Abstract
Among numerous drug-delivery approaches, reconstituted high-density lipoprotein (rHDL) nanocarriers have proven particularly applicable for delivering highly hydrophobic drugs. In this study, we have investigated the enhancement of the therapeutic impact of valrubicin (AD-32), an antineoplastic agent that has been limited to intravesicular application against bladder cancer, despite the encouraging original preclinical data. Earlier studies validated the superior therapeutic efficacy of AD-32 over doxorubicin. In the present study, rHDL/AD-32 nanoparticles were formulated and characterized with regard to encapsulation efficiency, physicochemical properties, selective toxicity, and receptor-mediated uptake. The half maximal inhibitory concentration values (IC50) for rHDL/AD-32 nanoparticles were 1.8 and 2.6 times lower than the free AD-32 for prostate (PC-3) and ovarian (SKOV-3) cancer cell lines, respectively, whereas nonmalignant cell lines demonstrated 5 and 1.48 times higher IC50 doses with rHDL/AD-32 formulations. The data obtained demonstrated effective receptor- mediated uptake of AD-32 from the rHDL nanocarriers by PC-3 and SKOV-3 cancer cells via a targeted drug-delivery process. The rHDL/AD-32 formulation was stable for 6 months when stored at 4°C or at −20°C, as 92% of the AD-32 was retained in the nanoparticles. The findings from this study show that the rHDL/AD-32 formulation can overcome the solubility barriers of AD-32 and thus serve as an effective systemically administered chemotherapeutic agent.
Introduction
Drug delivery via nanotechnology is one of the vanguards of the rapidly expanding field of cancer therapeutics.Citation1–Citation3 A variety of submicron drug carrier systems have been developed recently to improve the delivery and retention of pharmaceuticals by malignant cells and tumors.Citation4,Citation5 Lipid-containing nanoparticles (LNs) represent an alternative delivery system to traditional carriers, such as emulsions, liposomes, and polymeric nanoparticles, especially for the delivery of lipophilic drugs.Citation5,Citation6 While LNs have several advantages over other formulations, they also have serious limitations such as nonselective toxicity, instability, and limited drug payload.Citation6–Citation8 Encapsulation of drugs within the reconstituted high-density lipoprotein (rHDL) nanocarriers has shown great potential for cancer chemotherapy by minimizing limitations encountered with conventional formulations, including LNs and liposomes.Citation9–Citation11 The rHDL nanoparticles have been identified as an effective drug-delivery system for highly lipophilic drugsCitation11–Citation13 and for small interfering RNA (siRNA).Citation14 The proof of concept studies using rHDL nanoparticles loaded with paclitaxel, dilauryl-fluorescein, and siRNA have shown that the delivery of the drug to cancer cells and tumors occurs via a targeted, receptor-mediated mechanism involving the scavenger receptor class B type 1 (SR-B1).Citation13–Citation16
The current study was undertaken to evaluate the in vitro therapeutic efficacy of valrubicin [AD-32 (N-trifluoroacetyladriamycin- 14-valerate)], a derivative of doxorubicin, which was originally developed as a cancer therapeutic agent,Citation17–Citation19 leading to its restricted approval for treating Bacillus Calmette-Guerin (BCG)–resistant bladder cancers in 1998.Citation20,Citation21 Even though AD-32 has been found to be much less toxic to normal tissuesCitation16,Citation22 than doxorubicin during preclinical studies, its therapeutic application has been confined to nonsystemic administration due to its poor water solubility. This restriction remains in place except for recent attempts to extend the application of AD-32 to treat skin cancer and psoriasis via topical formulations.Citation23,Citation24 The highly hydrophobic nature of AD-32 and its proven antineoplastic properties make it a promising candidate to serve as an effective chemotherapeutic agent when used in conjunction with the rHDL nanoparticles as delivery vehicles. In our present work, we formulated AD-32 with rHDL nanocarriers using the cholate dialysis technique.Citation13 These particles were then characterized for their encapsulation efficiency and physicochemical properties. The rHDL/AD-32 nanocarriers were also evaluated for their effect on cell viability in malignant prostate (PC-3) and ovarian (SKOV-3) cell lines, and nonmalignant prostate (PZ-HPV) and ovarian (HiO 180) epithelial cells. Drug uptake through the SR-B1 receptors, the in vitro release profile, and the stability of formulations were also studied in detail.
Materials
Sodium cholate, egg yolk phosphatidyl choline (PC), free cholesterol, cholesteryl oleate, potassium bromide (KBr), isopropyl thiogalactoside (IPTG), dimethyl sulfoxide (DMSO), Triton X-100, and thrombin cleavage kit were purchased from Sigma-Aldrich Corporation, St Louis, MO. NZYCM was obtained from Teknova, Hollister, CA. Bacterial protein extraction reagent and bicinchoninic acid (BCA) protein assay kits were purchased from Thermo Scientific, Rockford, IL. A histidine-trap (His-Trap) affinity column was obtained from QIAGEN, Valencia, CA. Cholesterol and phospholipid estimation kits were obtained from Wako Pure Chemical Industries Ltd, Richmond, VA. Roswell Park Memorial Institute 1640 media, keratinocyte media and fetal bovine serum were obtained from Gibco, Invitrogen, Carlsbad, CA. AD-32 was provided by Dr Mervyn Israel, University of Tennessee Health Science Center, Memphis, TN. Low protein binding durapore membrane syringe filters were purchased from Millipore Ltd, Billerica, MA. Established cancer cell lines PC-3, SKOV-3, and PZ-HPV were obtained from American Type Culture Collection (ATCC), Manassas, VA. The ovarian epithelium cell line HiO 180 was provided by Dr Anil K Sood of MD Anderson Cancer Center, Houston, TX. The cell counting kit (CCK-8) was purchased from Dojindo Laboratories, Tabaru, Japan.
Methods
Preparation of the AD-32 containing nanoparticles
Isolation and purification of recombinant apoA-I
These were performed essentially as described by Ryan et al.Citation25 Briefly, BL21(DE3)pLysS cells bearing the pNFXex plasmid were cultured in 500 mL NZYCM media (+50 μg/mL ampicillin) at 37°C. When the culture optical density reached 0.6 at 600 nm, apoA-I synthesis was induced by the addition of IPTG to a final concentration of 0.5 mM. After 3 hours, the bacteria were pelleted by centrifugation and disrupted by lysis buffer (bacterial protein extraction reagent). The cell lysate was centrifuged at 20,000 g for 30 minutes at 4°C. The supernatant fraction was mixed with an equal volume of phosphate-buffered saline (PBS) containing 6 M guanidine hydrochloric acid (HCl), applied to a 5 mL bed volume His-Trap affinity column, and purified as per manufacturer’s instructions. The N-terminal His-Tag extension was removed using a thrombin CleanCleave kit (Sigma-Aldrich). The isolated apo A-I was then dialyzed against tris(hydroxymethyl)aminomethane (Tris)-buffered saline containing 1 mM benzamidine for 16 hours at 4°C. The dialyzed sample was filter-sterilized (0.2 μm) and stored at 4°C until use.
Preparation of rHDL/AD-32 complexes with apo A-I
This was accomplished by a procedure developed earlier in our laboratory.Citation10,Citation13 Briefly, a mixture of egg yolk PC in CHCl3 with free cholesterol (FC), and cholesteryl oleate (CE), was prepared with a molar ratio of ApoA1:FC:CE:PC = 1:5:1.3:1.15 M. The lipid mixture (PC, FC, and CE) and the drug (AD-32) were dried under N2 to a thin film and dispersed in 60 μL DMSO. To this mixture, apo A-I (5 mg) and 140 μL sodium cholate (from a stock of 100 mM) were added and the volume was made up to 2 mL with Tris-ethylenediaminetetraacetic acid (EDTA) buffer (10 mM Tris, 0.1 M KCl, 1 mM EDTA pH 8.0). (Final PC to cholate molar ratio was maintained at ~1:1.6). The lipid/protein/cholate mixture was then incubated for 12 hours at 4°C, followed by dialysis against 2 L of PBS, for 48 hours, with three buffer changes in the first 12 hours. The preparations were then centrifuged at 1000 rpm for 2 minutes and sterilized using a 0.2 μm syringe filter. The preparations were kept in the dark at 4°C until used.
Characterization of rHDL/AD-32 nanoparticles
Chemical composition
Cholesterol and phospholipids were determined by respective enzymatic reagent kits (cholesterol E and phospholipid C), using microtiter plate assays as per manufacturer’s suggestions. Protein determinations were carried out using a BCA protein assay kit. The percentage drug incorporation was determined by fluorescence spectroscopic measurements, in a Cary Eclipse Spectrofluorometer (Varian Inc, Mulgrave, Australia).
Determination of entrapment efficiency of AD-32 in the rHDL/AD-32 nanoparticles
The drug entrapment efficiency (DEE) was determined by measuring the amount of AD-32 in the formulations before the cholate dialysis step mentioned earlier (initial drug) and after filtration (final drug incorporated). DEE was calculated using the following formula: DEE = {Drug concentration after dialysis/Drug concentration before dialysis} ×100.
Drug-loading studies with rHDL/AD-32 nanoparticles
rHDL/AD-32 particles were prepared using increasing initial concentrations of AD-32 while keeping all other ingredients constant. Final drug concentration and DEE were calculated as described above.
Ultracentrifugation
KBr, 1.2 g/mL, was added to 3 mL of sample. The sample was layered with KBr solutions with the corresponding designated densities (1 mL, 1.22 g/mL; 4 mL, 1.063 g/mL; and 3 mL, 1.019 g/mL) and centrifuged in an Optima LE 80K ultracentrifuge (Beckman Coulter, Brea, CA) for 36 hours at 39,000 rpm at 4°C using a swinging bucket rotor (SW 40) in a Beckman.Citation26 Fractions (1 mL) were collected corresponding to very low-density lipoprotein (fraction 1–2, P = < 1.007 g/mL); intermediate density lipoprotein (fraction 3, P = 1.007–1.02 g/mL); low-density lipoprotein (fractions 4–6, P = 1.020–1.060 g/mL); high-density lipoprotein (HDL)2 (fractions 7–8, P = 1.060–1.15 g/mL): HDL3 (fractions 9–10, P = 1.15–1.235 g/mL) and lipoprotein deficient serum (fractions 11–13, P > 1.235 g/mL), and subsequently analyzed for protein, phospholipid, and cholesterol.
Estimation of the size and morphology of rHDL/AD-32 nanoparticles
Dynamic light scattering (DLS)
Particle size analysis of the AD-32-loaded rHDL nanoparticles was carried out using a Nanotrac system (Microtrac Inc, Montgomeryville, PA) as per manufacturer’s instructions. The nanoparticles were dispersed in aqueous buffer using an ultrasonic waterbath (Fisher Scientific, Pittsburgh, PA) for 2 minutes and then measured for particle size.
The results were reported as the average of three independent runs with duplicate observations in each run.
Atomic force microscopy (AFM)
The rHDL-AD-32 formulation was dialyzed at 4°C for 12–18 hours against sterile distilled and deionized water to remove the salts from the solution. It was further diluted 1:5 with sterile distilled water. Ten microliters of the sample was then placed on the microscope slide and was allowed to air dry. Sample was then processed with AFM on an NTEGRA Prima scanning probe microscope (NT-MDT, Santa Clara, CA). Closed-loop feedback semicontact mode was used at a rate of 0.6 Hz. Scanning started from the 50 μm area, going down to 5 μm. The images obtained were analyzed with NT-MDT image analysis software (v 2.2). At least 25 individual particles were measured from three different positions and the average diameter was reported.
Transmission electron microscopy (TEM)
Following dialysis in a volatile buffer (0.125 M ammonium acetate, 2.6 mM ammonium carbonate, 0.26 mM EDTA, pH 7.4), the isolated rHDL samples were negatively stained with 2% sodium phosphotungstate, pH 7.2 and placed on Formvar/Carbon-coated 200 mesh nickel grid support films (Ted Pella Inc, Redding, CA) as previously described.Citation27 The particles were visualized using a magnification of 50,000 on a Zeiss 910 transmission electron microscope (Carl Zeiss, Thornwood, NY). The pictures were enhanced and the particle diameter determined with Adobe ImageReady CS2 software (Adobe Systems, San Jose, CA).
Effect of free and rHDL encapsulated AD-32 on cell viability
Culturing of the malignant prostate cancer cell line (PC-3), malignant ovarian cancer cell line (SKOV-3) and nonmalignant ovarian epithelial cell line (HiO180) were carried out according to procedures and culturing conditions provided by the ATCC.Citation28,Citation29 Briefly, the cells were cultured in Roswell Park Memorial Institute 1640 containing 2.05 mM L-glutamine and 10% fetal bovine serum. A nonmalignant prostate epithelial cell line (PZ-HPV) was grown in keratinocyte medium supplemented with 10% fetal bovine serum medium containing human recombinant epidermal growth factor and bovine pituitary extract (both from Invitrogen) as per manufacturer’s instructions. All the cells were grown in 75 cm2 flasks and incubated at 37°C and 5% CO2. Cells were passaged using 0.25% trypsin to release the cells from the flasks, once 80%–90% confluency was reached.
Preparation of drug formulations
A stock solution (50 mg/mL) of AD-32 in DMSO was prepared by emulsifying the drug in 50% sterile bovine serum albumin (BSA)/PBS by stirring. Samples for the characterization studies were prepared by diluting the stock with PBS to achieve equivalent AD-32 molar concentration to that contained by the respective rHDL/AD-32 nanoparticle samples. (The unencapsulated AD-32 suspension in BSA is referred to as “free drug.”)
Determination of IC50 doses
The effect of AD-32 in free drug and rHDL encapsulated particles was studied using CCK-8 kit (Dojindo Molecular Technologies, Tabaru, Japan).Citation30 Briefly, the PC-3, SKOV-3, PZ-HPV, and HiO180 were grown according to procedures and culturing conditions provided by the ATCC in their respective media as stated earlier in 75 cm2 flasks and incubated at 37°C and 5% CO2. Citation28,Citation29 Cells were passaged using 0.25% trypsin to release the cells from the flasks, once 80%–90% confluency was reached. Cells were counted using hemocytometer and 5000 cells were seeded per well into 96-well microtiter plates and incubated at 37°C in 5% CO2 for 24 hours to allow the cells to attach to the plates. The free drug and the rHDL/AD-32 nanoparticles were diluted in serum-free medium to yield stock solutions of equivalent molar concentrations. Subsequently, aliquots of the stock solutions were added to microtiter plate wells to achieve the selected concentration range for the cell viability tests. Controls included cells with media (without rHDL/drug), cells with empty rHDL particles, and media without cells with same rHDL/drug and free drug of each concentration used. Cells were incubated at 37°C in 5% CO2 for 24 hours. After incubation, 10 μL of highly water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] stock solution (Dojindo) was added to each well. After 3 hours of incubation at 37°C, the absorbance at 450 nm was measured using a Bio-Rad 3550 microplate reader (Bio-Rad Laboratories, Hercules, CA). Each concentration was studied with six replicates.
Monitoring the uptake of the drug payload from the rHDL/AD-32 complex
Cells were plated in 24-well plates (100,000 cells/well) in their respective media. On the following day, the monolayers were washed with PBS, pH 7.4, and then incubated at 37°C with serum-free medium for 90 minutes. Cells were washed with PBS and incubated with a single concentration of the rHDL/AD-32 complex plus increasing amounts of HDL (0–120 μg) in serum-free medium for 90 minutes. The preparation was washed once with 1 × PBS, pH 3.0, and subsequently with 1 × PBS, pH 7.4. The cells were then lysed with lysis buffer (50 mm Tris-HCl [pH 8.0], 150 mM NaCl, 0.02% sodium azide, 100 μg/mL phenylmethanesulfonylfluoride, 1 μg/mL aprotinin, and 1% Triton X-100). The lysate was centrifuged at 10,000 rpm for 5 minutes. The protein and AD-32 content of the lysate was determined by BCA assays and spectrophotometric measurements respectively as described above.
In vitro release studies
These were performed using a dynamic dialysis technique,Citation31–Citation33 modified to maintain a sink condition for improved reproducibility. A 2 mL sample of AD-32-rHDL preparation was transferred to a dialysis bag with molecular weight cut off of 6000–8000 MW and placed into the preheated dissolution medium at 37°C containing 100 mL of PBS at pH 7.4. The suspension was stirred at 37°C ± 0.5°C using a gyratory shaker at 50 rpm. Five hundred microliters of the sample was withdrawn at fixed time intervals and replaced with the same volume of fresh buffer. Samples were scanned using Cary Winuv scan software (v 3.00) in a Cary Varian UV-visible spectrophotometer (Agilent Technologies, Santa Clara, CA). Free AD-32 in pH 7.4 PBS was used as a control. All the operations were carried out in triplicate. The amount of AD-32 released from the dialysis bags were measured using a standard graph with a known concentration range of AD-32 versus absorbance at 490 nm.
Stability studies
These were performed at 4°C and −20°C. The particles were stored at 4°C and 100 μL aliquots were removed at designated time periods. The samples were dialyzed using dialysis tubing with an 8000 MW cut off at 4°C for 18 hours. The AD-32 content and particle size before and after dialysis were determined spectrophotometrically and by DLS methods respectively as described above.
Stability to lyophilization
rHDL/AD-32 particles were dialyzed against 0.1 M PBS. The nanoparticles were freeze dried at −46°C/0.5 Mbar in a Labconco freeze drier (Labconco Corp, Kansas City, MO) until they were completely dry, producing a powder. The freeze-dried preparations were stored at −20°C for 24 hours and then reconstituted in 1 × PBS. The AD-32 content and particle size before and after dialysis were determined spectrophotometrically and by dynamic light scattering methods respectively as described above.
Results
Characterization of rHDL/AD-32 nanoparticles
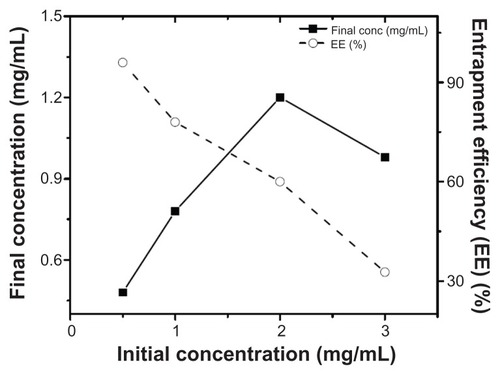
Assembly of the AD-32-containing nanoparticles was achieved by the cholate dialysis procedure, developed earlier in our laboratory.Citation13,Citation15 As shown in , the predominant component of the rHDL/AD-32 nanoparticles was phospholipid (62.0%), followed by protein (28.8%), cholesterol (0.96%) and AD-32 (8.3%). The phospholipid to protein ratio was 2.15:1 for the drug-loaded nanoparticles as compared with 1.96:1 for the empty particles (without drug). The entrapment efficiency of AD-32 in the rHDL nanoparticles was assessed by preparing particles with an increase in the initial concentration of AD-32 while keeping the amount and proportions of all the other components constant. As indicated in , the entrapment efficiency of AD-32 decreased from 96% to 32.7% with an increase in the initial concentration of AD-32 from 0.5 mg/mL to 3 mg/mL. The highest amount of drug was incorporated at the initial concentration of 2 mg/mL.
Table 1 Chemical composition of rHDL/AD-32 particles
Figure 1 Loading efficiency of AD-32 into rHDL nanoparticles via the cholate dialysis process (see methods for details).
Abbreviations: AD-32, valrubicin; rHDL, reconstituted high-density lipoprotein.
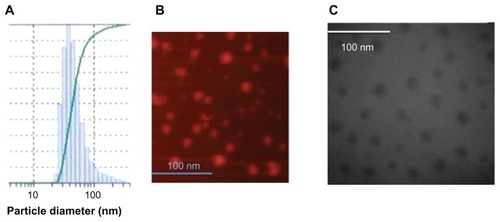
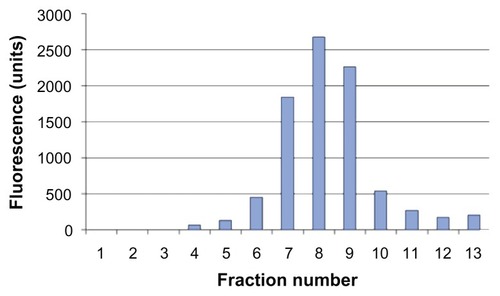
The size of the rHDL/AD-32 nanoparticles was determined using three methods: DLS, TEM, and AFM. The DLS data in depict a relatively narrow size distribution ranging from 29 to 120 nm with the mean particle size being 43.7 nm. The images obtained by AFM () and TEM () indicated the presence of spherical particles with a diameter ranging from 15 to 25 nm and mean particle size of 18 and 20 nm, respectively. The data from DLS show larger diameter particles (compared with the data from TEM and AFM) as it has during our previous studies with other drug-containing nanoparticles (unpublished data). The buoyant density pattern of the AD-32 containing rHDL preparation is shown in as the distribution of fluorescence in a density gradient. The centrifugation pattern developed for the rHDL/AD-32 particles shows a peak in fraction #8, coinciding with the position of circulating human HDL.
Determination of IC50
Cell viability assays (CCK-8) were performed using free AD-32, AD-32-loaded rHDL nanoparticles, and blank (empty) rHDL nanoparticles. Untreated cells and equivalent formulations without cells served as controls. The data for free AD-32 and AD-32 enclosed in rHDL nanoparticles depicted as the drug concentration required to inhibit cell growth by 50% (IC50), is shown in . The IC50 for the AD-32-loaded rHDL nanoparticles was found to be greater than 48 μM for both nonmalignant cell lines while the IC50 values for the free AD-32 were substantially lower (9.5 μM and 32.5 μM, respectively). These data indicate that the encapsulation in the rHDL nanoparticles protects normal (nonmalignant cells) from the impact of cytotoxic drugs. The IC50 values for the rHDL-enclosed and free AD-32 samples for the PC-3 cells were 8.1 and 19.3 μM, respectively. A similar trend was observed for the SKOV-3 cells, where the IC50 for rHDL loaded AD-32 particles and free drug were 11.9 and 21.5 μM, respectively. These data show that the encapsulation in the rHDL nanoparticles enhances the cytotoxic potential of AD-32 against malignant cells as we have shown earlier for paclitaxel.Citation13
Table 2 Cytotoxicity of AD-32, delivered by rHDL nanocarriers versus the free drug against malignant and normal cells
Uptake of AD-32 from rHDL particles by malignant and nonmalignant cells
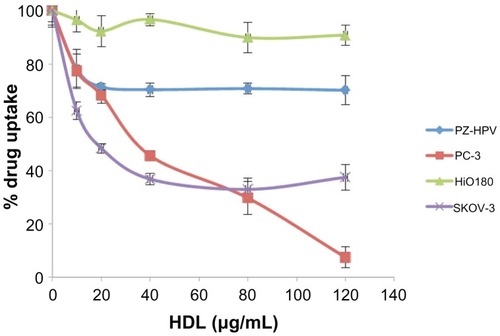
Uptake of AD-32 by the PZ-HPV, PC-3 (prostate), HiO180, and SKOV-3 (ovarian) cell lines was assessed in the presence and absence of increasing amounts of human HDL (a competitor for the SR-B1 receptor).Citation10,Citation11,Citation15 As shown in , there was a substantial decrease in the uptake of AD-32 from the AD-32 containing rHDL nanoparticles by the malignant cells (PC-3 and SKOV-3), in response to the progressively increasing HDL concentrations in the incubation mix. The nonmalignant cells (PZ-HPV and HiO180), however, showed a much more limited response to increasing HDL, consistent with their lower expression of the SR-B1 receptor.Citation10–Citation12,Citation14,Citation15.
Figure 4 Comparative drug uptake studies from rHDL/AD-32 nanoparticles with malignant and non-malignant ovarian and prostate cell lines.
Abbreviations: AD-32, valrubicin; rHDL, reconstituted high-density lipoprotein; PZ-HPV, nonmalignant prostate epithelial cell line; PC-3, prostate cancer cell line; HiO 180, nonmalignant ovarian epithelial cell line; SKOV-3, ovarian cancer cell line; HDL, high-density lipoprotein.
Stability studies of AD-32 loaded rHDL nanoparticles
As shown in , almost 90% of the AD-32 content of the rHDL nanoparticles was retained during 6 months storage at 4°C. Ninety-two percent of the original AD-32 was retained within the rHDL particles following 6 months storage at −20°C. Lyophilization was found to be the best approach for sustained storage for the drug-containing rHDL nanoparticles. The mean diameter of the stored particles was found to be very close to that of the freshly prepared particles ( and ) indicating minimum aggregation upon rehydration from the powdered form.
Table 3 The stability of AD-32/rHDL particles at different storage conditions
In vitro release profile of AD-32-loaded rHDL nanoparticles
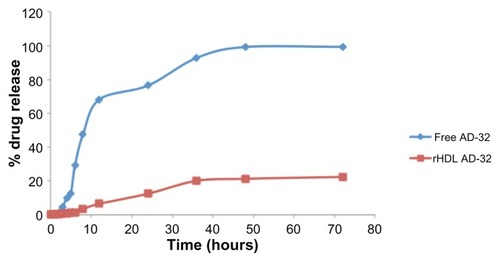
In vitro release studies were carried out by dynamic dialysis technique in a dialysis bag incubated at 37°C in PBS. About 80% of the integrity of rHDL/AD-32 nanoparticles was preserved up to 72 hours under these conditions. However, nearly 70% of the free drug was released within 8 hours and more than 90% of the drug was released after about 20 hours under the same dialysis conditions ().
Discussion
AD-32 has been shown to have antineoplastic activity, low cardiotoxicity, and 24-fold higher lipophilicity than its parent compound, doxorubicin.Citation17–Citation20 While the chemotherapeutic potential of AD-32 was recognized earlier during preclinical studies,Citation17,Citation18 its application has so far been limited to vesicular treatment of bladder cancer, primarily due to its poor water solubility.Citation21,Citation34 Here, we report an attempt to overcome this solubility barrier by encapsulating the AD-32 in rHDL nanoparticles.Citation11–Citation16
The advantageous characteristics of HDL-based drug carriers include small size, selective delivery via receptor targeting, stability during storage, and high payload capacity.Citation11–Citation16 Encapsulation of lipophilic drugs within nanocarriers has shown great potential as an alternative or an adjunct to existing chemotherapy regimens.Citation4,Citation5 In addition, the selective targeting of malignant cells through receptor-mediated uptake of anticancer drugsCitation13,Citation14 is a key concept in mediating or eliminating the side effects of conventional chemotherapy. The present study was undertaken to evaluate the potential of AD-32 as a chemotherapeutic agent upon encapsulation into biocompatible rHDL nanoparticles.
The rHDL/AD-32 particles were prepared by cholate dialysis, producing macromolecular structures that resembled circulating HDL with respect to physical and chemical characteristics. The rHDL/AD-32 nanoparticles, prepared by cholate dialysis, exhibited physico-chemical properties similar to that of native (circulating) human HDL10.Citation16 Overall, these particles exhibited similar shape, size, and chemical composition to formulations of rHDL/dilauryl fluorescein and rHDL/paclitaxel particles, described earlier.Citation13,Citation15 The discrepancy in the size by DLS and other methods is likely to be due to the bias of the light scattering instrument toward larger particles. The key findings of this study are that AD-32 could be efficiently encapsulated into rHDL nanoparticles yielding a stable (nonleaking) preparation that efficiently delivers its payload to cancer cells. A major advantage of the rHDL drug-delivery system is that the particles are composed of naturally occurring biodegradable ingredients found in the vascular system. Consequently, they are not likely to elicit any immunological response in the host. Thus, it is anticipated that the rHDL nanoparticles have a relatively extended residence time in the circulation (similar to HDL) compared with most traditional drug-delivery vehicles, including liposomes. These physicochemical properties of rHDL/AD-32 emphasize its candidacy as an effective delivery model for anticancer agents and other systemically administered drugs.
Doxorubicin alone or in combination with other drugs has been reported to be effective against a wide range of cancers including prostate, ovarian, and breast carcinoma.Citation35,Citation36 Despite its reduced cardiotoxicity, AD-32 has been mainly restricted to the treatment of bladder cancer. In order to evaluate the efficacy of AD-32 in the treatment of prostate and ovarian cancer, we have examined its effects on malignant and nonmalignant cells of prostatic and ovarian origin. Nonmalignant cells of both the PZ-HPV and HiO180 lines were more sensitive to the free AD-32 than to the rHDL-encapsulated drug preparation, suggesting better protection against cytotoxic effects of the encapsulated drugs provided by the rHDL carrier for normal cells and tissues.Citation14 The cancer cells, however, exhibited increased sensitivity to the rHDL encapsulated drug formulation as compared with the cytotoxic effect of the free drug, probably due to the higher expression of the SR-B1 receptor in malignant cells.Citation10–Citation12,Citation14,Citation15 These observations are consistent with our earlier studies with other chemotherapeutic agents on malignant cancer cells.Citation10–Citation12,Citation14,Citation15 Additional studies were conducted to probe the delivery mechanism of AD-32 by evaluating the effect of added human HDL on the drug uptake by the malignant and nonmalignant cells. Earlier, we showed that the majority of the drug molecules are taken up from the rHDL nanoparticles by cancer cells via the SR-B1 receptor, whose normal function includes the extraction of cholesteryl esters from HDL.Citation14,Citation15 This receptor has been shown to be markedly overexpressed in malignant cells and tissues, apparently because of their need for excess cholesterol, due to their high proliferative rates.Citation10–Citation12,Citation14,Citation15 A progressive decrease in the AD-32 uptake from rHDL nanoparticles was observed when the cancer cells were pre-incubated with human HDL. These findings suggest that the added HDL competes with rHDL for the receptor sites and that the uptake of AD-32 by cancer cells from rHDL nanoparticles is also facilitated by HDL/SR-B1 receptors.Citation11,Citation15 Previous studies have demonstrated that accumulation of the siRNA (delivered via the rHDL nanoparticles) was undetectable in nearly all normal tissues (except marginally in the liver) while it was substantial in tumor tissue, suggesting that selective drug delivery to tumors via rHDL is feasible.Citation11,Citation12,Citation14 The selective tumor delivery concept would make the rHDL nanoparticles outstanding drug-delivery candidates for the purposes of reducing off-target effects and avoiding drug resistance during cancer chemotherapy.
The stability studies of rHDL/AD-32 particles under different conditions demonstrated the ease of storage of these particles for long-term use. In particular, the stability subsequent to lyophilization enhances the feasibility of the AD-32/rHDL drug-delivery system for clinical applications. Furthermore, in vitro drug-release studies carried out at physiological pH conditions emphasize nonleaky formulations that could sustain these drug-loaded particles longer in circulation.
Conclusion
In this study, we prepared AD-32 containing rHDL nanoparticles that exhibited up to 96% entrapment efficiency and formed smooth, spherical particles with mean diameter size of 15–20 nm. The formulation was stable and nonleaking under several storage conditions. The findings from cytotoxicity and drug-uptake studies on malignant and normal (prostate and ovarian) cell lines suggest for the first time that the chemotherapeutic efficacy of AD-32 upon encapsulation in biocompatible nanoparticles may be markedly enhanced by the reduction (or elimination) of harmful side effects, due to selective tumor delivery of the anticancer agent, thus making it a potential candidate for alternate or adjuvant chemotherapeutic regimens.
Acknowledgments
This research was supported by Cowtown Cruisin’ for a Cure™ and seed funding for interdisciplinary projects in the field of women’s health focused on resources for health, education, and research from the University of North Texas Health Science Center. We thank Dr Zygmunt Gryczynski, for providing the spectrophotometer and fluorometer instrumentation and expertise for the AD-32 measurements. The atomic force microscopy studies were performed by Dr Irina Akapova.
Disclosure
The authors report no conflicts of interest in this work.
References
- CaruthersSDWicklineSALanzaGMNanotechnological applications in medicineCurr Opin Biotechnol2007181263017254762
- JainKKApplications of nanobiotechnology in clinical diagnosticsClin Chem200753112002200917890442
- DavisMEChenZShinDMNanoparticle therapeutics: an emerging treatment modality for cancerNat Rev Drug Discov20087977178218758474
- GuoDDXuCXQuanSJSynergistic anti-tumor activity of paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel in vitro and in vivoBiomaterials200930274777478519524293
- FreitasCMüllerRHCorrelation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phaseEur J Pharm Biopharm199947212513210234536
- UnerMYenerGImportance of solid lipid nanoparticles (SLN) in various administration routes and future perspectivesInt J Nanomedicine20072328930018019829
- YangSCLuLFCaiYZhuJBLiangBWYangCZBody distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brainJ Control Release199959329930710332062
- HuLDTangXCuiFDSolid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugsJ Pharm Pharmacol200456121527153515563759
- MüllerRHRadtkeMWissingSANanostructured lipid matrices for improved microencapsulation of drugsInt J Pharm20022421–212112812176234
- LackoAGNairMParanjapeSJohnsoSMcConathyWJHigh density lipoprotein complexes as delivery vehicles for anticancer drugsAnticancer Res20022242045204912174882
- LackoAGNairMParanjapeSMooberryLMcConathyWJTrojan horse meets magic bullet to spawn a novel, highly effective drug delivery modelChemotherapy200652417117316691026
- LackoAGNairMProkaiLMcConathyWJProspects and challenges of the development of lipoprotein-based formulations for anti-cancer drugsExpert Opin Drug Delivery200746665675
- McConathyWJNairMParanjapeSMooberryLLackoAGEvaluation of synthetic/reconstituted high density lipoproteins (rHDL) as delivery vehicles for paclitaxelAnticancer Drugs200819218318818176115
- ShahzadMMMangalaLHanHTargeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticlesNeoplasia201113430931921472135
- MooberryLKNairMParanjapeSMcConathyWJLackoAGReceptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrierJ Drug Target2010181535819637935
- McConathyWJParanjapeSMooberryLButtreddySNairMLackoAGValidation of the reconstituted high-density lipoprotein (rHDL) drug delivery platform using dilauryl fluorescein (DLF)Drug Deliv and Transl Res201112113120
- VecchiACairoMMantovaniASironiMSpreaficoFComparative antineoplastic activity of Adriamycin and N- trifluoroacetyladriamycin-14-valerateCancer Treat Rep1978621111117626989
- IsraelMModestEJFreiEIIIN-trifluoroacetyl adriamycin-14-valerate, an analog with greater experimental antitumor activity and less toxicity than adriamycinCancer Res1975355136513681054622
- KrishanAIsraelMModestEJDifferences in cellular uptake and cytofluorescence of Adriamycin and N-trifluoroacetyladriamycin-14-valerateCancer Res1976366210821091268863
- WilkinsonPMIsraelMPeggWJFreiE3rdComparative hepatobiliary metabolism and excretion of adriamycin (ADR) and N-trifluoroacetyladriamycin-14-valerate (AD82) in the ratCancer Res1978382365370620407
- WitjesJACurrent recommendations for the management of bladder cancer drug therapyDrugs19975334044149074842
- OnrustSVLambHMValrubicinDrugs Aging1999151697510459733
- AndersenSMRosadaCDagnaes-HansenFTopical application of valrubicin has a beneficial effect on developing skin tumorsCarcinogenesis20103181483149020554745
- RosadaCStenderupKde DarkóEDagnaes-HansenFKampSDamTNValrubicin in a topical formulation treats psoriasis in a xenograft transplantation modelJ Invest Dermatology20101302455463
- RyanROForteTMOdaMNOptimized bacterial expression of human apolipoprotein A-IProtein Expr Purif20032719810312509990
- LoneyWWKudchodkarBJWeissSClearfieldMBShoresJLackoAGEvaluation of gemfibrozil therapy: predictive response from lipoprotein sub fraction analysisAm J Ther199749–1030130910423623
- ForteTNorumKRGlomsetJANicholasAVPlasma lipoproteins in familial lecithin: cholesterol acetyltransferase deficiency: structure of low and high density lipoproteins as revealed by electron microscopyJ Clin Invest1971505114111485552411
- KaighnMENarayanKSOhnukiYLechnerJFJonesLWEstablishment and characterization of a human prostatic carcinoma cell line (PC-3)Invest Urol19791711623447482
- FoghJFoghJMOrfeoTOne hundred and twenty-seven cultured human tumor cell lines producing tumors in nude miceJ Natl Cancer Inst1977591221226327080
- MurakamiTWijagkanalanWHashidaMTsuchidaKIntracellular drug delivery by genetically engineered high-density lipoprotein nanoparticlesNanomedicine (Lond)20105686787920735223
- Venier-JulienneMCMathieuDFilmonRPhan-Tan-LuuRBenoitJPDevelopment of 5-iodo-2′-deoxyuridine milling process to reduce initial burst release from PLGA microparticlesInt J Pharm1999178225726810205645
- LeoECameroniRForniFDynamic dialysis for the drug release evaluation from doxorubicin-gelatin nanoparticle conjugatesInt J Pharm19991801233010089288
- YangCRZhaoXLHuHYPreparation, Optimization and Characteristic of Huperzine A Loaded Nanostructured Lipid CarriersChem Pharm Bull (Tokyo)201058565666120460792
- GreenbergRBahnsonRWoodDInitial report on intravesical administration of N-trifluoroacetyladriamycin-14-valerate (AD 32) to patients with refractory superficial transitional cell carcinoma of the urinary bladderUrology19974934714759123721
- ZhengJLeeHBin SattarMHuangYBianJCardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injuryEur J Pharmacol20116521–3828821114975
- IbsenSZahavyEWrasdiloWBernsMChanMEsenerSA novel doxorubicin prodrug with controllable photolysis activation for cancer chemotherapyPharm Res20102791848186020596761