Abstract
Background
Elemental selenium nanoparticles have emerged as a novel selenium source with the advantage of reduced risk of selenium toxicity. The present work investigated whether heat treatment affects the size, structure, and bioactivity of selenium nanoparticles.
Methods and results
After a one-hour incubation of solution containing 80 nm selenium particles in a 90°C water bath, the nanoparticles aggregated into larger 110 nm particles and nanorods (290 nm × 70 nm), leading to significantly reduced bioavailability and phase II enzyme induction in selenium-deficient mice. When a solution containing 40 nm selenium nanoparticles was treated under the same conditions, the nanoparticles aggregated into larger 72 nm particles but did not transform into nanorods, demonstrating that the thermostability of selenium nanoparticles is size-dependent, smaller selenium nanoparticles being more resistant than larger selenium nanoparticles to transformation into nanorods during heat treatment.
Conclusion
The present results suggest that temperature and duration of the heat process, as well as the original nanoparticle size, should be carefully selected when a solution containing selenium nanoparticles is added to functional foods.
Introduction
Selenium has multiple beneficial effects for human health, through regulation of at least 25 selenoproteins all containing selenocysteine, the 21st genetically encoded protein amino acid.Citation1 Among these selenoproteins, the glutathione peroxidase and thioredoxin reductase families are well characterized. Glutathione peroxidases catalyze the reduction of hydrogen peroxide and a variety of organic hydroperoxides, including phospholipid hydroperoxide, to water and corresponding alcohols, using glutathione as the hydrogen donor.Citation2 Thioredoxin reductases exert antioxidant actions through catalyzing the reduction of oxidized thioredoxin, using nicotinamide-adenine dinucleotide phosphate (NADPH) as the electron donor, or by directly reacting with hydrogen peroxide and hydroperoxides.Citation3 Variations in selenoprotein genes have been found to be associated with increased cancer risk.Citation4,Citation5 Transgenic mice with reduced selenoprotein expression manifest precancerous changes.Citation6,Citation7 Human studies have shown reduced cancer risk and DNA damage after selenium supplementation among those with lower basal plasma selenium.Citation8–Citation10 These studies argue for a cancer-preventive effect of selenium at nutritional levels in selenium-deficient populations.
Like various other naturally occurring chemopreventive agents, such as sulforaphane, curcumin, resveratrol, and epigallocatechin gallate, selenium at supranutritional levels (which are roughly 10–30-fold higher than nutritional levels) is also capable of inducing phase II detoxification enzymes, such as glutathi-one S-transferase and quinone reductase.Citation11–Citation15 The resulting enhanced detoxification many contribute to the powerful chemopreventive effects exhibited by supranutritional selenium, which have been observed in approximately 100 small animal studies.Citation8,Citation16,Citation17 Since supranutritional selenium levels are close to the toxic selenium levels in general,Citation8,Citation16,Citation17 the safety margin and potential toxic effects of different selenium compounds are critical considerations when evaluating their potential for supplementation.
Bulk red elemental selenium particles at the redox state of zero found in some bacteria are biologically inert.Citation18 It is known that elemental selenium atoms can be formed in the redox system of sodium selenite and glutathione at the molar ratio of 1:4.Citation19 We have demonstrated that the presence of a protein such as bovine serum albumin in the redox system can control the aggregation of neonatal elemental selenium atoms, thus inhibiting the formation of bulky red elemental selenium particles, and consequently leading to the formation of red elemental selenium nanoparticles.Citation20 Compared with several extensively studied selenium compounds, ie, sodium selenite, selenomethionine, and methylselenocysteine, selenium nanoparticles show markedly lower acute toxicity and significantly lower short-term and subchronic toxicities, but all these selenium compounds have equivalent efficacy in their ability to increase selenoenzymes.Citation20–Citation25 Furthermore, selenium nanoparticles appear to be more efficient than sodium selenite and selenomethionine in increasing glutathione S-transferase activity,Citation21,Citation25 and seem to be equally efficient in inducing apoptosis of certain types of cancer cells as methylseleninic acid, a metabolite of methylselenocysteine that is considered to be the most promising selenium compound in cancer prevention.Citation26 Selenium nanoparticles may enhance selenium permeation and retention in tumor tissues because their blood vessels contain enlarged pore sizes ranging from 100 nm to 800 nm, in stark contrast with the pore sizes of 2 nm to 6 nm in the vessels of healthy tissues.Citation27 In addition to cancer prevention, selenium nanoparticles also show potential as antimicrobial agents.Citation28
Selenium nanoparticles have emerged as a novel selenium source with the obvious advantage of reduced risk of selenium toxicity.Citation20–Citation25,Citation29,Citation30 Accordingly, a selenium nanoparticle solution may be added to functional foods, the final products of which may be subjected to heat during processing. An important feature of nanoparticles is their high surface to volume ratio. As the nanosize decreases, surface atomicity, surface energy, and surface binding energy all increase quickly; as a result, the surface atoms become more prone to diffusion, with an inherent tendency to combine with other atoms for energy dissipation. Hence, the thermodynamic properties and stability of nanoparticles should be affected by particle size and heat treatment.Citation31–Citation33 The thermal stability of selenium nanoparticles as a type of nanoscale biomaterial remains unknown. Herein, we investigated the impact of heat treatment on the size, structure, and bioactivity of selenium nanoparticles. We found that heat treatment causes selenium nanoparticles to aggregate into larger sizes and nanorods, leading to significantly reduced bioactivity in mice. The thermostability of selenium nanoparticles is size-dependent, smaller selenium nanoparticles being more resistant than larger selenium nanoparticles to transformation into nanorods during heat treatment.
Materials and methods
Chemicals
NADPH, dithio-bis-nitrobenzoic acid, glutathione, 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), insulin, thioredoxin (Escherichia coli), guanidine hydrochloride, bovine serum albumin, 1-chloro-2,4-dinitrobenzene, and 2,3-diaminonaphthalene were all purchased from Sigma (St Louis, MO). Other chemicals, including hexamethylene, hydroxylamine hydrochloride, nitric acid, and perchloric acid, were of the highest grade available.
Solution-based preparations of selenium nanoparticles of two sizes
One mL of 25 mM sodium selenite was mixed with 4 mL 25 mM of glutathione containing either 2 mg or 20 mg bovine serum albumin. The mixture pH was adjusted to 7.2 with 1.0 M sodium hydroxide to generate red elemental selenium and oxidized glutathione.Citation20 The red solution was dialyzed for 96 hours at 4°C against double distilled water which was replaced every 24 hours to separate oxidized glutathione from the selenium nanoparticles. The final solution containing selenium nanoparticles and bovine serum albumin was stored in a 4°C refrigerator, in which the selenium nanoparticle solution prepared using the low concentration of bovine serum albumin was stable for several months, and the selenium nanoparticle solution prepared by using the high concentration of bovine serum albumin was stable for several years.
Nanoparticle observation
A droplet of selenium nanoparticle solution was dropped onto copper grids and allowed to dry in air before observation under a high resolution transmission electron microscope. The average sizes of selenium nanoparticles prepared using the low and high concentrations of bovine serum albumin were 80 nm (45 nm ~ 95 nm) and 40 nm (20 nm ~ 55 nm), respectively.
Animals and treatments
Selenium-deficient male Kunming mice (21–22 g) and their selenium-deficient diet were all purchased from the animal center in Anhui Medical University. The mice were housed in plastic cages in a room with controlled temperature (22°C ± 1°C) and humidity (50% ± 10%) on a 12-hour light/dark cycle. The mice were allowed to obtain food and water ad libitum. All experiments involving the mice were performed in compliance with the ethical guidelines issued by the Anhui Agriculture University.
Nutritional level of selenium
In the bioavailability experiment, 15 mice were divided into three groups (n = 5): the control group was administered oral saline; the other two groups were orally given selenium nanoparticles, either unheated or heated, at a selenium dose of 100 μg/kg body weight for 7 days.
Supranutritional level of selenium
In the supranutritional dose experiment, 24 mice were divided into three groups (n = 8): the control group was administered oral saline; the other two groups were administered oral selenium nanoparticles, either unheated or heated, at a selenium dose of 2000 μg/kg body weight for 7 days.
Biomarkers
At the end of each set of experiments, the mice were sacrificed by cervical dislocation. Peripheral blood from the ophthalmic veins was collected into tubes to obtain blood and plasma after centrifugation. The livers were excised immediately and rinsed in ice-cold saline. The samples were stored at −30°C before assay. Liver tissues were homogenized in ice-cold 150 mM and pH 7.2 phosphate-buffered saline containing 1 mM EDTA-Na2 (1:9, w/v), and the homogenate was then centrifuged at 15,000 g and 4°C for 15 minutes. The resulting supernatants were used for determination of soluble protein levels with bovine serum albumin as standard,Citation34 and for determination of glutathione peroxidase, thioredoxin reductase, and glutathione S-transferase activity.
Glutathione peroxidase activity assay
Glutathione peroxidase was assayed using the method of Rotruck et al.Citation35 Glutathione peroxidase activity was expressed as units/mg protein or units/mL plasma; one unit of the activity was calculated in terms of 1 μmol of glutathione oxidized per minute.
Glutathione S-transferase activity assay
Glutathione S-transferase activity was chemically determined at 340 nm using 1-chloro-2,4-dinitrobenzene as the substrate. Glutathione S-transferase activity was expressed as units/minute/mg protein; one unit of activity was calculated in terms of nmol of 1-chloro-2,4-dinitrobenzene changed per minute.Citation36
Thioredoxin reductase activity assay
Thioredoxin reductase activity was measured based on the method of Holmgren and BjörnstedtCitation37 with some modifications. A stock mixture was composed of HEPES buffer (1.0 M, pH 7.6), EDTA (0.2 M), NADPH (40 mg/mL), and bovine insulin (10 mg/mL) in a volume ratio of 5:1:1:12.5. In a 96-well plate, 3 μL thioredoxin (2 mg/mL), 7 μL stock mixture, 40 μL HEPES (50 mM, pH 7.6), and 20 μL liver homogenate (with 20–30 μg protein) were added to each well. The enzymatic reaction was maintained at 37°C for 20 minutes, and then terminated by adding 240 μL stop solution containing 0.2 mg/mL dithio-bis-nitrobenzoic acid, 6 M guanidine hydrochloride, and 0.2 M Tris at pH 8.0. Each sample contained a nonenzymatic reaction as the control. The nonenzymatic reaction included all components except thioredoxin, which was replaced by the same volume of saline. The 96-well plates were read at 412 nm. The absorbance of the control was subtracted from the absorbance of the sample. A background control, which was the subtraction of absorbance with and without thioredoxin in the absence of liver homogenate, was further subtracted from all samples. Thioredoxin reductase activity unit was defined as A412 change × 1000 per minute and thioredoxin reductase activity was expressed as U/mg protein.Citation38
Selenium assay
Selenium was assayed by a fluorescence method.Citation39 Samples were digested using the mixture of nitric acid and perchloric acid at the ratio of 3:1 (v/v), then reacted with 2,3-diaminonaphthalene at 60°C for 30 minutes, then finally extracted with hexamethylene. The fluorescence intensity excited at 378 nm and recorded at 512 nm was used for calculating selenium concentration with sodium selenite as a standard.
Statistical analysis
Data are presented as the mean ± standard error of the mean. The differences between groups were examined by one-way analysis of variance post hoc Tukey test, using GraphPad Software (Prism version 5, San Diego, CA). P < 0.05 was considered to be statistically significant.
Results
Impact of heat treatment
Size and structure of 80 nm selenium nanoparticles
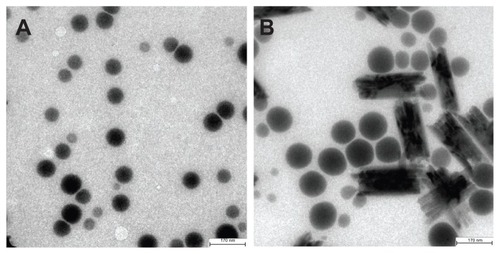
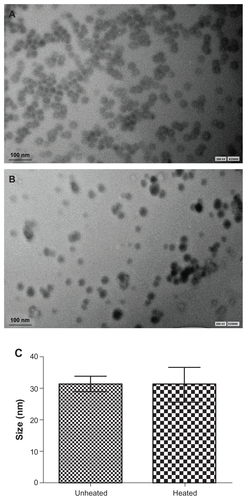
A sealed glass tube with 10 mL of the 80 nm selenium nanoparticle solution was incubated in a 1000 mL water bath at 90°C for one hour, and then the hot glass tube was cooled in an ice bath to room temperature. Under inspection by high resolution transmission electron microscopy, it was found that the selenium nanoparticles changed substantially due to heat treatment. As shown in , selenium particles in the precursor unheated selenium nanoparticle solution were spherical nanoparticles with an average size of 80 nm ranging from 45 nm to 95 nm, whereas in the heated selenium nanoparticle solution (), the precursor selenium nanoparticles were replaced by both enlarged nanoparticles with an average size of 110 nm ranging from 70 nm to 120 nm and nanorods, the average dimension of which was 290 nm × 70 nm.
Bioavailability of 80 nm selenium nanoparticles
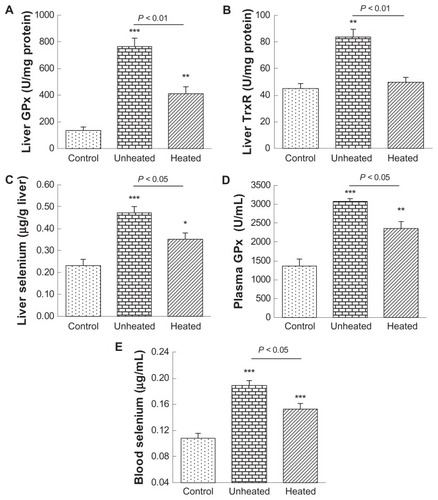
The primary purpose of this study was to investigate the impact of heat treatment on the bioactivity of selenium nanoparticles. One of the most fundamental properties of selenium compounds is bioavailability in terms of increasing selenoenzyme activity and tissue selenium levels. We have shown that oral administration of selenium nanoparticles to selenium-deficient mice at doses of 35, 70, and 1000 μg/kg for one week dose-dependently increased selenoenzyme activity and tissue selenium levels.Citation21 Thus, in the present study, we used selenium 100 μg/kg, which is a typical selenium nutritional dose, for one-week oral supplementation. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.001) increased hepatic glutathione peroxidase activity by 5.7-fold, whereas the heated selenium nanoparticles significantly increased the activity (P < 0.01), but only by 3.0-fold; accordingly, a significant difference (P < 0.01) existed between the two selenium nanoparticle groups. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.01) increased hepatic thioredoxin reductase activity by 1.9-fold. In contrast, the heated selenium nanoparticles did not significantly alter the activity; consequently, a significant difference (P < 0.01) existed between the two selenium nanoparticle groups. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.001) increased hepatic selenium by 2.0-fold, whereas the heated selenium nanoparticles significantly (P < 0.05) increased the selenium level, but only by 1.5-fold; accordingly, a significant difference (P < 0.05) existed between the two selenium nanoparticle groups. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.001) increased plasma glutathione peroxidase activity by 2.3-fold, whereas the heated selenium nanoparticles significantly (P < 0.01) increased the activity, but only by 1.7-fold; accordingly, a significant difference (P < 0.05) existed between the two selenium nanoparticle groups. Coincidentally, plasma selenium levels almost completely mirrored the alterations found in plasma glutathione peroxidase (). These results clearly demonstrate that the heat treatment significantly reduced bioavailability of the 80 nm selenium nanoparticles.
Figure 2 Heat treatment reduces bioavailability of 80 nm selenium nanoparticles at a nutritional level. Selenium-deficient mice were orally administered with saline as a control, or 80 nm selenium nanoparticles, either unheated or heated, at a selenium dose of 100 μg/kg for 7 days.
Notes Compared with the controls, *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: GPx, glutathione peroxidase; TxR, thioredoxin reductase.
Glutathione S-transferase induction and selenium retention of 80 nm nanoparticles at a supranutritional level
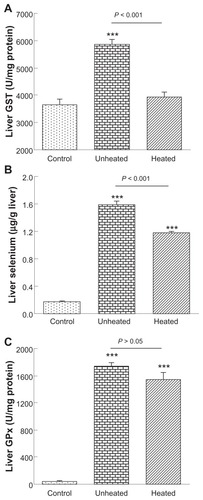
Another important bioactivity of selenium, in addition to bioavailability, is its impact on phase II enzymes. We have shown that oral administration of selenium nanoparticles to selenium-deficient mice at supranutritional selenium doses of 500 and 2000 μg/kg for one week dose-dependently increased glutathione S-transferase activity and tissue selenium levels.Citation40 Thus, in the present study, we used selenium 2000 μg/kg for one week as oral supplementation. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.001) increased hepatic glutathione S-transferase activity by 1.6-fold. In contrast, heated 80 nm selenium nanoparticles did not alter the activity; consequently, a significant difference (P < 0.001) existed between the two selenium nanoparticle groups. As compared with the control (), the unheated 80 nm selenium nanoparticles significantly (P < 0.001) increased hepatic selenium by 8.9-fold, whereas the heated selenium nanoparticles significantly (P < 0.001) increased the selenium levels, but only by 6.6-fold; accordingly, a significant difference (P < 0.001) existed between the two selenium nanoparticle groups. As compared with the control (), both the unheated and the heated selenium nanoparticles caused a highly significant (all P < 0.001) massive increase in hepatic glutathione peroxidase activity, by 38.8-fold and 34.2-fold, respectively. However, as expected, no significant differences were found between the two selenium nanoparticle groups, because selenoenzymes, including hepatic glutathione peroxidase, can be fully saturated at the upper end of nutritional selenium levels. Although the heated 80 nm selenium nanoparticles showed an approximately 50% reduced efficacy in increasing hepatic glutathione peroxidase activity at 100 μg selenium/kg as compared with the unheated material (), in this case a 20-fold increase of selenium amounts should fully compensate for this defect.
Figure 3 Heat treatment reduces the bioactivity of 80 nm selenium nanoparticles at a supranutritional level. Selenium-deficient mice were orally administered with saline as a control, or 80 nm selenium nanoparticles, either unheated or heated, at selenium dose of 2000 μg/kg for 7 days.
Note: Compared with the controls, ***P < 0.001.
Abbreviations: GST, glutathione; GPx, glutathione peroxidase.
Size and structure of 40 nm selenium nanoparticles
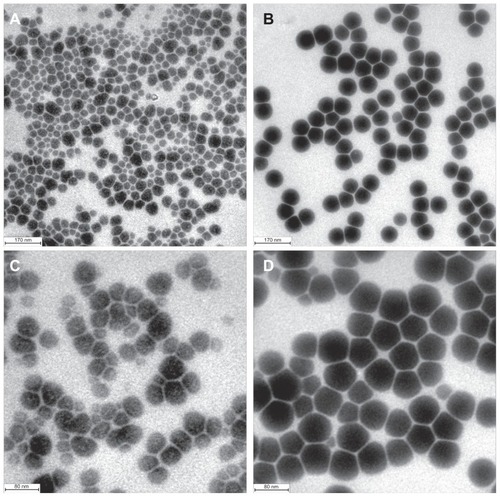
The 40 nm selenium nanoparticles were treated with heat under the same conditions and for the same duration as the 80 nm selenium nanoparticles. Under inspection by high resolution transmission electron microscopy, as shown in , the selenium particles in the unheated selenium nanoparticle solution were spherical, with an average size of 40 nm, ranging from 20 nm to 55 nm, whereas in the heated selenium nanoparticle solution (), enlarged nanoparticles with an average size of 70 nm were observed. In stark contrast with heat-activated evolution of the 80 nm selenium nanoparticles, in this case nanorods were not observed. Strikingly, the enlarged selenium nanoparticles had a surprisingly narrow size distribution ranging from 68 nm to 72 nm, highly faceted morphology, and uniform edge-to-edge distances between particles relative to the untreated precursor nanoparticles (). These contrasts could be more clearly seen from the photos with enhanced resolution ( for the unheated selenium nanoparticles and for the heated selenium nanoparticles).
Discussion
Elemental selenium nanoparticles can be formed naturally in certain bacteria,Citation41–Citation43 and can be prepared through inorganic selenium reduction in the presence of protein or polysaccharide.Citation20,Citation44–Citation46 Various sizes of selenium nanoparticles can be obtained by changing the concentration of a protein such as bovine serum albumin.Citation47,Citation48 Generally, the higher the bovine serum albumin concentration, the smaller the selenium nanoparticle size.Citation47,Citation48 Accordingly, in the present study, 40 nm and 80 nm selenium nanoparticles were prepared by adding low or high concentrations of bovine serum albumin to the redox system of sodium selenite and glutathione.
Nanoparticles have an inherent tendency to grow into larger particles. In general, a smaller nanoparticle with a lower melting point grows uniformly, until it becomes stable at a specific heat treatment temperature through thermodynamic control.Citation32,Citation33 Maye et al and Maye and Zhong, have investigated the effect of heat treatment on the manipulation of the size and shape of alkanethiol-protected gold nanoparticles in solution.Citation32,Citation33 The precursor gold nanoparticles they used showed an average core size of 2.0 nm with a wide size distribution; in addition, the particle shapes were not uniform. After heat treatment, the sizes of gold nanoparticles increased, and, in sharp contrast with the morphological features of the precursor nanoparticles, the enlarged nanoparticles evolved from heat treatment showed three striking features, ie, a much narrower size distribution, highly faceted outlines, and uniform spacing between the nanoparticles.Citation32,Citation33 Interestingly, in the present study, we observed generally similar evolutions using the 40 nm selenium nanoparticle solution. After the 40 nm selenium nanoparticle solution was subjected to 1 hour of heat treatment at 90°C, the enlarged nanoparticles displayed obvious alterations of shape and homogeneity in comparison with the precursor nanoparticles (). Three typical morphological features were evident: first, a much narrower size distribution; second, the predominance of faceted particles with pentagon or hexagon outlines exhibiting three-dimensional profiles; and third, uniform edge- to-edge distances.
However, the evolution of the 80 nm selenium nanoparticles on heat treatment was radical, with both faceted nanoparticles and nanorods being formed after 1 hour of heat treatment at 90°C (). Faceted nanoparticles may evolve, as with the 40 nm selenium nanoparticles, from the smaller 45 nm nanoparticles that are still present in the 90 nm selenium nanoparticles (), whereas the nanorods may evolve from the larger 95 nm nanoparticles in the 90 nm selenium nanoparticles (). Because bovine serum albumin concentration in the 80 nm selenium nanoparticles is 10-fold lower than that in the 40 nm selenium nanoparticles, it may be argued that the extra protein may prevent the formation of nanorods. However, this possibility was ruled out, because when we added bovine serum albumin to preformed 80 nm selenium nanoparticles at the same bovine serum albumin concentration as that in the 40 nm selenium nanoparticles, we found heat treatment at 90°C still resulted in formation of nanorods. Thus, the thermally-activated size and structure evolutions are considered to be dependent on precursor particle size, with smaller selenium nanoparticles being more resistant than larger selenium nanoparticles to transformation into nanorods during heat treatment.
In the present study, we only investigated the impact of heat treatment on bioactivity of the 80 nm selenium nanoparticles, without addressing the issue using the 40 nm selenium nanoparticles. This selection was based on two reasons. First, the bioactivity of nanorods generated from the 80 nm selenium nanoparticles is completely unknown. Second, we have elucidated size effects of selenium nanoparticles in vitro, in a cell model, and in vivo, using different sizes ranging from 10 nm to 200 nm,Citation40,Citation47,Citation48 and the sizes of both the precursor 44 nm selenium nanoparticles and the heated material fall into this range. Our previous studies have demonstrated that different sizes of selenium nanoparticles have no differences in bioavailability in both a cell model and in an in vivo model,Citation47 but selenium nanoparticles of small size gain advantages in their ability to scavenge multiple free radical species in vitro,Citation48 and to enhance selenium accumulation and glutathione S-transferase activity in vivo,Citation40 as summarized in and . Based on these studies, it can be inferred that transformation into nanorods is responsible for the observed reduction in bioavailability, and that heat treatment to the 44 nm selenium nanoparticles would reduce its bioactivity, other than bioavailability.
Table 1 Comparison of bioactivity of selenium nanoparticles with different sizes in vitro and in vivo at nutritional levels (small 5–15 nm, medium 20–60 nm, large 80–200 nm)
Table 2 Comparison of bioactivity of 36 nm and 90 nm selenium nanoparticles at supranutritional levels
As a novel supplemental agent with a reduced risk of selenium toxicity, selenium nanoparticle solution may be added to functional foods, for which the final products could require heat processing. The present results suggest that temperature and duration of heat processing, as well as original nanoparticle size, should be carefully selected when a selenium nanoparticle solution is added to functional foods, in order to conserve fully the bioactivity of the selenium nanoparticles. A selenium nanoparticle solution after heat treatment can be monitored for alterations in the size and structure of the nanoparticles, in addition to changes in nutritional parameters/biomarkers. Normally, selenium is supplemented in food at very low levels; it is not possible to observe selenium nanoparticles by transmission electron microscopy in foods at such low concentrations. Thus, the results obtained from studies of selenium nanoparticle solution as a surrogate for actual foods not only indicate alterations in nutritional parameters, but also link the alterations to changes in nanoscale size and structure. In addition, different foods would be processed by various types of heat treatment; we cannot address all the possible specific food processes in the present study. However, the results obtained using the selenium nanoparticle solution as a model can justify the general guideline that caution should be used when selenium nanoparticles are added to foods which need to be heated during processing. Based on the results of the present study, in regard to food that has been supplemented with selenium nanoparticles, the best general method for drying would be lyophilization. Given that the thermostability of selenium nanoparticles is size-dependent, smaller selenium nanoparticles being more resistant than larger selenium nanoparticles to transformation into nanorods during heat treatment, there is also the strong implication that the use of smaller size selenium nanoparticles in foods is essential if extensive heat exposure is unavoidable. On the other hand, some food heat processes involve lower temperature conditions than those required to affect selenium nanoparticle structure and bioavailability. For example, to prepare selenium capsules, 1 mL selenium nanoparticle solution (400 μg/mL) is added to 2 g starch, and this mixture could be dried at 60°C for 40 minutes with an airblast. Milk sterilization involves heat treatment at 62°C for 30 minutes. To mimic these processes, we observed the impact of heat treatment at 60°C for 40 minutes on a 31 nm selenium nanoparticle solution. As shown in Supplementary Figure 1, such a moderate heat process did not alter the size of the selenium nanoparticles. Thus, as compared with the use of inorganic selenium salts (where heat is not a consideration), the potential disadvantages of using selenium nanoparticles when a heat process is required can potentially be overcome, provided nanoscale size and heat treatment conditions are carefully selected.
Conclusion
Heat treatment has a significant impact on the size, structure, and bioactivity of selenium nanoparticles. In general, the sizes of selenium nanoparticles increase and bioactivities decrease when a selenium nanoparticle solution is subjected to heat treatment. However, the thermally-activated size and structure evolution are substantially dependent upon precursor particle size, with smaller selenium nanoparticles being more resistant than larger selenium nanoparticles to transformation into nanorods during heat treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 31170648 to JZ), the National Basic Research Program of China (973 Program, grant 2009CB930204), and by a grant from Anhui Agricultural University to JZ.
Disclosure
The authors have no financial conflict of interest in this work.
Supplementary material
Figure S1. Impact of heat treatment on size and structure of 31 nm selenium nanoparticles. (A) Unheated precursor selenium nanoparticles. (B) Precursor selenium nanoparticle solution after heating at 60°C for 40 minutes. (C) Size before and after heat treatment.
Note: Data are presented as the mean ± standard deviation (n = 70).
References
- SteinbrennerHSiesHProtection against reactive oxygen species by selenoproteinsBiochim Biophys Acta200917901478148519268692
- Brigelius-FlohéRTissue-specific functions of individual glutathione peroxidasesFree Radic Biol Med19992795196510569628
- ArnérESFocus on mammalian thioredoxin reductases – important selenoproteins with versatile functionsBiochim Biophys Acta2009179049552619364476
- MéplanCHughesDJPardiniBGenetic variants in selenoprotein genes increase risk of colorectal cancerCarcinogenesis2010311074107920378690
- PetersUChatterjeeNHayesRBVariation in the selenoenzyme genes and risk of advanced distal colorectal adenomaCancer Epidemiol Biomarkers Prev2008171144115418483336
- Diwadkar-NavsariwalaVPrinsGSSwansonSMSelenoprotein deficiency accelerates prostate carcinogenesis in a transgenic modelProc Natl Acad Sci U S A20061038179818416690748
- IronsRCarlsonBAHatfieldDLDavisCDBoth selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expressionJ Nutr20061361311131716614422
- RaymanMPSelenium in cancer prevention: a review of the evidence and mechanism of actionProc Nutr Soc20056452754216313696
- El-BayoumyKThe protective role of selenium on genetic damage and on cancerMutat Res200147512313911295158
- KowalskaENarodSAHuzarskiTIncreased rates of chromosome breakage in BRCA1 carriers are normalized by oral selenium supplementationCancer Epidemiol Biomarkers Prev2005141302130615894690
- XiaoHParkinKLInduction of phase II enzyme activity by various selenium compoundsNutr Cancer20065521022317044777
- El-SayedWMAboul-FadlTLambJGRobertsJCFranklinMREffect of selenium-containing compounds on hepatic chemoprotective enzymes in miceToxicology200622017918816451816
- El-SayedWMAboul-FadlTRobertsJCLambJGFranklinMRMurine hepatoma (Hepa1c1c7) cells: a responsive in vitro system for chemoprotective enzyme induction by organoselenium compoundsToxicol In Vitro20072115716417110078
- ‘t HoenPARooseboomMBijsterboschMKvan BerkelTJVermeulenNPCommandeurJNInduction of glutathione-S-transferase mRNA levels by chemopreventive selenocysteine Se-conjugatesBiochem Pharmacol2002631843184912034368
- ProkopczykBRosaJGDesaiDChemoprevention of lung tumorigenesis induced by a mixture of benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by the organoselenium compound 1,4-phenylenebis(methylene)selenocyanateCancer Lett2000161354611078911
- IpCLessons from basic research in selenium and cancer preventionJ Nutr1998128184518549808633
- WhangerPDSelenium and its relationship to cancer: an updateBr J Nutr200491112814748935
- CombsGFJrGarbisuCYeeBCBioavailability of selenium accumulated by selenite-reducing bacteriaBiol Trace Elem Res1996522092258811279
- GantherHESelenotrisulfides. Formation by the reaction of thiols with selenious acidBiochemistry19687289829055666757
- ZhangJSGaoXYZhangLDBaoYPBiological effects of a nano red elemental seleniumBiofactors200115273811673642
- WangHZhangJYuHElemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in miceFree Radic Biol Med2007421524153317448899
- ZhangJWangXXuTElemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in miceToxicol Sci2008101223117728283
- ZhangJWangHYanXZhangLComparison of short-term toxicity between Nano-Se and selenite in miceLife Sci2005761099110915620574
- JiaXLiNChenJA subchronic toxicity study of elemental Nano-Se in Sprague-Dawley ratsLife Sci2005761989200315707881
- ZhangJWangHPengDTaylorEWFurther insight into the impact of sodium selenite on selenoenzymes: high-dose selenite enhances hepatic thioredoxin reductase 1 activity as a consequence of liver injuryToxicol Lett200817622322918215477
- KongLYuanQZhuHThe suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosisBiomaterials2011326515652221640377
- TranPAWebsterTJNanotechnologies for cancer diagnostics and treatmentDixonCJCurtinesOWNanotechnology: Nanofabrication, Patterning, and Self AssemblyNew York, NYNova Science Publishers2011
- TranPAWebsterTJSelenium nanoparticles inhibit Staphylococcus aureus growthInt J Nanomedicine201161553155821845045
- ZhangJSpallholzJToxicity of selenium compounds and nano- selenium particlesCascianoDSahuSCHandbook of Systems ToxicologyWest Sussex, UKJohn Wiley and Sons2011
- ZhangJBiological properties of red elemental selenium at nano size (Nano-Se) in vitro and in vivoSahuSCCascianoDNanotoxicity: From In Vivo and In Vitro Model To Health RisksWest Sussex, UKJohn Wiley and Sons2009
- KaraARahmanTSVibrational dynamics and thermodynamics of surfaces and nanostructuresSurf Sci Rep200556159187
- MayeMMZhengWLeibowitzFLLyNKZhongCJHeating-induced evolution of thiolate-encapsulated gold nanoparticles: A strategy for size and shape manipulationsLangmuir200016490497
- MayeMMZhongCJManipulating core-shell reactivities for processing nanoparticle sizes and shapesJ Mater Chem20001018951901
- BradfordMMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem197672248254942051
- RotruckJTPopeALGantherHESwansonABHafemanDGHoekstraWGSelenium: biochemical role as a component of glutathione peroxidaseScience19731795885904686466
- HabigWHPabstMJJakobyWBGlutathione S-transferases. The first enzymatic step in mercapturic acid formationJ Biol Chem1974249713071394436300
- HolmgrenABjörnstedtMThioredoxin and thioredoxin reductaseMethods Enzymol19952521992087476354
- GantherHIpCThioredoxin reductase activity in rat liver is not affected by supranutritional levels of monomethylated selenium in vivo and is inhibited only by high levels of selenium in vitroJ Nutr200113130130411160550
- OlsonOEPalmerISCaryEEModification of the official fluorometric method for selenium in plantsJ Assoc Off Anal Chem197558117121
- PengDZhangJLiuQTaylorEWSize effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activityJ Inorg Biochem20071011457146317664013
- OremlandRSHerbelMJBlumJSStructural and spectral features of selenium nanospheres produced by Se-respiring bacteriaAppl Environ Microbiol200470526014711625
- MishraRRPrajapatiSDasJDangarTKDasNThatoiHReduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced productChemosphere2011841231123721664643
- DebieuxCMDridgeEJMuellerCMA bacterial process for selenium nanosphere assemblyProc Natl Acad Sci U S A2011108134801348521808043
- MishraBHassanPAPriyadarsiniKIMohanHReactions of biological oxidants with selenourea: formation of redox active nanoseleniumJ Phys Chem B2005109127181272316852575
- DobiasJSuvorovaEIBernier-LatmaniRRole of proteins in controlling selenium nanoparticle sizeNanotechnology20112219560521430311
- ZhangYWangJZhangLCreation of highly stable selenium nanoparticles capped with hyperbranched polysaccharide in waterLangmuir201026176171762320964304
- ZhangJWangHBaoYZhangLNano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and miceLife Sci20047523724415120575
- HuangBZhangJHouJChenCFree radical scavenging efficiency of Nano-Se in vitroFree Radic Biol Med20033580581314583345