Abstract
Background
Compared with titanium (Ti) and other metal implant materials, poly(ether-ether ketone) (PEEK) shows outstanding biomechanical properties. A number of studies have also reported attractive bioactivity for nano-TiO2 (n-TiO2).
Methods
In this study, n-TiO2/PEEK nanocomposites were prepared, taking advantage of the unique properties of both PEEK polymer and n-TiO2. The in vitro and in vivo bioactivity of these nanocomposites was assessed against a PEEK polymer control. The effect of surface morphology or roughness on the bioactivity of the n-TiO2/PEEK nanocomposites was also studied. n-TiO2/PEEK was successfully fabricated and cut into disks for physical and chemical characterization and in vitro studies, and prepared as cylindrical implants for in vivo studies. Their presence on the surface and dispersion in the composites was observed and analyzed by scanning and transmission electron microscopy and X-ray photoelectron spectroscopy.
Results
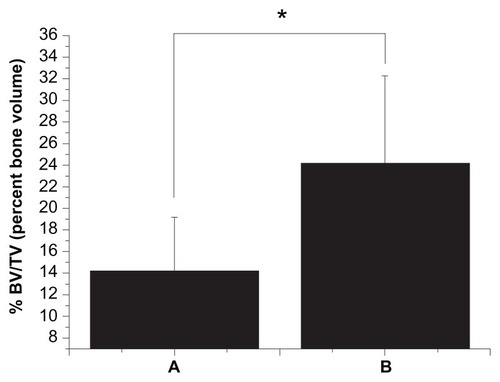
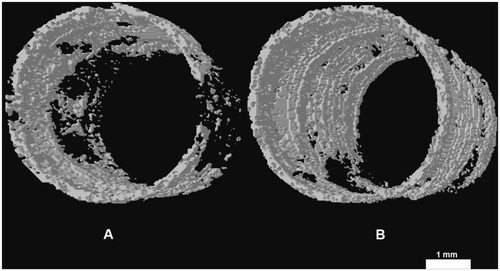
Bioactivity evaluation of the nanocomposites revealed that pseudopods of osteoblasts preferred to anchor at areas where n-TiO2 was present on the surface. In a cell attachment test, smooth PEEK showed the lowest optical density value (0.56 ± 0.07) while rough n-TiO2/PEEK exhibited the highest optical density value (1.21 ± 0.34, P < 0.05). In in vivo studies, the percent bone volume value of n-TiO2/PEEK was approximately twice as large as that of PEEK (P < 0.05). Vivid three-dimensional and histologic images of the newly generated bone on the implants further supported our test results.
Conclusion
Our study demonstrates that n-TiO2 significantly improves the bioactivity of PEEK, especially if it has a rough composite surface. A n-TiO2/PEEK composite with a rough surface could be a novel alternative implant material for orthopedic and dental applications.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Implants of titanium (Ti) and Ti alloy have been widely utilized as orthopedic and dental materials.Citation1–Citation3 However, a number of issues associated with metal implants, including Ti and Ti alloys, have been reported in long-term clinical studies.Citation4–Citation6 Two major issues are stress shielding and local inflammation. Stress shielding is due to stress mismatch between the metal implant material and surrounding bone tissue. Local inflammation is caused by metal implant debris from wear and corrosion. These two issues are considered to be the major causes of bone loss and implant failure.Citation5,Citation6
Poly(ether-ether-ketone) (PEEK), on the other hand, is considered to be one of the best choices to resolve stress shielding issues due to its outstanding biocompatibility and biomechanical properties,Citation6 such as a low modulus, compared with a metal implant and high strength compared with other polymers. However, the bioinert nature of PEEK is not conducive to fast bone cell attachment.Citation7–Citation9 There is a need to improve its bioactivity for orthopedic and dental applications.
The exceptional anticorrosive and biocompatibility properties of Ti and Ti alloy are due to a protective oxide layer (mainly TiO2) which forms rapidly on the Ti surface when it is exposed to the atmosphere.Citation10,Citation11 It was reported that calcium-phosphorus mineralization tended to occur on microgrooved TiO2 surfaces in the initial days.Citation12 Using an arc ion plating technique, a thin microsized TiO2 film was deposited onto a PEEK substrate, which promoted significant adhesion, proliferation, and differentiation of osteoblast cells, compared with a PEEK substrate without TiO2 coating.Citation13
It is believed that TiO2 nanoparticles have higher bioactivity than conventional (micron) particle sizes. When exposed to nanophase TiO2 particles, osteoblasts and chondrocytes show a well spread morphology and increased proliferation compared with cells exposed to particles of conventional size.Citation14 Compared with a micropit titanium surface, a micropit titanium surface with nanonodules promotes significant differentiation and proliferation of osteoblasts in in vitro studies.Citation15 Further, on biomechanical testing of implants, the strength of bone-titanium integration is three times greater for implants with micropits and 300 nm nanonodules than those with micropits alone. A TiO2 nanotube surface significantly accelerates osteoblast adhesion and shows strong bonding with bone.Citation16 TiO2 nanonetwork formation on the Ti surfaces significantly improves human bone marrow mesenchymal stem cell growth in vitro and in vivo.Citation10 Therefore, various kinds of n-TiO2 enhanced polymers have been fabricated for biomaterial applications such as g-TiO2/poly-L-lactide acid nanocompositesCitation17 and poly(lactic-co-glycolic acid)/TiO2 nanoparticle-filled composites.Citation18 It is reported that poly (D, L lactic acid) film containing 20 wt% TiO2 could improve the formation of hydroxyapatite (HA) after 21 days’ exposure to simulated body fluid and increase the relative metabolic activity of MG-63 cells after seven days of incubation.Citation19
All the aforementioned studies suggest that the excellent biocompatibility and bioactivity of n-TiO2 composites is due mainly to the favorable bioactivity of TiO2 nanoparticles in composites and the surface morphology of the TiO2 layer. The aim of this study was to utilize n-TiO2 to improve the bioactivity of PEEK and to investigate the bioactivity of n-TiO2/PEEK composites both in vitro and in vivo. Specific attention was also paid to the biologic effect of n-TiO2 on the composite surface as well as the biologic effect of the surface roughness of the n-TiO2/PEEK composite.
Materials and methods
Sample preparation
PEEK powder was obtained from Victrex (Lancashire, UK) and the TiO2 nanoparticle/PEEK composite (n-TiO2/PEEK) was fabricated by powder mixing and compression molding methodsCitation20 in the Key Laboratory for Ultrafine Material of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai. In this study, the amount of n-TiO2 in the n-TiO2/PEEK composite was 40 wt% (bending modulus 3.8 GPa; bending strength 93 MPa), because a value greater than this would have interfered with the mechanical properties of the composite (data not shown).
In brief, appropriate amounts of n-TiO2 and PEEK powder were codispersed using an electronic blender in alcohol to obtain a homogeneous powder mixture. When well dispersed, the mixture was dried in a forced convection oven at 90°C to remove the excess alcohol. The resulting powder mixture was placed in two specially designed molds, ie, disks (Φ 15 × 2 mm) for physical and chemical characterization and in vitro testing and cylindrical implants (Φ 4 × 7 mm) for in vivo testing. The moulds and powder mixtures were preheated to 150°C under a load of 35 MPa, and the temperature was increased to 400°C under a load of 15 MPa. After reaching the target temperature, the temperature and pressure were held for 10 minutes, and then the heater was turned off and the pressure was released after 10 minutes. The die and the samples were air cooled to 150°C and the samples were removed from the molds.
Disk samples of n-TiO2/PEEK composite were polished to 2000 and tested by a mechanical profilomoter (Dektek8 stylus profiler; Veeco, Plainview, NY). Disk samples with roughness average (Ra) below 0.1 μm were collected and considered as smooth groups. Some disk samples for the smooth groups were blasted by TiO2 particles and tested by profilomoter, and the disks with Ra 1.0–2.2 μm were collected and considered to be the rough groups. Unfilled PEEK samples were cut into the same shapes and prepared according to the same process as the control groups. Surface roughness is thought to be beneficial for the bioactivity of implants, and most implants utilized in the clinic are blasted. Therefore, all implant samples in this study were blasted for the animal experiments. In order to remove any potential free TiO2 nanoparticles, all samples were cleaned with deionized water using an ultrasonic cleaner for 8 hours. The deionized water was changed every 20 minutes during the ultrasonic cleaning process.
Characterization
TEM analysis of n-TiO2/PEEK composite
In order to characterize the n-TiO2 dispersion of the n-TiO2/PEEK composite, thin foil transmission electron microscope (TEM) specimens were prepared by microtome with a diamond knife and imaged using a FEI Tecnai F20 TEM (Philips Electron Optics, Eindhoven, the Netherlands) at 200 KeV.
SEM analysis of n-TiO2/PEEK composites
After treatment, the samples were sputter-coated with gold by a coating device (550X; Quorum, Hampshire, UK), and the surface morphology of the samples was observed using scanning electron microscopy (SEM; S-4800, Hitachi, Tokyo, Japan).
Composite surface analysis by XPS
An X-ray photoelectron spectroscopy (XPS; Axis-Ultra, Kratos Analytical, Manchester, UK) study was carried out for surface chemical analysis using monochromatic AL Kα radiation (225 W, 15 mA, 15 kV) and low-energy electron flooding for charge compensation. Binding energies were calibrated using a C 1 s hydrocarbon peak at 284.8 eV to compensate for surface charge effects. For manipulation and curve-fitting, the data were converted into a VAMAS file format and imported into CasaXPS software package.
Cytocompatibility in vitro
Cell attachment, cytotoxicity, cell morphology, and flow cytometric analysis were evaluated using MG-63 osteoblast cells obtained from the American Type Culture Collection. MG-63 was cultured at 37°C in a humidified, 5% CO2/95% air incubator, in modified Eagle’s medium with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/mL penicillin (Amresco, Cleveland, OH), and 0.1 mg/mL streptomycin (Amresco). Prior to in vitro testing, the samples were sterilized using gamma radiation at a total dose of 25 KGγ. The MG-63 cells were seeded at a density of 1 × 105 cells in each well of 24-well plates for cell attachment testing and at a density of 1.05 × 104 cells/cm2 for the other in vitro tests (n = 6). The culture period for cell attachment was 4 hours. There were three culture periods (3, 7, and 14 days) for cytotoxicity, cell morphology, and flow cytometric analysis.
Cell attachment
After culture on samples for 4 hours, the culture medium was removed and the specimen was rinsed with phosphate-buffered saline three times in order to remove the unattached cells. Cell viability of the adherent cells was measured by adding 1 mL/well culture medium containing 100 μL/well Cell Proliferation Reagent WST-1 (Roche Diagnostics, Mannheim, Germany). Adherent cells were incubated on samples at 37°C for another 4 hours, and 100 μL of culture medium was then transferred into each well of a 96-well plate from each corresponding well of a 24-well plate. Ultraviolet absorbance was measured using an enzyme-linked immunosorbent assay reader at 450 nm with the reference wavelength at 630 nm.
Cytotoxicity
After different culture periods (3, 7, and 14 days) cytotoxicity was evaluated using the WST-1 test on the rough and smooth surfaces of PEEK and n-TiO2/PEEK disks. At fixed detection times, the culture medium was replaced with a new 1 mL/well culture medium containing WST-1 reagent at 100 μL/well. Cells were cultured at 37°C for another 4 hours and ultraviolet absorbance was measured as described above. The relative growth rate was calculated.
Cell morphology
To observe the cell morphology, the samples were washed with phosphate-buffered saline at fixed experimental times (3, 7, and 14 days), and cells on the various materials were fixed with 4% glutaraldehyde in phosphate-buffered saline (pH 7.3) for 30 minutes and dehydrated in a graded series of alcohols. Prior to SEM observation, the cells on the samples were sputter-coated and observed as described above.
Flow cytometric analysis
To investigate the cell cycle of MG-63 osteoblasts further, the cells were stained using propidium iodide (Invitrogen, Carlsbad, CA) and analyzed by flow cytometry (BD FACSCalibur; Becton-Dickson, Franklin Lakes, NJ). In summary, the cells were initially cultured on samples for 3, 7, and 14 days. They were then collected, washed with phosphate-buffered saline (4°C), and resuspended in phosphate-buffered saline. Next, the cells were fixed in 75% ethanol and kept in ethanol at −20°C for 16 hours. Finally, they were collected and washed again, and resuspended in 1 mL RNaseA (1 μg/mL) at room temperature for 30 minutes. The sample cells were pelleted by centrifugation, propidium iodide (Invitrogen) was added, and the cells were incubated for 15 minutes at room temperature, collected, and suspended once again in 1 mL Tris-HCl buffer (pH 7.4), then analyzed by flow cytometry.
Biocompatibility in vivo
Surgery process
Surgical implantation was performed on three beagle dogs aged 1.5 years and weighing 11.4 ± 2.1 kg (mean ± standard deviation). All procedures were conducted according to the ethical principles of the Peking University Institutional Animal Care and Use Committee. The sample implants were sterilized prior to surgery as described above for in vitro testing. General anesthesia was achieved using an intravenous injection of 1% pentobarbital 80 mg/kg. For every composite sample, two cylindrical implants were placed on each animal on the medial surface of each tibia in the proximal diaphyseal region. For each group, ie, PEEK and n-TiO2/PEEK, there were six cylindrical implants placed. After surgery, three fluorochromes (Sigma, St Louis, MO), ie, calcein (100 mg/kg), calcein blue (100 mg/kg), and tetracycline (100 mg/kg) were administered to assess the osteogenic activity at weeks 1, 2, and 4, respectively. The dogs were sacrificed 4 weeks after surgery by an intracardiac injection of 10% kalium chloratum (0.5 mL/kg). The tibias were promptly fixed in 10% formalin and dehydrated in a graded series of alcohols.
Microcomputed tomography analysis
A scanner (1076, Skyscan) was utilized to evaluate all the tibias containing the implant at 40 kV (X-ray source voltage), 200 μA (beam current), 900 msec (exposure time), 9 μm (resolution), 0.8° (rotation step), and 180° (rotation angle). Using the CTan software package (Skyscan), percent bone volume was calculated choosing a serial polygonal region of interest in 100 slices with approximately 1 mm bonding to an implant, which represented the regenerated bone in the marrow only. Radiographs were also synthesized by the CTvol software package (Skyscan) to reconstruct three-dimensional regenerated bone of 0.5 mm width in the marrow bonding to the implants.
Histologic analysis
Following microcomputed tomographic analysis, ultrathin sections were obtained for histologic detection. In brief, the tibias were embedded in methyl methacrylate resin and sectioned after polymerization with a microtome (SP1600; Leica, Wetzlar, Germany) along the longitudinal axis of the cylindrical implants. The tissue sections were ground to a thickness of 30 μm and detected using confocal laser scanning microscopy (LSM710 NLO; Zeiss, Oberkochen, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation (n = 6). Analysis of variance was utilized for calculation of the significance level of the data, and P ≤ 0.05 was accepted as indicating statistical significance. All statistical analyses were carried out using OriginPro 8 SR3 software (OriginLab Corporation, Northampton, MA).
Results
Characterization of materials
TEM analysis of n-TiO2/PEEK composite
Particle size and size distribution as well as dispersion play critical roles in the mechanical performance of nanocomposites as well as their biocompatibility and bioactivity. TEM imaging of our n-TiO2/PEEK nanocomposite also showed relatively uniform dispersion of n-TiO2 in PEEK ().
SEM analysis of n-TiO2/PEEK composite
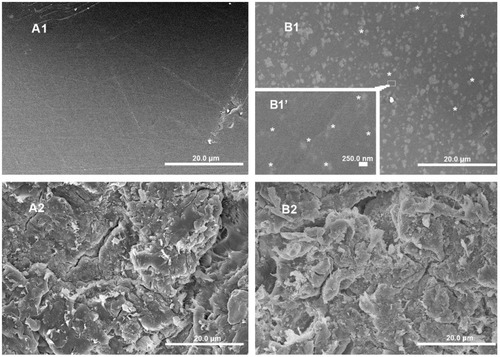
In order to understand the effect of implant surface morphology on cell culture and other biologic responses of PEEK with and without n-TiO2, SEM analysis was carried out on the smooth and rough surfaces of pure PEEK and the n-TiO2/PEEK composite. n-TiO2 particles were visualized on the smooth n-TiO2/PEEK surface (). A dramatic difference in surface morphology/tomography was observed between the smooth and rough sample surface prepared for bioactivity evaluation.
Figure 2 Scanning electron microscopy images of PEEK and n-TiO2/PEEK before and after blasted treatment. (A1) Smooth PEEK, (A2) rough PEEK, (B1) smooth n-TiO2/PEEK, and (B2) rough n-TiO2/PEEK.
Notes: *n-TiO2 particles exposed to the surface of smooth n-TiO2/PEEK. (A1, A2, B1, and B2 bars, 20.0 μm; B1 bar, 250.0 nm).
Abbreviation: PEEK, poly(ether-ether-ketone).
Composite surface analysis by XPS
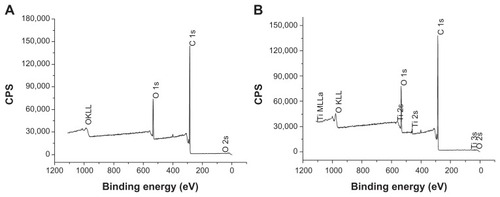
As discussed earlier, distribution of n-TiO2 in the composite, particularly on the surface, has a significant impact on composite tomography as well as bioactivity. XPS analysis demonstrated the presence of Ti on the surface of the n-TiO2/PEEK composite (), indicating the presence of n-TiO2 on the surface.
Cytocompatibility in vitro
Cell attachment
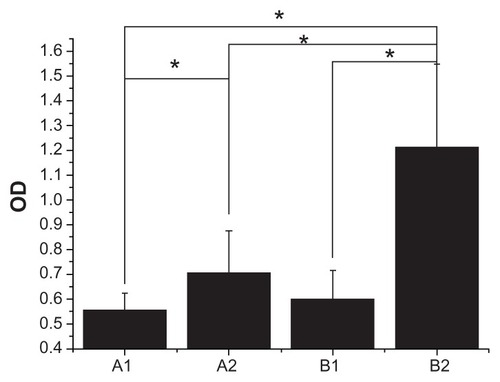
Cell attachment was measured by WST-1 testing after 4 hours of culture of the various samples, and the results are shown in . It is interesting to note that smooth PEEK showed the lowest optical density value (0.56 ± 0.07) among the groups (P < 0.05), while the rough n-TiO2/PEEK group had a significantly higher optical density value (1.21 ± 0.34, P < 0.05), indicating better cell attachment compared with the other groups. The study suggests the presence of n-TiO2 in the composites, especially those with a rough surface, with marked attachment of cells to the implant surfaces.
Cytotoxicity
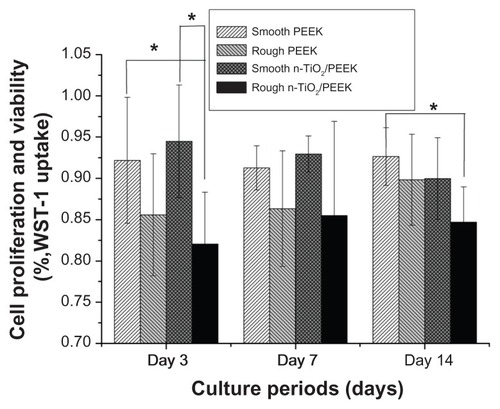
The cytotoxicity of the various materials with smooth or rough surfaces was evaluated in terms of cell proliferation and viability at 3, 7, and 14 days. The relative growth rate in the four groups was more than 80% with cytotoxicity grade 1, suggesting a lack of severe cytotoxicity in both the short term (3 days) and long term (7 and 14 days, ). The smooth n-TiO2/PEEK group showed significantly higher (P < 0.05) cell proliferation and viability in the short term (3 days), compared with rough PEEK. During the short culture period (3 days), the smooth PEEK showed higher cell proliferation and viability (P < 0.05) than the rough n-TiO2/PEEK group.
Cell morphology
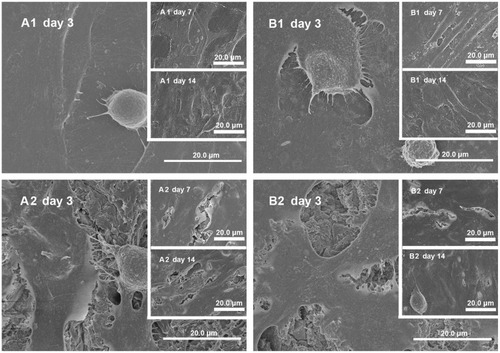
In general, cells in the rough groups seemed to spread more efficiently, with numerous pseudopods (), while cells on the smooth n-TiO2/PEEK exhibited better spreading than the smooth PEEK groups. Pseudopods seemed to prefer to anchor at the areas where n-TiO2 was exposed. After long-term culture (7 and 14 days), no significant difference could be found among the groups.
Figure 6 Cell morphology. (A1), (A2), (B1), and (B2) MG-63 cells cultured on disks of smooth PEEK, rough PEEK, smooth n-TiO2/PEEK and rough n-TiO2/PEEK for 3 days (A1, A2, B1 and B2), 7 days (A1, A2, B1 and B2) and 14 days (A1, A2, B1 and B2), respectively.
Note: Bar 20.0 μm.
Abbreviation: PEEK, poly(ether-ether-ketone).
Flow cytometric analysis
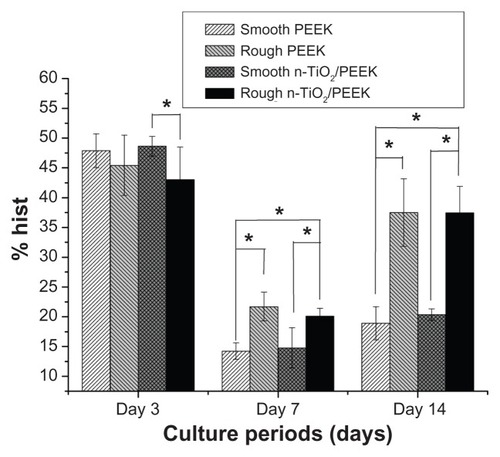
The cell cycle of the MG-63 cultures on the samples was further evaluated by measuring the DNA content of nuclei labeled with propidium iodide, and different cytodieresis phases (G0G1: pre-DNA synthesis of resting, S: DNA synthesis phase, G2M: post-DNA synthesis and mitosis) were analyzed. shows the total percentage of osteoblast MG-63 cells in the S and G2M phases for the four groups after the various culture periods (3, 7, and 14 days). For the short-term culture period (3 days), smooth n-TiO2/PEEK showed a larger percentage of cells in the S and G2M phases compared with the rough group (P < 0.05), with no significant difference found in the other groups. For the long-term culture periods (7 and 14 days), although there was no significant difference found between rough PEEK and rough n-TiO2/PEEK, these two groups showed a larger percentage than the other groups (P < 0.05).
Biocompatibility in vivo
Microcomputed tomography
Microcomputed tomography makes it possible to calculate not only the new bone bonding to the implants in terms of percent of bone volume/tissue volume but also to reconstruct three-dimensional images. As shown in , the bone volume/tissue volume of n-TiO2/PEEK was about twice as large as that of PEEK (P < 0.05). These results could be seen clearly in the three-dimensional reconstructions ().
Histologic analysis
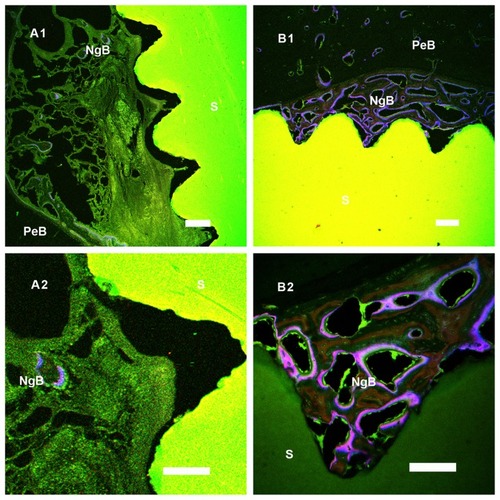
Fluorochrome bone marker labels (calcein, calcein blue, and tetracycline) were clearly observed for bone tissue bonding to the cylindrical implants. More bone deposition and remodeling was seen around the n-TiO2/PEEK implants (), suggesting a greater degree of bone regeneration than around the pure PEEK implants.
Figure 10 In histological analysis, new bone formation around the PEEK and n-TiO2/PEEK were detected by bone labelling (calcein, calcein blue, and tetracycline). Group A was PEEK and group B was n-TiO2/PEEK. Original magnification of (A1) and (B1) was 5× and (A2) and (B2) was 20×.
Notes: A1 and B1 bar, 200 μm; A2 and B2 bar, 100 μm.
Abbreviations: S, sample; NgB, newly grown bone deposition and remodeling zone; PeB, pre-existing bone tissue zone; PEEK, poly(ether-ether-ketone).
Discussion
While PEEK has exceptional biomechanical properties, there is a need to improve further its bioactivity for dental and orthopedic applications. The purpose of this study was to evaluate the in vitro and in vivo bioactivity of n-TiO2/PEEK with different surface morphologies. n-TiO2/PEEK composites were successfully fabricated. They did not cause severe cytotoxicity and there was no significant difference in nuclear DNA content compared with the other two materials. It was found that n-TiO2 significantly improved osteoblast cell attachment, and cells on n-TiO2/PEEK showed better spreading than on pure PEEK. These unique properties of n-TiO2 significantly enhanced new bone regeneration and bonding to the implants during in vivo testing.
In recent years, n-TiO2 particles have attracted considerable attention, not only because of their unique physicochemical properties, especially in biomedical applications, but also because of their inflammatory, proliferative, cytotoxic, and genotoxic effects. The chemistry and surface morphologic effect of the n-TiO2 nanostructure also significantly enhanced the proliferation and differentiation of osteoblasts.Citation1,Citation21,Citation22 A superthin (300 pm–6.3 nm) TiO2 coating fabricated by slow sputter deposition of molten TiO2 nanoparticles is sufficient to enhance biologic properties of microroughened titanium significantly, and at this thickness range it does not alter the previously created microtopography of the titanium surface.Citation1 On the other hand, some reports have shown that various cultured cell models show cytotoxicity when cultured with different doses of free TiO2 nanoparticles,Citation23–Citation25 and animal models have show an inflammatory response and tumorigenesis when exposed to free TiO2 nanoparticles.Citation23,Citation26,Citation27 Free TiO2 nanoparticles induce rapid phosphorylation in polymorphonuclear neutrophils, with a great potential to induce apoptosis.Citation24
Considering the aforementioned studies, we believe that the excellent biocompatibility of n-TiO2 nanocomposites is mainly due to their exceptional bioactivity and the surface morphologic effects of the TiO2 layer. Cytotoxicity, inflammatory response, or tumorigenesis are due to free TiO2 nanoparticles which could break into the intracellular environmentCitation23 and tissues from culture media or by administration via nasal administration, oral administration, or instillation.Citation26,Citation27 It is important to utilize the chemistry and surface morphologic effect of n-TiO2 and to limit free n-TiO2 particles when these particles are used to fabricate biomedical materials, especially orthopedic and dental implant materials.
In this study, a n-TiO2/PEEK composite was fabricated successfully. n-TiO2 could be visualized on the surface of n-TiO2/PEEK composites () which showed appropriate homogeneity throughout (). Elements of n-TiO2 were detected on the composite surface by XPS (). Therefore, the n-TiO2/PEEK composite could also be considered as a superthin n-TiO2 layer exposed on the PEEK surface in a safe way such that n-TiO2 was bound into the PEEK matrix, with only some parts of each nanoparticle exposed to the surface. Several kinds of successfully fabricated PEEK nanocomposites have been reported, such as strontium-containing (Sr)-HA/PEEK, nanosized SiO2/PEEK and nanosized Al2O3/PEEK.Citation20,Citation28 When reinforced with 0–30 vol% Sr-HA, the bending modulus of Sr-HA/PEEK composites increases with the volume ratio of Sr-HA (25 vol%, 9.6 GPa; 30 vol%, 10.6 GPa), while the bending strength of the composites decreases with addition of Sr-HA (25 vol%, 93.8 MPa; 30 vol%, 89.1 MPa).Citation20 The 25 vol% Sr-HA was chosen to produce the composites for in vitro testing, which was due to the desired mechanical performance. In this study, 40 wt% n-TiO2 was chosen to fabricate the n-TiO2/PEEK composite, which was based on an optimization between the bending modulus (3.8 GPa) and bending strength (93 MPa).
In vitro studies showed that n-TiO2 did not cause severe cytotoxicity or disturb cell cycle progression, but improved the bioactivity of PEEK. Although the rough n-TiO2/PEEK group showed lower cell proliferation and viability () than the other groups, n-TiO2 did not cause severe cytotoxicity in the n-TiO2/PEEK nanocomposite, and the relative growth rate of the n-TiO2/PEEK composites was more than 80%. Flow cytometric analysis () showed that n-TiO2 did not disturb cell cycle progression compared with the pure PEEK groups. After long-term culture (for 7 and 14 days), osteoblast cells on both rough PEEK and n-TiO2/PEEK surfaces contained a significantly larger portion in the S and G2M phases compared with cells on the smooth surface. This suggested that the rough-surfaced PEEK was beneficial to the proliferation of osteoblast cells. Other studies have reported that the rough surface of substrate materials improves proliferation and differentiation of osteoblasts.Citation3,Citation21,Citation29,Citation30
Furthermore, in cell attachment testing (), n-TiO2 significantly improved cell attachment to n-TiO2/PEEK compared with pure PEEK. This is consistent with the cell morphology test (). Cells on smooth n-TiO2/PEEK appeared better spread, with numerous pseudopods. The pseudopods of osteoblasts on the smooth n-TiO2/PEEK disks seemed to anchor exposed n-TiO2 particles to the surface of the composite and formed a superthin coating on the PEEK surface, indicating that the surface morphologic effect of n-TiO2 promotes the bioactivity of n-TiO2/PEEK. A similar effect was observed in other studies using n-TiO2. An n-TiO2 superthin coated Ti surface significantly improves osteoblast cell attachment.Citation1 Ti coating on PEEK also enhances cell attachment, proliferation, and differentiation of osteoblasts.Citation7 All of these studies suggest that the chemistry and surface morphologic effect of n-TiO2 particles plays an important role in the exceptional bioactivity of n-TiO2.
Our in vitro test results were consistent with those of in vivo testing. Microcomputed tomography is effective and reproducible for monitoring tumor-associated bone destruction,Citation31,Citation32 analyzing the three-dimensional structure of scaffolds,Citation33 measuring subtle differences in bone growth into three-dimensional scaffolds, and calculating new bone deposition around implants.Citation34,Citation35 In this study, the percent of bone volume/tissue volume of regenerated bone in the marrow bonding to the implants was calculated, and three-dimensional images were constructed. n-TiO2/PEEK showed higher bone volume/tissue volume than pure PEEK (). Three-dimensional () and histologic images () showed the same results, suggesting that n-TiO2 enhances the bioactivity of n-TiO2/PEEK. In terms of the potential risks of free n-TiO2 particles, the novel bioactivity of n-TiO2, such as chemistry and surface morphologic effect, is still attractive. It is important to utilize its outstanding bioactive properties and limit free n-TiO2 when it is used in biomaterials, especially orthopedic and dental implants.
Conclusion
In this study, n-TiO2/PEEK composites were fabricated and their in vitro and in vivo bioactivity was assessed. In in vitro testing, instead of causing cytotoxicity or disturbing cell cycle progression, n-TiO2 improved the bioactivity of PEEK. It promoted cell attachment and improved osteoblast cell spreading. In the in vivo studies, enhanced bone regeneration around the implants by n-TiO2 was indicated by higher bone volume/tissue volume and vivid visualization in three-dimensional and histologic images.
A special effect of n-TiO2 on the composite surface was also observed. Bioactivity evaluation of the nanocomposites revealed that pseudopods of osteoblasts preferred to anchor at areas where n-TiO2 presented at the surface. This study also showed that the surface roughness of n-TiO2/PEEK composites played a prominent role in promoting cell attachment and regeneration of new bones. Our study suggests that n-TiO2/PEEK composites utilize the attractive bioactivity of n-TiO2 as well as the outstanding mechanical properties of PEEK, and could be a potential substitute for metal implant material in orthopedic and dental applications.
Acknowledgments
This work was supported by the State Key Development Program for Basic Research of China (grant 2007CB936103), the Fundamental Research Funds for the Central Universities and Peking University 985 Grant, Nanospecial Program of Science and Technology Development of Shanghai (1052nm06600), and Key Medical Program of Science and Technology Development of Shanghai (09411954900). The authors express appreciation to Dr Dongsheng Wang (Institute of Stomatology, Chinese PLA General Hospital) for his technical assistance in histologic analysis. The authors also thank Xiaohong Wu for assistance in microcomputed tomography analysis, Feilong Nie for assistance with the animal experiments, and Qiuhong Li for assistance with the cell experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- SugitaYIshizakiKIwasaFEffects of pico-to-nanometer-thin TiO2 coating on the biological properties of microroughened titaniumBiomaterials201132338374838421840046
- Taxt-LamolleSFRubertMHaugenHJLyngstadaasSPEllingsenJEMonjoMControlled electro-implementation of fluoride in titanium implant surfaces enhances cortical bone formation and mineralizationActa Biomater2010631025103219778643
- Rausch-fanXQuZWielandMMatejkaMSchedleADifferentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfacesDent Mater200824110211017467048
- BulyRLHuoMHSalvatiEBrienWBansalMTitanium wear debris in failed cemented total hip arthroplasty: An analysis of 71 casesJ Arthroplasty1992733153231402950
- StadelmannVATerrierAPiolettiDPMicrostimulation at the bone-implant interface upregulates osteoclast activation pathwaysBone200842235836418006399
- RamakrishnaSMayerJWintermantelELeongKWBiomedical applications of polymer-composite materials: a reviewCompos Sci Technol200161911891224
- HanCMLeeEJKimHEThe electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological propertiesBiomaterials201031133465347020153890
- WongKLWongCTLiuWCMechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone compositesBiomaterials20093023–243810381719427032
- KurtzSMDevineJNPEEK biomaterials in trauma, orthopedic, and spinal implantsBiomaterials200728324845486917686513
- ChiangCYChiouSHYangWEFormation of TiO2 nano-network on titanium surface increases the human cell growthDent Mater20092581022102919329175
- LongMRackHJTitanium alloys in total joint replacement – a materials science perspectiveBiomaterials19981918162116399839998
- WuLNGengeBRWuthierREMicropatterned TiO2 effects on calcium phosphate mineralizationMater Sci Eng C200929823552359
- TsouH-KHsiehP-YChungC-JTangC-HShyrT-WHeJLLow-temperature deposition of anatase TiO2 on medical grade polyetheretherketone to assist osseous integrationSurf Coat Technol20092046–711211125
- GutweinLGWebsterTJOsteoblast and chondrocyte proliferation in the presence of alumina and titania nanoparticlesJ Nanopart Res200243231238
- KuboKTsukimuraNIwasaFCellular behavior on TiO2 nano-nodular structures in a micro-to-nanoscale hierarchy modelBiomaterials200930295319532919589591
- BrammerKSOhSCobbCJBjurstenLMvan der HeydeHJinSImproved bone-forming functionality on diameter-controlled TiO2 nanotube surfaceActa Biomater2009583215322319447210
- LuXLvXSunZZhengYNanocomposites of poly(l-lactide) and surface-grafted TiO2 nanoparticles: Synthesis and characterizationEur Polym J200844824762481
- TorresFGNazhatSNFadzullahSHMaquetVBoccacciniARMechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle- filled composites for tissue engineering scaffoldsCompos Sci Technol200767611391147
- WeiJChenQZStevensMMRoetherJABoccacciniARBiocompatibility and bioactivity of PDLLA/TiO2 and PDLLA/TiO2/Bioglass® nanocompositesMat Sci Eng C2008281110
- WongKLWongCTLiuWCMechanical properties and in vitro response of strontium-containing hydroxyapatite/polyether ether ketone compositesBiomaterials20093023–243810381719427032
- GittensRAMcLachlanTOlivares-NavarreteRThe effects of combined micron-/submicron-scale surface roughness and nano-scale features on cell proliferation and differentiationBiomaterials201132133395340321310480
- WangNLiHLüWEffects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigsBiomaterials201132296900691121733571
- HuangSChuehPJLinYWShihTSChuangSMDisturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposureToxicol Appl Pharmacol2009241218219419695278
- GonçalvesDMChiassonSGirardDActivation of human neutrophils by titanium dioxide (TiO2) nanoparticlesToxicol In Vitro20102431002100820005940
- BoccacciniARGerhardtLCRebelingSBlakerJJFabrication, characterisation and assessment of bioactivity of poly(D, L lactic acid) (PDLLA)/TiO2 nanocomposite filmsComposites Part A2005366721727
- WangJLiuYJiaoFTime-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticlesToxicology20082541–2829018929619
- WangYWertheimDFJonesASCoombesAGMicro-CT in drug deliveryEur J Pharm Biopharm2000741414919465120
- KuoMCTsaiCMHuangJCChenMPEEK composites reinforced by nano-sized SiO2 and Al2O3 particulatesMater Chem Phys2005901185195
- PeguerolesMAparicioCBosioMSpatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energyActa Biomater20106129130119635598
- ZhaoGRainesALWielandMSchwartzZBoyanBDRequirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topographyBiomaterials200728182821282917368532
- JohnsonLCJohnsonRWMunozSAMundyGRPetersonTESterlingJALongitudinal live animal micro-CT allows for quantitative analysis of tumor-induced bone destructionBone201148114115120685406
- SameiESaundersRSBadeaCTMicro-CT imaging of breast tumors in rodents using a liposomal, nanoparticle contrast agentInt J Nanomedicine20094127728220011244
- JonesACArnsCHSheppardAPHutmacherDWMilthorpeBKKnackstedtMAAssessment of bone ingrowth into porous biomaterials using Micro-CTBiomaterials200728152491250417335896
- AltVLipsKSHenkenbehrensCA new animal model for implant-related infected non-unions after intramedullary fixation of the tibia in rats with fluorescent in situ hybridization of bacteria in bone infectionBone20114851146115321281750
- ZhouWHanCSongYThe performance of bone marrow mesenchymal stem cell-implant complexes prepared by cell sheet engineering techniquesBiomaterials201031123212322120132981