 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Patients on mechanical ventilators for extended periods of time often face the risk of developing ventilator-associated pneumonia. During the ventilation process, patients incapable of breathing are intubated with polyvinyl chloride (PVC) endotracheal tubes (ETTs). PVC ETTs provide surfaces where bacteria can attach and proliferate from the contaminated oropharyngeal space to the sterile bronchoalveolar area. To overcome this problem, ETTs can be coated with antimicrobial agents. However, such coatings may easily delaminate during use. Recently, it has been shown that changes in material topography at the nanometer level can provide antibacterial properties. In addition, some metabolites, such as fructose, have been found to increase the efficiency of antibiotics used to treat Staphylococcus aureus (S. aureus) infections. In this study, we combined the antibacterial effect of nanorough ETT topographies with sugar metabolites to decrease bacterial growth and biofilm formation on ETTs. We present for the first time that the presence of fructose on the nanorough surfaces decreases the number of planktonic S. aureus bacteria in the solution and biofilm formation on the surface after 24 hours. We thus envision that this method has the potential to impact the future of surface engineering of biomaterials leading to more successful clinical outcomes in terms of longer ETT lifetimes, minimized infections, and decreased antibiotic usage; all of which can decrease the presence of antibiotic resistant bacteria in the clinical setting.
Introduction
Ventilator-associated pneumonia (VAP) is the most common hospital-acquired infection among patients requiring mechanical ventilation.Citation1–Citation3 Patients are intubated with endotracheal tubes (ETTs) which interact with the surrounding airway epithelium during the ventilation process. On the other hand, ETTs provide surfaces where bacteria can attach, colonize and proliferate from the contaminated oropharyngeal space to the sterile bronchoalveolar area.Citation4,Citation5 Prolonged intubation leads to the colonization of the upper respiratory tract by pathogens such as Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) and increases the risk of lung infection by six-fold to twenty-fold.Citation2,Citation3,Citation6–Citation8 In addition, the risk of VAP increases by 3% per day during the first 5 days of mechanical ventilation and by 2% between days 5 and 10.Citation5–Citation9 Therefore, there exists an unmet clinical need to create surfaces that resist bacterial attachment and growth to reduce the risk of infection.
Surfaces of invasive medical devices serve as substrates for bacterial attachment, growth, colonization, and biofilm formation.Citation10,Citation11 Bacterial biofilms are complex heterogeneous communities embedded in an extracellular polymeric substance (EPS) matrix which is composed of polysaccharides, nucleic acids, proteins, and lipids.Citation12,Citation13 The formation of these sessile (adherent, immobile bacteria) communities provides a resistance mechanism against host immune responses and antibacterial agents.Citation14 Inherent resistance of biofilms on material surfaces causes a real-world problem as many persistent and chronic bacterial infections occur in medical and industrial settings.
There is a growing interest in using nanotechnology (or the use of novel materials with at least one constituent dimension <100 nm) approaches for the treatment of bacterial infections.Citation11,Citation15–Citation17 Recently, many studies have shown that changes in the surface topography at the nanometer level can interfere with mammalian cell as well as bacterial cell functions.Citation4,Citation18–Citation33 Conventional biomaterials do not have nanoscale roughness. Nanoscale materials provide roughness and dimensions at the nano level which might influence bacteria behavior.Citation4,Citation25 An in vitro study recently reported decreased Staphylococcus epidermidis (S. epidermidis) attachment on nanostructured ZnO and titania (TiO2) compared to micro-structured surface features.Citation23 It was also demonstrated that the attachment of different pathogenic strains (S. aureus, S. epidermidis, and P. aeruginosa) could be controlled by changing titanium surface roughness and ultrafine crystallinity at the nano level.Citation22 Medical device surfaces can be modified with silver nanoparticles to improve their antibacterial effects.Citation34,Citation35 Silver nanoparticles also influenced the antibacterial properties of montmorillonite/chitosan bionanocomposites. Citation36 These nanotopographical effects on bacteria might be due to changes in surface energy leading to changes in the adsorbed protein layer which modulates intercellular interactions.Citation37,Citation38 For example, increased protein adsorption and bioactivity on nanostructured titanium surfaces resulted in improved functions of osteoblasts (bone-forming cells).Citation22,Citation27,Citation39–Citation42 Nanostructured hydroxyapatite-poly-lactideco- glycolide (PLGA) composites also promoted adhesion-induced osteogenic differentiation of human mesenchymal stem cells.Citation43 Moreover, an approximate 40% reduction in colony forming units was observed on nanorough polyvinyl chloride (PVC) ETT surfaces compared to conventional ETTs.Citation4,Citation24 These studies indicated that there is a great potential to create antibacterial surfaces via engineering surface roughness at the nanoscale.
In addition to the antibacterial effects of surface nanoroughness, eradication of biofilms can be controlled by changing the surrounding microenvironment and biological conditions. Persister bacteria represent a population of dormant cells which can form biofilms in chronic infections.Citation13,Citation44 Persisters enter a metabolic hibernation state and shut down their metabolism. This enables them to survive during the antibiotic treatment and become resistant. A recent study provided evidence that the addition of certain metabolites, such as fructose, could increase the efficiency of antibiotics which are used to treat both Gram-negative and Gram-positive persisters.Citation44 In this approach, persister bacteria are stimulated into an active state using fructose metabolites. Fructose molecules enter the bacterial metabolism through glycolysis and stimulate the shutdown metabolism of persister cells. As a result, changes in the bacterial metabolism stimulate the uptake of antibiotics. Therefore, fructose metabolites combined with an aminoglycoside antibiotic (ie, gentamicin) render biofilm forming bacteria vulnerable to antibiotic treatment and eradicate them from the medical device surfaces.Citation44 Metabolites which can trigger and enable the dispersal of biofilms have a great potential to treat infections in the clinical settings.
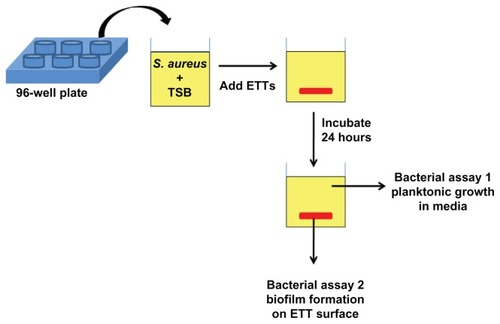
In this study, we combined the antibacterial effect of nanorough topographies with specific sugar metabolites to further decrease bacterial growth and biofilm formation on ETTs without using antibiotics. S. aureus was chosen as the target bacteria as it is one of the major causes of fatal nosocomial infections as well as community-associated infections. In addition, it is the most frequently isolated bacteria in patients whose episode of VAP was diagnosed during the first days of mechanical ventilation.Citation1,Citation45 The experimental strategy employed in our work is shown in . First, commercially available PVC ETTs were enzymatically degraded to create nanoscale surface roughness. Then, the nanorough (NanoR) ETTs were soaked in different concentrations of a fructose solution. Planktonic bacterial growth in the surrounding culture medium and biofilm formation of S. aureus on ETT surfaces were observed and quantified after 24 hours. We present for the first time that the presence of a specific metabolite, ie, fructose, on the nanorough PVC ETT surfaces decreases the number of planktonic bacteria in the solution and biofilm formation after 24 hours.
Figure 1 Schematic of bacteria assays. Commercially available PVC ETTs were enzymatically degraded to create nanoscale surface roughness. Then, control PVC and NanoR ETTs were soaked into different concentrations of a fructose solution. Bacterial survival in the medium (assay 1) and biofilm formation on the ETT surfaces (assay 2) were quantified.
Abbreviations: ETT, endotracheal tube; NanoR, nanorough; PVC, polyvinyl chloride; TBS, tryptic soy broth.
Materials and methods
PVC ETT material preparation and nanoroughening
Commercially available Sheridan® 6.0 mm ID, uncuffed ETTs (Hudson RIC, Temecula, CA) were cut vertically into 0.6 cm × 0.3 cm segments using a rectangular hole punch. Nanorough (NanoR) topographies on the PVC ETT surface were created using a lipase from Rhizopus arrhizus (Sigma- Aldrich, St Louis, MO) at a 0.1% concentration in 1M potassium phosphate buffer (PBS) at a pH of 7.2 (Fisher Scientific, Waltham, MA). PVC samples were soaked in the lipase solution at 37°C, 200 rpm for 24 hours. After 24 hours, the lipase solution was replaced with a fresh solution and the samples were soaked for an additional 24 hours. Then, the NanoR samples were removed and rinsed three times with double distilled water. The NanoR and untreated PVC samples were air dried overnight (approximately 12 hours) at room temperature, and then they were sterilized using ethylene oxide gas.
PVC surface modification with metabolites
D-Fructose (Fisher Scientific, Pittsburgh, PA) was dissolved in double distilled water. The fructose solution was sterilized via a polytetrafluoroethylene (PTFE) syringe filter with a 0.2 μm pore size (Fisher Scientific, Pittsburgh, PA). To coat surfaces with the fructose, sterilized PVC samples prepared above were soaked in 10 mM and 100 mM fructose solutions and incubated overnight (approximately 12 hours) in a sterile hood. Then, the samples were air dried in the sterile hood.
Surface characterization
Scanning electron microscopy (SEM)
Surface topography of the materials was visualized using an SEM (LEO 1530VP FE-4800 Field-Emission SEM, Carl Zeiss SMT, Peabody, MA) according to standard operating procedures. Samples were imaged with an accelerating voltage of 20 kV using variable pressure.
Atomic force microscopy (AFM)
Samples were further characterized using atomic force microscopy (AFM) with scan areas of 5 μm × 5 μm. The topography of the PVC substrates were evaluated with AFM (Asylum-1 MFP-3D AFM System, Santa Barbara, CA) under tapping mode using a 9 nm ± 2 nm AFM tip with a scan rate of 0.5 Hz. The root-mean-squared (RMS) roughness, which is the standard deviation of the height value in the selected line, was determined in five random fields per sample.
Contact angle measurements
Water contact angles were investigated using a drop shape analysis system (EasyDrop, Kruss, Hamburg, Germany). The contact angle from 1 μL of double distilled water sessile droplets (60 seconds after being dropped on the surface) was measured for each sample.
Bacteria assays
Bacteria culture
S. aureus (ATCC number 25923, American Type Culture Collection, Mannassas, VA) was hydrated and streaked for isolation on a tryptic soy agar plate. Following growth, a single isolated colony was selected and inoculated in 3 mL of tryptic soy broth (TSB) media (MP Biomedicals, Solon, OH). The bacteria culture was grown on an incubator shaker for 18 hours at 37°C, 200 rpm until it reached the stationary phase.
Bacterial growth and biofilm formation analyses
First, the optical density of the overnight culture at 562 nm (OD562) was adjusted to 0.52, which corresponds to a cell number of 109. Then, the cultures were serially diluted to obtain a 103 bacteria solution. One hundred microliters of a 103 bacteria solution was seeded onto a single well of a flat bottom 96-well plate (BD Biosciences, Franklin Janes, NJ) containing either NanoR or an untreated PVC sample soaked in a 10 mM and 100 mM fructose solution. Unsoaked NanoR and PVC samples were used as controls. The plate was then placed in a stationary incubator at 37°C with 5% CO2. After 24 hours, samples were removed with a pair of sterile forceps. Optical density of the plate was then measured at 562 nm using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) to analyze the planktonic bacteria growth. To remove sessile bacteria from the PVC surface, a vortexing method was used and a cell viability assay was performed to measure colony forming units/mL (CFU/mL). Specifically, each PVC sample was placed in 1 mL of PBS (Fisher Scientific) and vortexed at 3000 rpm for 10 minutes. The cells were then serially diluted in PBS (Fisher Scientific) over a 3-log range. Ten microliters of each dilution were plated on tryptic soy agar plates and incubated at 37°C overnight. After overnight incubation, colony forming units were counted for each sample to quantify biofilm formation on the ETT surfaces. CFU/mL values were calculated using the following formula:
Statistical analysis
All experiments were performed in triplicate and repeated at least three times to validate repeatability and reproducibility. Data were represented as the mean ± standard error of the mean (SEM). Results were analyzed for statistical significance using a Student’s t-test (unpaired) and Microsoft Excel software (Redmond, WA). Three different significance levels (P < 0.01, P < 0.05, and P < 0.1) were noted.
Results and discussion
Material characterization
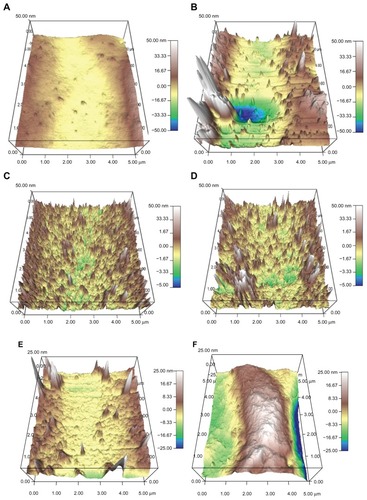
SEM analysis indicated that the lipase treatment degraded conventional PVC surfaces and created nanoscale features (). In addition, AFM micrographs revealed distinct topographies in control surfaces compared to NanoR PVC surfaces (). As expected, control PVC surfaces were smooth compared to NanoR PVC. Roughness of the untreated PVC was 0.704 nm ± 0.192 nm while it was 8.440 nm ± 1.282 nm for NanoR PVC as measured by an AFM with 5 μm × 5 μm scans (). SEM images also confirmed that PVC was smooth compared to the NanoR PVC surface. In addition, coating the surface with fructose decreased the nanoscale topography and nanoroughness for both the control PVC and NanoR PVC surfaces soaked in different fructose concentrations (). The decrease in the surface roughness was more apparent in the NanoR samples. Coating the NanoR surface with 10 mM fructose decreased the roughness from 8.440 nm ± 1.282 nm to 1.214 nm ± 0.144 nm. Surface roughness was significantly higher for NanoR ETT soaked in a 10 mM fructose solution than control PVC ETTs (P < 0.01). As the fructose concentration increased to 100 mM, the RMS values decreased from 8.440 nm ± 1.282 nm to 1.034 nm ± 0.175 nm. Surface roughness of NanoR ETTs soaked in a 100 mM fructose solution was also significantly higher than control PVC ETTs (P < 0.1). The rougher surfaces have more surface energy, area, and fructose, which decreased the roughness and made the surfaces smoother. Deposition of fructose crystals was also clearly visibly apparent on the surfaces, and more deposition was observed when NanoR surfaces were coated with 100 mM fructose (data not shown). The effect of the fructose coating on surface roughness was also observed for untreated PVC surfaces. The RMS values decreased on PVC soaked in 10 mM fructose from 0.704 nm ± 0.192 nm to 0.592 nm ± 0.037 nm. On the other hand, the RMS value of PVC soaked in 100 mM fructose was 0.702 nm ± 0.037 nm. This increase in roughness compared to PVC soaked in 100 mM could be due to the excessive deposition of fructose crystals on the surface (data not shown).
Table 1 RMS values of PVC and NanoR surfaces before and after soaking in two different concentrations (10 mM and 100 mM) of a fructose solution
Figure 2 SEM images of untreated PVC and NanoR PVC. Untreated PVC samples revealed a smooth surface (A, 10 KX; C, 30 KX; and E, 65 KX). On the other hand, NanoR PVC samples revealed a rough surface (B, 10 KX; D, 30 KX; and F, 65 KX).
Abbreviations: NanoR, nanorough; PVC, polyvinyl chloride; SEM, scanning electron microscope.
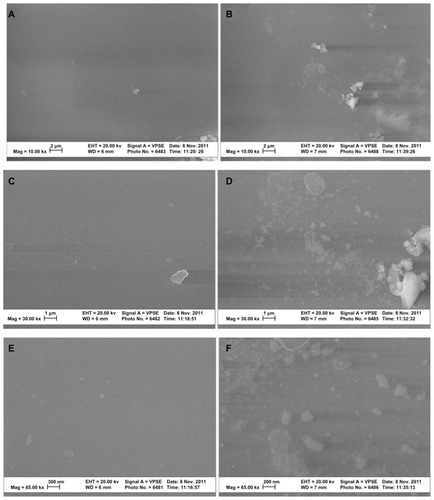
Figure 3 AFM micrographs showing the topography of (A and B) control PVC and NanoR PVC; (C and E) control PVC soaked in 10 mM and 100 mM fructose; and (D and F) NanoR PVC soaked in 10 mM and 100 mM fructose, respectively. Micrographs revealed distinct differences in nanotopographies between the control PVC and NanoR PVC surface soaked in different metabolite concentrations.
Abbreviations: AFM, atomic force microscopy; NanoR, nanorough; PVC, polyvinyl chloride.
Since surface nanoroughness can provide more surface area and make the surface more hydrophobic due to the water surface tension interactions,Citation46–Citation50 the hydrophobicity of the PVC and NanoR surfaces were characterized (). NanoR surfaces had a contact angle of 70.36° ± 1.18° and were significantly more hydrophilic than the control PVC surfaces, which had a contact angle of 88.62° ± 1.12° (P < 0.01). In this case, surface nanoroughness provided more wetting for the PVC ETTs. Moreover, coating surfaces with fructose decreased the hydrophobicity of both the NanoR and control PVC samples. A decrease in the contact angle was more apparent for the control PVC. Conventional PVC surfaces soaked in a 10 mM fructose solution had a contact angle of 72.98° ± 0.45°, while the PVC soaked in a 100 mM fructose solution had a contact angle of 69.02° ± 1.43°. In addition, contact angles on the NanoR PVC soaked in 10 mM and 100 mM solutions were significantly different than contact angles on control PVC (P < 0.01). The NanoR PVC surfaces soaked in a 10 mM fructose solution had a contact angle of 68.10° ± 3.19° while the contact angle of the NanoR soaked in a 100 mM fructose solution was 64.18° ± 3.46°.
Table 2 Water contact angles on PVC and NanoR PVC surfaces before and after soaking into two different concentrations (10 mM and 100 mM) of a fructose solution
Bacteria studies
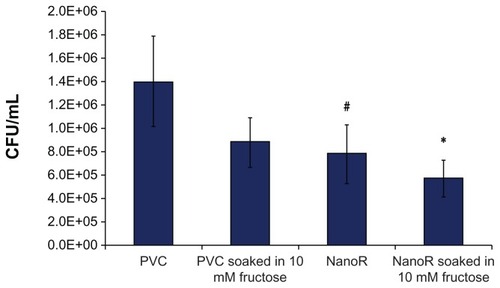
Most importantly, shows the effect of fructose treatment on planktonic S. aureus bacteria suspended in the surrounding culture medium. The growth of planktonic bacteria decreased on NanoR surfaces soaked into a 100 mM fructose solution compared to conventional PVC surfaces as well as PVC soaked in respective amounts of fructose. In addition, fewer planktonic bacteria were observed on NanoR PVC surfaces soaked in a 100 mM fructose solution compared to NanoR PVC surfaces soaked in a 10 mM fructose solution. Therefore, the fructose-reduced planktonic S. aureus bacteria growth was dependent on metabolite concentration on the nanorough surfaces. shows the S. aureus colony counts on PVC and NanoR surfaces soaked in a 10 mM metabolite solution. According to our results, the fructose treatment enabled the decrease in biofilm formation on both PVC and NanoR surfaces after 24 hours. We observed a 38% decrease in the growth of S. aureus on PVC surfaces soaked in a 10 mM fructose solution compared to unsoaked PVC surfaces. In addition, biofilm formation significantly decreased (45%) on NanoR surfaces compared to the control PVC surfaces (P < 0.1). In addition, a 60% decrease (approximately 0.4 log reduction) in biofilm formation was observed on NanoR surfaces soaked in a 10 mM fructose solution compared to conventional PVC surfaces (P < 0.05). More importantly, all these results were accomplished without using any antibiotics.
Figure 4 Staphylococcus aureus optical density measurements after 24 hours. Planktonic bacteria growth decreased on NanoR surfaces soaked in a fructose (10 mM and 100 mM) solution.
Notes: Data represent mean ± SEM, n = 3. *P < 0.05 NanoR compared to NanoR soaked in 100 mM fructose, NanoR soaked in 10 mM fructose compared to NanoR soaked in 100 mM fructose.
Abbreviations: NanoR, nanorough; OD, optical density; SEM, standard error of the mean.
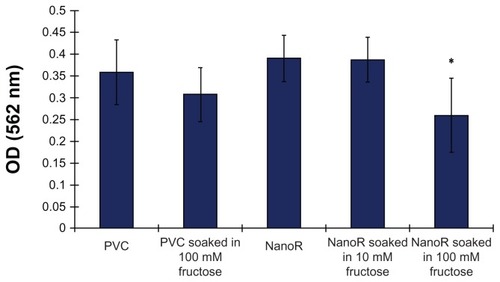
Figure 5 Staphylococcus aureus colony counts on PVC and NanoR surfaces soaked in a 10 mM fructose solution. Biofilm formation decreased on NanoR and PVC surfaces soaked in a 10 mM fructose solution after 24 hours.
Notes: Data represent mean ± SEM, n = 4. *P < 0.05 NanoR soaked in a 10 mM fructose solution compared to the PVC control, #P < 0.1 NanoR compared to the PVC control.
Abbreviations: CFU, colony forming units; NanoR, nanorough; PVC, polyvinyl chloride; SEM, standard error of the mean.
Biofilms develop a protected growth situation which allows survival in a hostile environment. As a result, bacterial persisters are a dormant population and they are tolerant to antibiotic treatment.Citation13,Citation14,Citation44 These persister structures within biofilms form channels in which nutrient circulation occurs. However, the uptake of some antibiotics, such as aminoglysocides, requires a promotion motive force (PMF). As the biofilm forming bacteria are in a dormant state, they do not uptake or metabolize antibiotics. Thus, bacterial resistance to antibiotics develops. If the metabolites which enter the upper glycolysis metabolism (ie, glucose, fructose, and mannitol) are added into the microenvironment as carbon sources, the efficiency of gentamicin increases. As the metabolism and PMF of the persisters are induced, metabolite stimulation increases the uptake of antibiotics and their killing efficiency. Citation44 In addition, these sessile biofilm communities can give rise to non-sessile bacterium and these planktonic bacteria can rapidly multiply and disperse within the solution. Planktonic bacteria must expose themselves to deleterious agents in their environment while the biofilm forming persisters protect themselves from harsh environmental conditions, such as antibiotics.Citation14 As the planktonic bacteria are dispersed within the surrounding medium due to the presence of fructose on the ETT surface, surface roughness at the nanoscale could minimize the contact between the bacteria cell wall and the NanoR ETT surface. This might inhibit the electrostatic interactions responsible for initial bacterial attachment on the surface.Citation4 Accumulation of proteins on nanorough surfaces might also affect initial bacterial adhesion though a thick protein layer.Citation51 This layer could decrease interactions at the bacteria–nanorough surface interface. Thus, this passivation effect could reduce bacterial attachment and biofilm formation. Citation51 Moreover, metabolites on both PVC and NanoR ETT surfaces might induce metabolism of biofilm forming S. aureus. All of these events might lead to the reduction of both planktonic bacteria growth and biofilm formation on NanoR surfaces that are soaked in fructose solutions, without the use of any antibiotics. Given the results of this study, the effect of combining nanostructured surfaces with other metabolites which enter bacterial metabolism through glycolysis (such as glucose, mannitol, and pyruvate) could be studied in future studies.
Conclusions
In summary, we present for the first time that the presence of fructose on nanorough PVC ETT surfaces decreases the number of planktonic S. aureus in the solution and biofilm formation on the surface, without using any antibiotics. In addition, we showed that engineered nanoscale topographies provide surfaces that are more resistant to bacterial growth than conventional PVC without using any antibiotics. Our results have important implications. First, the design of nanorough engineered surfaces decreases bacterial adhesion on surfaces. In addition, the reduction of biofilm forming bacteria can be improved through the use of specific metabolites on nanorough surfaces. We envision that this method has the potential to impact the future of surface engineering of ETTs as well as catheters and other invasive biomaterials leading to more successful clinical outcomes in terms of longer device lifetimes, minimized infections, and decreased antibiotic usage; all of these events will support the decreased presence of antibiotic resistant bacteria.
Acknowledgments
The authors thank the Hermann Foundation for funding.
Disclosure
The authors report no conflict of interest in this work.
References
- RelloJOllendorfDAOsterGEpidemiology and outcomes of ventilator-associated pneumonia in a large US databaseChest2002122621552121
- RichardsMJEdwardsJRCulverDHGaynesRPNosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance SystemCrit Care Med199927588789210362409
- VincentJLBihariDJSuterPMThe prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory CommitteeJAMA199527486396447637145
- SeilJTRubienNMWebsterTJTarquinioKMComparison of quantification methods illustrates reduced Pseudomonas aeruginosa activity on nanorough polyvinyl chlorideJ Biomed Mater Res B Appl Biomater20119811721634005
- RaadIIMohamedJAReitzelRAThe prevention of biofilm colonization by multidrug-resistant pathogens that cause ventilator-associated pneumonia with antimicrobial-coated endotracheal tubesBiomaterials201132112689269421295343
- FagonJYChastreJDomartYTrouilletJLGibertCMortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brushClin Infect Dis19962335385428879777
- AdairCGGormanSPFeronBMImplications of endotracheal tube biofilm for ventilator-associated pneumoniaIntensive Care Med199925101072107610551961
- SottileFDMarrieTJProughDSNosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubesCrit Care Med19861442652703956213
- RelloJTorresARicartMVentilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin- sensitive episodesAm J Respir Crit Care Med19941506154515497952612
- AntociVJrAdamsCSParviziJThe inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infectionBiomaterials2008354684469018814909
- TaylorEWebsterTJReducing infections through nanotechnology and nanoparticlesInt J Nanomedicine201161463147321796248
- XavierJBPicioreanuCRaniSAvan LoosdrechtMCStewartPSBiofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix – a modelling studyMicrobiology2005151Pt 123817383216339929
- DaveyMEO’tooleGAMicrobial biofilms: from ecology to molecular geneticsMicrobiol Mol Biol Rev200064484786711104821
- CostertonJWStewartPSGreenbergEPBacterial biofilms: a common cause of persistent infectionsScience199928454181318132210334980
- TrippRAAlvarezRAndersonBJonesLWeeksCChenWBioconjugated nanoparticle detection of respiratory syncytial virus infectionInt J Nanomedicine20072111712417722519
- ChenMYangZWuHPanXXieXWuCAntimicrobial activity and the mechanism of silver nanoparticle thermosensitive gelInt J Nanomedicine201162873287722131833
- TranPAWebsterTJSelenium nanoparticles inhibit Staphylococcus aureus growthInt J Nanomedicine201161553155821845045
- Mitik-DinevaNWangJMocanasuRCStoddartPRCrawfordRJIvanovaEPImpact of nano-topography on bacterial attachmentBiotechnol J20083453654418246568
- Mitik-DinevaNWangJTruongVKEscherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughnessCurr Microbiol200958326827319020934
- TruongVKRundellSLapovokREffect of ultrafine-grained titanium surfaces on adhesion of bacteriaAppl Microbiol Biotechnol200983592593719296098
- IvanovaEPTruongVKWangJYImpact of nanoscale roughness of titanium thin film surfaces on bacterial retentionLangmuir20102631973198219842625
- PuckettSDTaylorERaimondoTWebsterTJThe relationship between the nanostructure of titanium surfaces and bacterial attachmentBiomaterials201031470671319879645
- ColonGWardBCWebsterTJIncreased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2J Biomed Mater Res A200678359560416752397
- MachadoMCChengDTarquinioKMWebsterTJNanotechnology: pediatric applicationsPediatr Res201067550050420139795
- ScardinoAJZhangHCooksonDJLambRNde NysRThe role of nano-roughness in antifoulingBiofouling200925875776720183134
- SamarooHDLuJWebsterTJEnhanced endothelial cell density on NiTi surfaces with sub-micron to nanometer roughnessInt J Nanomedicine200831758218488418
- PuckettSParetaRWebsterTJNano rough micron patterned titanium for directing osteoblast morphology and adhesionInt J Nanomedicine20083222924118686782
- TranPWebsterTJEnhanced osteoblast adhesion on nanostructured selenium compacts for anti-cancer orthopedic applicationsInt J Nanomedicine20083339139618990948
- KeshelSHAzhdadiSNAsefnejadASadraeianMMontazeriMBiazarEThe relationship between cellular adhesion and surface roughness for polyurethane modified by microwave plasma radiationInt J Nanomedicine2011664164721556340
- ParkMRBanksMKApplegateBWebsterTJInfluence of nanophase titania topography on bacterial attachment and metabolismInt J Nanomedicine20083449750419337418
- RajyalakshmiAErcanBBalasubramanianKWebsterTJReduced adhesion of macrophages on anodized titanium with select nanotube surface featuresInt J Nanomedicine201161765177121980239
- ZhangLChunYWWebsterTJDecreased lung carcinoma cell density on select polymer nanometer surface features for lung replacement therapiesInt J Nanomedicine201013526927520517474
- KaragkiozakiVCLogothetidisSDKassavetisSNGiannoglouGDNanomedicine for the reduction of the thrombogenicity of stent coatingsInt J Nanomedicine20107523924820463940
- JuanLZhiminZAnchunMLeiLJingchaoZDeposition of silver nanoparticles on titanium surface for antibacterial effectInt J Nanomedicine2010526126720463942
- LiaoJAnchunMZhuZQuanYAntibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibilityInt J Nanomedicine201013533734220517478
- ShameliKAhmadMBYunusWMGreen synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV irradiation method and evaluation of antibacterial activityInt J Nanomedicine2010587588721116328
- LordMSFossMBesenbacherFInfluence of nanoscale surface topography on protein adsorption and cellular responseNanotoday2010516678
- TuttlePVRundellAEWebsterTJInfluence of biologically inspired nanometer surface roughness on antigen-antibody interactions for immunoassay-biosensor applicationsInt J Nanomedicine20061449750517722282
- WebsterTJErgunCDoremusRHSiegelRWBiziosRSpecific proteins mediate enhanced osteoblast adhesion on nanophase ceramicsJ Biomed Mater Res200051347548310880091
- WooKMChenVJMaPXNano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachmentJ Biomed Mater Res A200367253153714566795
- ErcanBWebsterTJGreater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulationInt J Nanomedicine20083447748519337416
- RaimondoTPuckettSWebsterTJGreater osteoblast and endothelial cell adhesion on nanostructured polyethylene and titaniumInt J Nanomedicine20107564765220856840
- LockJLiuHNanomaterials enhance osteogenic differentiation of human mesenchymal stem cells similar to a short peptide of BMP-7Int J Nanomedicine201162769277722114505
- AllisonKRBrynildsenMPCollinsJJMetabolite-enabled eradication of bacterial persisters by aminoglycosidesNature201173734621622021562562
- KuehnertMJKruszon-MoranDHillHAPrevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002J Infect Dis2006193217217916362880
- KhangDKimSYLiu-SnyderPPalmoreGTDurbinSMWebsterTJEnhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energyBiomaterials200728324756476817706277
- McHaleGShirtcliffeNJAqilSPerryCCNewtonMITopography driven spreadingPhys Rev Lett200493303610215323838
- LauKKSBicoJTeoKKBSuperhydrophobic carbon nanotube forestsNano Letters200331217011705
- ChowTSWetting of rough surfacesJ Phys Condens Matter19981027L445451
- BorgsCDe ConinckJKoteckýRZinqueMDoes the roughness of the substrate enhance wetting?Phys Rev Lett199574122292229410057891
- SinghAJVyasVPatilRQuantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formationPLos One.201169e2502921966403