Abstract
Background
The contribution of fibrinogen (FBN) to hemostasis acting on platelet aggregation and clot formation is well established. It has been suggested that FBN-coated liposomes could be useful in restoring hemostasis. In the present study, we evaluated the modifications induced by multilamellar raw liposomes (MLV) or fibrinogen-coated liposomes (MLV-FBN) on hemostatic parameters.
Materials and methods
Different experimental settings using whole blood or thrombocy-topenic blood were used. Thromboelastometry, aggregation studies, platelet function analyzer (PFA-100®) tests and studies under flow conditions were applied to detect the effect of MLV-FBN on hemostatic parameters.
Results
The presence of MLV-FBN in whole blood modified its viscoelastic properties, prolonging clot formation time (CFT) (226.5 ± 26.1 mm versus 124.1 ± 9.4 mm; P < 0.01) but reducing clot firmness (45.4 ± 1.8 mm versus 35.5 ± 2.3 mm; P < 0.05). Under thrombocy-topenic conditions, FIBTEM analysis revealed that MLV-FBN shortened clotting time (CT) compared to MLV (153.3 ± 2.8 s versus 128.0 ± 4.6 s; P < 0.05). Addition of either liposome decreased fibrin formation on the subendothelium (MLV 8.1% ± 4.7% and MLV-FBN 0.8% ± 0.5% versus control 36.4% ± 6.7%; P < 0.01), whereas only MLV-FBN significantly reduced fibrin deposition in thrombocytopenic blood (14.4% ± 6.3% versus control 34.5% ± 5.2%; P < 0.05). MLV-FBN inhibited aggregation induced by arachidonic acid (52.1% ± 8.1% versus 88.0% ± 2.1% in control; P < 0.01) and ristocetin (40.3% ± 8.8% versus 94.3% ± 1.1%; P < 0.005), but it did not modify closure times in PFA-100® studies. In perfusion experiments using whole blood, MLV and MLV-FBN decreased the covered surface (13.25% ± 2.4% and 9.85% ± 2.41%, respectively, versus control 22.0% ± 2.0%; P < 0.01) and the percentage of large aggregates (8.4% ± 2.3% and 3.3% ± 1.01%, respectively, versus control 14.6% ± 1.8%; P < 0.01).
Conclusion
Our results reveal that, in addition to the main contribution of fibrinogen to hemostasis, MLV-FBN inhibits platelet-mediated hemostasis and coagulation mechanisms.
Introduction
Liposomes are widely implemented for therapeutic use as drug carriers, as well as in drug targeting and other pharmacological applications, including basic research and applied technology.Citation1,Citation2 In previous studies, our group demonstrated the development of procoagulant activity in damaged vessels of synthetic phospholipids and liposomes,Citation3,Citation4 opening up new possibilities for using liposomes as platelet substitutes to improve hemostasis. Several groups have explored the use of modified liposomes and microcapsules as coagulants in order to improve hemostasis.Citation3–Citation9 Recently, the use of nanotechnological approaches to stop bleeding has been explored.Citation10 In this context, the effects of prohemostatic molecules, such as clotting factors in liposomes, could provide many advantages in controlling hemorrhages.
Fibrinogen (FBN) is a 340 kDa protein that is present in plasma at a concentration of 2 to 3 mg/mL. It has two identical disulfide-linked subunits composed of three nonidentical polypeptide chains: Aα, Bβ, and γ. In addition, a γ ′ chain is found in approximately 10% of FBN molecules. The four carboxyl-terminal amino acids found in the γA chain are substituted by a highly anionic 20-amino acid sequence in the γ ′ chain.Citation11 FBN plays an important role in blood coagulation as the substrate for thrombin, which is the final step in the coagulation cascade that results in fibrin formation.Citation12 Moreover, FBN mediates platelet aggregation by binding and bridging glycoprotein IIb–IIIa (GPIIb-IIIa).Citation13 Two regions of the FBN α chain that contain an arginine-glycine-aspartic acid motif, as well as the carboxyl-terminus of the FBN γ chain, represent potential binding sites for GPIIb-IIIa in the fibrinogen molecule.Citation14,Citation15
Thus, combining adequate sequences of adhesive proteins in the correct conformation on the appropriate carrier could improve primary hemostasis without enhancing fibrin formation. In the present study, we explored the effects of both raw multilamellar liposomes (MLV) and fibrinogencoated liposomes (MLV-FBN) on platelet function by using different experimental approaches: platelet aggregation induced by different agonists; experiments under flow conditions using the platelet function analyzer PFA-100® (Siemens Healthcare Diagnostics, Deerfield, IL) device; and perfusion systems to evaluate platelet interaction and fibrin formation on the subendothelium. Moreover, viscoelastic properties and the quality of clot formation were tested by thromboelastometric techniques.
Materials and methods
Experimental design
The present study was designed to evaluate the possible effect of liposomes containing FBN on different hemostatic parameters. The activity of liposomes was evaluated using whole blood and mild thrombocytopenic blood (30,000 platelets/μL) anticoagulated with low-molecular-weight heparin (LMWH) or citrate (19 mM), depending on the test used. Blood samples were incubated with MLV or MLV-FBN liposomes and the effects on the coagulation system, platelet activation, thrombus formation, and fibrin generation were assessed by different experimental settings.
Reagents
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (PE) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide] (N-MPB-PE) were purchased from Avanti Polar Lipids (Alabaster, AL); 1,2-dipalmitoyl-rac-glycero-3-phosphocholine (DPPC), cholesterol (CHOL), and FBN from human plasma protein from Sigma-Aldrich (St Louis, MO); and N-succinimidyl-S-acetylthioacetate (SATA) from Pierce (Rockford, IL). The buffer used was 50 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES), pH 7.5 (with and without 1 mM ethylenediaminetetraacetic acid for liposomes with and without FBN). Other reagents used were of analytical grade. The water used was ultrapure (MilliQ reverse osmosis system; Millipore, Billerica, MA) with a resistivity of 18.3 MΩ/cm. Arachidonic acid and ristocetin were purchased from Helena BioSciences Europe (Gateshead, UK), while collagen and adenosine diphosphate (ADP) were from Arkray Inc (Kyoto, Japan). ROTEM® reagents for the EXTEM and FIBTEM tests were from Pentapharm GmbH (Aesch, Switzerland).
Liposome preparation and characterization
Phospholipids (DPPC:CHOL:N-MPB-PE and DPPC:CHOL:PE at a molar ratio of 50:20:30) were dissolved in chloroform in a round-bottomed flask and dried in a rotary evaporator under reduced pressure at 50°C to form a thin film on the flask. The film was hydrated with a solution of 10 mM HEPES buffer (pH 7.4) and 145 mM NaCl to give a lipid concentration of 20 mM. Multilamellar liposomes were formed by constant vortexing for 4 minutes on a vortex mixer followed by sonication for 4 minutes in a Transsonic Digital T460/H bath sonicator (Elma®, Singen, Germany) to guarantee the complete suspension of lipids. Vesicle size, size distribution and ζ-potential were determined at 25°C by photon correlation spectroscopy and Doppler microelectrophoresis, using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK).Citation16
Preparation of thiolated fibrinogen and coupling to liposomes
The procedure for thiolating proteins with SATA has been previously reported.Citation17 The number of introduced thiol groups was assayed according to Ellman, using cysteine for the calibration curve.Citation18 According to this assay, the number of introduced thiol groups (expressed as mol of SH/mol of FBN) was 1.6 ± 0.1.
A volume of liposome suspension (DPPC: N-MPB-PE) was combined with an equal volume of fibrinogen solution. The resulting suspension was left to react overnight at room temperature while being centrifuged at 1,000 rpm.
The coupling reaction was stopped by adding 50 μL of N-ethylmaleimide (8 mM in HEPES buffer) and the liposomes were separated from free protein by two centrifugation steps in Optima L-100XP (Beckman-Coulter, Fullerton, CA) for 40 minutes at 100,000 g and 7°C. Liposomes (with or without FBM) were prepared in different batches. Measurements of size, ζ-potential, and amount of thiol groups introduced on the liposomal surface served as control parameters to ensure that the physicochemical properties were the same in all batches used.
Blood collection and preparation of thrombocytopenic blood
Blood was obtained from six healthy volunteers (4 males and 2 females, with an age range from 20 to 65 years) who had not taken drugs affecting platelets or coagulation mechanisms in the previous 10 days. Prior to the study, our local institutional ethics committee approved the protocol, which conformed to the ethical guidelines of the Helsinki Declaration. Written informed consent was obtained from all subjects. Blood was anticoagulated with LMWH (Fragmin®; Pfizer Inc, New York, NY) at a final concentration of 20 U/mL. This concentration of LMWH allows the generation of thrombin when blood is exposed to a denuded vascular segment.Citation19–Citation22 Blood platelets and leukocytes were reduced by a filtration procedure,Citation23 using an RC100 filter (PALL Corp, Glen Cove, NY). The platelet number was adjusted to 30,000 platelets/μL, mixing thrombocytopenic blood and whole blood. Before each experiment, samples were preincubated with liposomes coupled or uncoupled to FBN for 2 min at 37ºC. PFA-100® and aggregation experiments were performed with blood anticoagulated by citrate at a final concentration of 19 mM using standard laboratory procedures.
Thromboelastometric studies
To examine the kinetics and quality of clot formation, we used the ROTEM® thromboelastometry analyzer.Citation24 The technique was performed according to the manufacturer’s instructions. We used two different tests: EXTEM and FIBTEM. In the EXTEM test, tissue factor (TF) is used as an activator and is sensitive to changes in the extrinsic pathway of coagulation, FBN and fibrin polymerization, and platelet function. In the FIBTEM test, platelet function is inhibited by the platelet inhibitor cytochalasin D. While the clots obtained in EXTEM were formed by platelets and fibrin, the clot obtained in the FIBTEM assay was primarily a fibrin clot.
The study was carried out with whole and thrombocytopenic blood (30,000 platelets/μL) using 300 μL of blood containing 10% liposomes coupled or uncoupled to FBN. Measurements were recorded for 30 minutes, while controls were performed simultaneously by adding 10% of the corresponding buffer to the blood.
The main effect of liposomes was measured during clot formation by the standard thrombelastometric parameters: clotting time (CT) is the period of time from the start of the analysis until the clot reached 2 mm in amplitude; clot formation time (CFT) is the period until 20 mm amplitude was reached; and clot firmness was measured at 10-minute intervals (A10).Citation25 The CT and CFT, measured in seconds, indicate the dynamics of clot formation. The clot amplitude (mm) gives information about clot strength and stability, which largely depends on FBN and platelets.
Platelet aggregation
The experiments were performed in a four-channel aggregometer (APACT 4, Helena BioSciences) according to the classical turbidimetric technique.Citation26 Aliquots of 450 μL of platelet-rich plasma were transferred to the aggregometer cuvette and activated with different agonists: arachidonic acid and ristocetin (Helena BioSciences) at a final concentration of 1.4 mM and 1 mg/mL, respectively, and collagen and ADP at a final concentration of 2.5 μg/mL and 2 μM, respectively. The values of maximal platelet aggregation were recorded and expressed as percentages.
PFA-100® studies
PFA-100® evaluates the hemostatic capacity of platelets as a function of their ability to occlude an aperture in a membrane coated with collagen and epinephrine (EPI) or collagen and ADP under high shear stress (5000 s−1).Citation27 Normality values for closure times previously established for the PFA-100® in our laboratory were 113 ± 24 s (mean ± standard deviation (SD)) with cartridges containing collagen and epinephrin (COL-EPI) and 87 ± 18 s with cartridges containing collagen and adenosin diphosphate (COL-ADP).Citation21 Aliquots of blood incubated with MLV or MLV-FBN were used to study potential changes in closure time.
Perfusion studies
Rabbit abdominal aortic segments were prepared and processed as previously described.Citation28 Everted segments were mounted on a rod and inserted into the annular chamber. Two-mL aliquots of each liposome preparation were added to the blood samples. Control perfusions were carried out by adding equivalent amounts of the buffer used in the phospholipid preparations. The perfusates, with a total volume of 20 mL, were incubated at a constant temperature of 37°C for 2 minutes before starting the perfusion. Perfusions were conducted in annular chambers according to the method previously described.Citation3,Citation4,Citation28 Blood was recirculated through the chamber for 10 minutes at 37°C at a shear rate of 250 s−1, using a peristaltic pump. This low shear rate facilitates fibrin deposition onto the subendothelium.
At the end of the perfusion, vessel segments were histologically processed. Platelet interaction and fibrin deposition onto perfused subendothelium were analyzed morphometrically as previously described. Platelet interaction was expressed as the percentage of covered surface by platelets and the percentage of big platelet aggregates (>5 μm) classified as % thrombi (% T). The presence of fibrin was expressed as a percentage of fibrin.Citation20,Citation29,Citation30
Data analysis
Results are expressed as mean ± standard error of the mean (SEM). The number of experiments for each preparation was at least n = 6. Student’s t-test for paired data was used to compare differences between the control and the treated samples. The level of statistical significance was established at P < 0.05.
Results
Liposome characterization
The average size of liposomes, irrespective of the presence of FBN, ranged from 0.8 to 1.4 μm, and their polydispersity was close to 0.80. The incubation ratio of SATA to FBN of 10 (molar ratio) resulted in the introduction of 1.5 to 1.7 mol of thiol groups/mol of FBN. FBN was coupled at a ratio of 33 to 53 μg of FBN per μmol of phospholipid in the different FBN-MLV batches tested.
Measurements of the ζ-potential of raw liposomes in water afforded an average value of −8 mV, whereas after the coupling of fibrinogen, this value increased to −75 mV.
Effect of liposome preparation on hemostatic parameters
Thromboelastometric studies
The addition of MLV-FBN to whole blood did not modify the CT in comparison with the control in the EXTEM analysis. However, the presence of MLV-FBN led to a significant increase in the CFT (226.5 s ± 26.13 versus 124.17 s ± 9.46; P < 0.01), indicating a certain inhibition of clot formation. Moreover, MLV-FBN reduced clot firmness, measured as A10, with respect to control (35.50 ± 2.38 mm versus 45.40 ± 1.82 mm; P < 0.05). These results are summarized in . No differences were observed when the experiments were performed using thrombocytopenic blood.
Figure 1 Thromboelastometric results of EXTEM- (A–C) and FIBTEM (D and E) derived parameters (CT and CFT expressed in s, and A10 in mm). Experiments were performed in whole blood (WB) and thrombocytopenic blood (TPN) following in vitro addition of MLV and MLV-FBN.
Notes: Data presented as mean ± SEM, n = 6. *MLV-FBN versus control P < 0.01; #MLV-FBN versus MLV liposomes P < 0.01.
Abbreviations: CT, clotting time; CFT, clot formation time; CON, control; MLV, raw multilamellar liposomes; MLV-FBN, fibrinogen-coated liposomes; SEM, standard error of the mean.

The analysis of parameters using the FIBTEM tests indicated that the addition of MLV-FBN to whole blood did not induce differences compared to the control. However, in experiments performed with thrombocytopenic blood, MLV-FBN decreased the CT with respect to MLV (153.33 ± 2.82 versus 128.00 ± 4.62; P < 0.05), whereas no differences were found between MLV-FBN and control ().
As expected, the results using whole blood in the EXTEM test showed a reduction in clot formation and clot firmness. This test reflected the role of platelets and coagulation processes in hemostasis. In contrast, results obtained with the FIBTEM tests, which eliminated platelet function, showed significant differences in thrombocytopenic blood.
Platelet aggregation studies
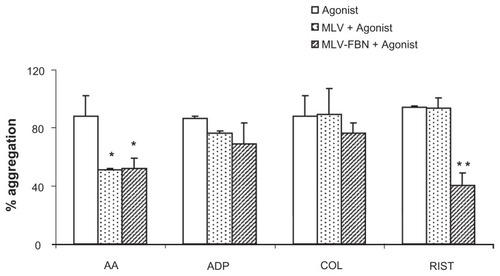
MLV modified aggregation profiles only when arachidonic acid was used as an agonist (50.9% ± 7.9% versus 88.0% ± 2.1% in control; P < 0.01). Interestingly, MLV-FBN also inhibited aggregation induced by arachidonic acid (52.1% ± 8.1% versus 88.0% ± 2.1% in control; P < 0.01) and ristocetin (16.7 ± 3.2 versus 68.3% ± 5.8%; P < 0.01). Aggregations induced by ADP and collagen were not modified by either MLV or MLV-FBN. However, we observed a reduction in the maximal aggregation induced by ADP in the case of MLV-FBN, but this was not statistically significant. These results are summarized in .
Figure 2 Bar diagram summarizing changes in platelet aggregation induced by different agonists (AA, ADP, COL, and RIST) in samples treated with MLV or MLV-FBN. Results are expressed as mean ± SEM; n = 6; *P < 0.05; **P < 0.01.
Abbreviations: AA, arachidonic acid; ADP, adenosine diphosphate; COL, collagen; RIST, ristocetin; MLV, raw multilamellar liposomes; MLV-FBN, fibrinogen-coated liposomes; SEM, standard error of the mean.
PFA-100® studies
The addition of liposomes to whole blood did not statistically modify closure times in the PFA-100® device. COL-EPI cartridges showed values of 171 ± 48.1 s for MLV, 194 ± 27.3 s for MLV-FBN, and 184 ± 27.4 s for control. Results using COL-ADP cartridges were 113.5 s ± 21.6 s for MLV, 124.3 ± 20.5 s for MLV-FBN, and 95 ± 16.9 s for control.
Perfusion experiments
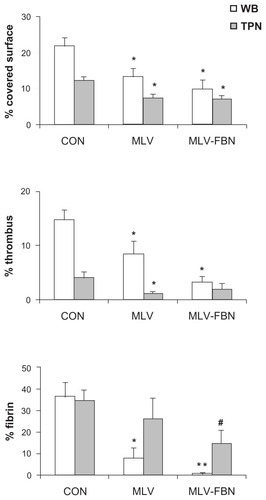
Perfusions with whole blood resulted in a platelet coverage surface of 22.01% ± 2.05%. Addition of MLV and MLV-FBN to whole blood samples decreased the coverage surface of platelets (13.25% ± 2.4% and 9.85% ± 2.41%, respectively, versus 22.01% ± 2.05%; P < 0.01). A more detailed analysis of platelet interaction also showed a decrease in the presence of large aggregates measured as % T (8.44% ± 2.33% and 3.36% ± 1.01%, respectively, versus 14.64% ± 1.89% in control experiments; P < 0.01). Moreover, a significant decrease in fibrin deposition was observed when liposomes were present in perfusates (MLV 8.12% ± 4.73% and MLV-FBN 0.84% ± 0.52% versus control 36.48% ± 6.76%; P < 0.01). Representative microscopic fields are shown in .
Figure 3 Light micrographs representative of changes induced by MLV-FBN in platelet interaction (p) and fibrin deposition (f) on the subendothelium observed in perfusions using whole blood (WB) and thrombocytopenic blood (TPN). (A) WB control; (B) TPN control; (C) WB + MLV-FBN; (D) TPN + MLV-FBN.
Abbreviations: MLV, raw multilamellar liposomes; MLV-FBN, fibrinogen-coated liposomes.

Experiments performed under conditions of moderate thrombocytopenia revealed that the presence of liposomes resulted in a significant reduction (P < 0.05) of the percentage of covered surface by platelets (MLV 7.30% ± 1.17% and MLV-FBN 6.99% ± 0.94% versus 12.50% ± 1.31% in thrombocytopenic blood; P < 0.05). MLV did not modify fibrin deposition on the subendothelium (34.58% ± 5.21%), but there was a significant decrease in fibrin deposition after the addition of MLV-FBN to thrombocytopenic blood (14.47% ± 6.34%; P < 0.05). These results are summarized in .
Figure 4 Bar diagram summarizing changes in platelet interaction in samples treated with MLV or MLV-FBN.
Notes: Results are expressed as % covered surface by platelets, % of large aggregates (thrombus), and % of fibrin deposition on the subendothelium. Mean ± SEM; n = 6. Comparisons with respect to control: *P < 0.01; **P < 0.005; comparison of MLV-FBN versus MLV – raw liposomes #P < 0.01.
Abbreviations: CON, control; MLV, raw multilamellar liposomes; MLV-FBN, fibrinogen-coated liposomes; SEM, standard error of the mean.
Discussion
The present study focused on the preparation of FBN-coated liposomes in order to improve hemostatic mechanisms, such as platelet reactivity and coagulation mechanisms. In recent years, several groups have explored the use of modified liposomes and microcapsules as coagulants to improve hemostasis.Citation5–Citation9,Citation31,Citation32 Our group demonstrated that raw preparations of phospholipids were able to activate the coagulation system and promote fibrin formation on damaged vascular surfaces under thrombopenic conditions.Citation3 A reduction of platelet counts is quite common in oncohematological patients who exhibit hemorrhagic episodes that sometimes may require platelet transfusion. Originally, the present study was designed to evaluate the possible effect of FBN-containing liposomes as a prohemostatic strategy in different conditions, such as normal platelet counts and thrombocytopenic conditions, in order to mimic patients potentially requiring platelet transfusion.
Under our experimental conditions, FBN-coated liposomes modified blood coagulation parameters, as indicated by the thromboelastometric and fibrin deposition results. In the FIBTEM thromboelastometric studies, in which platelets were inhibited, MLV-FBN reduced the CT in thrombocytopenic blood, indicating a positive effect on the coagulation mechanism. Interestingly, studies using EXTEM tests on whole blood, when both platelets and the coagulation mechanism are active, have demonstrated a partial inhibition of overall hemostatic parameters that includes a reduction in CFT and clot firmness. Moreover, our results of the experiments under moderate flow conditions indicate that MLV-FBN induced lower clotting activity, as demonstrated by a decrease in fibrin deposition on the subendothelium. Furthermore, MLV-FBN acted on primary hemostasis, as demonstrated by decreased platelet interactions in the perfusion experiments performed at a moderate shear rate. However, no differences were observed at a high shear rate, as shown by the closure time obtained with the PFA-100®. In fact, it is well established that the shear rate has a role in platelet adhesion and fibrin formation. Under high shear rates, platelet interactions predominate over fibrin formation, whereas under moderate and low shear rates, coagulation mechanisms prevail over platelet interactions.Citation33–Citation35 We also observed an inhibition in the aggregation profiles induced by ristocetin and arachidonic acid. In the presence of liposomes, this reduction could be explained by a direct effect of MLV-FBN on the GpIbα complexCitation32 in the ristocetin assay and by the phospholipidic composition of the liposomes. Indeed, it has been shown conclusively that the net charge of liposomes and the firmness of the membranes determine the effect on hemostasis.Citation4,Citation32,Citation36
Although the antihemostatic activity observed in our experiments with FBN-coated liposomes cannot be ascribed to the γ ′ chain of FBN, our results (reduced thrombin generation, inhibition of ristocetin-induced aggregation, decrease in fibrin density and inhibition of thrombus formation) show behavior similar to that described for the γ ′ chain.Citation11,Citation37 This could be explained by a global negative charge in our liposomesCitation17 and the use of thiolated FBN during the binding procedure, which could induce residue sulfation and generate conformational changes in the molecule similar to those observed in the γ ′ chain. Interestingly, the effect of MLV-FBN suggests that with a high number of platelets, FBN acts mainly in primary hemostasis, whereas its participation in coagulation is moderate.
Inconsistent effects of FBN have been described to date, probably because FBN can undergo several posttranslational modifications, such as glycosylation, glycation, nitration, oxidation, sulfation, phosphorylation and proteolytic degradation, and genetic differences among individuals.Citation32 In fact, contradictory results in studies on FBN binding to liposomes have been published. Whereas some studies show that conventional approaches to binding proteins to liposomes do not work effectively with FBN to provide a final product with hemostatic properties,Citation38 others demonstrate that the binding to liposomes of the dodecapeptide H12, corresponding to a fibrinogen γ-chain carboxy-terminal sequence, yields a product with demonstrated hemostatic activity.Citation31
The biological function of FBN can be modified by changes in protein folding.Citation39 It is accepted that structural features of fibrinogen are related to multiple biological functions, including binding to thrombin, fibrinolysis, regulation of FXIII activity, and interactions with blood cells, such as platelets and leukocytes. Residues A414 to L427 of the γ ′ chain have been reported to play a central role in thrombin binding because of their overall negative charge, which disrupts the platelet binding domain in the γA chain and introduces a binding site for zymogen factor XIII and thrombin.Citation10–Citation13 Moreover, the sulfation of residues Y418 and Y422 further enhances thrombin binding by increasing the net charge and inhibiting thrombin activity.Citation32,Citation40 On the other hand, γXL sites in FBN are also involved in the binding to FXIII and to the platelet integrin GPIIb/IIIa. It is not surprizing that when chemical modification occurs, the liposomalization of FBN gives liposomes the ability to bind to the GPIIb/IIIa of platelets, thus competitively inhibiting the formation of platelet aggregates. According to Sivaraman and Latour,Citation39 the conformation of FBN is the critical determinant of platelet adhesion. Adsorption-induced unfolding can expose two distinctly different types of platelet-binding sites in FBN: one induces platelet adhesion alone, and the other induces both platelet adhesion and activation.Citation11,Citation29,Citation41
The present study, in contrast to its initial purpose, offers the possibility of developing new clinical strategies for regulating arterial or venous thrombosis based on the use of liposomes. Our data suggest that the antihemostatic effect observed on liposomes is probably related to the conformational structure adopted by the FBN after binding to the liposome. These changes seem to play an important role not only in blood clot formation and stabilization, but also in primary hemostasis. Future investigations will need to address how the binding procedure may interfere with hemostasis.
Acknowledgments
This work was partially supported by grants PTR95/0890, PET2007_0169, SAF 2009-10365, FIS CP04-00112, FIS PS09/00664, MAT-2009-13155-C04-03, and Red HERACLES RD06/0009 from the Spanish government. Dr Galán belongs to the program of stabilization of researchers in the Instituto de Salud Carlos III, which is funded by the Spanish government, and the Direcció d’Estratègia i Coordinació del Departament de Salut, which is funded by the Generalitat de Catalunya.
Disclosure
The authors report no conflicts of interest in this work.
References
- StreicherPNassoyPBärmannMIntegrin reconstituted in GUVs: a biomimetic system to study initial steps of cell spreadingBiochim Biophys Acta200917882291230019665445
- YatuvRRobinsonMDayan-TarshishIBaruMThe use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophiliaInt J Nanomedicine2010558159120856833
- GalánAMHernándezMRBozzoJPreparations of synthetic phospholipids promote procoagulant activity on damaged vessels: studies under flow conditionsTransfusion199838100410109838928
- GalánAMCasalsEEstelrichJPossible hemostatic effect of synthetic liposomes in experimental studies under flow conditionsHaematologica20028761562312031918
- GuptaASHuangGLestiniBJSagnellaSKottke-MarchantKMarchantRERGD-modified liposomes targeted to activated platelets as a potential vascular drug delivery systemThromb Haemost20059310611415630499
- KitaguchiTMurataMIijimaKKamideKImagawaTIkedaYCharacterization of liposomes carrying von Willebrand factor-binding domain of platelet glycoprotein Ibalpha: a potential substitute for platelet transfusionBiochem Biophys Res Commun199926178478910441502
- LeeDHBlajchmanMANovel treatment modalities: New platelet preparations and substitutesBr J Haematol200111449650511552973
- LeviMFriederichPWMiddletonSFibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbitsNat Med199951071119883848
- RybakMERenzulliLAA liposome based platelet substitute, the plateletsome, with hemostatic efficacyBiomater Artif Cells Immobilization Biotechnol1993211011188318606
- BertramJPWilliamsCARobinsonRSegalSSFlynnNTLavikEBIntravenous hemostat: nanotechnology to halt bleedingSci Transl Med2009111ra22
- LovelyRSMoaddelMFarrellDHFibrinogen gamma′ chain binds thrombin exosite IIJ Thromb Haemost2003112413112871549
- TakeokaSTeramuraYOkamuraYHandaMIkedaYTsuchidaEFibrinogen-conjugated albumin polymers and their interaction with platelets under flow conditionsBiomacromolecules200121192119711777392
- SavageBBottiniERuggeriZMInteraction of integrin alpha IIb beta 3 with multiple fibrinogen domains during platelet adhesionJ Biol Chem199527028812288177499405
- BennettJSPlatelet-fibrinogen interactionsAnn N Y Acad Sci200193634035411460491
- KirschbaumNEMosessonMWAmraniDLCharacterization of the gamma chain platelet binding site on fibrinogen fragment DBlood199279264326481586714
- HunterRJZeta Potential in Colloid ScienceNew YorkAcademic Press1981
- CasalsEVerdaguerATondaREscolarGEstelrichJAtomic force microscopy of liposomes bearing fibrinogenBioconjugate Chem200314593600
- EllmanGLTissue sulphydryl groupsArch Biochem Biophys195982707713650640
- ZwagingaJJSixmaJJde GrootPGActivation of endothelial cells induces platelet thrombus formation on their matrix studies of new in vitro thrombosis model with low molecular weight heparin as anticoagulantArteriosclerosis19901049612297347
- AlemanyMHernándezMRBozzoJIn vitro evaluation of the hemostatic effectiveness of non-viable platelet preparations: studies with frozen-thawed, sonicated or lyophilized plateletsVox Sang19977336429269068
- EscolarGCasesAVinasMEvaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA-100™): Influence of hematocrit elevationHaematologica19998461461910406903
- GalánAMLopez-VilchezIDiaz-RicartMSerotonergic mechanisms enhance platelet-mediated thrombogenicityThromb Haemost200910251151919718472
- SirchiaGWenzBRebullaPParraviciniACarnelliVBertoliniFRemoval of white cells from red cells by transfusion through a new filterTransfusion19903030332296786
- AndersonLQuasimISoutarRStevenMMacfieAKorteWAn audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unitTransfus Med200616313916480437
- LakMScharlingBBlemingsAEvaluation of rFVIIa (Novo-Seven) in Glanzmann patients with thromboelastogramHaemophilia20081410311018070065
- BornJVRCrossMJThe aggregation of blood plateletsJ Physiol196316817819514056485
- KunduSKHeilmannEJSioRGarciaCOstgaardRADescription of an in vitro platelet function analyzer – PFA- 100(TM)Seminars in Thrombosis and Hemostasis1995211061127660150
- BaumgartnerHRPlatelet interaction with collagen fibrils in flowing blood. Reaction of human platelet with achymotry DPPS in digested subendotheliumThromb Haemost197737116576508
- EscolarGBastidaECastilloROrdinasADevelopment of a computer program to analyze the parameters of platelet-vessel wall interactionHemostasis198616814
- HernándezMRBozzoJMazzaraROrdinasAEscolarGPlatelet concentrates promote procoagulant activity: Evidence from experimental studies using a perfusion techniqueTransfusion1995356606657631406
- OkamuraYMaekawaITeramuraYHemostatic effects of phospholipid vesicles carrying fibrinogen gamma chain dodecapeptide in vitro and in vivoJ Thromb Haemost2009747047719143920
- WilligeSUStandevenKFPhilippouHAriënsRASThe pleiotropic role of the fibrinogen gamma′ chain in hemostasisBlood20091143994400119687509
- BeumerSIjsseldijkMJWde GrootPGSixmaJJPlatelet adhesion to fibronectin in flow: dependence on surface concentration and shear rate, role of platelet membrane glycoproteins GP IIb/IIIa and VLA-5, and inhibition by heparinBlood199484372437337949128
- TondaRLopez-VilchezINavalonFPlatelets interact with tissue factor immobilized on surfaces: effects of shear rateEur J Clin Invest200838344218173549
- WuYPVinkTSchiphorstMPlatelet thrombus formation on collagen at high shear rates is mediated by von Willebrand factor-glycoprotein Ib interaction and inhibited by von Willebrand factor-glycoprotein IIb/IIIa interactionArteriosclerosis Thrombosis and Vascular Biology20002016611667
- AnHNussioMRHusonMGVoelckerNHShapterJGMaterial properties of lipid microdomains: force-volume imaging study of the effect of cholesterol on lipid microdomain rigidityBiophysical Journal20109983484420682261
- CooperAVStandevenKFAriënsRAFibrinogen gamma-chain splice variant gamma′ alters fibrin formation and structureBlood200310253554012663453
- RetzingerGSDeanglisAPUniversity of CincinnatiFibrinogen-coated liposomes United States patent US 56585881996329
- SivaramanBLatourRAThe relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogenBiomaterials201031583283919850334
- MosessonMWUpdate on antithrombin I (fibrin)Thromb Haemost20079810510817597999
- FarrellDHThiagarajantPChungDWDavieEWBiochemistry role of fibrinogen a and y chain sites in platelet aggregationProc Nati Acad Sci U S A1992891072910732