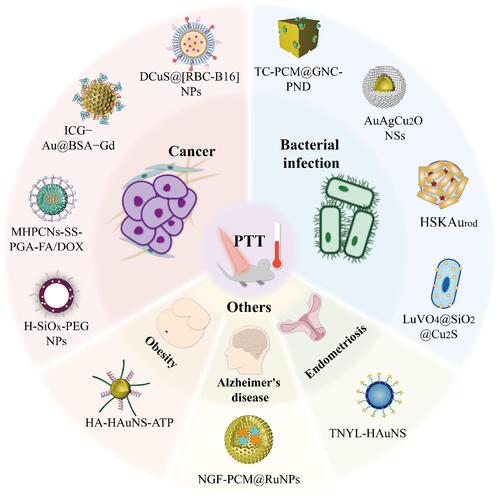
Abstract
Nanotechnology has prompted the development of hollow inorganic nanomedicine. These medicines are now widely investigated as photothermal-based therapies for various diseases due to their high loading capacity, tuneable wavelength, relatively small size and low density. We begin this review with a brief introduction, followed by a summary of the development of imaging-guided photothermal therapy (PTT) for cancer treatment during the last three years (from 2017 to 2020). We then introduce the antibacterial effects of these medicines on some bacterial infections, in which the pathogenic bacteria can be killed by mild photothermal effects, ions and antibiotic release. Other diseases can also be treated using hollow inorganic photothermal agents. Specifically, we discuss the use of PTT for treating Alzheimer’s disease, obesity and endometriosis. Finally, we share our perspectives on the current challenges and future prospects of using hollow inorganic materials in clinical PTT for various diseases.
Introduction
In the modern world, cancer is a predominant cause of death and is a very serious and challenging health problem currently faced by humanity. A recent study projected that over 1.8 million new cancer cases and over 0.6 million cancer deaths would occur in the US alone in 2020.Citation1 However, with the advancement of medical technology, many cancer therapies have been developed in the last 20 years, including surgery, radiotherapy and chemotherapy,Citation2,Citation3 resulting in a continuous decline in the mortality rate of cancer.Citation1 However, there are still some drawbacks to the clinical application of these therapies, including recurrence, non-selective targeting, low therapeutic indices, multiple drug resistance and serious side effects.Citation4,Citation5 In addition, bacterial infections are a serious health problem that account for almost one-third of global mortalityCitation6 and considerable financial losses.Citation7 Antibiotics are the most effective and frequently used treatments for bacterial infections, but the overuse of antibiotics in the clinical setting has contributed to pathogenic resistance.Citation8,Citation9 Multidrug resistance (MDR) is commonly seen in the context of both cancer and antibacterial therapies. Therefore, novel therapeutic strategies are urgently needed for combating both cancer and bacterial infections.
Recently, photothermal therapy (PTT) has been developed as a novel hyperthermia-based disease treatment strategy, in which photo-sensitive photothermal agents (PTAs) delivered at target sites in the body are used to convert near-infrared (NIR) light to heat in order to induce local hyperthermiaCitation10,Citation11 The photothermal effect can ablate aberrant cells and pathogenic bacteria, denaturing their proteins and causing cell death.Citation6,Citation12–Citation15 This laser-induced hyperthermia therapy is a robust and efficient therapeutic strategy for disease treatment, with the merits of high selectivity,Citation16 relatively low rates of side effectsCitation11 and negligible invasiveness.Citation17 Due to these beneficial features, PTT is believed to be a promising strategy for treating various diseases. Meanwhile, PTT is also deemed as a good helper for other therapies, more and more synergistic therapies combining PTT and other therapies such as chemotherapy,Citation18 photodynamic therapy,Citation19 immunotherapyCitation20 and starvation therapyCitation21 are developed for cancer treatment. For example, the effect of chemotherapy can be enhanced and the multidrug resistance can be reduced by using a chemo/photothermal synergistic therapy; moreover, the photothermal effect can also contribute to the targeted drug release from nano-carrier at tumor site. In other words, the synergistic therapies are more than simply putting two therapies together, the introduction of PTT can significantly enhance the effect of other therapies.
To date, various types of therapeutic PTAs have been discovered, including inorganic types such as gold nanomaterials,Citation22 carbon-based nanomaterials,Citation23 silica nanomaterialsCitation24 and metal chalcogenides,Citation25 and organic types such as conjugated polymersCitation26 and porphysomes.Citation27 Although organic nanomaterials are superior to their inorganic counterparts in terms of biocompatibility and biodegradability, they suffer from several limitations, such as unstable photothermal effects and low photothermal conversion efficiency (PCE). Owing to their excellent imaging capacity and PCE, inorganic PTAs are currently prioritised by researchers and have seen wide application in the diagnosis and treatment of diseases. Notably, the morphology of inorganic nanoscale PTAs exerts a great influence on their properties. Specifically, aggregates of nanoparticles (NPs) are usually limited by large size and instability, whereas solid NPs are limited by weak NIR absorption and narrow wavelength adjustability.Citation28 Thus, the emergence of nanomaterials with hollow structures could provide a solution to these obstacles. By changing the diameter and thickness of the shell, the optical properties of hollow NPs can be easily manipulated, with the absorption wavelength ranging from near-ultraviolet (UV) to infrared.Citation29,Citation30 In addition, hollow nanostructures possess lower mass than other nanostructures of the same size, thereby contributing to a relatively higher PCE per unit mass.Citation31 Moreover, the hollow interior endows hollow nanomaterials the capacity to be loaded with drugs, including imaging contrast agents (perfluorohexane for ultrasound [US] imaging) and therapeutic drugs (doxorubicin for cancer treatment), thus promoting both imaging and therapeutic efficacies. Owing to their features including high loading capacity, tuneable wavelength, relatively small size and low density,Citation32,Citation33 hollow inorganic nanostructures can be ideal agents in photothermal-based therapies for various diseases.
Reviews concerning the application of hollow or inorganic nanomedicines in PTT for cancer have been published.Citation29,Citation34–Citation36 However, reviews of the application of both hollow and inorganic nanomedicines in cancer PTT have not been published in recent three years. Moreover, their applications to other noncancerous diseases have never been summarized in a review. In this review, we focus on the recent advances in the development of hollow inorganic nanomaterials for PTT-based treatments for cancer, bacterial infections and other diseases, including Alzheimer’s disease (AD), obesity and endometriosis (). We conclude with our perspectives on the current challenges and future prospects of using hollow inorganic materials in clinical PTT for various diseases.
Photothermal-Based Therapy for Cancer Treatment
Hollow Gold Nanostructures
Due to special interactions with light, the free electrons of gold nanostructures undergo a collective coherent oscillation process known as localized surface plasmon resonance.Citation15,Citation37 Gold nanostructures can be endowed with optimal photothermal abilities, which are usually affected by the size, shape and dielectric constant of the nanostructure.Citation38 In gold hollow nanostructures in particular, the photothermal effects are likely to be affected by the thickness of the shell.Citation29 Recently, many hollow nanostructures have been developed for photothermal-based cancer therapy ().Citation18,Citation19,Citation39–Citation44
Table 1 Summary for Recently Developed Hollow Inorganic Nanostructures for PTT-Based Cancer Treatment
Hollow Gold Nanospheres or Nanoshells
Among all the hollow gold nanostructures, hollow gold nanospheres and nanoshells are frequently investigated. In 2003, Hirsch et al reported an SH-PEG-modified gold nanoshell for magnetic resonance (MR) temperature imaging-monitored photothermal therapy for transmissible venereal tumor.Citation45 In that study, a PEG-passivated nanoshell was injected interstitially into the tumour site and exposed to NIR light (820 nm, 4 W/cm2, 5-mm spot diameter, 6 min) for photothermal ablation under MRI monitoring. Fluorescence imaging based on the NIR light-absorbing effects of hollow gold nanospheres (HAuNS) has also shown potential in cancer diagnosis. Wang et al developed an NIR fluorophore, cypate-conjugated HAuNS, for tumour-specific imaging and PTT.Citation46 The fluorophore cypate was linked to HAuNS via a short spacer containing a urokinase-type plasminogen activator (uPA, an enzyme secreted by tumours) cleavable motif that could release the fluorophore to yield fluorescence in breast tumour cells. In 2017, You et al developed a hollow gold nanoshell (ICG-Au@BSA-Gd) for quad-model NIR fluorescence/photoacoustic (PA)/computed tomography (CT)/MR imaging-guided photodynamic (PD) and photothermal synergistic therapy for breast cancer.Citation19 In that study, the gold nanoshell was loaded with the common photosensitizer ICG to achieve photothermal (gold and ICG) and photodynamic (ICG) synergistic therapy under NIR light irradiation. As an FDA approved small molecule, ICG is widely applied in imaging or therapies of cancer. As mentioned, ICG can be solely used to achieve fluorescent and photoacoustic imaging and PTT/PDT. However, it is easily to aggregate and degrade in aqueous solution such as plasma, thus like many other small molecular drugs, the protection of ICG is usually carried out by nanoparticles encapsulation. In addition, this was the first study to synthesize a gadolinium (Gd)-based bovine serum albumin (BSA-Gd) hybrid to improve Gd-loading capacity, which endowed NPs with excellent MR/CT/PA imaging capacity. Coating with BSA guaranteed the biocompatibility and stability of the NPs. In subsequent in vivo experiments, quad model imaging was shown to be a precise and efficient method for the guidance of combined PTT and PDT.
Hollow Gold Nanocages
As another member of the hollow gold nanostructure (HGN) family, the biological application of gold nanocages, especially in cancer treatment, has been widely investigated. With their hollow interior and porous walls,Citation47,Citation48 gold nanocages are suitable for drug delivery, imaging and PTT.Citation49 In 2018, Zhao et al developed gold nanocages (AuNCs) for melanoma-targeted positron emission tomography/CT imaging with radiolabelled 64Cu.Citation50 The targeting ability stemmed from the conjugated α-melanocyte-stimulating hormone (α-MSH) peptide, which could specifically bind to the over-expressed melanocortin 1 receptor in tumour cells. Moreover, the targeting efficiency was improved by increasing the amount of α-MSH loaded on the AuNCs. Zhou et al also investigated a PA imaging-guided pH-sensitive gold nanocage (D-PGNC) for ovarian cancer PTT and the selective delivery of DOX to tumour cells, in which the pH value is lower than that in other cells.Citation18 The nanocage acted as a cargo and heat producer under NIR light, contributing to PTT and PA imaging. Inside the nanocage, DOX was covered by a pH-sensitive copolymer (PDPM) which released DOX at a low pH. Recently, Zhan et al developed a gold nanocluster-modified gold nanocage-based hybrid nanodrugCitation42 for multispectral optoacoustic tomography (MSOT) imaging-guided PTT for breast cancer and epidermal growth factor receptor (EGFR) pathway blockage. The nanodrug consisted of an EGFR inhibitor (EB)-loaded gold nanocage moiety and a gold nanocluster moiety. The former (gold nanocage moiety) acted as a drug carrier, MSOT imaging agent (functioned in sensing the nanodrug’s biodistribution and metabolic process) and photothermal generator. While the latter one (gold nanocluster moiety) was designed for stabilisation, fluorescence detection (an indication of drug release) and EB release.
Hollow Gold Nanorods
As a novel type of hollow structure, gold hollow nanorods (AuHNRs) were fabricated for the first time in 2018.Citation41 In the synthesis section, researchers utilized Se-doped Te nanorod as a template, followed by the modification of agents with sulfhydryl. The aspect ratio of AuHNRs was only 3, which endowed them with LSPR peak in NIR-II region. Then, under 1064 nm laser irradiation, a multimodel photothermal/photoacoustic/computed tomography imaging could be realized. With the hollow structure, AuHNRs were also used for drug-loading, and achieved targeted chemo/photothermal therapy in mouse model of squamous-cell carcinoma.
Hollow Metal Chalcogenides
Recently, metal chalcogenides have emerged as a novel tool for imaging-guided PTT. Examples include copper sulphide nanostructures, cobalt sulphide nanostructures, bismuth chalcogenides and manganese oxide nanostructures ().Citation51–Citation56 Similar to HGNs, hollow copper sulphide (CuS) NPs possess excellent NIR light-absorbing capacity, resulting in high light-to-heat transformation efficiency. However, unlike HGNs, the optical absorption of CuS NPs does not stem from surface plasmon resonance, but rather from the d-d bond transition of Cu ions, which is not affected by the size or shape of CuS NPs or the solvent.Citation57 Moreover, HGNs are inferior to hollow CuS NPs in terms of biodegradability, toxicity and cost.Citation58,Citation59 As mentioned above, toxicity is a persistent problem with HGNs. However, due to their biodegradability, CuS NPs have lower toxicity and are more suitable for biological application. In 2019, Qiu et al developed the first photodegradable CuS NPs for residual tumour surface-enhanced Raman scattering (SERS) imaging and PTT.Citation60 Under exposure to NIR light, the hollow CuS NPs disseminated into tiny clusters, thereby contributing to their clearance from tissues after imaging and preventing chronic toxicity. Furthermore, given that the photothermal efficiency is not affected by the shape and size of CuS NPs, they may serve as a robust nanoplatform for prostate tumor PTT. Zhang et al fabricated hollow CuS nanoflower for MRI-guided DOX-loaded chemo/photothermal liver cancer therapy.Citation61 In their study, both T1-weighed MRI and T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) MRI were effective for imaging in in vitro experiments. However, the latter technique was more robust than the former; therefore, T2-FLAIR MRI was utilised for subsequent in vivo experiments. Wang et al utilised hybrid membrane-coated DOX-loaded CuS NPs (DCuS) for homotypic-targeting chemo-PTT with a long circulation life.Citation52 As shown in , the hybrid membrane was composed of membranes from both red blood cells (RBCs) and melanoma cells (B16-F10 cells). The former helped DCuS NPs to evade capture by the immune system, whereas the latter helped DCuS NPs to homogeneously target melanoma cells due to the expression of surface adhesion molecules. The combination of these two membranes (namely, RBC-B16) prolonged the circulation life of the nanomedicine.
Figure 2 (A) Schematic of membrane fusion and coating. Membrane materials are derived from RBCs and B16-F10 cells and then fused together. The resulting hybrid membrane is used to camouflage DOX-loaded hollow copper sulfide nanoparticles (DCuS NPs) to produce DCuS@[RBC−B16] NPs. (B) Synergistic photothermal/chemotherapy of melanoma. Data from Wang et al.Citation52
![Figure 2 (A) Schematic of membrane fusion and coating. Membrane materials are derived from RBCs and B16-F10 cells and then fused together. The resulting hybrid membrane is used to camouflage DOX-loaded hollow copper sulfide nanoparticles (DCuS NPs) to produce DCuS@[RBC−B16] NPs. (B) Synergistic photothermal/chemotherapy of melanoma. Data from Wang et al.Citation52](/cms/asset/7b1a072a-3657-4ff7-aefd-b618fe6db132/dijn_a_12192177_f0002_c.jpg)
It is worth noting that the clinical application of CuS nanostructures has a number of limitations. First, the PCE of CuS NPs is relatively low. Thus, PTT agents with a high PCE are urgently needed. Second, some CuS NPs are not effective at low concentrations. Therefore, high-concentration CuS NPs are generally used to achieve PTT. The excessive amount of Cu used results in the overexpression of vascular EGF, followed by the formation of more microvessels and the activation of pro-tumour pathways, resulting in the promotion of cancer growth.Citation62–Citation64 To tackle this problem, Guan et al designed hollow porous cobalt sulphide nanospheres (PCSH NSs) for MRI-guided PTT with an unprecedented high PCE of 70.1% compared to existing binary chalcogenides, which made PTT possible even at a low PTT agent concentration.Citation65 In this study, the authors synthesized the nanostructures via a facile one-pot solvothermal synthetic method, where the molar ratio of thioacetamide to cobalt acetate was of vital significance as it was a decisive factor in its morphology. As the molar ratio was increased, the sizes of the nanostructures decreased, followed by the transformation from large nanosheets (molar ratio = 1) to nanospheres (molar ratio = 4) and finally to solid nanostructures (molar ratio = 20). The authors also proved that the concentration of S anions could affect the optical properties. Finally, in in vivo experiments, cervical tumour were eradicated with low doses of PCSH nanostructures (25 ppm) under NIR light (808 nm, 0.7 W/cm2), with the lower chronic toxicity of nanomaterials contributing to fewer side effects. Unlike the biodegradable materials approach mentioned in the last paragraph,Citation60 this approach was a more direct way of reducing chronic toxicity.
Manganese oxide, an excellent MRI contrast agent, has been widely used in MRI.Citation66 In 2008, An et al designed hollow manganese oxide (MnO) NPs of different sizes for use in MRI.Citation67 The authors found that the T1 relaxation was enhanced with increasing surface area, and that the hollow structures had a drug-loading capacity. In 2009, Shin developed hollow manganese oxide (Mn3O4) NPs for MRI and drug loading.Citation68 In another study, US imaging was combined with MRI for cancer imaging using some manganese oxide-based NPs.Citation69 Although manganese oxide nanosheetsCitation70 and mesoporous NPsCitation71 have been previously applied for PTT, hollow MnO2 NPs have only recently been used. For the first time, Zeng et al fabricated polydopamine (PDA)-coated photosensitiser chlorin e6-loaded hollow MnO2 NPs for photodynamic and photothermal synergistic breast cancer therapy.Citation53 Due to PDA’s stability at pH 7.4, the premature release of Ce6 into the blood was avoided. However, PDA was destroyed at pH 6.8 at tumour sites, resulting in the release of MnO2 and Ce6. MnO2 was subsequently decomposed to produce Mn2+ (generally used for MRI) and oxygen, which can release PDT by reacting with Ce6. Meanwhile, under NIR light (808 nm), PTT was achieved and the oxygen-burst and release of Ce6 was promoted. Recently, researchers started to focus on the combination of the two above mentioned metal chalcogenides (CuS and MnO2) to obtain various effects. Interestingly, in CuS@MnO2 nanocomplexes, MnO2 usually acted as a gatekeeper to prevent the release of loaded drugs in blood circulation and open the “gate” in the acid tumour microenvironment. In Lin’s work,Citation72 with DOX loaded inside the hollow interior of CuS NPs, multimodal (fluorescence, photothermal and MR) imaging-guided controlled release chemotherapy and PTT for liver cancer were achieved. Similarly, Xue et al achieved PA/FL/MR trimodal imaging-guided PDT/PTT synergistic breast cancer therapy with Ce6 loading.Citation54 In summary, these types of smart multifunctional nanoplatform have shown great potential for future clinical applications.
Hollow Carbon Nanostructures
As another promising PTT agent, carbon-based nanomaterials such as carbon nanotubes,Citation73 carbon nanohornsCitation74 and grapheneCitation23 have been widely utilized in cancer therapy. As shown in , more and more hollow carbon nanostructures have been developed for cancer PTT. Similar with gold nanoparticles, carbon nanomaterials generate heat from light via the relaxation of surface electrons. In addition, given the fact that the fluorescence of some carbon nanostructures are prone to be quenched by the mutual effect between different carbon layers or carbon layers with additional components, non-radiative relaxation dominated the de-excitation process of carbon nanostructures, thus most of the energy obtained from light can be converted into heat.Citation75 Compared to gold nanoparticles, carbon-based nanoparticles possess tunable photothermal effect in a wide spectral range.Citation76 Moreover, hollow carbon nanostructures are also superior in biocompatibility to other nanostructures mentioned, for their abundant surface functional groups and absent metal ions.Citation77
However, PTT when used alone is unable to eradicate tumour cells under some conditions. In these case, mesoporous carbon NPs (MCNs) are an ideal option to combine chemotherapy and PTT due to their large pore volume allowing them to serve as drug carriers. In 2017, Li et al developed a new kind of hollow MCN (HMCN) for chemotherapy/PTT for lung cancer and compared its efficacy with that of MCNs.Citation78 In that study, DOX was chosen to evaluate the drug loading and release process of the two NPs. With higher drug loading efficiency (up to 76.9%) and higher photothermal conversion efficiency, HMCNs are more suitable for cancer treatment and show great promise. However, due to their porous surface morphology, premature drug release is an obstacle. In the same year, Wang et al fabricated HMCNs.Citation79 To tackle the problem of premature drug release, they used PEI carbon dots as ‘doors’ for the mesoporous “gate”, with disulphide units acting as the ‘locks’. When the NPs reached breast cancer cells, GSH overexpressed in cancer cells acted as “keys” to the locks to cleave disulphide bonds, leading to the release of the loaded DOX. Meanwhile, the released CDPEI yielded fluorescence, indicating drug release. Then, NIR light irradiation showed that the release rate of DOX was accelerated. In the following year, Fang et al utilized graphene carbon dots to seal the pores, which also functioned as a robust PTT agent in the subsequent synergistic chemo/photothermal cervical cancer treatment.Citation80 A triple stimuli-response magnetic hollow porous carbon-based drug delivery system (MHPCNs–SS–PGA–FA/DOX) was developed by Wu et alCitation81. The poly-γ-glutamic acid-capped shell of hollow NPs included an inner Fe3O4 layer and a porous carbon outer layer. The authors used silica nanospheres and ferrocene to obtain SiO2@Fe3O4@C via the sacrificial template method. The silica core was then etched using NH3·H2O to form the MHPCNs. MRI-guided therapy was easily performed in mice with cervical cancer and the accumulation of NPs in vivo was monitored by MRI. Moreover, drug release was stimulated by GSH, pH and NIR light due to the unique properties of the MHPCNs. This guaranteed excellent tumour suppression capacity with negligible side effects in mice.
In addition, Fan et al fabricated nanozymes (Au@HCNs) with a yolk-shell structure to achieve catalytic-photothermal colon cancer therapy.Citation82 They used Au as the nanozyme, which functions as a peroxidase and oxidase to regulate reactive oxygen species (ROS). However, unsupported Au suffered from poor stability, which resulted in a decrease in catalytic efficiency. Thus, carbon shells were used to shelter Au, endowing the nanozymes with high stability in a harsh environment and long-term delivery. Moreover, an ROS burst was observed using irradiation with an 808-nm laser, indicating that the catalytic effect of Au@HCNs could be enhanced by the photothermal effect. The in vivo study verified the authors’ concept of catalytic-PTT as CT26 tumour growth was successfully inhibited by intravenous administration of Au@HCNs.
Hollow Silica Nanostructures
Silica-based NPs have been used as nanocarriers of photosensitisers and drugs in recent years due to their unrivalled biocompatibility (). As mentioned above, CuS NPs suffer from poor biocompatibility at high concentrations, leading to the development of the two methods described in the previous section. Here, we will introduce a third method: encapsulation by silica shells. In 2007, Li et al developed a dual-imaging-guided multifunctional platform (CuS@mSiO2-TD/ICG) to achieve PDT/PTT synergistic breast cancer therapy.Citation83 They coated the NPs with silica shells, which not only enhanced the biocompatibility, but also greatly improved the loading capacity. As a result, ICG, which shared a similar absorption wavelength at 808 nm with CuS, provided a synergistic photothermal effect with CuS. Moreover, as the temperature increased, the gatekeeper of the nanocarrier, 1-tetradecanol (TD), reached its melting point of 39°C, followed by a phase-change (solid TD to liquid TD) to allow the controlled release of ICG. ICG then underwent a reaction with oxygen in cancer cells to facilitate photodynamic treatment by yielding ROS. Simultaneously, ICG served as an indicator of the drug distribution in in vivo experiments by yielding fluorescence and PA signals. Early this year, Du et al developed a multimodal imaging-guided interventional therapy for pancreatic cancer.Citation84 In this study, Au star endowed the NP, named Gem-perfluorohexane-Au star-HMS (GN), with CT and PA imaging capacity. Meanwhile, Gem and perfluorohexane were loaded for chemotherapy and enhancing US imaging, respectively, and the GN was modified with IGF1 to form GN-T for targeted delivery. US, CT and PA imaging then demonstrated that the GN-based nanodrugs could selectively accumulate at the tumour site. Notably, to achieve sustainable release of GN-T, the authors utilised a thermosensitive gel, which solidified after being injected into the tumour site. GN-T was then slowly released with gel erosion. In summary, temperature-controlled PTT and single-dose administrated chemotherapy are promising methods for surgical resection and postoperative chemotherapy to prevent the recurrence of pancreatic cancer.
In 2017, Yu et al developed a novel type of hollow silicon oxide-based NP,Citation85 named H-SiOx NPs, with a non-stoichiometric ratio (x = 0.92). H-SiOx NPs were synthesised from hollow silica NPs through a magnesiothermic reduction process. This process endowed H-SiOx NPs with a considerable amount of oxygen vacancy, inducing free carriers. With such a high concentration of free carriers, a wide range of absorption from UV to NIR in the second NIR (II) window was ensured. Meanwhile, a high PCE was observed at 1064 nm (48.6%), which was higher than that of all silicon-based NPs. Given this property, efficient breast cancer PTT in vivo could be successfully achieved at a low power density (0.6 W/cm2) under 1064-nm NIR light irradiation. In conclusion, due to their high biocompatibility, silica-based NPs have become one of the safest options for clinical application.
Other Hollow Inorganic Nanostructures
Prussian Blue
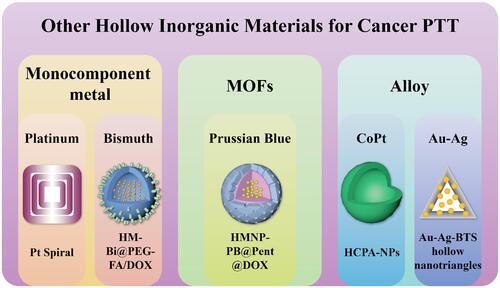
Other hollow inorganic nanostructures have also been designed for biological imaging and photothermal-based cancer therapy ().Citation86–Citation89 Among these, metal-organic frameworks are drawing significant attention due to their attractive properties, such as considerable pore volume and surface area.Citation86 Recently, Prussian blue (PB) NPs have been the research focus, since PB was approved for clinical application by the US Food and Drug Administration. That is to say, compared to other nanomaterials mentioned above, Prussian Blue is more likely to be applied to clinical treatment, with the clinical proved biosafety for human body. In 2017, Cai et al developed PB-based NPs (HMNP-PB@Pent@DOX) for trimodal imaging-guided controlled drug release and PTT.Citation90 The authors used polystyrene was used as a template, and coated it with a Fe3O4 shell to function as a T2-weighted MRI contrast agent, followed by coating with PB to form a drug carrier. The drug DOX was loaded/locked with 1-pentadecanol inside the carrier. Under NIR light, PB then converted light into heat, and when the temperature exceeded 42°C, 1-pentadecanol gradually melted and DOX was released at the liver tumour site. In 2018, Zhou et al fabricated glucose oxidase (GOx)-loaded hollow PB NPs (PHPBNs) for synergetic tumour starvation therapy and low-temperature PTT.Citation21 The proliferation of tumour cells requires a considerable amount of ATP, which is mainly generated via anaerobic glycolysis. Thus, to deprive cancer cells of the energy in order to inhibit their growth, GOx was used to consume glucose in tumour cells. Moreover, PHPBNs not only protected GOx from degradation in blood circulation but also produced oxygen, which is essential for the functionalisation of GOx. In addition, PHPBNs ameliorated the tumour resistance problem in a low hyperthermia environment by suppressing the expression of heat shock proteins (HSPs). As HSPs replace heat-denatured proteins and their expression is closely related to the ATP level in cancer cells,Citation91 inhibiting ATP production increases the efficiency of low-temperature PTT.Citation92 In an in vivo study, PHPBNs successfully inhibited liver tumour growth by the combination of starvation and mild PTT with almost no side effects in normal tissues.
Monocomponent Metal
Most recently, PTT using NIR-II window has gained popularity for its superior properties compared to PTT using the NIR-I region, including deeper tissue penetration, lower photon scattering and maximum permissible exposure.Citation93 Although the SPR wavelength can be tuned by adjusting the shape and size of the metal nanostructures, few metal NPs for NIR-II PTT have been developed. Notably, because the high-order longitudinal SPR mode of platinum (Pt) NPs is in the NIR zone, it is possible to extend their SPR wavelength to the NIR-II region.Citation94 In 2019, Wang et al fabricated hollow Pt nanoframes (Pt Spirals) for CT-guided PTT.Citation95 These Pt Spirals were smartly designed with a multilevel structure: first, 1D nanowires were tangentially assembled to produce 2D nanoshells. Then, 3D nanoframes were constructed by layer-by-layer assembling of 2D nanoshells. This smart design and SPR tuning paved the way for the design of other materials. The researchers proved that the absorption and PCE of the prepared Pt nanoframes were superior to those of solid Pt NPs, with the PCE (52.5%) representing the highest among all Pt-based NPs. In addition, Pt possesses robust X-ray attenuation, allowing the Pt Spirals to serve as a CT contrast agent. Under CT imaging, the cervix cancer cells were successfully ablated by PTT. Due to their negligible toxicity and low cost, nanomaterials based on bismuth, known as a “green metal”, are promising for clinical application.Citation96 Three months ago, Huang et al fabricated mono-component hollow bismuth nanoshells (HM-Bi@PEG-FA NSs) for controlled drug delivery and PTT.Citation97 When conjugated to folate, bismuth nanoshells could be targeted to tumour sites, where drug release was stimulated by the joint effects of pH and NIR light irradiation. The therapeutic efficacy of chemo/PTT for lung cancer was then confirmed in in vivo experiments. These nanoshells were found to be an ideal option for clinical application due to their extraordinary biological safety.
Alloy
In addition to monocomponent metal nanostructures, metal alloys are also a suitable alternative for imaging-guided PTT. Yang et al developed hollow CoPt alloy NPs (HCPA-NPs) for PA and MRI-guided liver cancer PTT via a facile, green synthesis route.Citation98 Plant polyphenols were used as assistive agents for the formation of HCPA-NPs, of which the sizes could be adjusted by changing the size of the polyphenol. This method could also be used to obtain other hollow alloy NPs and provided a novel green approach for synthesis in other fields such as electronics and catalysis. This year, Li et al fabricated Au-Ag hollow nanotriangles for gas therapy and PTT.Citation99 In their work, the SO2 prodrug benzothiazole sulfinate (BTS) was loaded onto the nanotriangles for effective deep tumour therapy. The SO2 gas was able to diffuse into deep breast tumour tissues despite tumour heterogeneity. After being stimulated in the acidic tumour microenvironment, BTS then released SO2, which, along with PTT, upregulated the expression of Bax in mitochondria and promoted cancer cell apoptosis. Overall, this work offers a promising strategy for deep tumour synergistic therapy.
Photothermal-Based Therapy for Antibacterial Treatment
Hollow Gold Nanostructures
As mentioned earlier, gold nanostructures possess excellent light-to-heat conversion efficiency and can thus be used in antibacterial treatment. Meeker et al developed PDA-coated gold nanocages (AuNCs) for chemo/photothermal synergistic treatment of Staphylococcus aureus infections.Citation100 The selectivity of the NPs was achieved by the conjugation of antibodies against staphylococcal protein A. Under NIR light, the consequent temperature rise was sufficient to kill the bacteria and was accompanied by the release of the loaded antibiotic daptomycin from PDA. The results of bactericidal experiments using biofilms showed that with PTT alone, the bacteria were initially eradiated but recovered within 24 hours. In contrast, the synergistic effect of antibiotics and PTT inhibited S. aureus growth completely and prevented regrowth. This study provided a robust method for the treatment of biofilm-associated infections to overcome the problem of MDR. In 2018, the authors developed PDA-coated AuNCs with various antibodies and antibiotics to evaluate the versatility of their previous method.Citation101 In this study, the authors successfully verified the versatility of the method by inhibiting the growth of the gram-negative pathogen Pseudomonas aeruginosa using a novel combination of antibiotics and antibodies. Further, to tackle the problem of MDR, Zhou et al fabricated gold-silver nanoshells (AuAgNSs) for wound healing.Citation102 As a bactericidal metal ion, Ag+ can be released from Ag NPs under laser irradiation and combined with PTT to exert a synergistic antibacterial effect. However, the photothermal efficiency of Ag is inferior to that of Au. As such, the authors developed nanoplatforms using both metals. They also conjugated 3.3′-diethylthiatricarbocyanine iodide to track residual bacteria by SERS for 8 weeks.
In recent years, macrophages have been used to achieve targeted drug delivery to tumour sites. This is achieved by coating nanodrugs with the membranes of macrophages.Citation103,Citation104 However, the application of this technique to antibacterial drug delivery is rare. Macrophages can be stimulated by various bacteria through different receptors expressed on the macrophage membrane. In one study, Wang et al coated gold-silver nanocages (GSNCs) with macrophage membranes pre-treated with S. aureus to form Sa-M-GSNCs,Citation105 and injected the Sa-M-GSNCs subcutaneously and intravenously to treat local and systemic (osteomyelitis) infections. Endowed with prolonged blood circulation time and retention at infected sites, this method paved the way for effective PTT-based antibacterial treatment.
Periodontitis is the main reason for tooth lossCitation106 and is generally caused by bacterial infections. Bacterial infections are also implicated in other diseases, such as diabetes and cardiovascular diseases.Citation107 To treat bacterial infections, Zhang et al fabricated a tetracycline (TC)-carrying nanoplatform (TC-PCM@GNC-PND) for light-controlled drug release and PTT.Citation108 They used phase-change material (PCM) and poly(N-isopropylacrylamide-co-diethylaminoethyl methacrylate) (PND) as two gatekeepers to prevent the premature release of TC. Under NIR laser irradiation, GNC converted light to heat, which then transformed the injectable solid into a gel at the infected site at 36°C as a result of the thermosensitive properties of PND. When the temperature rose to 45°C, PCM melted and released TC. The photothermal effect not only killed the bacteria but also improved the bactericidal efficiency of TC. In in vivo experiments, bone loss was used as an indicator for evaluating tissue destruction stemming from inflammation caused by bacteria. The results showed that the TC-PCM@GNC-PND-treated group experienced minimum bone loss, indicating the excellent therapeutic effect of this method.
Hollow Silica Nanostructures
Silica nanoshells have been shown to be excellent drug carriers in the chemo/photothermal treatment for cancer. However, due to their hollow interior and mesoporous walls, silica nanoshells can also be loaded with antibacterial drugs and combined with PTT to kill bacteria. Wang et al reported kanamycin-encapsulated gold nanorod-covered hollow silica NPs for antibacterial (E. coli BL21) chemo/PTT.Citation109 The gold nanorods served as photothermal agents and played the role of “gatekeepers”. Apart from the mechanism of gatekeepers mentioned above (phase-changeCitation83,Citation108 and bond-cleavage),Citation79 the authors reported an additional method. Under NIR light irradiation, apart from converting light to heat, the gold nanorods also underwent a shape-changing process before drug release. This synergistic mechanism reduced the drug dosage requirement and shortened the treatment time, providing greater bactericidal efficiency.
Similar to the structure of Sa-M-GSNCs developed by Wang,Citation105 silica-coated gold-silver nanocages (Au–Ag@SiO2 NCs) were fabricated by Xu et alCitation110. With Ag ion release, these nanocages achieved NIR-induced PTT both in an S. aureus biofilm model in vitro and in rats with wound infections. Interestingly, Ji et al produced a novel sandwich-like graphene-mesoporous silica (GS) nanoplatform (AA@GS@HA-MNPs) for targeted drug delivery. In this study, the •OH prodrug ascorbic acid (AA) was first introduced into the hollow pores, which were subsequently capped with hyaluronic acid-dopamine conjugates (HA-DA). The targeted release of AA was possible because HA was degraded by hyaluronidase (Hyal), which is overexpressed at bacterial infection sites.Citation111 Finally, Fe3O4 NPs were conjugated to GS to catalyse the •OH-generating effects of AA. The photothermal effect of graphene made synergistic chemo/PTT possible, dispersing stubborn biofilms and inhibiting bacteria growth. Moreover, the therapy was excellent for treating bacterial infections at tumour sites because cancer cells are the main producers of Hyal.Citation112,Citation113 The •OH produced could kill cancer cells and bacteria simultaneously.
Hollow Metallic Compound Nanostructures
As mentioned earlier, gold NPs have relatively high toxicity and low biocompatibility. However, in recent years, bismuth-based NPs have become popular due to their unrivalled biocompatibility.Citation56,Citation114,Citation115 This year, Qian et al reported a sea-urchin-like Bi2S3 hollow nanoplatform (TD/linalool@Bi2S3) for water sterilization.Citation116 In this study, Bi2S3 nanoplatforms encapsulated TD combined with the bactericidal agent linalool, the release of which could be controlled with an 808-nm laser to kill the bacteria (E. coli and S. aureus) in drinking water along with PTT.
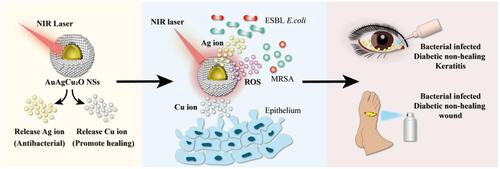
This year, Zhou et al developed dual-functional hollow AuAgCu2O nanospheres with both antibacterial and wound healing effects.Citation117 As shown in , the former was achieved using a combined therapy including photothermal effects, ROS generation and Ag ion release under laser irradiation to damage the cell walls, proteins and DNA of bacteria (MRSA and ESBL E.coli). The wound healing effects were derived from Cu ions released from AuAgCu2O nanostructures. The Cu ions were successfully used for promoting re-epithelialisation. The authors then verified the clinical potential of AuAgCu2O nanostructures in the treatment of diabetes-related chronic wounds and nonhealing keratitis. Later, the authors investigated ophthalmological diseases, and bromfenac sodium (BS) was conjugated to AuAgCu2O NSs to prevent post-cataract surgery endophthalmitis.Citation118 After intraocular injection of AuAgCu2O-BS NSs, the inflammatory symptoms were alleviated and the prognosis was improved due to a mild photothermal effect and the anti-inflammatory drug BS.
Figure 4 Near-Infrared (NIR)-Activated AuAgCu2O Nanoshells for Antibacterial-Resistant Bacterial Killing and Improved Wound Healing. The prepared AuAgCu2O NSs could release copper ions and silver ions under NIR laser irradiation. Consequently, multi-drug-resistant bacteria can be efficiently damaged through a synergistic antibiotic-photothermal strategy with silver ions and local high temperature, and the released copper ions could promote the re-epithelialization process, eventually accelerating the recovery of the nonhealing wound and keratitis. Data from Ye et al. Citation117
Other Hollow Nanostructures
Some other hollow nanostructures developed for photothermal-based antibacterial therapy have been relatively less reported. An example is hollow lanthanide-doped upconversion NPs (UCNPs), which emit visible light and even UV light under NIR light irradiation and provide relatively deep tissue penetration and low background autofluorescence.Citation119,Citation120 Due to these excellent properties, UCNPs are generally used in optical imaging. Given that antibacterial treatment is usually administered in vivo, moderate hyperthermia is crucial because overheating can harm healthy tissues. Thus, integrating a thermometer into a platform is a novel approach for temperature control. In 2017, Guo et al developed Nd3+/Yb3+/Er3+ co-doped yolk-shell-shaped upconverting thermometer-heater platforms (YS-GOF@Si) for antibacterial PTT.Citation121 In this study, the temperature of YS-GOF@Si rose under 808 nm irradiation. This effect was monitored in real time by fluorescence intensity ratio. However, the methods did not perform well in antibacterial experiments, with bacterial (E. coli) viability being 53.1% after treatment. Later, in 2018, the authors developed another hollow nanothermometer (LuVO4) that was also doped with lanthanides.Citation122 Furthermore, Cu2S was utilised to modify the olive-shaped NPs, which could absorb both incident light and NIR light from a luminescent core, providing excellent thermal sensitivity based on spectrally pure green emission. In addition, the nanothermometers prepared in this study possessed much higher bactericidal (E. coli and S. aureus) efficiency (~95%) than YS-GOF@Si. Overall, Guo et al provided a promising method for temperature-monitored, minimally invasive PTT.
In the cancer treatment section, we introduced Prussian blue as a member of the MOF family. Here, we introduce another member: HKUST-1. This year, Yu et al used HKUST-1 frameworks to encapsulate CuS NPs by converting trace amounts of Cu ions into CuS NPs. Under NIR light, the HKUST-1-supported CuS NPs could generate ROS and kill bacteria (E. coli and S. aureus) in combination with PTT.Citation123 Due to the protection provided by the framework, the aggregation and direct tissue contact of CuS NPs was avoided. By applying multimodal antibacterial therapy, including PTT, PDT and Cu ion release, a bactericidal effect of over 99% was achieved, demonstrating great potential for biomedical applications.
Photothermal-Based Therapy for the Treatment of Other Diseases
Alzheimer’s Disease
As a common neurodegenerative disease, AD is usually seen in patients with dementiaCitation124 and is considered a worldwide health problem.Citation125 The extracellular accumulation of amyloid plaques is one of the hallmarks of AD. These plaques are mainly composed of amyloid‐β peptides (Aβ).Citation126 Given this fact, a promising strategy for treating AD involves preventing Aβ aggregation and destroying the already-formed Aβ fibrils. To date, various therapies for inhibiting Aβ aggregation have been investigated.Citation127–Citation129 However, these methods often provide insufficient inhibition of aggregation and suffer from a poor disaggregating capacity. Therefore, localised PTT may be a robust tool and an ideal hyperthermia therapy for AD treatment, with minimal side effects to surrounding tissues.
In 2017, Ruff et al developed hollow Au NPs (HAuNS) conjugated with CLPFFD peptides for selectively binding Aβ structures.Citation130 They fabricated CLPFFD-PEG-HAuNS via two approaches, first by binding the CLPFFD peptides directly to the HAuNS and second by binding the peptides indirectly to a PEG ligand shell. The authors used in vitro blood-brain barrier (BBB) model to prove that the impedance of BBB passage caused by the negative charge on the peptide was countered by coupling peptides to the PEG ligand. The Aβ aggregation-inhibiting effect of CLPFFD peptide-modified HAuNS was demonstrated experimentally in a later workCitation131 and in in vivo work performed previously.Citation132,Citation133 These studies showed that HAuNS has potential for application in AD treatment.
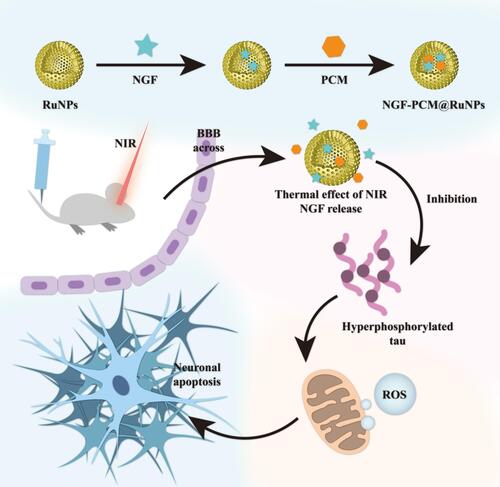
The hyperphosphorylation of tau protein is also a main culprit in AD, contributing to the aggregation of the protein followed by the production of ROS.Citation134 Abnormal production of ROS in the brain results in inflammatory reactions, which can impair neuron function and cause neuronal apoptosis, consequently influencing the basic functions of the brain such as learning and memory. In case of AD, repairing the impaired neurons and preventing tau protein hyperphosphorylation represent a promising treatment for AD. This year, Zhou et al fabricated nerve growth factor (NGF)-loaded hollow ruthenium (NGF-PCM@Ru) nanoflowers for AD treatment.Citation135 As a member of the neurotrophin family, NGF could serve as an inhibitor of tau hyperphosphorylation. However, the very low rate of BBB passage and short blood circulation time limited its biological application. As shown in , Ru NPs were utilised as carriers for NGF. Under NIR laser irradiation, BBB permeability was enhanced, helping the nanocarriers to enrich in brain tissue. Simultaneously, as the temperature rose, NGF was released via a phase-change process of PCM. Using the water maze test and nesting experiments, the authors proved the validity of NGF-PCM@Ru in rescuing memory loss in mice with AD.
Figure 5 NGF-PCM@Ru NPs were used to cross BBB under NIR irradiation, and then PCM triggered the release of NGF in response to thermal effects, thereby achieving reduction of ROS production and mitigation of neuronal damage by inhibiting tau hyperphosphorylation. Data from Zhou et al.Citation135
Obesity
In modern society, obesity is a fast-growing disease that can cause serious health impairments.Citation136 In children in particular, the obesity ratio dramatically increased to 47% in the 30+ years from 1980 to 2013.Citation137 In addition, obesity is implicated in many chronic diseases, including ischemic stroke,Citation138 type-2 diabetesCitation139 and fatty liver disease.Citation140 At present, liposuction and anti-obesity drugs are the two major treatment methods to combat obesity. However, discomfort and pain caused by liposuction and side effects stemming from drug abuse are the major drawbacks restricting their clinical application.
Recently, a novel obesity treatment that utilised NPs was developed.Citation141 The therapeutic strategy in most of these treatments was photothermal lipolysis. Han and Kim developed polypyrrole (PPy)-covered hollow gold nanoshells (HAuNS@PPy) for adipocyte ablationCitation142 and performed ex vivo experiments to evaluate their therapeutic efficiency. They found that subcutaneous adipose tissue was degraded, along with apoptosis of adipocytes. However, this method non-selectively ablated both adipose tissues and normal tissues, limiting its clinical application. To address this limitation, Lee et al developed HA-HAuNS-ATP for targeted transdermal delivery of the nanoshells.Citation143 In this study, hyaluronate was conjugated to HAuNS, endowing the nanoshells with transdermal ability, and the targeting capacity of the nanoshells was derived from the ATP sequence. The nanodrugs penetrated the epidermis and targeted adipocytes. Under laser irradiation, adipocytes were ablated by the photothermal effect, which could be visualised using PA imaging. The results showed that 20% of the initial lipid was eliminated, thus demonstrating a potential novel non-invasive therapy for obesity.
Endometriosis
Endometriosis is a common oestrogen-dependent gynaecological disease, which is defined as the growth of endometrial cells outside the uterine cavity.Citation144 Endometriosis is implicated in many health problems, including dysmenorrhea, dyspareunia, pelvic pain and infertility.Citation145,Citation146 The disease affects 10% of women of reproductive age. In patients with endometriosis, the endometrial cells exhibit a decreased rate of apoptosis and an increased rate of proliferation. The environment in the ectopic endometrial tissue can prompt the implantation of endometrial cells and help them escape immune clearance.Citation147,Citation148 At present, there is no cure for this disorder, and the most frequently used treatment strategy is surgical, which is associated with a high recurrence rate (>50%) due to from the presence of endometriotic residues after surgical excision.Citation149 Given that endometriosis is similar to solid cancer in many aspects and the diseases are usually concomitant,Citation150 NP-based PTT could be an ideal therapeutic strategy for endometriosis.
In 2017, Guo et al developed targeted HAuNS for photothermal-based endometriosis therapy.Citation151 Neovascularisation, a common feature of both cancer and endometriosis, is closely associated with the overexpression of Eph receptors. To achieve the targeting effect, HAuNS was conjugated with TNYL peptides, which possess remarkable binding efficiency to EphB4 receptors. In in vivo experiments, under NIR light irradiation, TNYL-HAuNS remarkably inhibited lesion growth by photothermal ablation, with negligible damage to normal tissues. However, patients whose uterus is in a congestive state (menses) cannot be treated with this method since EphB4 is also highly expressed in the uterus.
Conclusion and Perspectives
In this review, we discussed the recent biomedical applications of hollow inorganic nanomaterials in PTT for various diseases. PTT, along with the advancements in nanotechnology, has been widely investigated by scientists owing to the high therapeutic efficiency of PTT and the properties of hollow nanoplatforms, which can provide an ideal method for combining different therapies and enhance the treatment efficiency. Specifically, the hollow structures can significantly boost the loading capacity of nanostructures, allowing them to serve as perfect carriers for imaging contrast agents and therapeutic drugs. With the help of PTT, controlled drug release can be realised at laser-irradiated sites. Given these properties, many theranostic nanomedicines (based on gold, metal chalcogenides, carbon, silica, etc.) have been developed to treat intractable health problems. First, we introduced the application of hollow inorganic nanomedicines to PTT-based cancer treatment. By loading various drugs and agents on nanostructures, a combination therapy consisting of PTT and other therapies, such as chemotherapy (DOX-loaded) and photodynamic therapy (Ce6- or ICG-loaded) can be achieved to improve therapeutic efficacy and prevent cancer recurrence. Moreover, loading of contrast agents can allow precise monitoring using a combination of MR, PA, fluorescence and US imaging. Second, we discussed a promising strategy for combating infections caused by drug-resistant bacteria. The synergistic effects of antibiotics and hyperthermia can effectively inhibit bacterial growth, preventing “superbug” drug resistance. Third, we mentioned the application of hollow inorganic nanomedicines for the treatment of other diseases, including AD, obesity and endometriosis. As in the cases of cancer and bacterial infections, the mechanism underlying the treatment of obesity and endometriosis with hollow inorganic nanomedicine was the ablation of adipocytes and endometrial cells. However, apart from the ablative effect and controlled drug release, NIR light-induced hyperthermia had a novel usage in AD treatment, which was to increase the BBB permeability of nanodrugs. These studies suggest a novel approach for the development of drugs for brain diseases. Drugs for other diseases may also benefit from this enhanced permeability of biological barriers.
All of these achievements suggest the promise of applying hollow inorganic nanomedicines for treatment in clinical patients. However, to date, according to the data from ClinicalTrials.gov, only 19 photothermal therapies have been registered for clinical trials, of which only two nanomedicines have completed clinical trials. Different from traditional energy-based ablation therapies including high energy laser and focused ultrasound, nanoparticle-based tissue ablation can achieve high specificity towards solid tumor, thereby contributing to relative negligible side-effects to normal tissue. One of the two nanophotothermal therapies named NANOM-FIM (Identifier:NCT01270139) was successfully carried out. In this study, silica-gold nanoparticles were delivered by a bioengineered stem cell patch, with the help of minimally invasive cardiac surgery (MICS CABG), significant regression of coronary atherosclerosis was achieved through plasmonic photothermal therapy.Citation152 Moreover, the long-term outcome showed that NANOM-FIM was superior to stent XIENCE V in safety and mortality.Citation153 The clinical trial of the other nanomedicine was gold nanoshells, named Auroshell® (Identifier: NCT00848042) they were developed to treat head and neck and prostate cancers. Although their preclinical experiments demonstrated that Auroshell® showed low toxicity to Beagle dogs,Citation154 some patients still had serious side-effects during the clinical trial, where 11 people were involved and only 5 people completed the trial. We believe that the main clinical limitation of hollow inorganic PTAs is their safety and toxicity. However, these drawbacks can be overcome to some extent. In this review, we described three methods for alleviating chronic toxicity and improving safety, including using biodegradable materials, reducing the dose of NPs and encapsulating NPs in biocompatible materials such as silica. More experiments using cell or animal models should be performed to ensure the safety of nanomedicines, following these suggested approaches. Moreover, insufficient PCE and photothermal stability are further obstacles in the clinical application of hollow inorganic nanomedicines. Thus, scientists should focus on improving the photothermal stability and PCE of nanomedicines to ensure sufficient photothermal effects at lower doses. Notably, although robust photothermal ablation could efficiently eliminate the target cells, the surrounding normal tissues are also likely to undergo apoptosis. Thus, the PTT strategy for each disease should be designed to be disease-specific. Specifically, higher temperatures can be used to kill cancer cells, whereas only mild hyperthermia should be used at some bacterial infection sites, such as eyes. In addition, the clinical application of many photothermal therapies are facing challenges such as limited light penetration depth. For most NIR-I PTAs, light penetration depth is confined to several millimeters due to the tissue scattering; however, as mentioned, by using NIR-II PTAs the penetration depth would be deeper, along with higher maximum permissible exposure (MPE), the clinical application of noninvasive deep tissue PTT would be possible. At present, for some diseases including macular diseases, acne vulgaris, oral cancer, and gastric cancer, NIR laser can be easily delivered to the superficial lesion via direct irradiation or gastroscope. While, for some diseases whose lesions are deeply buried in abdomen, such as pancreatic cancer (PC), NIR light cannot be delivered to the lesion directly. Recently, a novel method named interventional PTT (IPTT) was employed by Tian’s group to ablate PC deep in the abdomen.Citation155 In IPTT, an NIR optical fiber runs through an 18-gauge (G) percutaneous transhepatic cholangiography (PTC) needle to form the IPTT device. By using this device, NIR light can be easily delivered to the deep-buried tumor site; moreover, precise PTT can also be achieved, thereby reducing the ablative effect on normal tissues.
At present, many researchers are focusing on combating cancer, for which a considerable number of hollow inorganic nanoscale PTAs have been developed compared to those for other diseases. Here, we described the potential of these materials in treating other health problems, including bacterial infections, AD, obesity and endometriosis. We hope that this review can arouse the interest of researchers in applying hollow inorganic nanoscale PTAs to treat these conditions, and that in the future, more diseases would be successfully treated using hollow inorganic nanomaterial-based PTT.
Acknowledgment
This work was supported by the Ministry of Science and Technology of China (NO.2017ZX09101001-005-003), the National Natural Science Foundation of China (NO.81972892 and NO.81673364) and the Applied Technology Research and the Development Project of the Inner Mongolia Autonomous Region (2019GG035).
Disclosure
The authors report no conflicts of interest related to this work.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
- Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med. 2010;61:359–373. doi:10.1146/annurev.med.60.052907.094936
- Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94(1):1–11. doi:10.1016/j.radonc.2009.12.008
- Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3(7). doi:10.1038/s41392-017-0004-3
- Xiao Q, Zheng X, Bu W, et al. A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J Am Chem Soc. 2013;135(35):13041–13048. doi:10.1021/ja404985w
- Ray PC, Khan SA, Singh AK, Senapati D, Fan Z. Nanomaterials for targeted detection and photothermal killing of bacteria. Chem Soc Rev. 2012;41(8):3193–3209. doi:10.1039/c2cs15340h
- Vikesland PJ, Wigginton KR. Nanomaterial enabled biosensors for pathogen monitoring - a review. Environ Sci Technol. 2010;44(10):3656–3669. doi:10.1021/es903704z
- Wright GD, McCarthy MI. Molecular mechanisms of antibiotic resistance. Chem Commun. 2011;47(14):4055–4061. doi:10.1039/c0cc05111j
- Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64. doi:10.4137/PMC.S14459
- Zou L, Wang H, He B, et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics. 2016;6(6):762–772. doi:10.7150/thno.14988
- Shanmugam V, Selvakumar S, Yeh C. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43(17):6254–6287. doi:10.1039/C4CS00011K
- Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. doi:10.1021/ja057254a
- Wang J, Wu X, Shen P, et al. Applications of inorganic nanomaterials in photothermal therapy based on combinational cancer treatment. Int J Nanomedicine. 2020;15:1903–1914. doi:10.2147/IJN.S239751
- Xu JW, Yao K, Xu ZK. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11(18):8680–8691. doi:10.1039/C9NR01833F
- Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts Chem Res. 2008;41(12):1578–1586. doi:10.1021/ar7002804
- Chen J, Ning C, Zhou Z, et al. Nanomaterials as photothermal therapeutic agents. Prog Mater Sci. 2019;99:1–26. doi:10.1016/j.pmatsci.2018.07.005
- Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–2108.
- Zhou G, Xiao H, Li X, et al. Gold nanocage decorated pH-sensitive micelle for highly effective photothermo-chemotherapy and photoacoustic imaging. Acta Biomater. 2017;64:223–236. doi:10.1016/j.actbio.2017.10.018
- You Q, Sun Q, Yu M, et al. BSA-bioinspired gadolinium hybrid-functionalized hollow gold nanoshells for NIRF/PA/CT/MR quadmodal diagnostic imaging-guided photothermal/photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9(46):40017–40030. doi:10.1021/acsami.7b11926
- Guo L, Yan DD, Yang D, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. Acs Nano Nano. 2014;8(6):5670–5681. doi:10.1021/nn5002112
- Zhou J, Li M, Hou Y, et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. Acs Nano Nano. 2018;12(3):2858–2872. doi:10.1021/acsnano.8b00309
- Kennedy LC, Bickford LR, Lewinski NA, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7(2):169–183. doi:10.1002/smll.201000134
- Yang K, Zhang S, Zhang G, Sun X, Lee S, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi:10.1021/nl100996u
- Cheng Y, Hu J, Qin S, Zhang A, Zhang X. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials. 2020;232:119738. doi:10.1016/j.biomaterials.2019.119738
- Huang X, Zhang W, Guan G, Song G, Zou R, Hu J. Design and functionalization of the NIR-responsive photothermal semiconductor nanomaterials for cancer theranostics. Accounts Chem Res. 2017;50(10):2529–2538. doi:10.1021/acs.accounts.7b00294
- Xu L, Cheng L, Wang C, Peng R, Liu Z. Conjugated polymers for photothermal therapy of cancer. Polym Chem-UK. 2014;5(5):1573–1580. doi:10.1039/C3PY01196H
- Lovell JF, Jin CS, Huynh E, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10(4):324–332. doi:10.1038/nmat2986
- Hartland GV. Coherent excitation of vibrational modes in metallic nanoparticles. Annu Rev Phys Chem. 2006;57(1):403–430. doi:10.1146/annurev.physchem.57.032905.104533
- Wang J, Li N. Functional hollow nanostructures for imaging and phototherapy of tumors. J Mater Chem B. 2017;5(43):8430–8445. doi:10.1039/C7TB02381B
- Hao E, Li S, Bailey RC, Zou S, Schatz GC, Hupp JT. Optical properties of metal nanoshells. J Phys Chem B. 2004;108(4):1224–1229. doi:10.1021/jp036301n
- Ren Q, Bai L, Zhang X, et al. Preparation, modification, and application of hollow gold nanospheres. J Nanomater. 2015;2015:534070. doi:10.1155/2015/534070
- You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. Acs Nano Nano. 2010;4(2):1033–1041. doi:10.1021/nn901181c
- Kwizera EA, Chaffin E, Shen X, et al. Size- and shape-controlled synthesis and properties of magnetic-plasmonic core-shell nanoparticles. J Phys Chem C Nanomater Interfaces. 2016;120(19):10530–10546. doi:10.1021/acs.jpcc.6b00875
- Bao Z, Liu X, Liu Y, Liu H, Zhao K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J Pharm Sci. 2016;11(3):349–364. doi:10.1016/j.ajps.2015.11.123
- Gao D, Guo X, Zhang X, et al. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Materials Today Bio. 2020;5:100035. doi:10.1016/j.mtbio.2019.100035
- Yu Z, Chan WK, Zhang Y, Tan T. Near-infrared-II activated inorganic photothermal nanomedicines. Biomaterials. 2020;120459. doi:10.1016/j.biomaterials.2020.120459
- Austin LA, Mackey MA, Dreaden EC, El-Sayed MA. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch Toxicol. 2014;88(7):1391–1417.
- Jain PK, Huang X, El-Sayed IH, El-Sayad MA. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2(3):107–118. doi:10.1007/s11468-007-9031-1
- Wang R, Deng J, He D, et al. PEGylated hollow gold nanoparticles for combined X-ray radiation and photothermal therapy in vitro and enhanced CT imaging in vivo. Nanomedicine-Uk. 2019;16:195–205. doi:10.1016/j.nano.2018.12.005
- Li Y, He D, Tu J, et al. The comparative effect of wrapping solid gold nanoparticles and hollow gold nanoparticles with doxorubicin-loaded thermosensitive liposomes for cancer thermo-chemotherapy. Nanoscale. 2018;10(18):8628–8641. doi:10.1039/C7NR09083H
- Cai K, Zhang W, Zhang J, Li H, Han H, Zhai T. Design of gold hollow nanorods with controllable aspect ratio for multimodal imaging and combined chemo-photothermal therapy in the second near-infrared window. ACS Appl Mater Interfaces. 2018;10(43):36703–36710. doi:10.1021/acsami.8b12758
- Zhan C, Huang Y, Lin C, Huang S, Zeng F, Wu S. A gold nanocage/cluster hybrid structure for whole-body multispectral optoacoustic tomography imaging, EGFR inhibitor delivery, and photothermal therapy. Small. 2019;15:190030933. doi:10.1002/smll.201900309
- Feng Y, Cheng Y, Chang Y, et al. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials. 2019;217:119327. doi:10.1016/j.biomaterials.2019.119327
- Huang S, Liu Y, Xu X, et al. Triple therapy of hepatocellular carcinoma with microRNA-122 and doxorubicin co-loaded functionalized gold nanocages. J Mater Chem B. 2018;6(15):2217–2229. doi:10.1039/C8TB00224J
- Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100(23):13549–13554. doi:10.1073/pnas.2232479100
- Wang J, Wheeler D, Zhang JZ, Achilefu S, Kang KA. NIR fluorophore-hollow gold nanosphere complex for cancer enzyme-triggered detection and hyperthermia. In: Welch WJ, Palm F, Bruley DF, Harrison DK, editors. Advances in Experimental Medicine and Biology. Springer; Vol. 765. 2013:323–328.
- Chen J, McLellan JM, Siekkinen A, Xiong Y, Li Z, Xia Y. Facile synthesis of gold-silver nanocages with controllable pores on the surface. J Am Chem Soc. 2006;128(46):14776–14777. doi:10.1021/ja066023g
- Yavuz MS, Cheng Y, Chen J, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8(12):935–939. doi:10.1038/nmat2564
- Chen JY, Wiley B, Li ZY, et al. Gold nanocages: engineering their structure for biomedical applications. Adv Mater. 2005;17(18):2255–2261. doi:10.1002/adma.200500833
- Zhao Y, Pang B, Detering L, et al. Melanocortin I receptor targeted imaging of melanoma with gold nanocages and positron emission tomography. Mol Imaging. 2018;17:153601211877582. doi:10.1177/1536012118775827
- Wang Y, An L, Lin J, Tian Q, Yang S. A hollow Cu9S8 theranostic nanoplatform based on a combination of increased active sites and photothermal performance in enhanced chemodynamic therapy. Chem Eng J. 2020;385:123925. doi:10.1016/j.cej.2019.123925
- Wang D, Dong H, Li M, et al. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. Acs Nano Nano. 2018;12(6):5241–5252. doi:10.1021/acsnano.7b08355
- Zeng W, Zhang H, Deng Y, et al. Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. Chem Eng J. 2020;389:124494. doi:10.1016/j.cej.2020.124494
- Li Q, Ren J, Chen Q, et al. A HMCuS@MnO(2)nanocomplex responsive to multiple tumor environmental clues for photoacoustic/fluorescence/magnetic resonance trimodal imaging-guided and enhanced photothermal/photodynamic therapy. Nanoscale. 2020;12(23):12508–12521. doi:10.1039/D0NR01547D
- Zhang C, Li D, Pei P, et al. Rod-based urchin-like hollow microspheres of Bi2S3: facile synthesis, photo-controlled drug release for photoacoustic imaging and chemo-photothermal therapy of tumor ablation. Biomaterials. 2020;237:119835. doi:10.1016/j.biomaterials.2020.119835
- Song Y, Wang Y, Zhu Y, et al. Biomodal tumor-targeted and redox-responsive Bi2Se3 hollow nanocubes for MSOT/CT imaging guided synergistic low-temperature photothermal radiotherapy. Adv Healthc Mater. 2019;8(16):1900250. doi:10.1002/adhm.201900250
- Sun S, Li P, Liang S, Yang Z. Diversified copper sulfide (Cu2-xS) micro-/nanostructures: a comprehensive review on synthesis, modifications and applications. Nanoscale. 2017;9(32):11357–11404.
- Dong K, Liu Z, Li Z, Ren J, Qu X. Hydrophobic anticancer drug delivery by a 980 nm laser-driven photothermal vehicle for efficient synergistic therapy of cancer cells in vivo. Adv Mater. 2013;25(32):4452–4458. doi:10.1002/adma.201301232
- Guo L, Panderi I, Yan DD, et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. Acs Nano Nano. 2013;7(10):8780–8793. doi:10.1021/nn403202w
- Qiu Y, Lin M, Chen G, et al. Photodegradable CuS SERS probes for lntraoperative residual tumor detection, ablation, and self-clearance. ACS Appl Mater Inter. 2019;11(26):23436–23444. doi:10.1021/acsami.9b00469
- Zhang H, Chen Y, Cai Y, et al. Paramagnetic CuS hollow nanoflowers for T-2-FLAIR magnetic resonance imaging-guided thermochemotherapy of cancer. Biomater Sci-UK. 2019;7(1):409–418. doi:10.1039/C8BM01412D
- Brady DC, Crowe MS, Turski ML, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509(7501):492. doi:10.1038/nature13180
- Martin F, Linden T, Katschinski DM, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105(12):4613–4619. doi:10.1182/blood-2004-10-3980
- Jain S, Cohen J, Ward MM, et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann Oncol. 2013;24(6):1491–1498. doi:10.1093/annonc/mds654
- Guan G, Wang X, Huang X, et al. Porous cobalt sulfide hollow nanospheres with tunable optical property for magnetic resonance imaging-guided photothermal therapy. Nanoscale. 2018;10(29):14190–14200. doi:10.1039/C8NR01926F
- Ding B, Zheng P, Ma P, Lin J. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv Mater. 2020;32:190582310. doi:10.1002/adma.201905823
- An K, Kwon SG, Park M, et al. Synthesis of uniform hollow oxide nanoparticles through nanoscale acid etching. Nano Lett. 2008;8(12):4252–4258. doi:10.1021/nl8019467
- Shin J, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew Chem Int Ed. 2009;48(2):321–324. doi:10.1002/anie.200802323
- Chen Y, Yin Q, Ji X, et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials. 2012;33(29):7126–7137. doi:10.1016/j.biomaterials.2012.06.059
- Wang L, Guan S, Weng Y, et al. Highly efficient vacancy-driven photothermal therapy mediated by ultrathin MnO2 nanosheets. ACS Appl Mater Inter. 2019;11(6):6267–6275. doi:10.1021/acsami.8b20639
- Li X, Feng X, Sun C, Liu Y, Zhao Q, Wang S. Mesoporous carbon-manganese nanocomposite for multiple imaging guided oxygen-elevated synergetic therapy. J Control Release. 2020;319:104–118. doi:10.1016/j.jconrel.2019.12.042
- Lin X, Fang Y, Tao Z, et al. Tumor-microenvironment-induced all-in-one nanoplatform for multimodal imaging-guided chemical and photothermal therapy of cancer. ACS Appl Mater Inter. 2019;11(28):25043–25053. doi:10.1021/acsami.9b07643
- Kam N, O’Connell M, Wisdom JA, Dai HJ. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA. 2005;102(33):11600–11605. doi:10.1073/pnas.0502680102
- Chen D, Wang C, Jiang F, Liu Z, Shu C, Wan L. In vitro and in vivo photothermally enhanced chemotherapy by single-walled carbon nanohorns as a drug delivery system. J Mater Chem B. 2014;2(29):4726–4732. doi:10.1039/C4TB00249K
- Crochet J, Clemens M, Hertel T. Quantum yield heterogeneities of aqueous single-wall carbon nanotube suspensions. J Am Chem Soc. 2007;129(26):8058–8059. doi:10.1021/ja071553d
- Lu G, Shang W, Deng H, et al. Targeting carbon nanotubes based on IGF-1R for photothermal therapy of orthotopic pancreatic cancer guided by optical imaging. Biomaterials. 2019;195:13–22. doi:10.1016/j.biomaterials.2018.12.025
- Sabella S, Carney RP, Brunetti V, et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale. 2014;6(12):7052–7061. doi:10.1039/c4nr01234h
- Li X, Yan Y, Lin Y, et al. Hollow mesoporous carbon as a near-infrared absorbing carrier compared with mesoporous carbon nanoparticles for chemophotothermal therapy. J Colloid Interf Sci. 2017;494:159–169. doi:10.1016/j.jcis.2017.01.090
- Wang X, Lin Y, Li X, et al. Fluorescent carbon dot gated hollow mesoporous carbon for chemo-photothermal synergistic therapy. J Colloid Interf Sci. 2017;507:410–420. doi:10.1016/j.jcis.2017.08.010
- Fang J, Liu Y, Chen Y, Ouyang D, Yang G, Yu T. Graphene quantum dots-gated hollow mesoporous carbon nanoplatform for targeting drug delivery and synergistic chemo-photothermal therapy. Int J Nanomed. 2018;13:5991–6007. doi:10.2147/IJN.S175934
- Wu F, Zhang M, Lu H, et al. Triple stimuli-responsive magnetic hollow porous carbon-based nanodrug delivery system for magnetic resonance imaging-guided synergistic photothermal/chemotherapy of cancer. ACS Appl Mater Inter. 2018;10(26):21939–21949. doi:10.1021/acsami.8b07213
- Fan L, Xu X, Zhu C, et al. Tumor catalytic-photothermal therapy with yolk-shell gold@carbon nanozymes. ACS Appl Mater Inter. 2018;10(5):4502–4511. doi:10.1021/acsami.7b17916
- You Q, Sun Q, Wang J, et al. A single-light triggered and dual-imaging guided multifunctional platform for combined photothermal and photodynamic therapy based on TD-controlled and ICG-loaded CuS@mSiO(2). Nanoscale. 2017;9(11):3784–3796. doi:10.1039/C6NR09042G
- Xing L, Li X, Xing Z, et al. Silica/gold nanoplatform combined with a thermosensitive gel for imaging-guided interventional therapy in PDX of pancreatic cancer. Chem Eng J. 2020;382:122949. doi:10.1016/j.cej.2019.122949
- Yu X, Yang K, Chen X, Li W. Black hollow silicon oxide nanoparticles as highly efficient photothermal agents in the second near-infrared window for in vivo cancer therapy. Biomaterials. 2017;143:120–129. doi:10.1016/j.biomaterials.2017.07.037
- Chen W, Zeng K, Liu H, et al. Cell membrane camouflaged hollow prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv Funct Mater. 2017;27:160579511.
- Cai X, Gao W, Ma M, et al. A prussian blue-based core-shell hollow-structured mesoporous nanoparticle as a smart theranostic agent with ultrahigh pH-responsive longitudinal relaxivity. Adv Mater. 2015;27(41):6382. doi:10.1002/adma.201503381
- Song G, Liang C, Yi X, et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv Mater. 2016;28(14):2716–2723. doi:10.1002/adma.201504617
- Liu Z, Cheng L, Zhang L, Yang Z, Liu Z, Fang J. Sub-100 nm hollow Au-Ag alloy urchin-shaped nanostructure with ultrahigh density of nanotips for photothermal cancer therapy. Biomaterials. 2014;35(13):4099–4107. doi:10.1016/j.biomaterials.2014.01.053
- Li J, Zhang F, Hu Z, et al. Drug “pent-up” in hollow magnetic prussian blue nanoparticles for nir-induced chemo-photothermal tumor therapy with trimodal imaging. Adv Healthc Mater. 2017;6:170000514.
- Ungelenk S, Moayed F, Ho C, et al. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun. 2016;7:13673.
- Chen W, Luo G, Lei Q, et al. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. Acs Nano Nano. 2017;11(2):1419–1431. doi:10.1021/acsnano.6b06658
- Jiang Y, Li J, Zhen X, Xie C, Pu K. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Adv Mater. 2018;30:170598014. doi:10.1002/adma.201705980
- Zhang N, Han C, Xu Y, et al. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat Photonics. 2016;10(7):473–482. doi:10.1038/nphoton.2016.76
- Wang Q, Wang H, Yang Y, et al. Plasmonic Pt superstructures with boosted near-infrared absorption and photothermal conversion efficiency in the second biowindow for cancer therapy. Adv Mater. 2019;31:190483646. doi:10.1002/adma.201904836
- Mohan R. Green bismuth. Nat Chem. 2010;2(4):336. doi:10.1038/nchem.609
- Huang Y, Xue Z, Zeng S. Hollow mesoporous Bi@PEG-FA nanoshell as a novel dual-stimuli-responsive nanocarrier for synergistic chemo-photothermal cancer therapy. ACS Appl Mater Inter. 2020;12(28):31172–31181. doi:10.1021/acsami.0c07372
- Song XR, Yu SX, Jin GX, et al. Plant polyphenol-assisted green synthesis of hollow CoPt alloy nanoparticles for dual-modality imaging guided photothermal therapy. Small. 2016;12(11):1506–1513. doi:10.1002/smll.201503250
- Xu M, Lu Q, Song Y, Yang L, Li J, Li N. Enhanced Bax upregulating in mitochondria for deep tumor therapy based on SO(2) prodrug loaded Au-Ag hollow nanotriangles. Biomaterials. 2020;250:120076. doi:10.1016/j.biomaterials.2020.120076
- Meeker DG, Jenkins SV, Miller EK, et al. Synergistic photothermal and antibiotic killing of biofilm-associated staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis. 2016;2(4):241–250. doi:10.1021/acsinfecdis.5b00117
- Meeker DG, Wang T, Harrington WN, et al. Versatility of targeted antibiotic-loaded gold nanoconstructs for the treatment of biofilm-associated bacterial infections. Int J Hyperther. 2018;34(2):209–219. doi:10.1080/02656736.2017.1392047
- He J, Qiao Y, Zhang H, et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials. 2020;234:119763. doi:10.1016/j.biomaterials.2020.119763
- Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–9618. doi:10.1021/acsami.6b00853
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi:10.1016/j.immuni.2011.05.006
- Wang C, Wang Y, Zhang L, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30(46):e1804023. doi:10.1002/adma.201804023
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
- Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017;11(2):72–80.
- Zhang L, Wang Y, Wang C, et al. Light-activable on-demand release of nano-antibiotic platforms for precise synergy of thermochemotherapy on periodontitis. ACS Appl Mater Interfaces. 2020;12(3):3354–3362. doi:10.1021/acsami.9b17335
- Hu B, Zhang LP, Chen XW, Wang JH. Gold nanorod-covered kanamycin-loaded hollow SiO2 (HSKAu(rod)) nanocapsules for drug delivery and photothermal therapy on bacteria. Nanoscale. 2013;5(1):246–252. doi:10.1039/C2NR32457A
- Wu S, Li A, Zhao X, et al. Silica-coated gold-silver nanocages as photothermal antibacterial agents for combined anti-infective therapy. ACS Appl Mater Interfaces. 2019;11(19):17177–17183. doi:10.1021/acsami.9b01149
- Hynes WL, Walton SL. Hyaluronidases of gram-positive bacteria. FEMS Microbiol Lett. 2000;183(2):201–207. doi:10.1111/j.1574-6968.2000.tb08958.x
- Choi KY, Yoon HY, Kim J, et al. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. Acs Nano Nano. 2011;5(11):8591–8599. doi:10.1021/nn202070n
- Lee Y, Lee H, Kim YB, et al. Bioinspired surface immobilization of hyaluronic acid on monodisperse magnetite nanocrystals for targeted cancer imaging. Adv Mater. 2008;20(21):4154.
- Ren J, Zhang L, Zhang J, et al. Light-activated oxygen self-supplied starving therapy in near-infrared (NIR) window and adjuvant hyperthermia-induced tumor ablation with an augmented sensitivity. Biomaterials. 2020;234:119771. doi:10.1016/j.biomaterials.2020.119771
- Sun L, Li Q, Zhang L, et al. Stimuli responsive PEGylated bismuth selenide hollow nanocapsules for fluorescence/CT imaging and light-driven multimodal tumor therapy. Biomater Sci. 2019;7(7):3025–3040. doi:10.1039/C9BM00351G
- Wang W, Zhang C, Zhang M, et al. Precisely photothermal controlled releasing of antibacterial agent from Bi2S3 hollow microspheres triggered by NIR light for water sterilization. Chem Eng J. 2020;381: 122630. doi:10.1016/j.cej.2019.122630
- Qiao Y, He J, Chen W, et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. Acs Nano Nano. 2020;14(3):3299–3315. doi:10.1021/acsnano.9b08930
- Ye Y, He J, Qiao Y, et al. Mild temperature photothermal assisted anti-bacterial and anti-inflammatory nanosystem for synergistic treatment of post-cataract surgery endophthalmitis. Theranostics. 2020;10(19):8541–8557. doi:10.7150/thno.46895
- Rodriguez-Sevilla P, Zhang Y, Haro-Gonzalez P, et al. Thermal scanning at the cellular level by an optically trapped upconverting fluorescent particle. Adv Mater. 2016;28(12):2421–2426. doi:10.1002/adma.201505020
- Del Rosal B, Ximendes E, Rocha U, Jaque D. In vivo luminescence nanothermometry: from materials to applications. Adv Opt Mater. 2017;5(1):16005081. doi:10.1002/adom.201600508
- Suo H, Zhao X, Zhang Z, Guo C. 808 nm light-triggered thermometer-heater upconverting platform based on Nd(3+)-sensitized yolk-shell GdOF@SiO(2). ACS Appl Mater Interfaces. 2017;9(50):43438–43448. doi:10.1021/acsami.7b12753
- Suo H, Zhao X, Zhang Z, Wu Y, Guo C. Upconverting LuVO(4):Nd(3+)/Yb(3+)/Er(3+)@SiO(2)@Cu(2)S hollow nanoplatforms for self-monitored photothermal ablation. ACS Appl Mater Interfaces. 2018;10(46):39912–39920. doi:10.1021/acsami.8b18184
- Yu P, Han Y, Han D, et al. In-situ sulfuration of Cu-based metal-organic framework for rapid near-infrared light sterilization. J Hazard Mater. 2020;390:122126. doi:10.1016/j.jhazmat.2020.122126
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi:10.1016/S0140-6736(06)69113-7
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi:10.1016/j.jalz.2012.11.007
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi:10.1152/physrev.2001.81.2.741
- Poduslo JF, Curran GL, Kumar A, Frangione B, Soto C. Beta-sheet breaker peptide inhibitor of Alzheimer’s amyloidogenesis with increased blood-brain barrier permeability and resistance to proteolytic degradation in plasma. J Neurobiol. 1999;39(3):371–382. doi:10.1002/(SICI)1097-4695(19990605)39:3<371::AID-NEU4>3.0.CO;2-E
- Hudson SA, Ecroyd H, Dehle FC, Musgrave IF, Carver JA. (-)-epigallocatechin-3-gallate (EGCG) maintains kappa-casein in its pre-fibrillar state without redirecting its aggregation pathway. J Mol Biol. 2009;392(3):689–700. doi:10.1016/j.jmb.2009.07.031
- Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, et al. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J Am Chem Soc. 2008;130(46):15437–15443. doi:10.1021/ja8041806
- Ruff J, Hüwel S, Kogan MJ, Simon U, Galla HJ. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood-brain barrier. Nanomedicine-Uk. 2017;13(5):1645–1652. doi:10.1016/j.nano.2017.02.013
- Ruff J, Hassan N, Morales-Zavala F, et al. CLPFFD-PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J Mater Chem B. 2018;6(16):2432–2443. doi:10.1039/C8TB00655E
- Guerrero S, Herance JR, Rojas S, et al. Synthesis and in vivo evaluation of the biodistribution of a 18F-labeled conjugate gold-nanoparticle-peptide with potential biomedical application. Bioconjug Chem. 2012;23(3):399–408. doi:10.1021/bc200362a
- Guerrero S, Araya E, Fiedler JL, et al. Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine (Lond). 2010;5(6):897–913. doi:10.2217/nnm.10.74
- Alavi NS, Soussi-Yanicostas N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid Med Cell Longev. 2015;2015:151979.
- Zhou H, Gong Y, Liu Y, et al. Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer’s disease. Biomaterials. 2020;237:119822. doi:10.1016/j.biomaterials.2020.119822
- Chan RS, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010;7(3):765–783. doi:10.3390/ijerph7030765
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8
- Mitchell AB, Cole JW, McArdle PF, et al. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46(6):1690–1692. doi:10.1161/STROKEAHA.115.008940
- McCarthy MI, Feero WG, Guttmacher AE. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–2350. doi:10.1056/NEJMra0906948
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi:10.1002/hep.23280
- Li J, Cha R, Luo H, Hao W, Zhang Y, Jiang X. Nanomaterials for the theranostics of obesity. Biomaterials. 2019;223:119474. doi:10.1016/j.biomaterials.2019.119474
- Han S, Kim Y. Polypyrrole-coated hollow gold nanoshell exerts anti-obesity effects via photothermal lipolysis. Coll Surf A. 2019;570:414–419. doi:10.1016/j.colsurfa.2019.03.063
- Lee JH, Jeong HS, Lee DH, et al. Targeted hyaluronate-hollow gold nanosphere conjugate for anti-obesity photothermal lipolysis. ACS Biomater Sci Eng. 2017;3(12):3646–3653. doi:10.1021/acsbiomaterials.7b00549
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. doi:10.1038/nrendo.2013.255
- Wilson ML, Fleming KA, Kuti MA, Looi LM, Lago N, Ru K. Pathology and laboratory medicine in low-income and middle-income countries 1: access to pathology and laboratory medicine services: a crucial gap. Lancet. 2018;391(10133):1927–1938. doi:10.1016/S0140-6736(18)30458-6
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. doi:10.1016/S0889-8545(05)70302-8
- Kyama CM, Overbergh L, Mihalyi A, et al. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril. 2008;89(2):301–310. doi:10.1016/j.fertnstert.2007.02.057
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi:10.1016/j.fertnstert.2012.06.029
- Kikuchi I, Takeuchi H, Kitade M, Shimanuki H, Kumakiri J, Kinoshita K. Recurrence rate of endometriomas following a laparoscopic cystectomy. Acta Obstet Gyn Scan. 2006;85(9):1120–1124. doi:10.1080/00016340600627154
- Oral E, Aydin O, Kumbak BA, et al. Concomitant endometriosis in malignant and borderline ovarian tumours(*). J Obstet Gynaecol. 2018;38(8):1104–1109. doi:10.1080/01443615.2018.1441815
- Guo X, Li W, Zhou J, et al. Specific photothermal ablation therapy of endometriosis by targeting delivery of gold nanospheres. Small. 2017;13(15):1603270. doi:10.1002/smll.201603270
- Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7(17):8003–8015. doi:10.1039/C5NR01050K
- Kharlamov AN, Feinstein JA, Cramer JA, Boothroyd JA, Shishkina EV, Shur V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017;13(4):345–363. doi:10.2217/fca-2017-0009
- Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold-silica nanoshells). Int J Toxicol. 2012;31(6):584–594. doi:10.1177/1091581812465969
- Hu Y, Chi C, Wang S, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv Mater. 2017;29(33):1700448. doi:10.1002/adma.201700448