −14, this immunization was repeated. On day 0, the blood was respectively collected from the orbits vein of the immunized and nonimmunized mice. The blood was coagulated at room temperature for 15 min and centrifuged (2000 rpm, 30 min). Finally, the supernatant antiserum and blank serum was respectively collected, pooled, and stored at −80°C before use.
In order to evaluate the ability of APC to protect Ad5 evading from NAb, the antiserum or blank serum was complement inactivated at 56°C for 30 min in advance. Naked Ad5 (50 μL), and complexes of Ad5/PEI-2k or Ad5/APC at the optimal ratio containing 1.25 × 107 pfu of Ad5-LacZ, were respectively incubated with 200 μL of diluted antiserum or blank serum at 37°C for 1 hour, making a neutralizing effect and the final dilution of the serum was 1:50 and 1:250. Then 250 μL per well of the mixture was respectively treated to A549 cells in 24 wells tissue culture plate, for which a confluence of 80%~90% had been achieved. The following processes were the same as the in vitro transfection. According to comparison of gene expression efficiency among naked Ad5, complexes of Ad5/PEI-2k and Ad5/APC in A549 at the presence of antiserum or blank serum, the ability of APC protecting Ad5 evading NAb were relatively evaluated.
Statistical analysis
All the above assays were performed at least in triplicate. The data are shown as the mean ± standard deviation (SD). Statistical analysis was carried out by Student’s t-test. The value of P < 0.05 was considered to be statistically significant.
Results
Synthesis and characterization of APC
APC was prepared by two steps of ring-opening copolymerization of EO and AGE, with Boc-aminoethanol as the initiator, followed by deportation of the Boc group and addition of 2-mercaptamine to the pendant of allyl groups. The main routes of synthesis are given in . The synthesis of APC was confirmed by 1H-NMR as follows.
Figure 2 Synthesis scheme of APC. (a) (Boc)2O, NaOH, MeOH:H2O = 1:1 (v/v), RT, 12 hours. (b) NaH, EO, dioxane, 60°C, 4 days. (c) NaH, AGE, 60°C, 3 days. (d) Trifluoroacetic acid:dichloromethane = 1:4 (v/v), RT, 2 hours. (e) MeOH, RT, 2 days.
Abbreviations: APC, cationic PEG derivative; EO, ethylene oxide; AGE, allyl glycidyl ether; RT, room temperature.
Firstly, on the spectra of Boc-aminoethanol, occurrence of peaks of δ (CH3CO–) = 1.448, δ (–CH2OH) = 3.285 indicated that Boc group had been successfully congjugated to the amine of aminoethanol. Then the polymerization of EO with Boc-aminoethanol as an initiator has been characterized by the typical peaks of δ (–O–CH2–CH2–O) = 3.740~3.382 and δ (–O–CH–CH2–) = 3.803. The following polymerization of AGE was confirmed by the double bond peak of δ (–CH = CH2–) = 5.867 and δ (–CH = CH2–) = 5.216. (The chemical shift of hydrogen atoms is shown in bold in this paragraph.) Next, deprotection of Boc group was achieved due to disappearance of the methyl peak of Boc group, and the spectra of final product, Amino-(EO)n/(AGE)m-Cys, showed no alkenyl peaks, demonstrating a complete addition.
The MW of APC was estimated to be 19571 Da by GPC with PEG as standard substance. Elemental analysis of APC showed that nitrogen took up 2.38%, carbon 48.76%, hydrogen 8.21%, and sulfur 4.88%. From the mass percentage of nitrogen and carbon together with the MW and molecular formula of APC, it can be calculated that n was 36 and m was 288, respectively, indicating the number of monomers of EO and AGE. In the following, the linear PEG on APC was inferred to 12.6 kd with 36 amines per molecular APC.
Enhanced transgenic expression efficiency by adenovirus complexed with APC
To evaluate the optimal ratio of polymer (APC, PEI-2k) and Ad5-LacZ, Ad5-LacZ (1.25 × 107 pfu) coated with a serial dose of PEI-2k or APC over the range from 50 ng to 1000 ng were transfected in CAR-rich A549 cells. As shown in , the peaks of the two curves, indicating the relation of gene transfection efficiency and polymer weight, revealed the optimal proportion of adenovirus and polymers (APC, PEI-2k), 1.25 × 107 pfu of adenovirus-LacZ corresponded to 600 ng for both PEI-2k and APC (P < 0.05). Excitingly, APC-mediate Ad5 gene transfection at the optimal ratio achieved as efficiently as PEI-2k (P > 0.05). Both of the polymers enhanced gene expression of naked Ad5 by approximately 10 times. The results may suggest a CAR-independent transfection for polymer shielded adenovirus.
Figure 3 In vitro transfection assays of Ad5 or complexes. To determine the best ratio of Ad5 and polymers, transfection assays were performed in A549 cells treated with Ad5-LacZ (1.25 × 107 pfu/well) complexed with a serial dose of polymers, PEI-2k or APC (50~1000 ng). Following incubation for 24 hours after transfection, the cells was lyzed for quantity of β-galactosidae (A). Transfection assays in A549, B16, and SKOV3 treated with Ad5 alone (1.25 × 107 pfu/well) or complexes (Ad5/PEI-2k or Ad5/APC) at the optimal ratio (B).
Notes: Data are represented as mean ± SD (n = 3), ***P < 0.001.
Abbreviations: Ad5, adenovirus vectors type 5; APC, cationic PEG derivative; PEI, polyethylenimine; Ad5/APC, adenovirus complexed with APC; Ad5/PEI-2k, adenovirus complexed with PEI-2k; pfu, plaque forming units; SD, standard deviation.
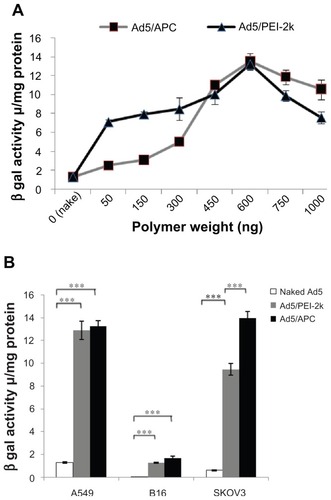
In the following, in order to confirm superior gene transfection efficiency of APC and PEI-2k in a CAR-ablate way, in vitro transfection assays of the complexes at the optimal ratio were performed as above in CAR-negative SKOV3 and B16 cells. As represented in , naked Ad5 showed far lower expression of β-gal in B16 cells and SKOV3 cells than in A549 cells. Greatly increased expression of β-gal was observed for Ad5 complexed with APC or PEI-2k. The gene expression efficiency of Ad5 was improved about 40 times in B16 cells and about 23 times in SKOV3 cells by the complexation with APC. Interestingly, there was no significant difference between APC and PEI-2k for Ad5-mediated gene therapy in A549 or B16 cells. While in SKOV3 cells, APC exhibited significantly higher gene expression than PEI-2k (P < 0.001). The results further confirmed that Ad5 with cationic polymers modification enabled improved gene expression in CAR-positive or negative cells. As expected, APC can achieve as efficient transduction as commercial PEI-2k.
Investigation of physical properties and morphology of Ad5 alone or complexes
Particle sizes and surface potential of naked adenovirus or complexes (Ad5/PEI-2k, Ad5/APC) at the optimal ratio were measured by dynamic light scattering and electrophoretic light scattering respectively. As shown in , the surface potential of the complexes turned positive, 9.64 ± 0.67 mV for Ad5/PEI-2k and 31.23 ± 0.40 mV for Ad5/APC while naked Ad5 remained negatively charged, −17.56 ± 0.53 mV. This result suggested that the copolymers had been ionically anchored on the viral surface, forming newly cationic hybrid vectors. Compared with naked Ad5 (115.6 ± 5.46 nm), Ad5/APC (130.2 ± 0.60 nm) had a slightly increased particle diameter. But the particle diameter of Ad5/PEI-2k was over 1 micrometer (1382 ± 79.9 nm) suggesting a severe aggregation induced by PEI-2k. Low polydispersity index value indicated all formulations had uniform distribution. The study demonstrated that APC and PEI-2k were accessible to complex with adenovirus by ionic interaction. Moreover, APC modification makes adenovirus relatively stable to commercial PEI-2k, which may facilitate gene delivery in vivo. showed the morphology of naked Ad5 or APC-coated Ad5 complex at an optimal ratio by TEM. Both naked Ad5 and Ad5/APC complex showed regular morphology with uniform distribution. But compared to Ad5 alone, Ad5/APC complex exhibited a notable shell-core structure. This result indicated that APC polymer had been ionically anchored onto the surface protein of Ad5, forming a coated Ad5.
Table 1 Zeta potential and size distribution of naked Ad5 and complexesTable Footnotea
Cytotoxicity
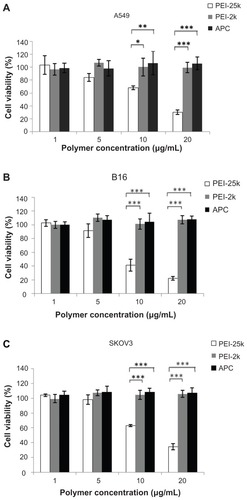
Cytotoxicity of adenovirus coated with a serial concentration of APC, PEI-2k, or PEI-25k (1, 5, 10, 20 μg/mL) were determined on A549, SKOV3, and B16 cells, using MTT assay. As shown in , the cytotoxicity of PEI-25k was concentration- dependent in the three cell lines. Ad5 complexed with a low concentration of PEI-25k (1 μg/mL) showed little cytotoxicity while the cell viability decreased along with the increased concentration of PEI-25k. When the concentration of PEI-25k rose to 20 μg/mL, the relative cell viability decreased to 30.3% ± 3.66% for A549 cells, 22.0% ± 2.62% for B16 cells, and 34.8% ± 6.90% for SKOV3 cells. However, over the range of designed concentration (1 μg/mL ~20 μg/mL), there was no significant difference between APC and PEI-2k (P > 0.05), both of which exhibited no cytotoxicity to the three cell lines. The result demonstrated that the aminated PEG derivative (APC), as well as low MW PEI-2k, was a relatively safe polymer to PEI-25k, giving APC potential for further study in vitro or in vivo.
Figure 5 Relative viability of (A) A549, (B) B16, and (C) SKOV3 cells treated with Ad5 (1.25 × 106 pfu/well) complexed with a serial concentration of APC, PEI-25k, and PEI-2k over the range from 1 μg/mL to 20 μg/mL. Each sample has five repeated wells.
Notes: Data are represented as mean ± SD (n = 5), *P < 0.05, **P < 0.01, ***P < 0.001.
Abbreviations: Ad5, adenovirus vectors type 5; pfu, plaque forming units; PEI, polyethylenimine; APC, cationic PEG derivative; SD, standard devation.
In vitro neutralizing assays
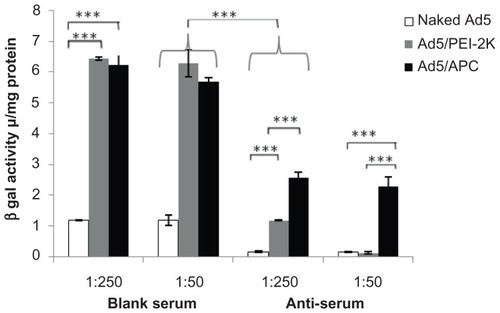
To evaluate the ability of Ad5 complexed with APC evading anti-Ad5 neutralizing antibody, naked Ad5, Ad5/APC, or Ad5/PEI-2k complexes were respectively incubated at 37°C for 1 hour with blank serum or antiserum isolated from immunized mice or nonimmunized mice. The incubated mixtures were then treated with A549 cells for transfection assays. As shown in , for the three samples treated with blank serum, there were no significant differences between 1:50 dilution and 1:250 dilution. Wherein, Ad5/APC complex achieved gene transduction as efficient as Ad5/PEI-2k, significantly more efficient than naked Ad5. The result almost matched in vitro transfection assays in . When the samples were exposed to antiserum at the dilution 1:250, the gene transfection efficiency of naked Ad5 decreased by about 7.1 times, from 1.18 ± 0.014 u/mg to 0.17 ± 0.02 u/mg, revealing that anti-Ad5 neutralizing antibody had been successfully generated by intramuscular administration in mice. Comparatively, at the same dilution, Ad5/PEI-2k showed a decrease by 5.4 times, from 6.44 ± 0.056 u/mg to 1.19 ± 0.016 u/mg, which was still a higher transduction than naked Ad5. At the 1:50 dilution of antiserum, PEI-2k-mediate gene expression almost decreased to the same level as naked Ad5, while Ad5/APC still exhibited significantly efficient gene transfer. Compared with Ad5/APC at the 1:50 dilution of antiserum, there was just a 2.4 times decrease from 5.70 ± 0.13 u/mg to 2.28 ± 0.32 u/mg. Furthermore, no significant difference was found for Ad5/APC exposed to 1:50 versus 1:250 diluted antiserum. The above results revealed that, (1) immunized serum containing NAb against Ad5 could inactive naked Ad5 in a titer-related way; (2) PEI-2k could partially protect Ad5 evading NAb with a low titer relative to Ad5 alone; and, most importantly, (3) APC was significantly better at allowing Ad5 to escape from NAb than PEI-2k, which may be due to the effect of PEG being present on the APC polymer.
Figure 6 Neutralizing assays in A549 cells treated with Ad5 alone (1.25 × 107 pfu), Ad5/PEI-2k or Ad5/APC at the optimal ratio. Before transfection, a neutralizing effect was processed by incubation of naked Ad5 or freshly prepared complexes (Ad5/PEI-2k or Ad5/APC) and complement-inactivated blank serum or antiserum which respectively isolated from nonimmunized or immunized C57BL/6 N mice at 37°C for 1 hour. The following processes were performed as described in in vitro transfection assays.
Notes: Data are represented as mean ± SD (n = 3), ***P < 0.001.
Abbreviations: Ad5, adenovirus vectors type 5; PEI, polyethylenimine; APC, cationic PEG derivative; pfu, plaque forming units; Ad5/PEI-2k, adenovirus complexed with PEI-2k; Ad5/APC, adenovirus complexed with APC; SD, standard deviation.
Discussion
To construct a safe and efficient Adv-based vector system which is robust in the presence of NAb and permits repeated administration in clinical trials, a number of polymers have been extensively investigated to incorporate onto the surface of adenovirus. Thus, a shielded vector was formed to prevent Adv from interaction with proteins or cells in blood, induce low immune response, and facilitate Adv evading Nab.Citation39–Citation42 In our study, the cationic PEG derivative (APC), a linear PEG with one side blocked by amines, was designed to modify the surface protein of Ad5 by physical adsorption. The complex (Ad5/APC) with PEG extending outside achieved a satisfiable protection of Adv from NAb as well as highly enhanced gene expression efficiency.
Prior to the synthesis of APC, the size of PEG and the number of amine pendants had been taken into serious consideration. In general, 3~5k MW of PEG were frequently used to form a compact coating. But recently, some reports revealed that the size and density of PEG anchored on to the surface protein of adenovirus played a crucial part in the biodistribution in vivo.Citation25,Citation43,Citation44 Large size of PEG (20 kd) not small size (3–5 kd) remarkably facilitated detargeting from liver thus relatively reduced hepatotoxicity and made systemic administration of adenovirus possible as well as allowing evasion from NAb. Therefore, APC (approximately 20 kd) with linear PEG (approximately 13 kd) was synthesized in the current study. Meanwhile, the number of primary amines mounting on one side of APC, which was closely related to the density of APC anchored onto Ad5, was evaluated as 36 according to GPC and elementary analysis. Branched PEI-2k contains a total of about 50 amines, of which primary amines account for 25% and secondary amines 50%. Comparatively, APC may bear more positive charge than PEI-2k. Additionally, we had tried to reduce the number of amines by a third via a lower AGE mole ratio, but weaker protection ability from NAb was obtained (data not shown). The optimal number of amines requires further study.
In the past, cationic polymers have been explored to carry plasmid or viruses for gene delivery, with achievement of improved gene transduction. One explanation for the enhanced effect was that positively charged complexes (Adv/polymers) facilitated interaction with negatively charged cells. At a starting point to select a control to APC in our study, PEI-600, PEI- 2k, and PEI-25k were respectively complexed with Ad5 at the optimal ratio then applied to A549 cells. We found there was no significant difference between PEI-25k and naked Ad5 for Adv-based gene expression. Interestingly, PEI-2k was far superior to PEI-600 or PEI-25k, and had 10 times enhanced gene expression than naked Ad5 (data not shown). Commercially branched PEI-25k as one of the most efficient polymers in gene transduction, was usually used as a benchmark to other biomaterials especially newly synthesized polymers for carrying gene delivery.Citation45,Citation46 Similarly, authors have shown that PEI-25k exhibited somewhat lower gene expression of Adv than naked Adv in A549 cells and C2C12 cells.Citation45 A recent report indicated that PEI-25k significantly enhanced Adv uptake but had no effect on transgenic expression, compared to naked Adv.Citation47 Normally, Adv-mediated gene delivery begins with binding and internalization into cells. During endocytosis, fibers are detached. Afterwards, hexons, pentons, and other structural proteins are dissociated, resulting in Adv escaping from endosome into cytosol and subsequent import to nucleus via interaction with cytoplasmic dynein.Citation22,Citation48 Each step may influence a successful delivery. Since PEI-25k facilitates Adv internalization but not transgenic expression, this suggests that PEI-25k bears more positive charge, affording a strong electrostatic force for Adv/PEI-25k complex and a difficult release for Adv from the polymer. Comparatively, PEI-2k may mediate a better Adv gene delivery among the PEI polymers.
In our study, PEI-2k showed 10 times more efficient gene expression than naked Adv in A549 cells. To the best of our knowledge, the enhanced transduction of Adv mediated by PEI-2k has not been reported previously. In our study, Ad5/APC complex enhanced Ad5-mediated gene transfer to the same extent as PEI-2k in CAR-positive cells (A549) or CAR-negative cells (B16), and interestingly, significantly more greatly than PEI-2k in CAR-deficient cells (SKOV3). The significant difference of gene expression between Ad5/PEI-2k and Ad5/APC in SKOV3 needs further exploration. The observed enhanced gene expression by APC was meaningful: (1) It makes lower doses of Adv available – higher doses of Adv induce a severe immunoresponse and hepatotoxicity.Citation49 (2) Adv infect cells mainly via CAR-dependent endocytosis,Citation50,Citation51 leaving a limit to be internalized by CAR-poor tumor cells. Polymer coatings gifted Adv with CAR-ablated infection ability for nonspecific cell uptake.Citation52 (3) A ligand can be added to retarget for in vivo study and prevent unwanted binding of Ad5 to human erythrocytes which were found to be present in CAR.Citation53
The physical property of Ad5 alone or Ad5/APC complex was characterized by measurements of particle size and zeta potential. Ad5/PEI-2k complex had an average diameter of 1382 ± 79.9 nm. The result was in agreement with previous reports that cationic polymers without exception induced severe aggregation of viral vectors.Citation27 The aggregation enabled a greater contact of adenovirus to cell monolayer, which was another mechanism proposed for enhanced gene transduction by cationic polymers.Citation52,Citation54 But complexes up to 1 micrometer in size may afford poor permeability into tumor vessels where the maximum diameter size of 400~600 nm was required.Citation55 The unstable complex may potentially interact with negatively charged protein or cells, inducing immediate cytotoxicity. While APC makes a stable hybrid vector, 130.2 ± 0.60 nm is a slightly increased diameter than adenovirus alone. Ad5/APC complexes with such a size may benefit from enhanced permeability and retention effect when applied in vivo for cancer treatment.Citation56 The TEM image of Ad5/APC supported that Ad5/APC complexes have been formed in shell core structure.
Furthermore, cytotoxicity is the bottleneck for cationic polymers such as PEI to be utilized in clinics. The polymer cytotoxicity is closely related to factors such as MW, concentration of free cationic polymers, ionic strength, and the processes after entry into cells.Citation39,Citation57 For plasmids, gene expression efficiency is in proportion to polymeric size. High gene expression efficiency needs a large size but is accompanied by severe cytotoxicity. Furthermore, in vivo studies require highly increased doses of complex, thus the release of nonbiodegradable polymer from the complex after entry into cells is a nondesirable side effect. To reduce cytotoxicity, biodegradable biomaterials have been widely investigated. Another effective strategy is the introduction of PEG into cationic polymers. As reported, PEG can prevent cationic complexes from interaction with other proteins, thus stabilizing the complex and reducing cytotoxicity.Citation58,Citation59 For example, it was demonstrated that PLL (24 kd) showed cytotoxicity to KB cells from 1 μg/mL, but PLL (24 kd) with a PEG grafting ratio of 8.4% (PLL-g-PEG) showed little cytotoxicity over the range of 1 μg/mL to 10 μg/mL.Citation59 The low cytotoxity of APC in our study warrants further investigation of the complex.
Although APC enabled a stable, low toxic Ad5-mediated system with high gene transduction efficiency, Ad5-mediated gene therapy has also been hindered by the neutralization of NAb towards Adv, making readministration problematic. Shielding antigen epitope of Adv and avoidance of recognition by NAb are anticipated. Therefore, it is key to develop a stable and robust Adv-shielded vector before endocytosis into cells. In the present study, APC-based protection ability of Ad5 from NAb was evaluated. The results indicated that commercial PEI-2k offered weak protection of Ad5 from NAb. But the Ad5/APC complex showed relatively good resistance to NAb. This superior protection effect was mainly contributed by the PEG segments present on APC. The unstable Ad5/PEI-2k complex may be more susceptible to NAb or other negatively charged proteins in serum than the Ad5/APC complex. In addition, polymer coating by physical adsorption permitted a systemic administration by reducing hepatic uptake and extending blood half-life.Citation41,Citation60 Therefore, APC may be a potential Adv-carrier for gene therapy.
Taken together, we present a novel polymer-coated Adv delivery system, which is simple, stable, has low toxicity, and is highly efficient in transgenic expression. More importantly, this system offered good protection of Adv from NAb in vitro. This Adv delivery system is based on a newly designed cationic PEG derivative. If necessary, PEG length and amines pendants or positive charge present on the copolymer can be easily optimized by adjusting the molar ratio among Boc-aminoethanol, EO, and AGE. Meanwhile, the amines of the copolymer afford many conjunctive sites for the conjugation of ligands or active proteins, thus it is more susceptible to reconstruct a specifically desired copolymer. In future studies, we will investigate in vivo delivery of APC under the establishment of a pre-existing immunized animal model.
Acknowledgments
We are thankful for the financial support of the National Natural Science Foundation of China (No. 81173011) and Program for New Century Excellent Talents in University (No. NTEC-10-0601) and the National Science & Technology Major Project of China (No. 2011ZX09401-304[4-3]) We also thank Miss Mengtian Zhang and Mr Sanjun Shi for their help with this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- HaismaHJBelluARPharmacological interventions for improving adenovirus usage in gene therapyMol Pharm201181505520979428
- MatthewsQLCapsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approachMol Pharm20118131121047139
- RenteriaSSClemensCCCroyleMADevelopment of a nasal adenovirus-based vaccine: Effect of concentration and formulation on adenovirus stability and infectious titer during actuation from two delivery devicesVaccine20102892137214820044048
- Gene therapy clinical trials worldwide [database on the Internet]J Gene Med Available from: http://www.wiley.com//legacy/wileychi/genmed/clinical/Accessed January 25, 2011
- HorowitzJAdenovirus-mediated p53 gene therapy: overview of preclinical studies and potential clinical applicationsCurr Opin Mol Ther19991450050911713766
- MaGShimadaHHiroshimaKTadaYSuzukiNTagawaMGene medicine for cancer treatment: commercially available medicine and accumulated clinical data in ChinaDrug Des Devel Ther20092115122
- SinghRKostarelosKDesigner adenoviruses for nanomedicine and nanodiagnosticsTrends Biotechnol200927422022919251331
- WuTLErtlHCImmune barriers to successful gene therapyTrends Mol Med2009151323919101205
- ZaissAKMachadoHBHerschmanHRThe influence of innate and pre-existing immunity on adenovirus therapyJ Cell Biochem2009108477879019711370
- ZhongZHanJWanYZhangZSunXAnionic liposomes enhance and prolong adenovirus-mediated gene expression in airway epithelia in vitro and in vivoMol Pharm20118367368221510701
- SunCZhangYFengLEpidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern ChinaVaccine201129223837384121447314
- MastTCKiersteadLGuptaSBInternational epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type- 36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trialsVaccine201028495095719925902
- PilankattaRChawlaTKhannaNSwaminathanSThe prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccinesJ Med Virol201082340741420087930
- SinghRTianBKostarelosKArtificial envelopment of nonenveloped viruses: enhancing adenovirus tumor targeting in vivoFASEB J20082293389340218556649
- EtoYYoshiokaYIshidaTOptimized PEGylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancerBiol Pharm Bull20103391540154420823571
- MokHPalmerDJNgPBarryMAEvaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responsesMol Ther2005111667915585407
- EtoYGaoJQSekiguchiFNeutralizing antibody evasion ability of adenovirus vector induced by the bioconjugation of methoxypolyethylene glycol succinimidyl propionate (MPEG-SPA)Biol Pharm Bull200427693693815187452
- CroyleMAChirmuleNZhangYWilsonJM“Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lungJ Virol200175104792480111312351
- KimPHSohnJHChoiJWActive targeting and safety profile of PEG-modified adenovirus conjugated with herceptinBiomaterials20113292314232621227505
- LeeGKMaheshriNKasparBSchafferDVPEG conjugation moderately protects adeno-associated viral vectors against antibody neutralizationBiotechnol Bioeng2005921243415937953
- O’RiordanCRLachapelleADelgadoCPEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivoHum Gene Ther19991081349135810365665
- EtoYYoshiokaYMukaiYOkadaNNakagawaSDevelopment of PEGylated adenovirus vector with targeting ligandInt J Pharm20083541–23817904316
- JungYParkHJKimPHRetargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cellsJ Control Release2007123216417117854941
- Suzuki-KouyamaEKatayamaKSakuraiFHexon-specific PEGylated adenovirus vectors utilizing avidin-biotin interactionBiomaterials20113261724173021106235
- WortmannAVöhringerSEnglerTFully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodiesMol Ther200816115416217848961
- WangIJJhuangMCChenYHYehLKLiuCYYoungTHChitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cellsPLoS One201058e1208520711466
- FasbenderAZabnerJChillónMComplexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivoJ Biol Chem199727210647964899045673
- SteelJCCavanaghHMBurtonMADingwallDJKalleWHModification of liposomal concentration in liposome/adenoviral complexes allows significant protection of adenoviral vectors from neutralising antibody, in vitroJ Virol Methods20051261–2313615847916
- DoddsEPiperTAMurphySJDicksonGCationic lipids and polymers are able to enhance adenoviral infection of cultured mouse myotubesJ Neurochem19997252105211210217291
- MoselhyJSarkarSChiaMCEvaluation of copolymers of N-isopropylacrylamide and 2-dimethyl(aminoethyl)methacrylate in nonviral and adenoviral vectors for gene delivery to nasopharyngeal carcinomaInt J Nanomedicine20072346147818019844
- LvHZhangSWangBCuiSYanJToxicity of cationic lipids and cationic polymers in gene deliveryJ Control Release2006114110010916831482
- AhnCHChaeSYBaeYHKimSWSynthesis of biodegradable multi-block copolymers of poly(L-lysine) and poly(ethylene glycol) as a non-viral gene carrierJ Control Release200497356757415212887
- LiangBHeMLXiaoZPSynthesis and characterization of folate- PEG-grafted-hyperbranched-PEI for tumor-targeted gene deliveryBiochem Biophys Res Commun2008367487488018201560
- LaiWFLinMCNucleic acid delivery with chitosan and its derivativesJ Control Release2009134315816819100795
- EtoYGaoJQSekiguchiFPEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion abilityJ Gene Med20057560461215543536
- YamashitaMInoAKawabataKSakuraiFMizuguchiHExpression of coxsackie and adenovirus receptor reduces the lung metastatic potential of murine tumor cellsInt J Cancer200712181690169617546646
- KimJSLeeSHChoYSChoiJJKimYHLeeJHEnhancement of the adenoviral sensitivity of human ovarian cancer cells by transient expression of coxsackievirus and adenovirus receptor (CAR)Gynecol Oncol200285226026511972385
- KoyamaYItoTMatsumotoHNovel poly(ethylene glycol) derivatives with carboxylic acid pendant groups: synthesis and their protection and enhancing effect on non-viral gene transfection systemsJ Biomater Sci Polym Ed200314651553112901435
- HanSMahatoRISungYKKimSWDevelopment of biomaterials for gene therapyMol Ther20002430231711020345
- FisherKDSeymourLWHPMA copolymers for masking and retargeting of therapeutic virusesAdv Drug Deliv Rev201062224024520005911
- SubrVKostkaLSelby-MilicTCoating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood componentsJ Control Release2009135215215819166885
- HofherrSEMokHGushikenFCLopezJABarryMAPolyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopeniaHum Gene Ther200718983784817767399
- DoroninKShashkovaEVMaySMHofherrSEBarryMAChemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirusHum Gene Ther200920997598819469693
- HofherrSEShashkovaEVWeaverEAKhareRBarryMAModification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expressionMol Ther20081671276128218461056
- KimP-HKimTIYockmanJWKimSWYunCOThe effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapyBiomaterials20103171865187419962189
- HanJZhaoDZhongZZhangZGongTSunXCombination of adenovirus and cross-linked low molecular weight PEI improves efficiency of gene transductionNanotechnology20102110105106
- KasmanLMBaruaSLuPRegeKVoelkel-JohnsonCPolymer-enhanced adenoviral transduction of CAR-negative bladder cancer cellsMol Pharm2009651612161919655763
- KelkarSAPfisterKKCrystalRGLeopoldPLCytoplasmic dynein mediates adenovirus binding to microtubulesJ Virol20047818101221013215331745
- MorralNO’NealWKRiceKLethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboonsHum Gene Ther200213114315411779418
- Medina-KauweLKEndocytosis of adenovirus and adenovirus capsid proteinsAdv Drug Deliv Rev200355111485149614597142
- MeierOGreberUFAdenovirus endocytosisJ Gene Med20046Suppl 1S15216314978758
- DavisHEMorganJRYarmushMLPolybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranesBiophys Chem2002972–315917212050007
- CarlisleRCDiYCernyAMHuman erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1Blood200911391909191819131551
- DavisHERosinskiMMorganJRYarmushMLCharged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregationBiophys J20048621234124214747357
- YuanFDellianMFukumuraDVascular permeability in a human tumor xenograft: molecular size dependence and cutoff sizeCancer Res19955517375237567641188
- FisherKDGreenNKHaleASubrVUlbrichKSeymourLWPassive tumour targeting of polymer-coated adenovirus for cancer gene therapyJ Drug Target2007157–854655117671901
- GodbeyWTWuKKMikosAGPoly(ethylenimine) and its role in gene deliveryJ Control Release1999602–314916010425321
- WeissSISieverlingNNiclasenMUronic acids functionalized polyethyleneimine (PEI)-polyethyleneglycol (PEG)-graft-copolymers as novel synthetic gene carriersBiomaterials200627102302231216337267
- ParkJWMokHParkTGPhysical adsorption of PEG grafted and blocked poly-L-lysine copolymers on adenovirus surface for enhanced gene transductionJ Control Release2010142223824419913577
- GreenNKHerbertCWHaleSJExtended plasma circulation time and decreased toxicity of polymer-coated adenovirusGene Ther200411161256126315215884