Abstract
Background
The objective of this study was to prepare, characterize, and evaluate a folate-modified self-microemulsifying drug delivery system (FSMEDDS) with the aim to improve the solubility of curcumin and its delivery to the colon, facilitating endocytosis of FSMEDDS mediated by folate receptors on colon cancer cells.
Methods
Ternary phase diagrams were constructed in order to obtain the most efficient self-emulsification region, and the formulation of curcumin-loaded SMEDDS was optimized by a simplex lattice experiment design. Then, three lipophilic folate derivatives (folate-polyethylene glycol-distearoylphosphatidylethanolamine, folate-polyethylene glycol-cholesteryl hemisuccinate, and folate-polyethylene glycol-cholesterol) used as a surfactant were added to curcumin-loaded SMEDDS formulations. An in situ colon perfusion method in rats was used to optimize the formulation of FSMEDDS. Curcumin-loaded FSMEDDS was then filled into colon-targeted capsules and the in vitro release was investigated. Cytotoxicity studies and cellular uptake studies was used in this research.
Results
The optimal formulation of FSMEDDS obtained with the established in situ colon perfusion method in rats was comprised of 57.5% Cremophor® EL, 32.5% Transcutol® HP, 10% Capryol™ 90, and a small amount of folate-polyethylene glycol-cholesteryl hemisuccinate (the weight ratio of folate materials to Cremophor EL was 1:100). The in vitro release results indicated that the obtained formulation of curcumin could reach the colon efficiently and release the drug immediately. Cellular uptake studies analyzed with fluorescence microscopy and flow cytometry indicated that the FSMEDDS formulation could efficiently bind with the folate receptors on the surface of positive folate receptors cell lines. In addition, FSMEDDS showed greater cytotoxicity than SMEDDS in the above two cells.
Conclusion
FSMEDDS-filled colon-targeted capsules are a potential carrier for colon delivery of curcumin.
Introduction
Curcumin, a polyphenol of turmeric, is derived from the rhizomes of Curcuma longa, which can suppress the tumorigenic activity of various cancers, such as the colon, duodenum, esophagus, forestomach, stomach, liver, breast, leukemia, oral cavity, and prostate.Citation1,Citation2 Five Phase I clinical trials have demonstrated the safety and tolerability of curcumin in colorectal cancer patients.Citation3 In spite of this huge potential of curcumin as an effective chemotherapy agent against colon cancer, it has poor systemic availability due to its low aqueous solubility, efficient first-pass effect, and some degree of intestinal metabolism when administered via the oral route. It has been demonstrated that a large daily oral dose of 3.6 g of curcumin resulted in pharmacologically efficacious levels in colorectal tissue.Citation4
Current trends of curcumin in the treatment of colon cancer have concentrated on the development of potential delivery systems to increase its aqueous solubility and bioavailability as well as controlled delivery of curcumin at or around cancer tissues.Citation5 Recently, much attention has been given to self-microemulsifying drug delivery systems (SMEDDS) to enhance the solubility, dissolution, and oral absorption for poorly water-soluble drugs, such as itraconazole, oridonin, coenzyme Q10, and zedoary essential oil.Citation6–Citation9 SMEDDS is an isotropic and thermodynamically stable solution consisting of oil, surfactant, cosurfactant, and drug, which can spontaneously form oil-in-water microemulsion when mixed with water under gentle stirring. The microemulsion presents the drug in a dissolved form, and the small droplet size (less than 100 nm) provides a large interfacial surface area favoring drug absorption.
For efficient drug delivery, a carrier system should facilitate not only tumor localization but also intracellular access. Folate receptor is a glycosylphosphatidylinositol-linked membrane glycoprotein of 38 kDa, which is overexpressed on the surface of most solid tumors, including colorectal carcinoma.Citation10 In recent years, it has been demonstrated that folate receptor-mediated uptake of the vitamin folic acid could be exploited to facilitate entry of an attached liposome or emulsion into cells.Citation11,Citation12
In the present study, an attempt was made to improve the solubility and targeted-delivery of curcumin to the colon by formulating it as folate-modified SMEDDS (FSMEDDS) and filling the formulation into colon-targeted capsules coated by Eudragit® S 100 (Evonik Industries AG, Essen Germany), a coating material for colonic delivery because of it will not dissolve under the condition of pH < 7.Citation5 Under conditions of colon fluid, curcumin-loaded SMEDDS (CUR-SMEDDS) containing folate conjugated material can form a microemulsion and then it can bind with folate receptors at the surface of colorectal tissues or colon cancer cells to increase the absorption in the colon or induce the endocytosis of CUR-FSMEDDS.Citation10,Citation13
The main objectives of this study were to develop and characterize a CUR-SMEDDS formulation for colon targeting and to evaluate its anticancer effects. An efficient CUR-SMEDDS formulation was developed using solubility and phase diagrams. The formulation optimization of CUR- FSMEDDS was conducted with the established in situ colon perfusion method in rats. The developed formulations were characterized by assessing morphology, particle size, and zeta potential. Cytotoxicity of SMEDDS and FSMEDDS was evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma Chemical Co, St Louis, MO), and cellular uptake of the two formulations was investigated with fluorescence microscopy and flow cytometry.
Materials and methods
Materials
For preparation of the SMEDDS formulation, curcumin was purchased from Sigma-Aldrich Corporation (St Louis, MO). Capryol™ 90, Labrafil® M1944CS, Labrasol®, Plurol® Oleique CC 497, and Transcutol® HP were purchased from Gattefosse (Saint-Priest, France). Cremophor® EL was obtained from BASF (Ludwigshafen, Germany). Emulsifier OP, 1,2-propanediol, and Tween® 80 were purchased from Guangcheng Chemical Co, Ltd (Tianjin, China). Ethyl oleate and polyethylene glycol (PEG) 400 were purchased from Shanghai Chemical Co, Ltd (Shanghai, China). Ethanol was obtained from Fuyu Fine Chemical Co, Ltd (Tianjin, China). Castor oil and isopropyl myristate were purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). Folate-PEG-cholesterol (F-PEG-Chol) was presented by Professor Guangya Xiang (School of Pharmacy, Huazhong University of Science and Technology, Wuhan, Hubei, China). For the cell culture experiment, folate-free Roswell Park Memorial Institute 1640 medium and Gibco® trypsin-ethylenediaminetetraacetic acid were purchased from Life Technologies (Carlsbad, CA). Characterized fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Materials Co, Ltd (Hangzhou, China). Hela (human uterine cervix cancer cell line) and HT-29 (human colon carcinoma cell line) were purchased from the cell bank of the Chinese Academy of Science (Beijing, China). The MTT assay was purchased from Sigma-Aldrich.
SMEDDS formulation optimization
Solubility studies
Solubility studies were conducted by placing an excess amount of curcumin in a 2 mL tube containing 1 mL of oil, surfactant, and cosurfactant, as shown in . The mixture was then shaken in a water bath shaker at room temperature for 48 hours to get to equilibrium. The equilibrated sample was centrifuged at 12,000 rpm for 10 minutes, followed by filtration through a membrane filter (0.45 μm) to remove the undissolved curcumin. The supernatant was taken and diluted with methanol for quantification of curcumin by high-performance liquid chromatography system (Agilent 1200 Infinity series; Agilent Technologies, Santa Clara, CA). The high-performance liquid chromatography method used a phenomenex-C18 column (5 μm, 4.6 mm × 250 mm) at room temperature, and the mobile phase was a mixture of methanol:water (containing 3.6% glacial acetic acid) (73:27 volume/volume). The flow rate was set at 1.0 mL/minute and the analysis wavelength was at 428 nm.
Table 1 Solubility of curcumin in various vehicles
Construction of ternary phase diagrams
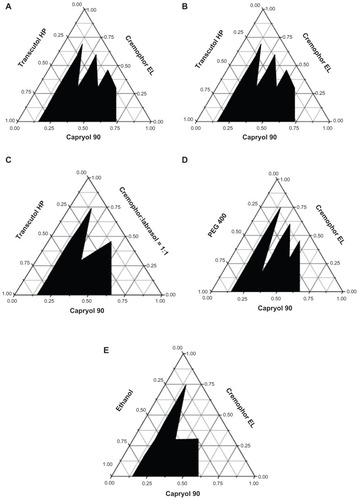
Ternary phase diagrams were constructed in order to obtain the concentration range of components for the existing region of microemulsions.Citation14 The effect of various oils on the microemulsion formation was studied by constructing ternary phase diagrams with Capryol 90 and Labrafil M1944CS at the oil phase, with Cremophor EL as the surfactant, and Transcutol HP as the cosurfactant. When Capryol 90 was fixed at the oil phase, the influence of various surfactants on the microemulsion formation with Transcutol HP as cosurfactant and the influence of various cosurfactants on the microemulsion formation with Cremophor EL as surfactant were evaluated by constructing a ternary phase diagram.
Preparation and optimization of CUR-SMEDDS formulations
Blank SMEDDS formulations were obtained by mixing the oil with surfactant and cosurfactant, which were selected by ternary phase diagrams under a magnetic stirring at 400 rpm/minute for 3 minutes at room temperature. Curcumin was added to blank SMEDDS and mixed by gentle vortexing until a transparent preparation was obtained, then the CUR-SMEDDS was prepared. In this study, a simplex lattice experiment design was used to optimize the compositions of SMEDDS formulation. The Simplex–Lattice experiment design for a three-component system was represented by an equilateral triangle in a two-dimensional space.Citation15,Citation16 Seven batches of CUR-SMEDDS were prepared as shown in .
Table 2 Actual and transformed values, mean particle size, and solubility of seven different formulations per Simplex–Lattice design
Synthesis of lipophilic folate derivatives and optimization of CUR-FSMEDDS
Two lipophilic derivatives, folate-PEG-distearoylphosphatidylethanolamine (F-PEG-DSPE) and folate-PEG-cholesteryl hemisuccinate (F-PEG-CHEMS), were synthesized based on established methods.Citation13,Citation17,Citation18 F-PEG-DSPE, F-PEG-CHEMS, or F-PEG-Chol was added to CUR-SMEDDS formulations as a surfactant. It has been previously reported that due to the existence of receptors such as lectin or transferrin receptors on the intestinal mucosa, relative ligands such as lectin and transferrin-modified nanoparticulate delivery systems could enhance the intestinal absorption of the drug through specific binding.Citation19–Citation21 Thus, it could be inferred that the folate-modified carriers would improve the absorption in the colon by the encapsulated drug binding with folate receptors on normal colorectal mucosa.Citation10 The effect of different folate derivatives on the absorption in the colon of curcumin in FSMEDDS formulations was thus studied using the established in situ colon perfusion method in rats.Citation22,Citation23
Rats were fasted overnight with free access to water before the experiment. The rat was anesthetized by an intraperitoneal injection of 1% sodium pentobarbital (0.4 mL/ 100 g body weight) and was placed under an infrared lamp to keep body temperature normal. Upon verification of the loss of pain reflexes, the abdomen was opened with a middle longitudinal incision about 3 cm long. After the abdominal cavity was opened, the colon segment in perfusion was exposed and incisions were made on both sides of the segment. It was rinsed with physiological saline and then connected to a constant- flow pump (BT00–100M; Baoding Longer Precision Pump Co, Ltd, Baoding, China) with catheters to the perfusion system. Curcumin-loaded microemulsion (25 mL) was prepared by dispersing CUR-SMEDDS or CUR-FSMEDDS containing different types and concentrations of lipophilic folate derivatives in a Krebs-Ringer buffer solution (Sigma- Aldrich) at 37°C. Perfusion with the curcumin dose of 50 mg/kg was introduced into colon segment loops with the pump at a flow rate of 2.5 mL/minute; both ends of the loop were ligated. The entire surgical area was covered with a piece of sterilized absorbent gauze wetted with normal saline. After 6 hours, the effluent solution in the loop was collected and diluted by Krebs-Ringer buffer solution for determination.
Characterization of CUR-FSMEDDS
Morphology
The morphology of CUR-FSMEDDS was observed under transmission electron microscope (JEM-1200EX; JEOL Ltd, Tokyo, Japan). CUR-FSMEDDS was diluted with distilled water (1:100) and gently mixed, and then stained with a 2% aqueous solution of phosphotungstic acid and allowed to dry before observation under the transmission electron microscope.
Droplet size and zeta potential determination
CUR-FSMEDDS formulation (1 mL) was diluted with 100 mL of deionized water under constant stirring. The droplet size distribution and zeta potential of the resultant microemulsion was determined by a particle size and zeta potential analyzer (Delsa™Nano; Beckman Coulter Inc, Brea, CA).
Release of curcumin from FSMEDDS-filled colon-targeted capsules
CUR-FSMEDDS equivalent to 1 mg of curcumin were filled into colon-targeted capsules for rats (Qiangji Pharmaceutical Factory, Chaozhou, China) without shell damage. Release studies of CUR-FSMEDDS capsules in vitro were carried out in 150 mL of pH progression medium at 37°C ± 0.5°C, with the paddle operated at 50 rpm.Citation24
The drug release from FSMEDDS was designed as follows: hour 0–2: drug release was carried out in the simulated gastric fluid (0.1 N hydrochloride) with pH 1.2 as dissolution medium; hour 2–5: drug release was performed in the simulated intestinal fluid (6.8045 g monopotassium phosphate, 118 mL of 0.2 N sodium hydroxide in 1000 mL of distilled water) with pH 6.8 as dissolution medium; hour 5–24: drug release was performed in the simulated colonic fluid (6.8 g monopotassium phosphate, 291 mL of 0.1 N sodium hydroxide in 1000 mL of distilled water) with pH 7.4.
Samples (3 mL) were withdrawn and replaced with fresh media at 60, 120, 180, 240, 300, 305, 310, 320, 330, 345, 360, and 390 minutes. Samples were then filtered using a 0.45 μm filter and analyzed by an ultraviolet spectrophotometer (UV-2102; Shanghai Instrument Ltd, Shanghai, China). Three separate replicate studies were conducted for each of the formulations and data presented as mean ± standard deviation (n = 3).
Cell culture
HT-29 and Hela cell lines were maintained in folate-free Roswell Park Memorial Institute 1640 media supplemented with 10% fetal bovine serum. The cells were incubated at 37°C with 5% carbon dioxide. After reaching confluence, the cells were detached from the flask with trypsin-ethylenediaminetetraacetic acid. The cell suspension was centrifuged at 800 rpm for 3 minutes and then resuspended in the growth medium for further experiments.
Cytotoxicity studies
Cytotoxicity of SMEDDS was determined by an MTT assay.Citation11 Hela and HT-29 cells (5000/well) were seeded in a 96-well plate and allowed to attach for 24 hours. The medium was replaced with 100 μL of fresh folate-free medium and the cells were treated with 100 μL of different concentrations of free curcumin (curcumin-dimethyl sulfoxide), CUR- SMEDDS, and CUR-FSMEDDS with or without 1 mM of free folate (1, 5, 10, 20, 40 μM curcumin/ well). After being incubated for 24 hours at 37°C in a carbon dioxide incubator, the medium was removed and then fresh medium was added after cells were washed three times with phosphate buffered saline. Then, 20 μL of MTT (5 mg/mL in phosphate buffered saline) was added to the cells and incubated for 4 hours at 37°C. The medium was then removed and replaced with 150 μL of dimethyl sulfoxide to dissolve the blue formazan crystals converted from MTT by live cells. The optical density of each well was measured using a microplate reader (Model 680; Bio-Rad Laboratories, Hercules, CA) equipped with a 570 nm filter. Triplicate samples were analyzed for each experiment.
Cellular uptake studies
Qualitative cellular uptake study
Coumarin 6 (Sigma-Aldrich) was used as a model hydrophobic fluorescent probe to be encapsulated in SMEDDS formulation. HT-29 and Hela cells were seeded in a 24-well plate at a density of 105 cells/well and allowed to attach for 24 hours. By replacing the contents with fresh medium, the cells were incubated with nontargeted Coumarin 6-loaded SMEDDS (Coumarin 6-SMEDDS) or Coumarin 6-FSMEDDS at 37°C for 0.5, 1, 2, 5, and 24 hours, respectively. To determine the role of folate receptor binding, 1 mM of free folate was added to the media in the folate receptor blocking group (Coumarin 6-FSMEDDS) and incubated for 1 hour before adding drugs at different time intervals. All the wells were treated with 15 μg/mL of Coumarin 6 for each formulation.Citation25 At the end of incubation, the cells were washed three times with cold phosphate buffered saline to remove residual drugs, and the fluorescence images of Coumarin 6 in different cells were captured under a fluorescence microscope (BX 51; Olympus Corporation, Tokyo, Japan) using a blue filter.
Cellular uptake by flow cytometry
The cellular uptake of Coumarin 6 in SMEDDS and FSMEDDS formulations (with or without 1 mM folate) was formed by fluorescence-activated cell sorting analysis. Cells were seeded in a 6-well plate at a density of 106 cells/well. After reaching 90% confluence, 15 μg/mL of Coumarin 6 in different formulations was added and incubated at 37°C for 2 hours. Intracellular Coumarin 6 fluorescence was analyzed with a flow cytometer (FACSCalibur™ system; Becton, Dickinson, and Company, Franklin Lakes, NJ).
Statistical analysis
Statistical data analyses were performed using Student’s t-test with the aid of IBM SPSS (v16.0; SPSS Inc, Chicago, IL). A value of P < 0.05 was considered statistically significant.
Results and discussion
Solubility studies
To develop a SMEDDS formulation for oral delivery of poorly water-soluble curcumin, a suitable oil, surfactant, and cosurfactant needed to be chosen. The solubility of curcumin in various vehicles are presented in . As shown in , curcumin showed good solubility in the oils Capryol 90 (9.43 ± 0.80 mg/mL) and Labrafil M1944CS (3.17 ± 0.13 mg/mL).
Surfactants Labrasol and Cremophor EL showed maximum drug solubilization (99.30 ± 1.73 mg/mL and 65.83 ± 4.80 mg/mL, respectively). Cosurfactants Transcutol HP (140.56 ± 3.42 mg/mL) and PEG 400 (159.78 ± 0.48 mg/mL) exhibited higher solubility for curcumin. The high solubility in PEG 400, Cremophor EL, and Labrasol might be due to the ability of curcumin to form a hydrogen bond with the polyethylene oxide groups in above materials.Citation26 The above excipients with high solubility for curcumin were used to construct the ternary phase diagrams.
Construction of ternary phase diagrams
Drug loading capability is the main factor when screening the oil phase. Curcumin showed higher solubility in Capryol 90 and Labrafil M1944CS. Their phase behaviors were compared by constructing ternary phase diagrams. The phase diagrams of the systems containing Cremophor EL as surfactant, Transcutol HP as cosurfactant, and different oils (Capryol 90 and Labrafil M1944CS) are shown in and B. A larger self-microemulsifying region was found, as shown in . Hence, Capryol 90 was selected at oil phase. Two phase diagrams were constructed with Capryol 90 as the oil, Transcutol HP as cosurfactant, and different surfactants (Cremophor EL or Cremophor EL:Labrasol [1:1]). As shown in , the self-microemulsion region of the formulation containing Cremophor EL was larger than that containing Cremophor EL:Labrasol (1:1). Therefore, the desirable surfactant should be Cremophor EL.
As to the selection of a cosurfactant, Capryol 90 and Cremophor EL were used as oil phase and surfactant, respectively. As shown in , the solubility of curcumin in PEG 400 was higher than that in Transcutol HP or in ethanol. However, compared with Transcutol HP, PEG 400 had relatively higher hydrophilic properties, which could increase the risk of destroying the microemulsion.Citation26 In addition, the self-microemulsion region of Transcutol HP was larger compared with that of PEG 400 or ethanol. Therefore, Transcutol HP was selected as the cosurfactant.
Preparation and optimization of CUR-SMEDDS formulations
A Simplex–Lattice design was used to optimize the composition of CUR-SMEDDS. In view of the feasibility of SMEDDS formation, according to the ternary phase diagrams shown in , the ranges of the three factors were selected as follows: surfactant percentage (X1), 30%–60%; cosurfactant percentage (X2), 30%–60%; oil percentage (X3), 10%–40%. Seven formulations containing curcumin were conducted (). In order to achieve a high drug loading and a uniform droplet size (<100 nm), solubility and mean particle size of the seven formulations were selected as evaluation indexes.
The results were processed with a MATLAB® 7.0 data-processing system (MathWorks, Natick, MA) and the equations for solubility and mean particle size were as follows:
Ysolubility = 50.16 + 14.79[CoS] − 543.59[O] − 88.89[S] [CoS] + 1665.8[S][O] + 1533.6[CoS][O] − 4971.1[S] [CoS][O], (R2 = 0.9999)
Yparticle size = 242.3 + 647.9222[CoS] + 39.0667[O] − 8268.4[S][CoS] − 10358[S][O] − 13758 [CoS] [O] + 61727[S][CoS][O], (R2 = 0.9999).
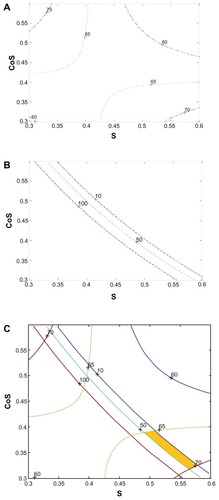
In the two equations, CoS represents the cosurfactant, S the surfactant, and O the oil. All the obtained coefficients R2 are larger than 0.95, indicating that over 95% of the variation in response could be explained with this model, and the agreement of fit to the model was confirmed. As shown in , particle size increased and the solubility of curcumin decreased with the increase of the ratio of oil in the formulation. In order to obtain suitable particle size and high solubility of curcumin, the percentage of oil was set at 10%.Citation27 Based on the two equations, contour plots of solubility and mean particle size were constructed (). Trace contours were constructed when Ysolubility was equal to 60, 65, and 70 mg/g as shown in . In , three mean particle size curves stood for 10 nm, 50 nm, and 100 nm. The optimized formulation was chosen by superimposing the contour plots of the two responses (). The common section between Yparticle size (located at the field of 10–50 nm) and Ysolubility (located at the scope of 65–70 mg/g) was the best area for the concentrations of surfactant and cosurfactant.
Figure 2 The contour plots of response for the contour plot of mean particle size (A), the contour plot of solubility (B), and the mixed contour plot of mean particle size and solubility (C).
Abbreviations: S, surfactant; CoS, cosurfactant.
Based on solubility for curcumin and the mean particle size of the formed microemulsion, the composition of optimized formulation was chosen as follows: 57.5% surfactant (Cremophor EL), 32.5% cosurfactant (Transcutol HP), and 10% oil (Capryol 90). To confirm the model’s adequacy for prediction, three batches of CUR-SMEDDS under the optimum composition were prepared, and the two responses (Ysolubility and Yparticle size) were evaluated. The results presented in show the predicted values of mean particle size and solubility of SMEDDS calculated by equations were close to those obtained from the experiment.
Table 3 The predicted values and the experimental results of the curcumin-loaded self-microemulsifying drug delivery system prepared under the optimum conditions
Synthesis of lipophilic folate derivatives
The structures of the synthesized F-PEG-DSPE and F-PEG-CHEMS were confirmed by hydrogen-1 nuclear magnetic resonance, and the spectrograms for the two materials are shown in . For F-PEG-CHEMS, hydrogen-1 nuclear magnetic resonance analysis showed principal peaks (in ppm) related to the folate moiety (8.11 [double peaks; d], 7.63 [d], 6.93 [three peaks; t], 6.63 [d], 4.48 [d], 4.30 [mixed peaks; m]), the PEG moiety (3.65 [m]) and the CHEMS moiety (5.34 [d], 4.5 [m], 0.65–2.30 [m]), which was similar to a previous report.Citation18 Moreover, the structure of F-PEG-DSPE was corroborated by hydrogen-1 nuclear magnetic resonance, and the main peaks (in ppm) for the folate moiety (8.63 [small peaks; s], 7.68 [d], 6.62 [d], 4.47 [d]), the PEG moiety (3.65 [m]) and the DSPE moiety (0.85 [t], 1.23 [s], 1.49 [d], 2.24 [d]) confirmed the structure of F-PEG-DSPE, which was in accordance with a previous study.Citation28
Optimization of CUR-FSMEDDS
Folate receptor is overexpressed in many tumors and enables the endocytosis of folate-conjugated carriers. Thus, folate is an attractive tumor-targeting ligand in the cell-specific delivery systems.Citation29 Currently, lipophilic folate derivatives, such as F-PEG- DSPE, F-PEG-CHEMS, and F-PEG-Chol were used to enhance the accumulation of formulations in the tumor.Citation12,Citation17,Citation30 Except for these, folate receptor was proven to exist in normal colorectal mucosa, which could lead to the endocytosis of folate material and an increase in the absorption of drug in the colon.Citation10 In order to evaluate the effect of different types and concentrations of lipophilic folate derivatives in SMEDDS on the absorption of curcumin in colon mucosa, a colon perfusing experiment in rats was performed.Citation27
CUR-FSMEDDS was prepared by adding lipophilic folate derivatives into the mixture of surfactant, cosurfactant, and oil. The absorption percentage of curcumin in the colon for 6 hours was calculated using the formula: P = (C0V0/ C1V1) × 100%, where C0V0 is the original concentration and volume and C1V1 is the resulting concentration and volume. When the weight ratio of folate conjugated materials to Cremophor EL was at 3:100, the absorption percentage of the formulation containing F-PEG-CHEMS showed no significant difference compared with that containing F-PEG-DSPE or F-PEG-Chol (P > 0.05), but showed a significant difference compared with CUR-SMEDDS (P < 0.05) (). Moreover, the two negative charges in F-PEG-DSPE and a carbamate linker in F-PEG-Chol could limit their hydrolytic stability.Citation18 Thus, F-PEG-CHEMS was chosen for concentration screening. Three weight ratios of F-PEG-CHEMS to Cremophor EL (0.5:100, 1:100, and 3:100) were evaluated, and the formulation in which the weight ratio of F-PEG-CHEMS to Cremophor EL was 1:100 had the maximum absorption percentage among the studied preparations (P < 0.05). Therefore, the final formulation of FSMEDDS was comprised of 57.5% Cremophor EL, 32.5% Transcutol HP, 10% Capryol 90 with a small amount of F-PEG-CHEMS (the weight ratio of folate materials to Cremophor EL was 1:100). After the incorporation of F-PEG-CHEMS, the solubility of curcumin in FSMEDDS was 66.59 ± 1.21 mg/g, which was not significantly different compared with that in SMEDDS (67.32 ± 0.92 mg/g) (P > 0.05). However, the solubility of curcumin in the aqueous medium was about 11 ng/g.Citation31,Citation32 Therefore, it could be concluded that FSMEDDS increased the solubility of curcumin in water more than 6 × 106-fold.
Table 4 The absorption percentage of curcumin in different formulations with the established in situ colon perfusion method in rats for 6 hours
Characterization of CUR-FSMEDDS
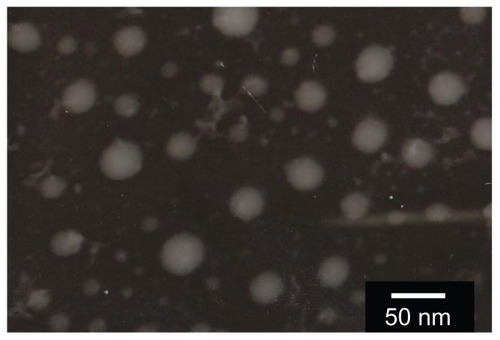
CUR-FSMEDDS can form into microemulsion when diluted with distilled water. As shown in the transmission electron microscope picture (), the microemulsion droplets appeared spherical without aggregation. The parameters for physicochemical characters of the optimized formulation are shown in . For blank FSMEDDS, droplet size was significantly increased with the incorporation of F-PEG-CHEMS (P < 0.05). The long PEG-spacer in F-PEG-CHEMS dispersing into the emulsifying membrane layer (composed of surfactant and cosurfactant) might lead to increased droplet size. As seen in , the addition of curcumin in SMEDDS and FSMEDDS significantly increased droplet size after diluting with distilled water (P < 0.05), which was in accordance with another study.Citation27 The zeta potential is an electrostatic value measured by surface electrostatic double layer of droplets. As shown in , the absolute zeta potential value of CUR-SMEDDS was significantly higher than that of blank SMEDDS (P < 0.05). The possible reason being that the formation of intermolecular hydrogen bonds among the hydroxyl groups in curcumin and some relative groups containing oxygen or nitrogen atoms in surfactant, cosurfactant, and oil produced the change in the surface electrostatic double layer of droplets.Citation33
Table 5 Mean particle size and zeta potential of different self-microemulsifying drug delivery system formulations
Release of curcumin from FSMEDDS-filled capsules for colon targeting
The optimized SMEDDS formulations were filled volumetrically in colon-targeted gelatin capsules coated with Eudragit® S 100 (Evonik Industries, Essen, Germany).
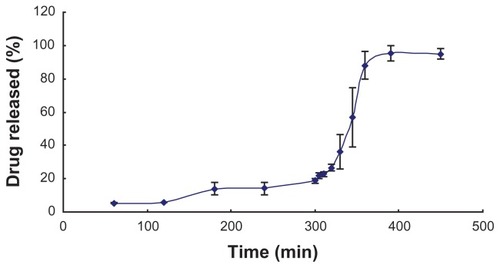
In vitro release studies were carried out in order to evaluate the effect of colon-targeted capsules with the objective of releasing most of the drug in the colon. An ideal formulation for site-specific delivery of the drug to the colon should suppress the drug’s release in the upper gastrointestinal tract and once transported to the colon, the drug should release rapidly. shows the drug release from the colontargeted capsules filled with CUR-FSMEDDS in simulated gastrointestinal tract fluids. At pH 1.2 about 5% of the drug amount was released from the capsule in the following 3 hours and (in simulated intestinal fluid with pH 6.8), about 13% of curcumin was released from the capsule. In conclusion, in the first 5 hours, less drug was released from the capsules, which was attributed to the insolubility of Eudragit S 100 in low pH. However, after the capsules were transferred into simulated colonic fluid (pH 7.4) for 150 minutes, about 77% of the drug was released from the capsules, which was due to the dissolution of capsules and the formation of curcumin microemulsion. The results indicated that drug was protected from the upper gastrointestinal tract by the colon-targeted capsules, and this formulation of curcumin could reach the colon efficiently and release the drug immediately.
Cytotoxicity studies
Cytotoxicity of blank SMEDDS, blank FSMEDDS, CUR-SMEDDS, CUR- FSMEDDS, free folate + CUR-SMEDDS, and free curcumin were studied using MTT assay, and their concentrations leading to 50% cell death were determined from concentration-dependent cell viability curves. As shown in , the 50% cell death value for CUR-FSMEDDS was lower than that for CUR-SMEDDS and free curcumin (P < 0.05), which may be because the receptor-mediated endocytosis increased the accumulation in the cell of curcumin from FSMEDDS.Citation13 The 50% cell death value for CURFSMEDDS increased when 1 mM folate was first added in both Hela and HT-29 cells (P < 0.05). The results suggested that free folate in the medium could prevent CUR-FSMEDDS transport into cells by competitively binding to folate receptors on the cell surface. The much higher cytotoxicity of free curcumin compared with CUR-SMEDDS may be a result of when cells were exposed to free curcumin in dimethyl sulfoxide or CUR-SMEDDS for the same amount of time, leading to more rapid free curcumin uptake.Citation13
Table 6 Cytotoxicities of various curcumin to Hela and HT-29 cells
Cellular uptake studies
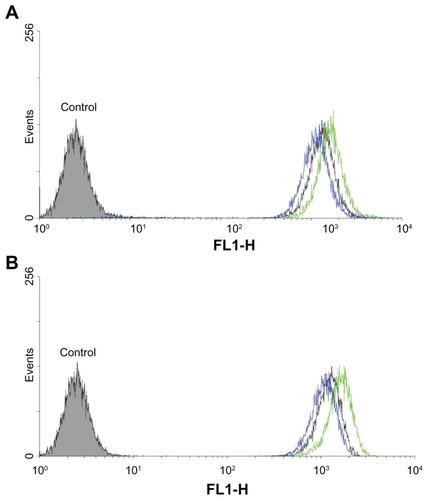
Although curcumin demonstrates autofluorescence, its fluorescence intensity was weak in the preliminary experiment. Therefore, Coumarin 6 was used as a model hydrophobic fluorescent probe in this study.Citation34 Uptake by HT-29 and Hela cells of microemulsions formed from SMEDDS in culture media was analyzed with fluorescence microscopy and flow cytometry. As shown in , more FSMEDDS fluorescently-labeled cells could be clearly visualized compared with SMEDDS and the intensity of fluorescence decreased when 1 mM folate was added to the medium, indicating folate-mediated endocytosis of FSMEDDS could be blocked by competitively binding to folate receptors on the cell surface. These microemulsions formed from SMEDDS were found to rapidly associate with folate receptor-positive HT-29 and Hela cells in the initial 30 minutes. The fluorescence intensity increased along with the extension of incubation time, and remained almost steady even after 48 hours, suggesting the retention of Coumarin 6 from SMEDDS localized inside the cells.Citation35 Flow cytometric analysis was performed after incubation at 37°C for 2 hours. Similarly, the flow cytometry results in show that the cellular uptake of folate receptor-targeted SMEDDS was much greater than that of nontargeted SMEDDS and the uptake was partly blocked by 1 mM free folate. All of these results indicated that the CUR-FSMEDDS formulation was transported into Hela and HT-29 cells via a folate receptor-mediated endocytosis process.
Figure 5 Fluorescence microscopy images showcasing the time dependent (0.5, 1, 2, 5, and 24 hours) intracellular uptake of Coumarin 6 from Coumarin 6-loaded self-microemulsifying drug delivery system, Coumarin 6-loaded folate-modified self-microemulsifying drug delivery system, and Coumarin 6-loaded folate-modified self-microemulsifying drug delivery system+1mM folic acid by HT-29 (human colon carcinoma cell line) (A) and Hela (human uterine cervix cancer line) cells (B), the concentration of Coumarin 6 is 15 μg/mL.
Abbreviations: FA, folic acid; FSMEDDS, folate-modified self-microemulsifying drug delivery system; h, hours; SMEDDS, self-microemulsifying drug delivery system.
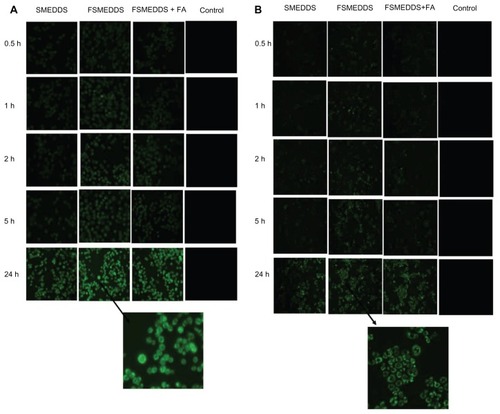
Figure 6 Flow cytometry results of HT-29 (human colon carcinoma cell line) (A) and Hela (human uterine cervix cancer cell line) (B) cells incubated with Coumarin 6-loaded folate-modified self-microemulsifying drug delivery system + 1 mM folate (black), Coumarin 6-loaded folate-modified self-microemulsifying drug delivery system (green), Coumarin 6-loaded self-microemulsifying drug delivery system (blue) at an equivalent Coumarin 6 concentration of 15 μg/mL for 2 hours at 37°C.
Conclusion
In this study, an FSMEDDS formulation was developed which could improve the solubility of curcumin and target the folate receptor in colon cancer cells. Compositions of SMEDDS were selected with ternary phase diagrams, and the formulation of CUR-SMEDDS was optimized by a Simplex–Lattice experiment design. The effect of different folate derivatives such as F-PEG-CHEMS, F-PEG-DSPE, and F-PEG-Chol in SMEDDS on the absorption of curcumin in colon mucosa was studied with a colon perfusing experiment in rats. The results showed the absorption percentage of SMEDDS containing F-PEG-CHEMS in rat colons was better than that containing F-PEG-DSPE or F-PEG-Chol at the same concentration, and CUR-FSMEDDS with the weight ratio of F-PEG-CHEMS to Cremophor EL at 1:100 was higher compared with when the ratio was at 0.5:100 or 3:100. In addition, cytotoxicity and cellular uptake studies indicated that due to folate-mediated endocytosis, FSMEDDS showed a higher ability to actively target tumor cells with overexpressed folate receptors on the cell surface in comparison with SMEDDS. In addition, the in vitro release of CUR-FSMEDDS in colon-targeted capsules showed this formulation of curcumin can reach the colon efficiently and release the drug immediately. It could be concluded that FSMEDDS-filled colon-targeted capsules are a potential carrier for colon delivery of curcumin.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No 30973646).
Disclosure
The authors report no conflicts of interest in this work.
References
- BasileVFerrariELazzariSBellutiSPignedoliFImbrianoCCurcumin derivatives: molecular basis of their anti-cancer activityBiochem Pharmacol200978101305131519580791
- GoelAKunnumakkaraABAggarwalBBCurcumin as “Curecumin”: from kitchen to clinicBiochem Pharmacol200875478780917900536
- JohnsonJJMukhtarHCurcumin for chemoprevention of colon cancerCancer Lett2007255217018117448598
- SharmaRAGescherAJStewardWPCurcumin: the story so farEur J Cancer2005411319951968
- PrajaktaDRatneshJChandanKCurcumin loaded pH-sensitive nanoparticles for the treatment of colon cancerJ Biomed Nanotechnol20095544545520201417
- HongJYKimJKSongYKParkJSKimCKA new self-emulsifying formulation of itraconazole with improved dissolution and oral absorptionJ Control Release2006110233233816297483
- ZhangPLiuYFengNXuJPreparation and evaluation of self-microemulsifying drug delivery system of oridoninInt J Pharm20083551–226927618242895
- BalakrishnanPLeeBJOhDHEnhanced oral bioavailability of dexibuprofen by a novel solid self-emulsifying drug delivery system (SEDDS)Eur J Pharm Biopharm200972353954519298857
- ZhaoYWangCChowAHSelf-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studiesInt J Pharm20103831–217017719732813
- ShiaJKlimstraDSNitzkorskiJRImmunohistochemical expression of folate receptor alpha in colorectal carcinoma: patterns and biological significanceHum Pathol200839449850518342661
- KimSHKimJKLimSJParkJSLeeMKKimCKFolate-tethered emulsion for the target delivery of retinoids to cancer cellsEur J Pharm Biopharm200868361862517949957
- XiongSYuBWuJLiHLeeRJPreparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMSBiomed Pharmacother20116512821177069
- LeeRJLowPSFolate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitroBiochim Biophys Acta1995123321341447865538
- CraigDQMBarkerSABanningDBoothSWAn investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopyInt J Pharm19951141103110
- SubramanianNRaySGhosalSKBhadraRMoulikSPFormulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxibBiol Pharm Bull200427121993199915577219
- RispoliFShahVA new efficient mixture screening design for optimization of mediaBiotechnol Prog200925498098519533739
- HanXLiuJLiuM9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: preparation and evaluation in vitroInt J Pharm20093721–212513119166923
- XiangGWuJLuYLiuZLeeRJSynthesis and evaluation of a novel ligand for folate-mediated targeting liposomesInt J Pharm20083561–2293618258394
- ZhangNPingQHuangGXuWChengYHanXLectin-modified solid lipid nanoparticles as carriers for oral administration of insulinInt J Pharm20063271–215315916935443
- XiaCQShenWCTyrphostin-8 enhances transferrin receptor-mediated transcytosis in Caco-2-cells and increases hypoglycemic effect of orally administered insulin-transferrin conjugate in diabetic ratsPharm Res200118219119511405290
- YinYChenDQiaoMLuZHuHPreparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentinJ Control Release2006116333734517097180
- LiuYChenZQZhangXAn improved formulation screening and optimization method applied to the development of a self-microemulsifying drug delivery systemChem Pharm Bull (Tokyo)2010581162220045959
- YaoJLuYZhouJPPreparation of nobiletin in self-microemulsifying systems and its intestinal permeability in ratsJ Pharm Pharm Sci2008113222918801304
- NekkantiVKaratgiPPrabhuRPillaiRSolid self-microemulsifying formulation for candesartan cilexetilAAPS Pharm Sci Tech2010111917
- HuKLiJShenYLactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: in vitro and in vivo evaluationsJ Control Release20091341556119038299
- SetthacheewakulSMahattanadulSPhadoongsombutNPichayakornWWiwattanapatapeeRDevelopment and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in ratsEur J Pharm Biopharm201076347548520659556
- GaoYWangYMaYFormulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsionColloids Surf B Biointerfaces200971230631419375897
- PradhanPGiriJRiekenFTargeted temperature sensitive magnetic liposomes for thermo-chemotherapyJ Control Release2010142110812119819275
- LinJJChenJSHuangSJFolic acid-Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applicationsBiomaterials200930285114512419560199
- ZhaoXBMuthusamyNByrdJCLeeRJCholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomesJ Pharm Sci20079692424243517588260
- YuHLHuangQREnhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starchFood Chem20101192669674
- KaminagaYNagatsuAAkiyamaTProduction of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseusFEBS Lett2003555231131614644434
- TønnesenHHMássonMLoftssonTStudies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stabilityInt J Pharm20022441–212713512204572
- XuYJinXPingQA novel lipoprotein-mimic nanocarrier composed of the modified protein and lipid for tumor cell targeting deliveryJ Control Release2010146329930820580913
- MulikRSMönkkönenJJuvonenROMahadikKRParadkarARTransferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosisInt J Pharm20103981–219020320655375