Abstract
Boron nitride nanotubes (BNNTs) have attracted huge attention in many different research fields thanks to their outstanding chemical and physical properties. During recent years, our group has pioneered the use of BNNTs for biomedical applications, first of all assessing their in vitro cytocompatibility on many different cell lines. At this point, in vivo investigations are necessary before proceeding toward realistic developments of the proposed applications. In this communication, we report a pilot toxicological study of BNNTs in rabbits. Animals were injected with a 1 mg/kg BNNT solution and blood tests were performed up to 72 hours after injection. The analyses aimed at evaluating any acute alteration of hematic parameters that could represent evidence of functional impairment in blood, liver, and kidneys. Even if preliminary, the data are highly promising, as they showed no adverse effects on all the evaluated parameters, and therefore suggest the possibility of the realistic application of BNNTs in the biomedical field.
Introduction
Boron nitride nanotubes (BNNTs), similar to carbon nanotubes (CNTs), have attracted wide attention thanks to their potentially unique and important properties in structural and electronic applications.Citation1,Citation2 A BNNT is a structural analog of a CNT, but, despite this similarity, it presents many different chemical and physical properties. For instance, BNNTs display excellent mechanical properties, with a measured Young’s modulus of 1.22 ± 0.24 TPa. Moreover, while CNTs exhibit either a semiconductive or conductive behavior depending on chirality and diameter, BNNTs have only a constant band gap of about 5.5 eV.Citation3 Ab initio calculationsCitation4 and experimental data,Citation5 moreover, demonstrated that they work as excellent piezoelectric systems, with response values larger than those of piezoelectric polymers and comparable to those exhibited by wurtzite semiconductors.
BNNTs have also attracted attention in the fields of nanomedicine, both as nanovectors for drug delivery purposesCitation6 and as intracellular nanotransducers.Citation7 Data on cytocompatibility of these innovative nanovectors come from studies of BNNTs incubated with different cell types, like human embryonic kidney cells, HEK 293; Chinese hamster ovary cells, CHO;Citation8 human osteoblasts or mouse macrophages;Citation9 human neuroblastoma cells, SH-SY5Y;Citation10 mouse myoblasts, C2C12;Citation11 and neuronal-like PC12 cells.Citation7
Results reported by many groups strongly agree about the optimal cytocompatibility of BNNTs, as their administration did not affect cell proliferation, metabolism, and differentiation. However, a recent report by Horvath and coworkers outlined possible adverse effects of BNNTs on fibroblasts, macrophages, and lung cells.Citation12 A possible explanation for such different results may deal with the considerable length (tens of microns) of the tested BNNTs: the toxicity of nanomaterials is, in fact, well-known as being connected to the aspect ratio of tubular nanostructures.Citation13
At this point, as extensive cytocompatibility investigations of BNNTs have been performed, in vivo testing (ie, biocompatibility studies) is fundamental to answer open questions and direct scientific efforts toward realistic applications of BNNTs in the biomedical fields. Here, we report the first toxicology investigation of BNNTs in animals. Although preliminary, our results suggest a good response of the subjects to intravenous injections of BNNT dispersions, with no adverse effects on blood cells, liver, and kidneys.
Materials and methods
Preparation of BNNT dispersions
BNNTs were purchased from the Nano and Ceramic Materials Research Center, Wuhan Institute of Technology, China.Citation14 Details about production and characterization of the samples, and about the preparation of the dispersions, can be found elsewhere.Citation10 Briefly, glycol chitosan (G-chitosan, G7753; Sigma-Aldrich Co, St Louis, MO) was used for the stabilization of BNNTs in aqueous medium, and nanotube noncovalent coating was achieved through sonication at 20 W for 12 hours with a Bransonic Sonicator 2510 (Danbury, CT). In this study, dispersions were prepared in physiological solution (0.9% NaCl; Eurospital, Trieste, Italy) by mixing polymer and BNNTs at a 1:1 (w/w) ratio, thus achieving a final concentration of 1 mg/mL BNNTs, coated with 1 mg/mL of G-chitosan. Before injection, dispersions were autoclaved for sterilization.
Characterization of BNNT dispersions
Microphotographs of the final dispersions of BNNTs were obtained through focused-ion beam microscopy (FIB) with a FEI 200 microscope (FEI HQ, Hillsboro, OR). Transmission electron microscopy (TEM) was performed with a Zeiss 902 TEM (Carl Zeiss, GmbH, Germany) dropping a small quantity of BNNT aqueous suspension (diluted 1000×) on a copper grid. For atomic force microscopy (AFM) imaging, 50 μL of BNNT solution (diluted 2500×) was deposited on freshly cleaved, highly ordered pyrolitic graphite (HOPG; Bruker AXS, Inc, Madison, WI). Graphite substrates were incubated for 6 minutes and rinsed five times with 100 μL of pure water. Samples were then vacuum dried using a Laboport vacuum pump (KNF Neuberger, Inc, Trenton, NJ). Imaging was performed on a Multimode scanner with a Nanoscope V controller, using the Scan-Assyst mode (Bruker AXS). Imaging scan rate was 1.95 Hz for a scan size of 250 nm. Images were recorded at 512 × 512 pixels and stripe noise was evaluated using the DeStripe server.Citation15 V-shaped silicon nitride cantilevers with silicon tips (SNL; Bruker AXS) with a nominal spring constant of 0.35 N/m were used. Imaging forces were maintained below 100 pN.
Particle size distribution and Z-potential of the dispersions were analyzed with a Nano Z-Sizer 90 (Malvern Instruments Ltd, Malvern, UK). For both analyses, each acquisition was performed three times, using samples appropriately diluted in physiological solution.
Animal treatment
Five male New Zealand rabbits, aged 8–9 months, and weighing 2.0 ± 0.1 kg, were used in the present study. Animal care and handling were performed according to the provisions of Council Directive 86/609 EEC, recognised and adopted by the Italian Government (DL 27/1/1992, n 16). The experimental protocol was approved by the Ethics Committee of the University of Pisa and by the Italian Ministry of Health (authorization n 4886, 04/04/2011). Animal health was clinically assessed through physical examination and complete hematological analyses before experimental sessions.
Rabbits were housed in single cages, under conventional conditions of ventilation, temperature (18°C–22°C) and lighting (16-hour light:day cycle). Animals were allowed to adjust to their environment for 1 week. Food and water were available ad libitum during the whole trial. Animals had a diet of complete premixed food for rabbits (Consorzio Agrario della Maremma Toscana, Grosseto, Italy) and alfalfa hay. For the whole experimental period, general rabbit health was daily monitored by qualified personnel supervised by a veterinary physician.
Animals were randomly assigned to two groups: a control group (I, n = 2) and an experimental group (II, n = 3). Each subject in group I and group II received a single intravenous dose (1 mg/kg) of plain G-chitosan and G-chitosan-coated BNNTs in the morning. The two solutions, having a concentration of 1 mg/mL, allowed for injections of about 2 mL volume. Solutions were slowly injected into the marginal ear vein of animals placed in restraining cages. Objective symptoms such as sweating, excitement, trembling, and head nodding were scored. Body temperature was also assessed during the study. Blood samples were collected for analysis at intervals of 0, 2, 24 and 72 hours by the contralateral marginal ear vein used for administration, and they were placed in collection tubes containing appropriate anticoagulants. Blood samples were processed and analyzed at the Biochemistry Clinical Veterinary Laboratory of the Veterinary Teaching Hospital, Pisa, Italy, within 2 hours of collection.
Results and discussion
BNNTs were produced by using an annealing method from boron-containing precursors. Details of sample, provided by the supplier included a boron nitride content > 98.5 wt% and yield of the procedure > 80%. A more detailed characterization of BNNTs and of their dispersions was reported in a previous work.Citation10
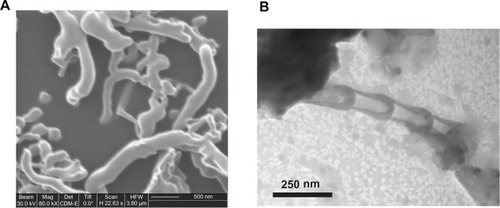
shows the results of FIB and TEM imaging on the G-chitosan-coated BNNTs used in animal experiments. The presence of tubular structures with random orientation and quite dispersed size distribution (tube diameters were between 30 nm and 100 nm and lengths between 0.5 μm and 2.0 μm) can be seen in the FIB image (), whereas the typical bamboo-like structure of BNNTs,Citation16 associated with a thin G-chitosan coating (around the BNNT walls), can be appreciated in the TEM image ().
Figure 1 (A) Focused-ion beam microscopy image of G-chitosan-coated boron nitride nanotubes (BNNTs) and (B) transmission electron microscopy analysis showing the typical BNNT structure.
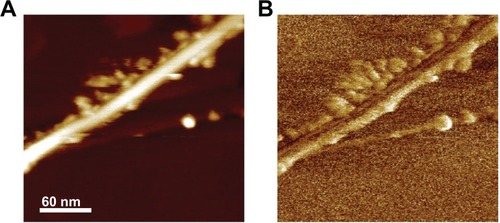
AFM imaging shows an isolated nanotube: in the height image (), the nanotube appears as a bright line with edges decorated by globular structures, whereas it appears as a dark line decorated by brighter globular structures in the phase image (). The latter once more confirms the efficiency of G-chitosan-coating procedure: the phase delay introduced by the globular structures, which can be appreciated through the brighter color, is due to the G-chitosan lower stiffness with respect to that of BNNTs, which in fact display a darker color.Citation17
Figure 2 (A) Height and (B) phase atomic force microscopy images of G-chitosan coated boron nitride nanotubes.
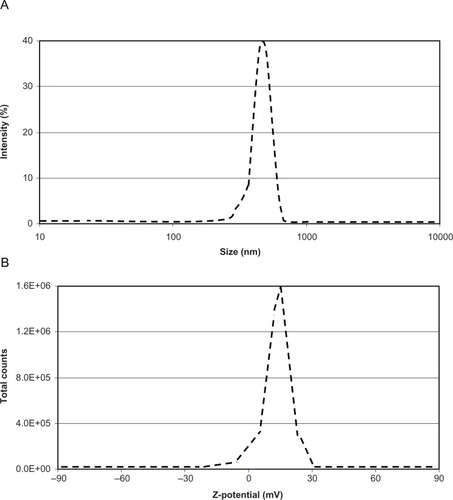
To characterize BNNT assembling in aqueous medium and to gain an idea of the actual sizes of the nanocomplexes in the injected dispersions (FIB, TEM, and AFM analyses were carried out on dried samples), dynamic light scattering and Z-potential analyses were performed in physiological solution. Dynamic light scattering revealed a 96.5% peak at a size of about 560 nm, with a polydispersion index of 0.250 (). Moreover, a Z-potential of about 15 mV denoted a good stability of the obtained dispersion ().
Figure 3 (A) Size distribution and (B) Z-potential analyses of the injected boron nitride nanotube dispersions.
During the whole study period, neither unusual behavior, such as sweating, excitement, trembling, and head nodding, nor differences could be observed between the two groups. Moreover, no significant changes in body temperature were observed up to 72 hours following the injection.
Results on hematological analyses are reported in detail in . Typical blood values (white cell count, red cell count, platelet count, etc) are not significantly different between control (I, ie, treated with G-chitosan) and experimental (II, ie, treated with G-chitosan-coated BNNTs) groups. Only platelet count seems to be increased at 72 hours after injection in the BNNT-treated group, but this value is still in the healthy range tabulated for rabbits.Citation18
Table 1 Blood analyses to evaluate hematic parameters and liver and kidney functionality
Blood biochemical parameters quantifying both renal (urea and creatinine) and hepatic (alkaline phosphatase, γ-glutamyl transferase, aspartate transferase, and alanine amino transferase) functions were also satisfactory: no substantial differences can be appreciated between the two groups at each time-point analysis.
Conclusion
This study demonstrates the first in vivo toxicity evaluation of BNNTs following intravenous injection. Single administrations of high doses did not produce evident toxicity in rabbits within the 72 hours following injection. Because of the small number of animals, our findings must be considered preliminary; nonetheless, the absence of negative effects on blood parameters, as well as liver and kidney functions is highly promising for further biocompatibility exploration of BNNTs. Future investigations will consist of dose-response and long-term studies. Particular attention will be also given to the investigation of the BNNT biodistribution and pharmacokinetics that are strictly dependent on the physicochemical characteristics of the nanoparticles, such as size, shape, aggregation, chemical composition, surface functionalizations, and solubility.Citation19
Acknowledgements
AFM imaging was performed during the 3rd International AFMBioMed summer school in Marcoule in cooperation with the European COST Action TD1002 (Chair: Dr Pierre Parot). The authors thank Dr Matilde Masini, Department of Experimental Pathology BMIE, University of Pisa, Pisa, Italy, for TEM technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChopraNGLuykenRJCherreyKBoron nitride nanotubesScience1995269522696696717807732
- TerronesMRomo-HerreraJMCruz-SilvaEPure and doped boron nitride nanotubesMaterials Today2007103038
- GolbergDBandoYTangCZhiCBoron nitride nanotubesAdv Mater20071924132432
- DaiYGuoWZhangZZhouBTangCElectric-field-induced deformation in boron nitride nanotubesJ Phys D Appl Phys200942085403
- BaiXDGolbergDBandoYZhiCYTangCMitomeMDeformation-driven electrical transport of individual boron nitride nanotubesNano Lett20077363263717288485
- CiofaniGPotential applications of boron nitride nanotubes as drug delivery systemsExpert Opin Drug Deliv20107888989320632897
- CiofaniGDantiSD’AlessandroDEnhancement of neurite outgrowth in neuronal-like cells following boron nitride nanotube-mediated stimulationACS Nano20104106267627720925390
- ChenXWuPRousseasMBoron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cellsJ Am Chem Soc200913189089119119844
- LahiriDRouzaudFRichardTBoron nitride nanotube reinforced polylactide-polycaprolactone copolymer composite: mechanical properties and cytocompatibility with osteoblasts and macrophages in vitroActa Biomater2010693524353320226282
- CiofaniGDantiSD’AlessandroDMoscatoSMenciassiAAssessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assayBiochem Biophys Res Commun2010394240541120226164
- CiofaniGRicottiLDantiSInvestigation of interactions between poly-L-lysine-coated boron nitride nanotubes and C2C12 cells: up-take, cytocompatibility, and differentiationInt J Nanomedicine2010528529820463944
- HorváthLMagrezAGolbergDIn vitro investigation of the cellular toxicity of boron nitride nanotubesACS Nano2011553800381021495683
- PolandCADuffinRKinlochICarbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot studyNat Nanotechnol2008342342818654567
- WangJGuYZhangLZhaoGZhangZSynthesis of boron nitride nanotubes by self-propagation high-temperature synthesis and annealing methodJ Nanomater2010 article ID:540456.
- ChenSWPellequerJLDeStripe: frequency-based algorithm for removing stripe noises from AFM imagesBMC Struct Biol201111721281524
- TangCCLamy de la ChapelleMLiPLiuYMDangHYFanSSCatalytic growth of nanotube and nanobamboo structures of boron nitrideChem Phys Lett20013425–6492496
- JandtKAtomic force microscopy of biomaterials surfaces and interfacesSurface Science2001491303332
- MooreDMHematology of rabbits and hematology of the guinea pigFeldmanBFZinklJGJainNCSchalm’s Veterinary Hematology5th edBaltimore, MDLippincott Williams & Wilkins200011001110
- LacerdaLBiancoAPratoMKostarelosKCarbon nanotubes as nanomedicines: from toxicology to pharmacologyAdv Drug Deliv Rev200658141460147017113677