Abstract
A nanohybrid was prepared with an inorganic clay material, montmorillonite (MMT), for taste masking of sildenafil (SDN). To further improve the taste-masking efficiency and enhance the drug-release rate, we coated the nanohybrid of SDN–MMT with a basic polymer, polyvinylacetal diethylaminoacetate (AEA). Powder X-ray diffraction and Fourier transform infrared experiments showed that SDN was successfully intercalated into the interlayer space of MMT. The AEA-coated SDN–MMT nanohybrid showed drug release was much suppressed at neutral pH (release rate, 4.70 ± 0.53%), suggesting a potential for drug taste masking at the buccal cavity. We also performed in vitro drug release experiments in a simulated gastric fluid (pH = 1.2) and compared the drug-release profiles of AEA-coated SDN–MMT and Viagra®, an approved dosage form of SDN. As a result, about 90% of SDN was released from the AEA-coated SDN–MMT during the first 2 hours while almost 100% of drug was released from Viagra®. However, an in vivo experiment showed that the AEA-coated SDN–MMT exhibited higher drug exposure than Viagra®. For the AEA-coated SDN–MMT, the area under the plasma concentration– time curve from 0 hours to infinity (AUC0-∞) and maximum concentration (Cmax) were 78.8 ± 2.32 μg · hour/mL and 12.4 ± 0.673 μg/mL, respectively, both of which were larger than those obtained with Viagra® (AUC0-∞ = 69.2 ± 3.19 μg · hour/mL; Cmax = 10.5 ± 0.641 μg/mL). Therefore, we concluded that the MMT-based nanohybrid is a promising delivery system for taste masking of SDN with possibly improved drug exposure.
Introduction
There have been many attempts to explore new nanohybrids with the desired physicochemical and biological properties, such as inorganic–organic nanohybrids with two-dimensional limited high-temperature superconductivity,Citation1 bio–inorganic nanohybrids with enhanced gene delivery characteristics,Citation2–Citation4 intravenous injectable drug-delivery systems,Citation5 and various therapeutic agent-delivery systems with high efficiency.Citation6–Citation13 Among various inorganic nanohybrids, the most widely studied for pharmaceutical application were smectite clays as formulation additives. For taste masking in this study, we employed a smectite clay material that can swell and encase a drug.
The unpleasant taste of drugs has been one of the major limitations for designing oral drug formulations since swallowing a tablet may be the only option for the drug to bypass taste perception in the buccal cavity. However, such methods for drug administration still require the patients’ cooperation to a large extent and may be inconvenient, especially for drugs needing punctual or sudden dosing schedules.
For this reason, sildenafil (SDN) citrate may be a drug that needs a delivery strategy other than a tablet formulation for swallowing. SDN, an inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase type 5, has been used for treatment of erectile dysfunction and is already approved as a tablet dosage form for oral delivery (Viagra®; Pfizer, New York, NY). The nature of this specific drug therapy implies that the dosing moments are mostly unexpected or sudden, and for patient convenience, an easier administration method other than swallowing is needed. Redesigns of the formulations, such as chewable or dissolvable tablets,Citation14–Citation17 or rapidly dissolving film,Citation18–Citation20 would be desirable, but may not be practical for SDN delivery due to its unpleasant taste.Citation21
Therefore, taste masking of SDN may be needed for orally disintegrating formulations to ensure patient compliance and improve acceptability of medication. Previously, numerous attempts have been made in the field of taste masking, where most approaches were based on encapsulation of drugs with polymeric materials, such as cellulose, Eudragit® (Evonik Industries AG, Essen, Germany), or polyethylene glycol, among others.Citation22–Citation24 The purpose of encapsulation was primarily focused on suppressed release of drugs in biological fluids with no (or almost no) free drug molecules available at the buccal cavity. However, for those conventional approaches, the diffusion barrier formed by polymeric materials would still be in effect while the formulations pass through the gastrointestinal tract, where the drug release would be undesirably suppressed. This is not beneficial, especially for SDN delivery, since the drug requires rapid systemic absorption for its own therapeutic purpose. To resolve this problem, the delivery profile of SDN should be specifically tailored: the drug release should be highly suppressed at the buccal cavity and then released rapidly in the gastrointestinal fluid.
Some previous studies utilized polymers with pH-dependent solubility as an encapsulant, where the barrier polymer dissolves only at low pH, with controlled release of the drug in the gastric cavity, not in the buccal cavity.Citation25–Citation27 Although this selective delivery profile may benefit from both taste masking and rapid drug release to some extent, the formulations may not yet be optimized for the following reasons. The drug molecules may be distributed on the surface of the polymeric encapsulant slightly, which may still cause a bitter taste. The delayed dissolution of polymers may still inhibit rapid onset of drug release and the systemic absorption of drugs may be retarded altogether.
In this study, we prepared a bentonite-based nanohybrid coated with an acid-soluble polymer to better achieve both taste masking and rapid delivery of SDN. Bentonite, mainly composed of montmorillonite (MMT), consists of tetrahedral sheets of SiO4 units and octahedral sheets of Al3+ ions.Citation28,Citation29 The isomorphous substitution of Al3+ with Mg2+ can generate a negative surface charge on bentonite. To balance the excess negative charge, the interlayer cations are stabilized within the layers and as a result, bentonite possesses cation-exchange capacity (CEC). Therefore, the cationic drug SDN could be encased in the layered space of MMT with strong ionic bonding, producing an SDN–MMT nanohybrid with taste-masking functionality. The nanohybrid was also coated with an acid-soluble polymer, polyvinylacetal diethylaminoacetate (AEA), to further prevent drug release in the buccal cavity and aid rapid release in the stomach. For the latter, we reported that the presence of a large molecule possessing the same polarity as the intercalated compound facilitated the diffusion of the intercalated compound by enlarging the entrance of layered structures of MMT.Citation30–Citation32 The carrier material, MMT clay, employed in this work is approved by the US Food and Drug Administration (FDA) as a diluting agent for oral delivery and is widely used in medicine and pharmacology.Citation33,Citation34 The polymer used for coating, AEA, is also accepted as a food additive by the FDA.Citation35
In this study, we developed an SDN–MMT nanohybrid with taste-masking functionality and compared its drug-release profile with that of Viagra®, an SDN medication available on the market. We aimed to prove that our SDN–MMT nanohybrid formulation was advantageous in its therapeutic efficacy as well as in administration strategy. Our SDN–MMT nanohybrid was characterized with powder X-ray diffraction (PXRD), Fourier transform infrared (FT-IR) spectra, and thermogravimetry (TG) analysis to examine the properties of SDN intercalated in MMT. The release profiles of SDN with both noncoated and AEA-coated SDN–MMT nanohybrids were also examined in simulated biological fluids, using high-performance liquid chromatography (HPLC). To assess the drug bioavailability, the SDN–MMT formulations were compared with Viagra® through both in vitro and in vivo experiments.
Material and methods
Materials
Montmorillonite (MMT, Kunipia-F; CEC = 110 mequiv/100 g) and polyvinylacetal diethylaminoacetate (AEA) were obtained from Kunimine Industries Co, Ltd (Tokyo, Japan) and Sankyo (Tokyo, Japan), respectively and used without further purification. Sildenafil (SDN) was a kind gift from Daewoong (Seoul, Korea). Acetonitrile, methanol, and glacial acetic acid of high performance liquid chromatography (HPLC) grade were purchased from JT Baker (Cleveland, OH).
Preparation of SDN–MMT and AEA-coated SDN–MMT
The SDN–MMT nanohybrid was prepared by ion exchange reaction. Briefly, 20 g MMT was dispersed in 2 L deionized water for 3 hours at room temperature to give a 1.0 wt% MMT suspension. To prepare an SDN solution, 14.7 g SDN, which is equivalent to the CEC of 20 g MMT, was first dissolved in 1200 mL of a 1% phosphoric acid solution, and 800 mL ethanol was added afterwards. The resulting solution was then mixed with the MMT suspension and stirred for 4 hours at room temperature to facilitate an ion exchange reaction. The solid product, SDN–MMT, was then filtered and washed twice with 50% ethanol solution and pure ethanol, respectively, to remove the residual drug. The resulting SDN–MMT slurry (200 g) was dispersed again in a mixture of methylene chloride (MC) and ethanol (MC: ethanol, 200 mL: 400 mL, v/v) and spray-dried with an SD-1000 spray dryer (Eyela, Tokyo, Japan) under the following conditions: inlet temperature, 80°C; blower speed, 0.30 m3/minute, and atomizing pressure, 125 ± 5 kPa.
To prepare the AEA-coated SDN–MMT, the SDN–MMT slurry (200 g) was dispersed in a mixture of MC and ethanol (MC: ethanol, 200 mL: 400 mL, v/v), where 10 g AEA was dissolved. The resulting suspension was then spray-dried with a spray dryer under the same conditions stated above.
Characterization of nanohybrids
The PXRD patterns of both SDN–MMT and AEA-coated SDN–MMT were collected using a Rigaku D/MAX-2200 Ultima diffractometer (Rigaku International Corporation, Tokyo, Japan) equipped with Ni-filtered Cu-Kα radiation (λ = 1.5418 Å). The patterns were recorded at 40 kV and 30 mA. TG analysis was performed at a heating rate of 5°C/minute from room temperature to 1000°C under ambient atmosphere (SDT Q600; TA Instruments, New Castle, DE). FT-IR spectra (Figure S1) were recorded with a JASCO FT/IR-6100 spectrophotometer (JASCO, Easton, MA) by the standard KBr disk method. The particle size (Figure S2) of SDN–MMT and AEA-coated SDN–MMT were obtained with dynamic light-scattering method (ZetaSizer; Nano ZetaSizer, Malvern Instruments, Malvern, UK).
Determination of SDN content
To determine the amount of SDN, SDN needs to be completely extracted from the SDN–MMT nanohybrids. Thus, the solution was first prepared with an aqueous solution buffered at pH 4.5 (KH2PO4), acetonitrile and phosphoric acid (400:600:1, v/v/v). Then, each of the samples containing the equivalent amount of 6 mg SDN was dispersed in 100 mL of the resulting solution and sonicated for 40 minutes. The suspension was filtered by a polypropylene membrane with a pore size of 0.45 μm (Pall, Port Washington, NY), which was then measured with HPLC (1100 Series Instrument; Agilent Technologies, Santa Clara, CA) using a column, Zorbax Eclipse XDB-C18 (4.6 mm × 250 mm, 5 μm; Agilent Technologies). The mobile phase was prepared with an aqueous solution buffered at pH 4.5 (KH2PO4) and acetonitrile (40:60, v/v). The samples were analyzed under the following conditions: flow rate, 1 mL/minute; injection volume, 10 μL; column temperature, 35°C; and UV wavelength, 230 nm.
In vitro drug release experiment
The in vitro drug release tests were conducted with intact SDN, SDN–MMT, AEA-coated SDN–MMT, and Viagra®, following the paddle-stirring method using a DST-810 dissolution tester (LabFine, Seoul, Korea).Citation36 The bath temperature was maintained at 37°C ± 0.5°C and the impeller was set at 50 rpm. Each sample containing the equivalent amount of 50 mg SDN was dissolved in 900 mL of the release media, the aliquot of which was sampled at scheduled intervals. The aliquot was then filtered with a polypropylene membrane with a pore size of 0.45 μm (Pall), which was measured with the HPLC as described above in ‘Determination of SDN content’. In this work, two distinct release media were employed to simulate the buccal and gastric cavities, respectively. To simulate the buccal condition and evaluate the taste-masking ability of the nanohybrids, the release test was performed at neutral pH for 2 minutes, following the International Pharmaceutical Federation/American Association of Pharmaceutical Sciences guidelines.Citation37 To simulate the gastric conditions, the release test was performed at pH 1.2 for 2 hours.
In vivo experiment
Preparation of standard solutions and animals
The stock solution of SDN was prepared at a concentration of 50 μg/mL in methanol and was further diluted to 0.1~4.0 μg/mL for plasma calibration standards. The solutions of six different concentrations, covering the expected ranges of 2~200 ng/mL, were prepared. Six male beagle dogs, 8.5 months old and weighing 12~15 kg, were purchased from Central Lab Animal (Seoul, Korea). The animals were fasted overnight with free access to water. Viagra® or AEA-coated SDN–MMT was orally administered to each dog at a dose of 20 mg/kg (n = 3). The SDN dose was selected on the basis of the pharmacological and pharmacokinetic data published previously.Citation38 The Viagra® tablets were ground into powder, which was weighed to match the dose of SDN. OASIS HLB 96-well plates (10 mg; Waters Corporation, Milford, MA) were utilized for solid-phase extraction (SPE) of the analytes in plasma samples. In all instances, animals were humanely handled in accordance with Institutional Animal Care and Use Committee guidelines.
Analytical method development
Blood sampling and HPLC-ESI-MS/MS analyses
Approximately 5 mL of blood was collected from a right front leg vein at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16, and 24 hours after oral dosing. The cannula was filled with heparinized saline (500 IU mL−1) to prevent blood clotting, and then the blood samples were taken after centrifugation and stored at −70°C until analysis.
An OASIS HLB 96-well SPE plate (Waters Corporation) was used for the extraction of the analyte from the plasma samples. Aliquots of 200 μL of 6% perchloric acid were added into the 300 μL of blank or plasma sample in a 1.5 mL microtube to precipitate the protein. The mixture was vortexed for 10 seconds, then centrifuged at 15,000 × g for 10 minutes. The supernatant was immediately loaded onto each well of OASIS HLB plate, previously conditioned with 600 μL of methanol followed by 600 μL of water. The whole wells were washed with 600 μL of water and then air-dried for about 30 seconds. The attached analyte was eluted twice with 200 μL of 5% methanolic ammonium hydroxide (conc NH4OH/methanol, 5:95, v/v), then evaporated to dryness at room temperature by vacuum centrifugation. The residues were reconstituted to 200 μL with methanol, and a twentieth (10 μL) of the solution was subjected to HPLC-electrospray ionization mass spectrometry/mass spectrometry (ESI-MS/MS) analysis.
All HPLC-ESI-MS/MS experiments were carried out using a Waters/Micromass Quattro micro/MS interface consisting of a Waters 2695 separation module connected directly to a Micromass Quattro micro/MS (Waters Corporation). Separation was performed on a 30 mm × 2.1 mm Xterra MS C18 (3.5 μm; Waters Corporation) reversed-phase column. The analytes were eluted at a flow rate of 180 μL/minute with an isocratic system of 24% (v/v) aqueous acetonitrile with 0.1% acetic acid for 7 minutes. The column effluent was directed to the ESI-MS, which was operated in the positive ion mode without splitting. The instrument was tuned by the direct infusion of a stock solution of SDN (2 μg/mL in methanol) in the ion source at 40 μL/minute. The optimization parameters of the ESI-MS/MS system were based on the maximum generation, first of the protonated molecular (parent) ions, and then of the corresponding fragment (product) ions. The following tuning parameters were retained for the optimum ESI-MS/MS detection of SDN: the capillary voltage and cone voltage were 2 kV and 35 V, respectively; the source and desolvation temperatures were 130°C and 250°C, respectively; the desolvation gas and cone gas flow rates were 500 L/hour and 50 L/hour, respectively. The collision energy in the MS/MS mode, concurring with full argon-induced fragmentation of the parent molecules, was found to be 0.27 V. The quantification of SDN was conducted using MS/MS in multiple reaction monitoring (MRM) mode. This was done by choosing the two mass ions set to detect a transition of the parent ion to the product ion specific to SDN (474.7 > 99.9). The molecular structures and major fragmentation pattern for SDN are shown in .
HPLC-ESI-MS/MS analytical method validation for SDN in in vivo samples
Linearity of calibration was assessed by analyzing six standards ranging from 2~200 ng/mL in plasma. The calibration curve was based on drug peak area and was analyzed by weighted linear regression using the Sigmaplot program (v. 8.0; SPSS Inc, Chicago, IL). The correlation coefficient was calculated. The limit of quantification (LOQ) was defined as the drug concentration producing at least ten times the response compared to the blank response (S/N > 10). Quality control (QC) samples were prepared at low (5 ng/mL), medium (50 ng/mL), and high concentration (150 ng/mL) in the same way as the plasma calibration samples. Intra- and Interday precision and accuracy were assessed by analyzing the above-mentioned QC samples with five replicates on three different days, and they were presented as relative standard deviations (RSD). Recoveries of the analytes spiked into plasma samples at three different concentrations (2, 20, and 100 ng/mL) were calculated by comparing the peak area of the extracted sample to that of the unextracted standard solution prepared with the same solvent, and each experiment at the same concentrations was done in triplicate. Stability of SDN was conducted using QC samples after three freeze–thaw cycles, at room temperature, and after post-preparation procedures.
Pharmacokinetic study
Pharmacokinetic parameters were determined from the plasma SDN concentration–time data by noncompartmental analysis using WinNonlin Professional software (v. 2.0; Pharsight Co, Mountain View, CA). The pharmacokinetic parameters estimated from the data were area under the plasma concentration–time curve from 0 hours to infinity (AUC0-∞), elimination half-life (t1/2), apparent volume of distribution (Vd/F), and total clearance (Cl/F). The maximum plasma concentration (Cmax) and the time required to reach Cmax (Tmax) were determined from the individual plasma concentration–time curve by visual inspection.
Statistical analysis
All the results were presented as means ± standard deviations. An unpaired Student’s t-test was used to determine significant differences between the SDN and AEA-coated nanohybrid data. Differences were considered to be significant when P < 0.05.
Stability test
The shelf-life of SDN for SDN–MMT and AEA-coated SDN–MMT nanohybrids were examined through an accelerated stability test.Citation39 Each of the samples was put in a glass vial and sealed, which was then placed in a constant temperature and humidity chamber (LH-1000; New Power Engineering, Seoul, Korea) for 6 months. The temperature and humidity were maintained at 40°C and 75%, respectively. The amount of nondegraded SDN was measured with HPLC as described above in “Determination of SDN content”. For each of the samples, the fraction of nondegraded SDN was calculated by percentage based on the SDN content measured initially before the stability test.
Results and discussion
Powder X-ray diffraction analysis
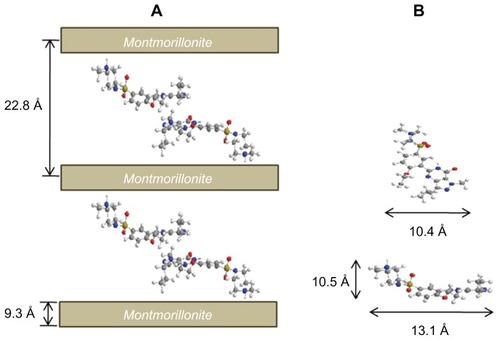
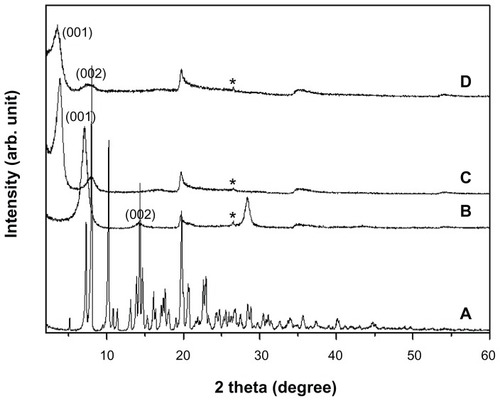
shows the PXRD patterns of intact SDN, pristine MMT, SDN–MMT, and AEA-coated SDN–MMT. For pristine MMT, the characteristic peak of (001) was clearly seen at 6.9° (), which was shifted to 3.8° for both SDN–MMT and AEA-coated SDN–MMT nanohybrids (). This could be ascribed to the expanded basal spacing of MMT from 12.5 Å to 22.8 Å after intercalation of SDN. There was no difference in the PXRD patterns between the SDN–MMT and the AEA-coated SDN–MMT nanohybrids, implying that SDN still resided in the interlayer space of the MMT after the AEA coating. The peaks of intact SDN (namely, crystalline SDN) were not observed with SDN–MMT and AEA-coated SDN–MMT nanohybrids, indicating that SDN molecules were distributed in the MMT interlayer space with a molecular level as reported previously in other nanohybrid systems.Citation32,Citation40
Figure 2 Powder X-ray diffraction patterns of (A) sildenafil citrate, (B) montmorillonite (MMT), (C) sildenafil–montmorillonite (SDN–MMT), and (D) polyvinylacetal diethylaminoacetate (AEA)-coated SDN–MMT.
Note: *Quartz.
Subtracting the layer thickness of MMT (9.3 Å) from the basal spacing of SDN–MMT, the gallery height was estimated to be 13.5 Å. Considering that the longitudinal and lateral molecular dimension of SDN are 5 Å and 16 Å, respectively, it became very likely that the SDN molecules should be stabilized in a double-layer arrangement. To explain this, the steric limitations between the intercalated SDN and charged sites of MMT layers were examined. The steric limitations are generally expressed by the equivalent area (Ae) of clay lattices and the area demand (Ac) of intercalated molecules. The Ae can be estimated from the equation Ae = ab/2ξ, where a and b are lattice parameters and ξ is the layer charge. The Ae of MMT was estimated to be 28.85 Å2, calculated using a negative charge density, 0.27e−/46.5 Å2.Citation41 The area demand (Ac) of SDN was about 136 Å2, calculated from the molecular model in . Thus, since Ac is larger than 2Ae, SDN molecules should have a tilted bilayer arrangement to avoid steric hindrance.Citation31,Citation42 The protonation sites of SDN should be only at N22 as shown in since protonation in combined rings of pyrimidine and pyrazole is unlikely due to resonance and steric effects.Citation43 As a result, the SDN molecules should be arranged in the interlayer space of MMT as depicted in .
Thermogravimetric analysis
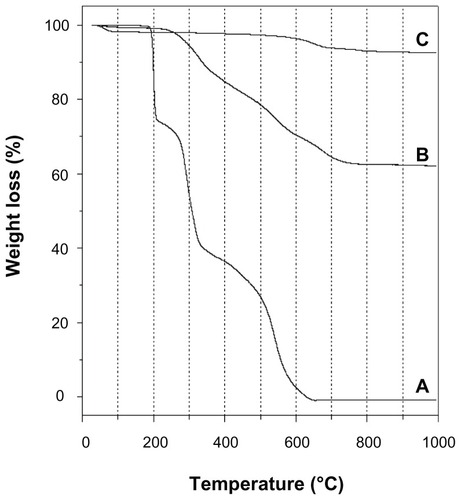
depicts the TG curves of pristine MMT, intact SDN, and SDN–MMT. For pristine MMT, the first weight loss up to 100°C was due to the evaporation of water adsorbed on the MMT surface (). The weight loss observed at 600°C was attributed to dehydroxylation and phase transition of MMT. The intact SDN exhibited three consecutive weight losses (). The first weight loss at 188°C was due to the decomposition of citrate and the second weight loss at 270°C was ascribed to the decomposition of the SDN molecule itself. The intact SDN was completely decomposed above 326°C, corresponding to the last weight loss. For SDN–MMT (), the weight loss due to dehydration was seen up to 200°C. The following weight loss from 250°C to 750°C was due to the decomposition of intercalated SDN. Interestingly, the decomposition temperature of SDN in the nanohybrid (250°C) was lower than that of intact SDN (270°C). This result could be due to the molecular distribution of SDN upon intercalation, which would possibly lead to the enhancement of SDN solubility.Citation31,Citation44
SDN content in nanohybrids
The SDN content of the SDN–MMT and AEA-coated SDN–MMT nanohybrids was 30.61% ± 0.55% and 16.59% ± 0.44%, respectively. The decrease in SDN content with AEA-coated SDN–MMT was due to incorporation of the AEA polymer through the coating process employed in this work (MMT:AEA = 1:1 w/w).
In vitro drug release test
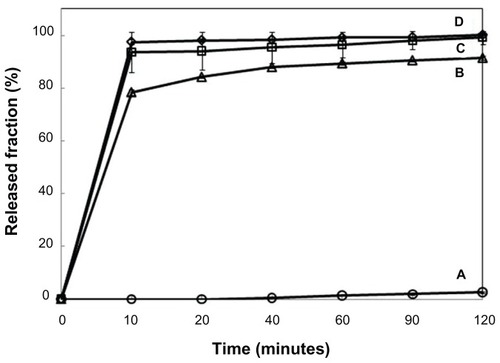
We prepared our nanohybrid system to mask the taste of SDN. To assess this quality, we conducted an in vitro drug-release experiment in deionized water for 2 minutes, mimicking the condition in the buccal cavity.Citation37 As shown in , for intact SDN, more than 73% of the drug was dissolved rapidly into the release media as expected. Almost half of SDN was released from Viagra® during the first 2 minutes since the tablet is designed to rapidly release the drug in the biological fluid. In contrast, the nanohybrids (ie, SDN–MMT and AEA-coated SDN–MMT) exhibited greatly suppressed release under the test condition employed in this work. The SDN release from the nanohybrids was more than tenfold less than that from intact SDN and Viagra®: the SDN–MMT exhibited no release, and for AEA-coated SDN–MMT, only a slight portion of the drug (<5%) was released during the first 2 minutes. The strong ionic interaction between the SDN molecules and the MMT interlayers appeared to hinder freeing the drug molecules, resulting in very low drug release. A slight drug release (<5%) from AEA-coated SDN–MMT could be due to slight dissolution and ionization of the AEA even in the neutral media to deintercalate the drug in the MMT to some extent.
Table 1 In vitro drug dissolution profiles under a simulated buccal condition
In addition to taste masking, the SDN needs to be released rapidly in the gastric cavity for satisfactory therapeutic efficacy. Thus, we examined the drug-release profiles of SDN–MMT and AEA-coated SDN–MMT nanohybrids in a simulated gastric condition and compared them with those of intact SDN and Viagra® (; Table S4). The drug release from intact SDN and Viagra® was very rapid as expected. More than 90% of SDN was released in 10 minutes and 99% during the first 2 hours, which could be attributed to high solubility of SDN in acidic fluid.Citation45 However, the drug was hardly released from SDN–MMT (<3%) again due to the strong ionic interaction between the intercalated SDN and MMT. In spite of its excellent taste-masking properties (; Table S3), the SDN–MMT by itself may not be useful for effective delivery of SDN. On the other hand, drug release increased dramatically with the AEA-coated SDN–MMT nanohybrid. About 75% of SDN was released in 10 minutes and up to 90% during the first 2 hours, which was only 10% less than Viagra®. The cationic macromolecule, AEA, when dissolved and ionized selectively at low pH, could replace the intercalated drug molecules effectively and enlarge the interlayer spacing to facilitate drug diffusion out towards the release media.Citation31 This result is meaningful in a sense that AEA-coated SDN–MMT nanohybrids could provide both taste masking and effective in vitro release of SDN relatively similar to proven medications like Viagra®.
SDN analytical method validation
Under the HPLC-ESI-MS/MS conditions described in the experimental section, no interfering peak was detected in the plasma samples. MRM in a positive mode was used to quantify SDN (see Supplementary information). Figure S3 shows the typical product ion spectra of SDN, and the typical chromatograms of SDN detected with the MRM mode of HPLC-ESI-MS/MS are demonstrated in Figure S4. The calibration curve for SDN was generated by linear regression of peak area ratios against the injected amount of the analyte. The curves show good linearity over the calibration ranges in plasma (R2 = 0.9999). The LOQ, defined as a signal-to-noise ratio of 10, was estimated at 1.2 ng/mL.
The intra- and interday precision and accuracy using the QC samples are shown in Table S1. The precisions (RSDs) are all less than 5%, and accuracy ranged from 91.7% to 96.2% for intraday and 91.0% to 93.6% for interday, respectively. This indicates the method is accurate and precise enough to apply in pharmacokinetic study.
The mean recoveries of SDN spikes in plasma at three different concentrations ranged from 91.4% to 93.6%, and the percentage of RSDs were all less than 7% (n = 3) (see Table S2 for supplementary information), demonstrating that the employed OASIS HLB method for SPE coupled with HPLC-ESI-MS/MS analysis seems to be suitable for detection of SDN in plasma samples. The stabilities of SDN spikes in plasma were studied under different storage conditions including three cycles of freeze-thawing, room temperature for 5 hours, and post-preparation for overnight. The deviation of the mean quantification data was within 5% in all stability tests for QC samples, and there seems no significant effect (P < 0.5) on the quantification of SDN in the plasma samples.
Pharmacokinetics of SDN after oral administration of Viagra® and AEA-coated SDN–MMT to dogs
shows the mean plasma concentration–time curve of SDN after oral administration of Viagra® and AEA-coated SDN–MMT to the beagle dogs. The relevant pharmacokinetic parameters are presented in . The AUC0-∞ and Cmax of SDN significantly increased in AEA-coated SDN–MMT nanohybrid compared with Viagra® (P < 0.05). Such a difference in drug exposure could be explained by individual pharmacokinetics variability, but the AUC0-∞ and Cmax values were still statistically meaningful between two groups, even though the number of the animals studied was small (n = 3). The increased drug exposure observed in AEA-coated SDN–MMT seems to be most likely due to the decreased oral clearance of SDN (4.34 L/hour in Viagra® vs 3.81 L/hour in AEA-coated SDN–MMT; P < 0.05). According to the in vitro drug-release profiles (), after the initial burst release (~80%), 10% of drug was released slowly for the remaining 110 minutes. After 2 hours, the remaining 10% of drug was still entrapped in the AEA-coated SDN–MMT nanohybrid, which was not released under a simulated in vitro condition. However, many different types of cations possibly present in the living body may continuously replace the entrapped drug molecules to facilitate drug diffusion out.Citation46–Citation48 Therefore, the remaining 10% of SDN would be released in a sustained manner after the nanohybrid passed the gastric cavity. In the intestine, the polymeric barrier of AEA would not be present due to its complete dissolution in a gastric fluid.
Table 2 Pharmacokinetic parameters of SDN after oral administration of Viagra® and AEA-coated nanohybrids to the beagle dogs
Figure 6 Mean plasma concentration–time curves of sildenafil after oral administration of Viagra® (●) and polyvinylacetal diethylaminoacetate (AEA)-coated hybrid (○) to the beagle dogs.
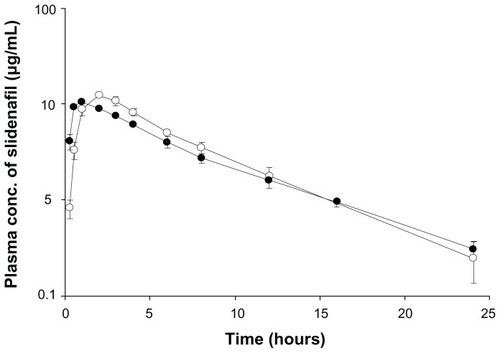
Viagra® released almost all drug in 10 minutes, which would allow high systemic absorption at the early stage, resulting in a faster Tmax (1 hour), while the absorption of SDN was delayed in the AEA-coated SDN–MMT because the drug entrapped in the interlayer of MMT was released slowly during its passage through the gastrointestinal tract (Tmax = 2.33 hours). For the specific drug, SDN, a faster onset of pharmacodynamic response (ie, a faster Tmax) would be still desirable. For this reason, our nanohybrid systems need to be further improved to achieve faster drug release at an early stage in addition to taste masking of the drug. In this sense, we envision using more AEA or employing other cationic macromolecules for coating our nanohybrid systems to facilitate deintercalation of the drug and enhance drug release in the gastric cavity.Citation32
Stability of SDN
The drug molecules in nanohybrids could be more sensitive to degradation due to their distribution at a molecular level, giving a higher chance of interaction with the surrounding environment. Therefore, we assessed the shelf-life of SDN–MMT and AEA-coated SDN–MMT nanohybrids under an accelerated stability test condition (temperature, 40°C; humidity, 75%).Citation49 As shown in , the changes in nondegraded SDN content during 6 months were minimal (<±5%) for all samples, indicating that the SDN molecules could be well protected in the interlayer space of MMT due to their strong ionic interaction.
Table 3 Fraction of nondegraded SDN content in the nanohybrids after incubation under an accelerated stability test condition (40°C, 75% RH)
Conclusion
In this study, we suggest nanohybrids as a potential oral disintegrating formulation for delivery of SDN, a drug for treatment of erectile dysfunction. For this purpose, the drug was intercalated into MMT by cation exchange reaction to produce an SDN–MMT nanohybrid for taste masking of SDN. In order to improve the release rate in the simulated gastric fluid, the SDN–MMT nanohybrid was further coated with AEA, a cationic polymer, which exhibited suppressed release under a simulated buccal condition. For in vitro drug release in a simulated gastric fluid, AEA-coated SDN–MMT exhibited relatively fast drug release, ie, 80% during the first 10 minutes and 10% during the remaining 110 minutes. However, the total percentage of drug release was about 10% lower than Viagra®. The in vivo pharmacokinetic study revealed that the AUC0-∞ and Cmax values of SDN were significantly increased with AEA-coated SDN–MMT nanohybrid compared with Viagra®. Therefore, we conclude that the nanohybrid system suggested in this work has potential for taste masking of SDN as well as possibly increased drug exposure.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) (SRC program: 2012-0000650, and WCU program: R31-2008-000-10010-0), the Ministry of Knowledge Economy (10030036) and partly by the Ewha Global Top 5 Grant 2011 of Ewha Womans University.
Disclosures
Some authors are inventors of a relevant patent.Citation50 The authors declare no other conflicts of interest in this work.
Supplementary information
Table S1 Accuracy and precision of SDN analyses in plasma samples
Table S2 Recovery of SDN spiked into plasma samples
Table S3 In vitro drug dissolution profiles under a simulated buccal and gastric fluid condition
Table S4 Release profiles of sildenafil from (A) SDN–MMT, (B) AEA-coated SDN–MMT, (C) Viagra®, and (D) sildenafil citrate. The release experiments were performed at pH = 1.2
Figure S1 Fourier transform infrared spectra of (A) sildenafil citrate, (B) montmorillonite (MMT), (C) sildenafil–montmorillonite (SDN–MMT), and (D) polyvinylacetal diethylaminoacetate (AEA)-coated SDN–MMT.
Notes: The circles (●) and the dashed vertical lines show the characteristic peaks seen with intact SDN and MMT.
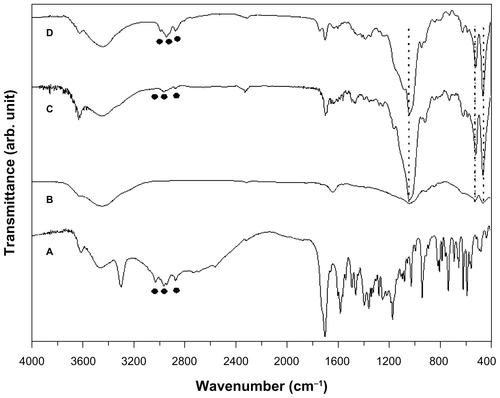
Figure S2 The particle size distribution of (A) sildenafil–montmorillonite (SDN–MMT) and (B) polyvinylacetal diethylaminoacetate (AEA)-coated SDN–MMT.
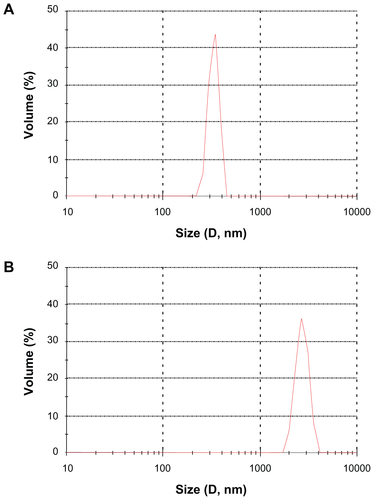
Figure S3 Typical electrospray ionization mass spectrometry/mass spectrometry spectra of authentic sildenafil.
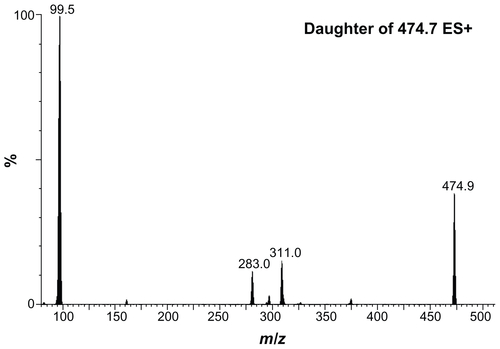
Figure S4 Representative chromatograms of (A) sildenafil detected in a plasma sample collected for 2 hours after sildenafil administration, and (B) blank plasma sample collected just before sildenafil administration.
Abbreviations: ES, electrospray; MRM, multiple reaction monitoring; TIC, total ion count.
References
- ChoyJHKwonSJParkGSHigh-Tc superconductors in the two-dimensional limit: [(Py-CnH2n+1)2HgI4]-Bi2Sr2Cam−1CumOy (m = 1 and 2)Science19982805369158915929616119
- ChoyJHKwakSYParkJSJeongYJPortierJIntercalative nano-hybrids of nucleoside monophosphates and DNA in layered metal hydroxideJ Am Chem Soc1999121613991400
- ChoyJHKwakSYJeongYJParkJSInorganic layered double hydroxides as nonviral vectorsAngew Chem Int Ed2000392240424045
- DesigauxLBelkacemMBRichardPSelf-assembly and characterization of layered double hydroxide/DNA hybridsNano Letters20066219920416464034
- KwakSYKrivenWMWalligMAChoyJHInorganic delivery vector for intravenous injectionBiomaterials200425285995600115183614
- ChoyJHJungJSOhJMLayered double hydroxide as an efficient drug reservoir for folate derivativesBiomaterials200425153059306414967539
- OhJMChoiSJKimSTChoyJHCellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: enhanced efficacy due to clathrin-mediated endocytosisBioconjugate Chem200617614111417
- ChoiSJOhJMChoyJHHuman-related application and nanotoxicology of inorganic particles: complementary aspectsJ Mater Chem2008186615620
- ChoiSJChoiGEOhJMOhYJParkMCChoyJHAnticancer drug encapsulated in inorganic lattice can overcome drug resistanceJ Mater Chem2010204294639469
- KhanAILeiLNorquistAJO’HareDIntercalation and controlled release of pharmaceutically active compounds from a layered double hydroxideChem Commun (Camb).2001222342234312240066
- WilliamsGRO’HareDTowards understanding, control and application of layered double hydroxide chemistryJ Mater Chem2006163030653074
- LerouxFTaviot-GuéhoCFine tuning between organic and inorganic host structure: new trends in layered double hydroxide hybrid assembliesJ Mater Chem20051535–3636283642
- KikuchiMMatsumotoHNYamadaTKoyamaYTakakudaKTanakaJGlutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocompositesBiomaterials2004251636914580909
- KumarVYangTYangYInterpolymer complexation. II. Entrapment of ibuprofen by in-situ complexation between polyvinyl acetate phthalate (PVAP) and polyvinylpyrrolidone (PVP) and development of a chewable tablet formulationPharm Dev Technol200161718111247277
- SuzukiHOnishiHTakahashiYIwataMMachidaYDevelopment of oral acetaminophen chewable tablets with inhibited bitter tasteInt J Pharm20032511–212313212527182
- AzarmiSRoaWLöbenbergRCurrent perspectives in dissolution testing of conventional and novel dosage formsInt J Pharm20073281122117084051
- DouroumisDOrally disintegrating dosage forms and taste-masking technologies; 2010Expert Opin Drug Deliv20118566567521438776
- LiangACChenLHFast-dissolving intraoral drug delivery systemsExpert Opin Ther Patents2001116981986
- MashruRCSutariyaVBSankaliaMGParikhPPDevelopment and evaluation of fast-dissolving film of salbutamol sulphateDrug Dev Ind Pharm2005311253415704855
- CilurzoFCuponeIEMinghettiPSelminFMontanariLFast dissolving films made of maltodextrinsEur J Pharm Biopharm200870389590018667164
- SinghSAminDGoudMAjanta Pharma Ltd, assigneeTaste masked chewable compositions of sildenafil citrate Patent Cooperation Treaty WO 09/0749956182009
- MapelliLGMarconiMGRZemaMEurand International SpA, assigneePharmaceutical Formulations Patent Cooperation Treaty WO 91/01604310311991
- SohiHSultanaYKharRKTaste masking technologies in oral pharmaceuticals: recent developments and approachesDrug Dev Ind Pharm200430542944815244079
- ObeidatWMPriceJCPreparation and in vitro evaluation of propylthiouracil microspheres made of Eudragit RL 100 and cellulose acetate butyrate polymers using the emulsion-solvent evaporation methodJ Microencapsul200522328128916019914
- Lorenzo-LamosaMLCunaMVila-JatoJLTorresDAlonsoMJDevelopment of a microencapsulated form of cefuroxime axetil using pH-sensitive acrylic polymersJ Microencapsul19971456076169292436
- YajimaTItaiSTakeuchiHKawashimaYOptimum heat treatment conditions for masking the bitterness of the clarithromycin wax matrixChem Pharm Bull200351111223122614600362
- XuJBovetLLZhaoKTaste masking microspheres for orally disintegrating tabletsInt J Pharm20083591–2636918455893
- LuckhamPFRossiSThe colloidal and rheological properties of bentonite suspensionsAdv Colloid Interface Sci1999821–34392
- ErenEAfsinBAn investigation of Cu(II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA studyJ Hazard Mater20081512–368269117644249
- JungHKimHMChoyYBHwangSJChoyJHItraconazole-laponite: kinetics and mechanism of drug releaseAppl Clay Sci2008401–499107
- JungHKimHMChoyYBHwangSJChoyJHLaponite-based nanohybrid for enhanced solubility and controlled release of itraconazoleInt J Pharm20083491–228329017888597
- ParkJKChoyYBOhJMKimJYHwangSJChoyJHControlled release of donepezil intercalated in smectite claysInt J Pharm20083591–219820418502063
- CaiYMengXFCaoYXLuHZhuSFZhouLZMontmorillonite ameliorates hyperthyroidism of rats and mice attributed to its adsorptive effectEur J Pharmacol20065511–315616117027745
- LinFHLeeYHJianCHWongJMShiehMJWangCYA study of purified montmorillonite intercalated with 5-fluorouracil as drug carrierBiomaterials20022391981198711996039
- GoelHRaiPRanaVTiwaryAKOrally disintegrating systems: innovations in formulation and technologyRecent Pat Drug Deliv Formul2008225827419075912
- BaxterJLKukuraJMuzzioFJHydrodynamics-induced variability in the USP apparatus II dissolution testInt J Pharm20052921–2172815725550
- SiewertMDressmanJBrownCKShahVPFIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage formsAAPS Pharm Sci Tech200341E7
- WalkerDKAcklandMJJamesGCPharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and manXenobiotica199929329731010219969
- KuuWYChilamkurtiRChenCEffect of relative humidity and temperature on moisture sorption and stability of sodium bicarbonate powderInt J Pharm19981662167175
- ChoyJHChoiSJOhJMParkTClay minerals and layered double hydroxides for novel biological applicationsAppl Clay Sci2007361–3122132
- StulMSMortierWJThe heterogeneity of the charge density in montmorillonitesClay Clay Miner197422391396
- YangJHHanYSParkMParkTHwangSJChoyJHNew inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rateChem Mater2007191026792685
- DineshNDNagarajaPGowdaNMMRangappaKSExtractive spectrophotometric methods for the assay of sildenafil citrate (Viagra) in pure form and in pharmaceutical formulationsTalanta200257475776418968678
- MelnikovPCorbiPPCuinACavicchioliMGuimarãesWRPhysicochemical properties of sildenafil citrate (Viagra) and sildenafil baseJ Pharm Sci200392102140214314502553
- ElshafeeyAHBendasERMohamedOHIntranasal microemulsion of sildenafil citrate: in vitro evaluation and in vivo pharmacokinetic study in rabbitsAAPS Pharm Sci Tech2009102361367
- BarakatNSElbagoryIMAlmurshediASFormulation, release characteristics and bioavailability study of oral monolithic matrix tablets containing carbamazepineAAPS Pharm Sci Tech200893931938
- LingGZhangPZhangWDevelopment of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibitionJ Control Release2010148224124820727928
- JijunFXiaoliWLishuangXPreparation and in vitro–in vivo evaluation of double layer coated and matrix sustained release pellet formulations of diclofenac potassiumInt J Pharm20114061–2849021219996
- LiuJWilliamsROIIILong-term stability of heat-humidity cured cellulose acetate phthalate coated beadsEur J Pharm Biopharm200253216717311879999
- ChoyJHLeeJHParkMCChangHCDaewoong Pharmaceutical Co, LTD, assigneeSildenafil-bentonite-AEA nanohybrid, pharmaceutical composition containing the same and method for preparing the same Republic of Korea patent 20110028178 March 172011