Abstract
Background/objective
The size-dependent mucosal immunoresponse against nanomaterials (nanoimmunoresponse) is an important approach for mucosal vaccination. In the present work, the size-dependent nanoimmunoresponse of mouse Peyer’s patches (PPs) and immunoglobulin A (IgA) level was investigated using fluorescent thiol-organosilica particles.
Methods
Various sizes of fluorescent thiol-organosilica particles (100, 180, 365, 745, and 925 nm in diameter) were administered orally. PPs were analyzed histochemically, and IgA levels in PP homogenates, intestinal secretions around PPs, and bile were analyzed biochemically.
Results
When compared with the larger particles (745 and 925 nm), oral administration of smaller thiol-organosilica particles (100, 180, and 365 nm) increased the number of CD11b+ macrophages and IgA+ cells in the subepithelial domes of the PPs. Additionally, administration of larger particles induced the expression of alpha-L-fucose and mucosal IgA on the surface of M cells in the follicle-associated epithelia of PPs and increased the number of 33D1+ dendritic cells in the subepithelial domes of the PPs. IgA contents in the bile and PP homogenates were high after the administration of the 100 nm particles, but IgA levels in the intestinal secretions were high after the administration of the 925 nm particles. Two size-dependent routes of IgA secretions into the intestinal lumen, the enterohepatic route for smaller particles and the mucosal route for larger particles were proposed.
Conclusion
Thiol-organosilica particles demonstrated size-dependent nanoimmunoresponse after oral administration. The size of the particles may control the mucosal immunity in PPs and were useful in mucosal vaccination approaches.
Introduction
Nanoparticles (NPs) have the following advantages for mucosal vaccination: controlled release, specific targeting, and efficient immunization.Citation1,Citation2 The size-dependent immunoresponse against nanomaterials in various immune tissues (nanoimmunoresponse) is a new and attractive field, and mucosal immunity induction and enhancement using nanomaterials is a nanoimmunoresponse that is important to understand for developing new applications for nanomaterials.
The initiation of adaptive mucosal immunity occurs in organized, mucosal lymphoid tissues such as the Peyer’s patches (PPs) of the small intestine.Citation3 The PP immune cells play an important role in the immune defense against pathogens and antigen-coated NPs.Citation3,Citation4 A main immunological function of macrophages is to remove NPs from the body, especially from gut lymphoid tissue or from other organs.Citation5,Citation6 Additionally, dendritic cells (DCs) have a primary role in the immune defense against mucosal pathogens and antigen-coated NPs and in the uptake of NPs from gut-associated lymphoid tissue.Citation7,Citation8
Analysis of the size-dependent nanoimmunoresponses of immune cells in PPs is important for understanding the mucosal immunity. Some previous studies detected a change in the numbers of immune cells in PPs after oral administration of bacteria and viruses such as Lactobacillus paracasei and Cowpea Mosaic virus.Citation9–Citation13 But studies on the nanoimmunoresponse, such as those on the change in the number of PP immune cells after oral administration of nanomaterials, are very rare. In a previous study, a powerful intestinal secretagogue and induction of an abnormal mucin composition in the intestinal mucosa were observed after oral administration of silver NPs (average diameter of 60 nm). The silver NPs decreased the number of mucus cells, and mucus was released from the goblet cells in the intestine. Moreover, rectum and colon mucosa exhibited a higher amount of sialomucins.Citation14 It was also reported that fluorescent polystyrene particles (200 nm) were efficiently transported by M cells to the subepithelial dome (SED) of the PPs and were ingested by CD11c+ cells and that a small number of microparticles were ingested with CD11b+ macrophages after oral administration.Citation4
Locally produced immunoglobulin A (IgA) is considered to be among the most important of protective humoral immune factors.Citation15–Citation17 Recently, mucosal and systemic nanoimmunoresponses after oral administration of particles have been actively investigated.Citation4,Citation18–Citation20 It has been previously reported that a mucosal nanoimmunoresponse occurred after using functionalized NPs of a single particle size.Citation2,Citation19–Citation21 Also, intestinal IgA and serum HBsAg-specific total antibodies are specific mucosal nanoimmunoresponses against antigens and have been induced after oral administration of poly lactic-co-glycolic acid (PLGA) microparticles (2–9 μm) plasmid DNA, which encoded HBsAg,Citation19 and PLGA NPs (390 nm), which encapsulated the hepatitis B surface antigen.Citation18 Additionally, the IgA, size-dependent nanoimmunoresponse in serum after oral administration of functionalized particles of various sizes has been reported.Citation22–Citation24 In another study, PLGA NPs (1–5 μm), which encapsulated the hepatitis B surface antigen (HBsAg), induced secretory IgA levels in salivary, intestinal, and vaginal secretions.Citation25 However, no report has been published on the changes in the numbers of immune cells from PPs that was based on the size-dependent nanoimmunoresponse against NPs. Therefore, studies regarding the size-dependent nanoimmunoresponse against nanomaterials in the gastrointestinal tract require further investigation.
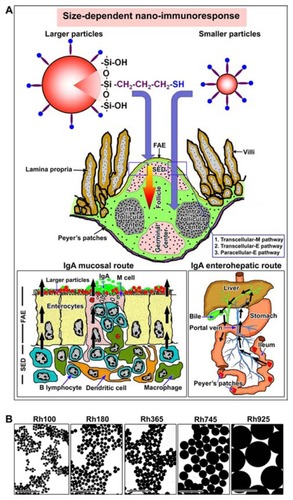
The authors of this present study recently developed new nanomaterials, ie, organosilica particles, for nanomedicine applications.Citation26–Citation32 Various sizes of fluorescent, thiol-organosilica particles can be synthesized with a narrow size distribution, high dispersion, and unique surface charge.Citation28 Recently it was reported that thiol-organosilica particles have excellent adhesion ability to mucosal surfaces, possibly due to effects of thiol residue.Citation33 The authors of this present study previously administered various sizes of thiol-organosilica particles orally into mice and demonstrated the size-dependent uptake and novel pathways in PP (). In the present study, histochemical studies of PP were performed to quantitatively investigate the size-dependent nanoimmunoresponse enhancement of surface molecules on M cells and the immune cell number. Furthermore, the size-dependent nanoimmunoresponse enhancement of IgA in PP tissue homogenates and in bile and intestinal secretions surrounding the PPs was biochemically investigated. The outcomes from this study could provide important information for enhancing mucosal and systemic immunity against nanomaterials and could improve mucosal vaccination efficiency and nanomedicine applications through intestinal mucosa.
Figure 1 (A) Schemes for two size-dependent nanoimmunoresponses induced by smaller thiol-organosilica particles and larger thiol-organosilica particles, and two possible routes, enterohepatic route and mucosal route, of two size-dependent IgA secretions. (B) Electron micrograph of different sizes of fluorescent thiolorganosilica particles.
Note: Scale bar = 1 μm.
Abbreviations: FAE, follicle associated epithelium; IgA, immunoglobulin A; paracellular-E pathway, paracellular pathway between enterocytes; Rh, rhodamine B; SED, subepithelial dome; transcellular-E pathway, transcellular pathway through enterocytes; transcellular-M pathway, transcellular pathway through M cell.
Materials and methods
Materials
3-Mercaptopropyltrimethoxysilane, rhodamine B (Rh), lectin Ulex europaeus agglutinin I (UEA I) conjugated with fluorescein isothiocyanate (FITC), and anti-mouse IgA (alpha chain-specific) antibody conjugated with FITC were purchased from Sigma–Aldrich Chemical Co (Saint Louis, MO). Anti-mouse CD11b (Mac 1 alpha chain) antibody and affinity-purified antimouse DC marker (33D1) were purchased from eBioscience, Inc (Kobe, Japan). A sheet of polyvinylidene difluoride (PVDF) membrane was obtained from Amersham Pharmacia Biotech UK Limited (Buckinghamshire, England).
Preparation and characterization of thiol-organosilica particles
The particles were prepared from 3-mercaptopropyltrimethoxysilane as the silica source, and five different sizes of particles containing Rh B (100 nm, 180 nm, 365 nm, 745 nm, and 925 nm in diameter) were prepared according to previous methods with slight modificationCitation28,Citation32 ( and ). The images of particles were obtained with a Hitachi H7650 electron microscope (Tokyo, Japan), and the sizes and standard deviations of about 200 particles were analyzed by Image-Pro Plus software (Media Cybernetics, Inc, Bethesda, MD).
Table 1 Average sizes with standard deviations and coefficient variants of fluorescent thiol-organosilica particles containing Rh
Oral administration of different sizes of thiol-organosilica particles
Female C57 BL/6J mice (6–8 weeks old) were used for this study, and all experiments were conducted according to the guidelines for the Care and Use of Animals that was approved by the Animal Experiments Committee at the University of Tokushima. Before administration of the particles, the mice were fasted for 4 hours with free access to water, and the animals were then separated into six groups (four mice per group). Each group of mice was orally administered 5 mg (100 μL of 50 mg/mL) of various sizes of particles. The control group was orally administered 5 mg (100 μL of 50 mg/mL) of food that had been dissolved in phosphate-buffered saline solution. After oral administration, the mice were left with free access to water. Five to seven PPs were dissected from the intestines of the mice, fixed with 4% paraformaldehyde, and processed for cryostat sectioning.
Immunohistochemical and quantitative analysis of PPs
Sections were incubated with lectin UEA I conjugated with FITC (20 μg/mL), anti-mouse IgA antibody conjugated with FITC (68.75 μg/mL), anti-mouse CD11b antibody (0.625 μg/mL), or anti-mouse 33D1 (5 μg/mL). All sections were observed with an inverted TE 2000 fluorescence microscope (Nikon, Kanagawa, Japan) equipped with a 100 W mercury lamp as a light source and a CCD camera. For quantitative analysis of PP immune cells, about 20 representative sections (n = 20) were selected from each group, and a survey to count IgA+ cells, 33D1+ DCs, and CD11b+ macrophages for each PP SED was performed. The number of PP immune cells was normalized with an averaged area of the PP SED (0.154 mm2).
Western blotting of PP homogenates, bile, and intestinal secretions IgA
A control and two experimental groups, which were administrated either Rh100 or Rh925, were used for this study. Collected bile from gall bladder and intestinal secretions surrounding PPs and PP tissues were applied to Western blotting. A suitable and equal amount of protein from each sample (5 μg of bile, 15 μg of PP homogenates, and 10 μg of intestinal secretions) was applied to sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins on the gel were transferred to a PVDF membrane using a semi-dry transfer unit electrophoretically. The membrane was incubated with anti-IgA antibody conjugated to FITC (11 μg/mL, 1:100 dilution) for 1 hour, and scanned using a Typhoon scanner 9400 (GE Healthcare, Tokyo, Japan). IgA bands were analyzed using ImageQuant TL software, and the IgA value was calculated from the means ± standard deviations of four mouse groups (n = 4).
Results
Histochemical and immunohistochemical analysis of the nanoimmunoresponse of PPs after oral administration of thiol-organosilica particles
Localization of thiol-organosilica particles with immune cells of PPs
After 4 hours of oral administration, the co-localization of thiol-organosilica particles and some immune cells from PPs was observed. The uptake of thiol-organosilica particles by PPs was size-dependent, ie, the fluorescence area from smaller particles (Rh100, Rh180, and Rh365) in the SEDs of PPs was larger than that from larger particles (Rh745 and Rh925) (–). To identify the precise localization of the particles in the SEDs of the PPs, lectin UEA I labeling of follicle-associated epithelium (FAE) M cells, and immunohistochemical staining of macrophages, DCs, and IgA+ cells were performed. As shown in , PP FAE contains UEA I− enterocytes and UEA I+ M cells that are scattered between enterocytes. UEA I+ M cells expressed an alpha-L-fucose residue only on the surface and upper cytoplasm of the cells (, arrowheads). Only a few of the smaller particles were localized with the green fluorescence that represented UEA I (); however, for the larger particles, nearly all red fluorescence from the particles was observed to localize with the green fluorescence of UEA I (). The particles localized on the surface and in the cytoplasm of UEA I+ M cells, but they were then translocated into the SEDs of the PPs. Immunohistochemical staining with a CD11b antibody, a specific marker for macrophages, revealed that CD11b+ macrophages (, arrowheads) were scattered in the SEDs of the PPs of the control. As shown in , CD11b+ macrophages with smaller particles (Rh100, Rh180, and Rh365) fluoresced more than those with larger particles (Rh745 and Rh925) (). In some cases, the particles diffused freely into the SED of the PPs or into intercellular spaces, as shown by fluorescence (, , and ) and electron microscopy (Supplementary figures 1 and 2). Histochemically, to identify DCs and IgA+ cells in PPs, 33D1 and IgA antibodies (, arrowheads; , bold arrows) were used, respectively. A small number of particles co-localized with 33D1+ DCs, compared with CD11b+ macrophages (, bold arrows), and in some cases, a small amount of particles co-localized with 33D1+ DCs (, arrowheads). Furthermore, co-localization of particles and IgA+ cells (, arrowheads) was rare. These data revealed that a main role of UEA I+ M cells and CD11b+ macrophages is to take up particles into the SEDs of PPs.
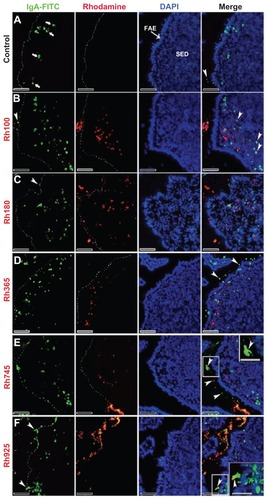
Figure 2 Fluorescent microscope images showing the FAE of PPs that had been stained with lectin UEA I (a marker for alpha-L-fucose residue on M cells) after 4 hours of oral administration of different sizes of fluorescent thiol-organosilica particles. (A) Control, M cells were scattered in the FAE, and alpha-L-fucose residue was expressed on the surface and upper cytoplasm of M cells. PP after administration of (B) Rh100 particles, (C) Rh180 particles, (D) Rh365 particles, (E) Rh745 particles, and (F) Rh925 particles. The expression of alpha-L-fucose residue on the surface and cytoplasm of M cells increased more in animals that had been administered Rh745 or Rh925 than in animals that had been administered the smaller particles Rh100, Rh180, or Rh365 or the control.
Note: Scale bar = 50 μm.
Abbreviations: FAE, follicle-associated epithelium; PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome; UEA I, Ulex europaeus agglutinin I.
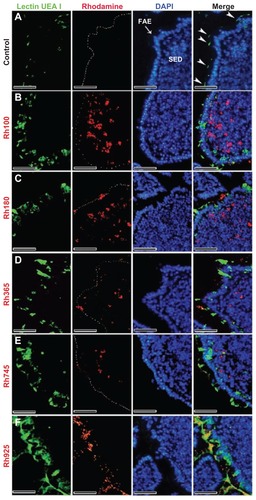
Figure 3 Fluorescent microscope images showing the SED of PPs stained with CD11b (Mac 1α chain) antibody (CD11b) (a marker for macrophages) after 4 hours of oral administration of different sizes of fluorescent thiol-organosilica particles. (A) Control, macrophages scattered in the SEDs of PPs. PPs after administration of (B) Rh100 particles, (C) Rh180 particles, and (D) Rh365 particles; there was co-localization of particles and Cd11b+ macrophages, and the number of CD11b+ macrophages increased compared with the control. PPs after administration of (E) Rh745 particles and (F) Rh925 particles; the number of CD11b+ macrophages were similar to that of the control. CD11b+ macrophages aggregated under the FAE of PPs after administration of all particle sizes.
Note: Scale bar = 50 μm.
Abbreviations: FAE, follicle associated epithelium; PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
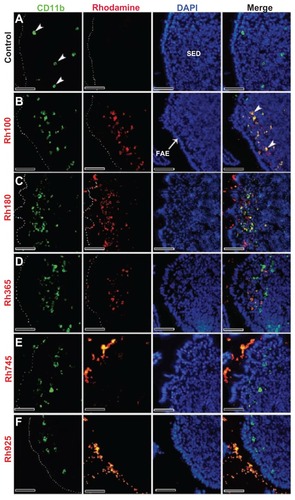
Figure 4 Fluorescent microscope images showing the SED of PPs stained with 33D1 antibody (a marker for DCs) after 4 hours of oral administration of differently sized fluorescent thiol-organosilica particles. (A) Control, 33D1+ DCs scattered in the SED of the PPs. PPs after administration of (B) Rh100 particles, (C) Rh180 particles, and (D) Rh365 particles; there was no co-localization of particles and 33D1+ DCs. The number of 33D1+ DCs was similar to the control. PPs after administration of (E) Rh745 particles and (F) Rh925 particles; the number of 33D1+ DCs increased when compared with the control. 33D1+ DCs aggregated under the FAE of the PPs after administration of all particle sizes.
Note: Scale bar = 50 μm.
Abbreviations: DCs, dendritic cells; FAE, follicle associated epithelium; PPs, Peyer’s patches; Rh, rhodamine B, SED, subepithelial dome.
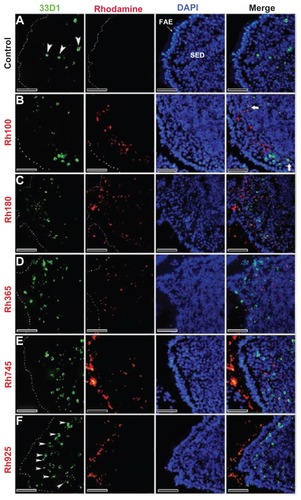
Figure 5 Fluorescent microscope images showing PPs at high magnifications after 4 hours of oral administration of Rh100 fluorescently labeled thiol-organosilica particles. (A) CD11b+ macrophages containing particles scattered in the SED of the PPs and aggregated under the FAE of the PPs; large numbers of particles co-localized with CD11b+ macrophages. (B) 33D1+ DCs scattered in the SED of the PPs; there was little co-localization of the particles with 33D1+ DCs.
Note: Scale bar = 25 μm.
Abbreviations: DCs, dendritic cells; FAE, follicle associated epithelium; PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
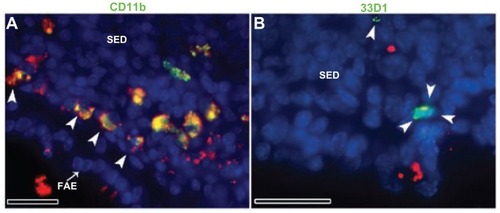
Figure 6 Fluorescent microscope images showing the SED of PPs stained with IgA antibody after 4 hours of oral administration of different sizes of fluorescently labeled thiolorganosilica particles. (A) Control, IgA+ cells scattered in the SED of the PPs. PPs after administration of (B) Rh100 particles, (C) Rh180 particles, and (D) Rh365 particles; there was no co-localization of the particles and IgA+ cells. The number of IgA+ cells in animals treated with the smaller particles (Rh100, Rh180, and Rh365) increased compared with the control. PPs after administration of (E) Rh745 particles and (F) Rh925 particles; the number of IgA+ cells were similar to that of the control, but IgA expression on the surface of M cells increased when compared with the smaller-sized particles or the control (white quadrates and enlarged parts of merged (E) and (F)).
Notes: Scale bar = (A–F), 50 μm; enlarged sections of merged (E) and (F), 10 μm.
Abbreviations: DCs, dendritic cells; FAE, follicle associated epithelium; IgA, immunoglobulin A; PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
Nanoimmunoresponse of immune cells of PPs after oral administration of thiol-organosilica particles
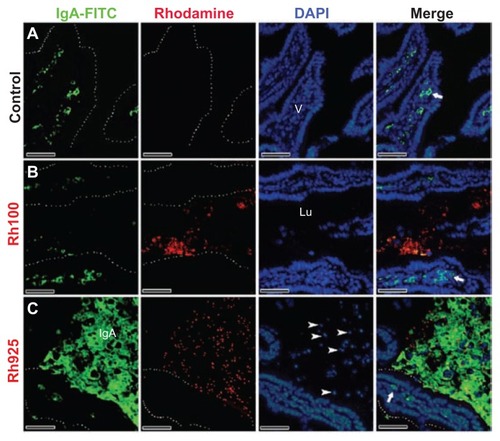
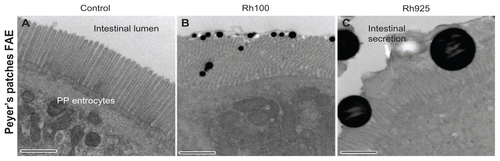
In contrast to the M cells from the PPs of the control group (, arrowheads), the smaller particles (Rh100, Rh180, and Rh365) increased the expression of alpha-L-fucose residue on the surface of M cells and redistributed it into the cytoplasm and the apical region of the cells (). However, uptake and mechanical adhesion of the larger particles (Rh745 and Rh925) () with the FAE and M cells of PPs increased the expression of alpha-L-fucose residue and redistributed it to the apical region of the cell, in contrast to what occurred with smaller particles (Rh100, Rh180, and Rh365) and M cells from the PPs of the control group. Additionally, electron microscopy analysis showed that mechanical contact between the larger particles (Rh925) and cells deformed the microvilli of the FAE of the PPs, in contrast to the control group or to the smaller particles (Rh100), which smoothly dispersed between the microvilli (Supplementary figure 3). In addition, the expression of IgA on the surface of M cells increased after administration of the larger particles (Rh745 and Rh925) (enlarged parts in , arrowheads), in contrast to what was observed with the smaller particles (Rh100, Rh180, and Rh365) and the control group (, arrowheads). Moreover, larger particles (Rh925) () increased IgA induction in the intestinal lumen more readily than the smaller particles (Rh100) () or the control group ().
Figure 7 Fluorescent microscope images showing intestinal lumen (Lu) stained with IgA antibody after 4 hours of oral administration of fluorescently labeled Rh100 or Rh925 thiol-organosilica particles. (A) Control, IgA+ cells (bold arrows) in the villi (V) lamina propria; and (B) Rh100 particles inside the intestinal lumen; no IgA expression inside the lumen was observed, similar to the control. (C) The Rh925 particles inside the intestinal lumen; IgA proteins that were secreted into the lumen and the leukocyte (arrow heads) number increased when compared with that of Rh100-administered animals.
Note: Scale bar = 50 μm.
Abbreviations: IgA, immunoglobulin A; Lu, lumen; Rh, rhodamine B; V, villi.
An increased number of CD11b+ macrophages, 33D1+ DCs, and IgA+ cells in the SEDs of the PPs were observed after oral administration of the particles. Namely, the number of CD11b+ macrophages that contained smaller particles (Rh100, Rh180, and Rh365) in the SEDs of the PPs was greater (, arrowheads) than that of the control group (, arrowheads). The ratios of CD11b+ macrophages that contained Rh100 or Rh180 particles to those without particles were higher than the corresponding ratio calculated with CD11b+ macrophages that contained Rh365 particles. Furthermore, the number of CD11b+ macrophages in the SEDs of PPs after administration of the larger particles (Rh745 and Rh925) was similar or slightly higher () than that of the control group. The uptake of smaller particles (Rh100) by PPs induced CD11b+ macrophages to migrate under the FAE of PPs (, arrowheads); and similar to CD11b+ macrophages, the uptake of smaller particles (Rh100, Rh180, and Rh365) (, arrowheads) increased the number of IgA+ cells in the SEDs of PPs more readily than larger particles (Rh745 and Rh925) () or the control group (). In contrast to CD11b+ macrophages and IgA+ cells, the mechanical contact of larger particles with the FAE of PPs (Rh745 and Rh925) increased the number of 33D1+ DCs in the SEDs (, arrowheads), when compared with the control group () or to the uptake of smaller particles (Rh100, Rh180, and Rh365) (, bold arrows). These data indicate that the uptake of smaller particles increases the number of CD11b+ macrophages and IgA+ cells. In contrast, the uptake and mechanical adhesion of larger particles increase the number of 33D1+ DCs.
Quantitative analysis of the nanoimmunoresponse of immune cells of PPs after oral administration of thiol-organosilica particles
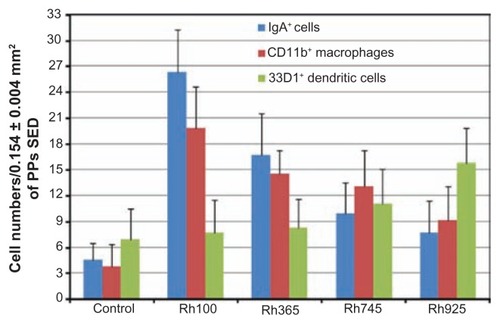
To evaluate and quantitatively compare the number of CD11b+ macrophages, 33D1+ DCs, and IgA+ cells, the number of PP immune cells in the SEDs were counted and that number normalized to the average area (0.154 mm2) (). After administration of smaller particles (Rh100), the number of CD11b+ macrophages in the SED of PPs was five times higher than that of the control group or two times higher than that of larger sized particles (Rh925). Furthermore, the number of IgA+ cells after administration of smaller particles (Rh100) was seven times higher than that of control or four times higher than that of larger particles (Rh745 and Rh925). In contrast, the number of 33D1+ DCs that accumulated after administration of larger particles (Rh925), within the same area, was two times higher than that of the control and other particle sizes. These quantitative data confirmed the immunohistochemical analysis of the nanoimmunoresponse of PP immune cells against thiol-organosilica particles.
Figure 8 Quantitative analysis of the nanoimmunoresponse of immune cells from PPs 4 hours after oral administration of different-sized, fluorescently labeled, thiolorganosilica particles. Immune cells of PPs were counted in 0.154 ± 0.004 mm2 sections of the SEDs of PPs. The uptake of smaller particles (Rh100) increased the number of CD11b+ macrophages and IgA+ cells two- and fourfold, respectively, when compared with larger particles (Rh925) or five- and sevenfold, respectively, when compared with the control. In contrast, mechanical adhesion of larger particles increased the number of 33D1+ DCs twofold when compared with smaller particles or the control.
Note: Bars represented means ± standard deviations (n = 20).
Abbreviations: DCs, dendritic cells; IgA, immunoglobulin A; PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
Western blotting of the IgA nanoimmunoresponse after oral administration of thiol-organosilica particles
Because an increase in the number of IgA+ cells was observed after oral administration of smaller particles, the protein level of IgA was measured by Western blotting using an anti-IgA antibody in bile, PP homogenates, and intestinal secretions. In , it can be observed that Western blotting detected a band of 55 kDa, which is consistent with the molecular weight of IgA. In the bile sample (5 μg/lane), the intensity of the 55 kDa protein band after the administration of smaller or larger particles was significantly higher than that of the control group. Similarly, the intensity of the same protein band after administration of Rh100 was significantly higher than that after Rh925 administration ( and ). For PP homogenates (15 μg/lane), the intensity of the 55 kDa protein after Rh100 administration was higher than that of the control or after the administration of Rh925. Furthermore, after Rh925 administration, IgA levels were slightly higher or similar to that of the control group ( and ). In contrast, Western blotting analysis of intestinal secretions (10 μg/lane) indicated that the intensity of the 55 kDa protein after the administration of Rh925 was higher than that after the administration of Rh100, which, in turn, was slightly higher than that of the control group ( and ). These data revealed that IgA induction increased after a 4-hour administration of smaller particles (Rh100) in bile secretions and PP homogenates, when compared with the control group or the administration of larger particles (Rh925). In contrast, larger particles increased the induction of IgA in intestinal secretions, when compared with the control group or administration of smaller particles ().
Figure 9 Western blotting analysis of IgA induction after 4 hours of oral administration of Rh100 or Rh925 particles. Western blotting analysis of IgA induction in (A) the bile (5 μg/each lane), (B) the intestinal secretions (10 μg/each lane), and (C) in PP homogenates (15 μg/each lane). Smaller thiol-organosilica particles (Rh100) induced IgA in both PP homogenates and bile secretions when compared with larger particles (Rh925); however, the opposite was true for intestinal secretions.
Abbreviations: IgA, immunoglobulin A; PPs, Peyer’s patches; Rh, rhodamine B.
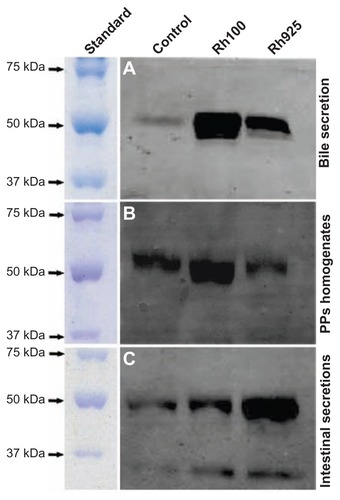
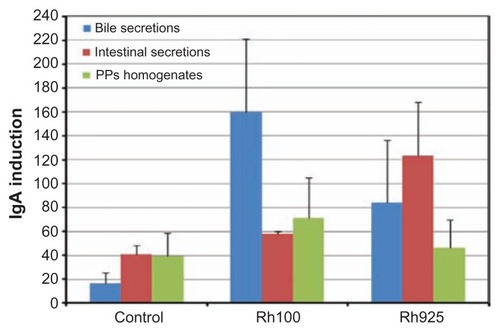
Figure 10 Densitometric analysis of IgA induction in the bile, intestinal secretions and PP homogenates after 4 hours of oral administration of Rh100 or Rh925 particles.
Note: Bars represent means ± standard deviations (n = 4).
Abbreviations: IgA, immunoglobulin A; PPs, Peyer’s patches; Rh, rhodamine B.
Discussion
In the present study, a mucosal, size-dependent nanoimmunoresponse, ie, a change in mouse PP immune cell numbers and expression of alpha-L-fucose and IgA secretion, using various sizes of thiol-organosilica particles was demonstrated. These results were unexpected because the particles were not immunologically functionalized. Although the smaller (Rh100) and larger (Rh925) particles were prepared from the same silica source, they showed different patterns of nanoimmunoresponse. In the present study, M cells of the PPs did not take up many of the larger particles (Rh925). Unlike the smaller particles, larger particles localized to the surface of the FAE of PPs and increased the amount of alpha-L-fucose residue and IgA expression on the surface of M cells. Additionally, the larger particles increased the numbers of 33D1+ DCs more than the smaller particles. Alpha-L-fucose is an important cell surface carbohydrate and fucose-containing glycan, and it has important roles in host-microbe interactions.Citation34 Also, IgA has an important role in the humoral immune response against pathogens and antigen-coated NPs,Citation22–Citation24 and in the mouse intestine, IgA was found to bind selectively to M cells of the PPs.Citation35,Citation36 Therefore, the authors of this present study conclude that larger particles can stimulate the mucosal nanoimmunoresponse.
Larger particles that are similar in size to bacteria or spores have stronger surface tension forces and heavier and higher gravitational effects than smaller particles;Citation37 therefore, these particles might induce mechanotransduction in the FAE of PPs. Previous studies have revealed that mechanotransduction is a physiological process that allows cells to sense and respond to mechanical stress,Citation38 and several molecules and receptors that have intermediary roles in mechanotransduction have been identified.Citation3,Citation39–Citation46 One of these receptors is the Toll-like receptor-2 (TLR2),Citation42,Citation43 which is expressed on the villus epithelium and on the FAE and in M cells of PPs. This receptor plays a critical role in the early, innate immunoresponse and invasion of pathogens by sensing microorganisms.Citation3,Citation45–Citation49 Furthermore, the TLR2 induces B-cell homing to the gastrointestinal tract and IgA production.Citation48,Citation49 Therefore, it is possible that the adhesion of larger particles to the FAE stimulates mechanotransduction receptors, such as the TLR2, which then induce mucosal nanoimmunoresponses such as IgA and M cell alpha-L-fucose.
DCs play a primary role in oral tolerance and defense against mucosal pathogens,Citation8 by maintaining IgA production and regulating the activity of T, B, and plasma cells.Citation8,Citation15,Citation16 An increased number of 33D1+ DCs in the SEDs of PPs was observed when larger particles were administrated; however, there was no direct contact between the larger particles and DCs. It was reported that enterocytes in the FAE of PPs could release chemokines that attract DCs,Citation50–Citation52 and one of the chemokines was the macrophage inflammatory protein (MIP-3 alpha), which was expressed by the FAE of PPs and attracted DCs into the SEDs of those PPs.Citation50 Therefore, it is possible that larger particles increase the number of 33D1+ DCs by releasing chemokines that are produced in the FAE. However, no report describing the induction of chemokines by mechanotransduction has been published. The authors of this present paper hypothesize that mechanotransduction due to larger particles induces the release of chemokines from the FAE, and those chemokines attract 33D1+ DCs into the SEDs of PPs.
In this study, it was demonstrated that, smaller thiolorganosilica particles were taken up mainly by CD11b+ macrophages rather than by 33D1+ DCs. In contrast to these findings, a previous study reported that orally administrated, hydrophobic, fluorescent polystyrene particles (200 nm), were taken up mainly by CD11c+ DCs rather than by CD11b+ macrophages.Citation4 Also, previously it was reported that, if the surface charge of the NPs is positive or uncharged, it has an affinity for adsorptive enterocytes through hydrophobic interactions, whereas a negatively charged and hydrophilic surface shows greater affinity for adsorptive enterocytes and M cells.Citation32,Citation53 It is possible that this difference in cellular uptake was brought about by the surface properties of the particles, eg, thiol-organosilica particles are hydrophilic and naturally possess mercaptopropyl residues that contain alkyl chains and thiol residues with highly negative surface charges (). Therefore, the particles could have adsorbed antigenic molecules that were derived from commensal microbes in the intestinal lumen, which could explain why thiol-organosilica particles have an increased ability to bind proteins, as reported previously.Citation28 Therefore, orally administered, smaller particles could be significantly taken up by CD11b+ macrophages from PPs, rather than by 33D1+ DCs, but further studies are needed to clarify the mechanism of uptake of thiol-organosilica particles by PP cells.
The present study demonstrated that the uptake of smaller particles increased the number of CD11b+ macrophages and IgA+ cells in the SEDs of PPs, and this uptake was dependent on the size of the particle. In contrast, after the administration of larger particles, the number of CD11b+ macrophages and IgA+ cells was similar to that of the control cells. Macrophages have an important role in the uptake and clearing of nanomaterials and pathogens from the body,Citation54–Citation56 and it has been reported that after oral administration of the Scrapie virus, the uptake of the virus and the number of Mac-3+ macrophages increased in the SEDs of the PPs.Citation14 IgA+ cells function in the small intestine to enhance the immune system against some infections by producing IgA. In a previous study, when mice were orally administered Lactobacillus paracasei subsp,Citation9 or reovirus (70–80 nm in diameter), IgA+ cells increased in number in the SEDs of the PPs and villi lamina propria.Citation57 In the present authors’ previous work, size-dependent uptake of particles by the M cells of PPs was reported, and this uptake was achieved by transcytosis of PPs M cells and enterocytes and a paracellular pathway in the FAE of PPs. The uptake of six different sizes of particles by M cells of PPs using simultaneous, dual administration was also compared, and it was found that the uptake of the smaller particles by immune cells of the PPs was higher than that of the larger particles.Citation32 Smaller particles were easily transported to the SED of PPs, and the presence of more of the smaller particles in the SED increased the number of CD11b+ macrophages and IgA+ cells. In that study, the size-dependent nanoimmunoresponse that alters the number of CD11b+ macrophages and IgA+ cells in the SEDs of PPs were described, and the results indicate that smaller particles enter into PPs easily via three pathways,Citation32 and could increase the number of CD11b+ macrophages and IgA+ cells with respect to the number of particles that are taken up. However, larger particles, which are transported to PPs inefficiently, do not increase the number of immune CD11b+ macrophages and IgA+ cells in the PPs.
In this present study, an increase in IgA secretion in bile and PP homogenates after the administration of smaller particles (Rh100) was observed. Recently, some studies reported a size-dependent nanoimmunoresponse in serum after oral administration of various sizes of surface-functionalized particles. Citation22–Citation24,Citation58 An increase in serum IgA and IgG was observed after the administration of 500 or 100 nm tetanus toxoid-loaded PLGA NPs but not after 1 μm NPs or tetanus toxoid solution alone.Citation23 It has also been reported that serum IgG induction by ovalbumin PLGA microspheres show the following dependence on particle size: 4.0 μm > 1.3 μm = 7.5 μm > 14 μm. Also, particle surface area may be the most appropriate parameter to evaluate the pulmonary inflammatory potential.Citation58 The size-dependent nanoimmuneresponse and pulmonary inflammation come from the larger surface area of smaller particles as compared with larger particles.Citation24,Citation58 These reports indicated that inductions of serum IgA and IgG by orally administering particles depended not only on the size of the particles but also on the type of particle, antigen, and surface area. In this present study, the adhesion of larger particles to the FAE of PPs increased mucosal IgA secretion and IgA expression on the surface of M cells; however, the uptake of smaller particles by the SED of PPs increased systemic IgA in bile and PP homogenates 4 hours after oral administration. These results allow us to hypothesize that there are two routes of size-dependent IgA nanoimmunoresponse in the small intestine, from enterohepatic circulation into lumen (enterohepatic route) and directly via mucosal membrane into the lumen (mucosal route) (). In the enterohepatic route, many macrophages take up commensal antigens from the intestinal lumen and process and present antigenic information to B and plasma cells. As reported previously, smaller particles can be taken up by PPs through PP M cells as well as two novel pathways (through PP enterocytes and between PP enterocytes). The smaller particles were taken up by PPs more, as compared with larger particles.Citation32 Smaller particles with higher surface area as compared with larger particles, probably introduce more antigenic molecules into the SED of PPs or other lymphoid tissues such as villi lamina propria. A portion of the IgA is released, binds to the IgA receptor on epithelial cells, and is transported into the intestinal lumen. The other portion of IgA that is produced in the lymphoid tissues enters the portal flow, is transported into the liver, and then released into the bile.Citation59,Citation60 This mechanism could explain the induction of IgA by smaller particles via enterohepatic route. In the mucosal route, larger particles mechanically stimulate the FAE, including M cells, and IgA molecules are released from M cells and are then secreted into the lumen directly. In this report, the authors proposed two possible routes, enterohepatic route and mucosal route, of two size-dependent of IgA secretion into the intestine lumen using organosilica particles.
Conclusion
Size-dependent nanoimmunoresponses in PPs were demonstrated by orally administering various sizes of thiol-organosilica particles to mice. As shown in , larger particles (745 and 925 nm) increased the expression of alpha-L-fucose on the surface of M cells of the FAE and the secretion of mucosal IgA into the intestinal lumen. In addition, larger particles increased the number of 33D1+ DCs in the SED of PPs. Smaller particles (100, 180, and 365 nm) were transported to the SED and taken up by CD11b+ macrophages, which then increased in number and increased the content of systemic IgA in both PP homogenates and bile. The novel findings in this study indicated that there are two size-dependent routes of IgA secretions into the intestinal lumen: the mucosal route by larger particles and the enterohepaic route by smaller particles. However, the mechanisms for size-dependent enhancement of the immunological responses of thiol-organosilica particles must be clarified. Additionally, in this study, size-dependent nanoimmunoresponse after 4 hours of oral administration was evaluated. The nanoimmunoresponse may be altered with time, and further studies, such as those on the time-dependent change in IgA induction after administration, are required to understand the mucosal and systemic nanoimmunoresponse enhancement that occurs when using thiol-organosilica particles. Furthermore, an investigation of the enhancement of the size-dependent nanoimmunoresponse and its mechanism are important for the development of nanomedicine, mucosal vaccination enhancement, and protective immunity.
Table 2 A summary of the size-dependent co-localization of thiol-organosilica particles and the immune cells from PPs.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Younger Scientists (No 17790356) (to M Nakamura) and a Grant-in-Aid for Scientific Research (C) (Nos 19510117 and 21500409) (to M Nakamura) from the Ministry of Education, Science, Sports, and Culture of Japan as well as a Grant for Practical Application of University R&D Results under the Matching Fund Method (to M Nakamura) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan. Financial support of the Egyptian Ministry of Higher Education and Scientific Research, Missions Sector (to A Awaad) is gratefully acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary figures
Figure S1. Electron photomicrographs showing the colocalization of Rh130 thiol-organosilica particles and PP SED cells.
Note: Scale bar = (A) 2 μm; (B) 1 μm; (C) 200 nm.
Abbreviations: PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
Figure S2. Electron photomicrographs showing, (A–E) The colocalization of Rh130 particles with PP SED cells. (G and H) The diffusion of Rh130 thiol-organosilica particles between the PP SED cells.
Note: Scale bar = (A), (D), (G), 2 μm; (B), 1 μm; (E), (H), 500 nm; (C), (F), (I) 200 nm.
Abbreviations: PPs, Peyer’s patches; Rh, rhodamine B; SED, subepithelial dome.
Figure S3. Electron photomicrographs showing the PP FAE enterocytes and the mechanical adhesion of thiol-organosilica particles. (A) The control of PP FAE, (B) mechanical adhesion of Rh100 on PP FAE, (C) mechanical adhesion of Rh925 on PP FAE.
Note: Scale bar = (A); (B); and (C), 700 nm.
Abbreviations: FAE, follicle associated epithelium; PPs, Peyer’s patches; Rh, rhodamine B.
References
- SalmanHHIracheJMGamazoCImmunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccinationVaccine200927354784479019539576
- SalmanHHGamazoCAgüerosMIracheJMBioadhesive capacity and immunoadjuvant properties of thiamine-coated nanoparticlesVaccine200725488123813218029067
- ChabotSWagnerJSFarrantSNeutraMRTLRs regulate the gatekeeping functions of the intestinal follicle-associated epitheliumJ Immun200617674275428316547265
- ShreedharVKKelsallBLNeutraMRCholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-Cell areas of Peyer’s patchesInfec Immun200371150450912496201
- SemeteBBooysenLIKalomboLIn vivo uptake and acute immune response to orally administered chitosan and PEG coated PLGA nanoparticlesToxicol Appl Pharmacol2010249215816520851137
- KaewamatawongTShimadaAOkajimaMAcute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillationToxicol Path200634795896517178696
- SenDDeerinckTJEllismanMHParkerICahalanMDQuantum dots for tracking dendritic cells and priming an immune response in vitro and in vivoPLoS ONE200839e329018820727
- JohanssonCKelsallBLPhenotype and function of intestinal dendritic cellsSemin Immun2005174284294
- TsaiYTChengPCLiaoJWPanTMEffect of the administration of Lactobacillus paracasei subsp. paracasei NTU 101 on Peyer’s patch-mediated mucosal immunityInt Immunopharmacol2010107792798
- GonzalezMJPlummerEMRaeCSManchesterMInteraction of Cowpea Mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivoPLoS ONE2009411e798119956734
- ZuercherAWCebraJJStructural and functional differences between putative mucosal inductive sites of the ratEur J Immunol200232113191319612555664
- Romero-TrevejoJLGómez-VillamandosJCPedreraMBlancoABautistaMJSánchez-CordónPJImmunohistochemical study of macrophage and cytokine dynamics in the gut of scrapie-infected miceHistol Histopathol20102581025103820552553
- UmesakiYOkadaYImaokaASetoyamaHMatsumotoMInteractions between epithelial cells and bacteria, normal and pathogenicScience199727653149649659139662
- JeongGNJoUBRyuHYKimYSSongKSYuIJHistochemical study of intestinal mucins after administration of silver nanoparticles in Sprague–Dawley ratsArch Toxicol2010841636919756516
- MacphersonAJUhrTInduction of protective IgA by intestinal dendritic cells carrying commensal bacteriaScience200430356641662166515016999
- TezukaHAbeYIwataMRegulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cellsNature2007448715692993317713535
- MagistrisMTDMucosal delivery of vaccine antigens and its advantages in pediatricsAdv Drug Del Rev20065815267
- MishraNTiwariSVaidyaBAgrawalGPVyasSPLectin anchored PLGA nanoparticles for oral mucosal immunization against hepatitis BJ Drug Target201019111220233082
- HeXWangFJiangLLiJLiuSXiaoZInduction of mucosal and systemic immune response by single-dose oral immunization with biodegradable microparticles containing DNA encoding HBsAgJ Gen Virol200586Pt 360161015722520
- AkagiTUenoMHiraishiKBabaMAkashiMAIDS vaccine: Intranasal immunization using inactivated HIV-1-capturing core-corona type polymeric nanospheresJ Control Release20051091–3496116256237
- GuptaPNMahorSRawatAKhatriKGoyalAVyasSPLectin anchored stabilized biodegradable nanoparticles for oral immunization. 1. Development and in vitro evaluationInt J Pharm20063181–216317316621367
- NagamotoTHattoriYTakayamaKMaitaniYNovel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine deliveryPharm Res200421467167415139524
- JungTKammWBreitenbachAHungererKHundtEKisseTTetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): evaluation of antibody response after oral and nasal application in micePharm Res200118333523360
- TakahiroUShigeruGOral delivery of poly(lactide-co-glycolide) microspheres containing ovalbumin as vaccine formulation: particle size studyBio Pharm Bull1994179127212767841952
- ThomasCGuptaVAhsanFInfluence of surface charge of PLGA particles of recombinant hepatitis B surface antigen in enhancing systemic and mucosal immune responsesInt J Pharm20093791415019524654
- NakamuraMIshimuraKSynthesis and characterization of organosilica nanoparticles prepared from 3-mercaptopropyltrimethoxysilane as the single silica sourceJ Phys Chem C2007111511889218898
- NakamuraMIshimuraKOne-pot synthesis and characterization of three kinds of thiol-organosilica nanoparticlesLangmuir20082495099510818366224
- NakamuraMOzakiSAbeMDoiHMatsumotoTIshimuraKSize-controlled synthesis, surface functionalization, and biological applications of thiol-organosilica particlesColloids Surf B Biointerfaces2010791192620417071
- NakamuraMIshimuraKSize-controlled, one-pot synthesis, characterization, and biological applications of epoxy-organosilica particles possessing positive zeta potentialLangmuir20082421122281223418823130
- NakamuraMOzakiSAbeMMatsumotoTIshimuraKOne-pot synthesis and characterization of dual fluorescent thiol-organosilica nanoparticles as non-photoblinking quantum dots and their applications for biological imagingJ Mater Chem2011211246894695
- HayashiKNakamuraMIshimuraKIn situ synthesis and photoresponsive rupture of organosilica nanocapsulesChem Commun (Camb)20114751518152021103535
- AwaadANakamuraMIshimuraMImaging of size-dependent uptake and identification of novel pathways in mouse Peyer’s patches using fluorescent organosilica particlesNanomedicine Epub September 1, 2011
- IrmukhametovaGSMunGAKhutoryanskiyVVThiolated mucoadhesive and PEGylated nonmucoadhesive organosilica nanoparticles from 3-mercaptopropyltrimethoxysilaneLangmuir2011159551955621707076
- BeckerDJLoweJBFucose: biosynthesis and biological function in mammalsGlycobiology200313741R53R
- MantisNJCheungMCChintalacharuvuKRReyJCorthésyBNeutraMRSelective adherence of IgA to murine Peyer’s patch M cells: Evidence for a novel IgA receptorJ Immun200216941844185112165508
- WeltzinRLucia-JandrisPMichettiPFieldsBNKraehenbuhlJPNeutraMRBinding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteinsJ Cell Biol19891085167316852541137
- MijailovichSMKojicMTsudaAParticle-induced indentation of the alveolar epithelium caused by surface tension forcesJ Appl Physiol201010941179119420634359
- KhanKMScottAMechanotherapy: how physical therapists’ prescription of exercise promotes tissue repairBr J Sports Med200943424725219244270
- SchwartzMADeSimoneDWCell adhesion receptors in mechanotransductionCurr Opin Cell Biol200820555155618583124
- WangYMaciejewskiBSDrouillardDA role for caveolin-1 in mechanotransduction of fetal type II epithelial cellsAm J Physiol Lung Cell Mol Physiol20102986L77578320172952
- ImaiYKondohSKatoSMechanotransduction via estrogen receptors in boneClin Calcium20081891272127718758032
- KimHGKimJYGimMGLeeJMChungDKMechanical stress induces tumor necrosis factor-α production through Ca2+ release-dependent TLR2 signalingAm J Physiol Cell Physiol20082952C43243918550705
- GeigerBSpatzJPBershadskyADEnvironmental sensing through focal adhesionsNat Rev Mol Cell Biol2009101213319197329
- ZhangXShephardFKimHBTILRR, a novel IL-1RI co-receptor, potentiates MyD88 recruitment to control Ras-dependent amplification of NF-kappaBJ Biol Chem2010285107222723219940113
- TohnoMShimosatoTKitazawaHToll-like receptor 2 is expressed on the intestinal M cells in swineBiochem Biophys Res Commun2005330254755415796917
- ShimosatoTTohnoMKitazawaHToll-like receptor 9 is expressed on follicle-associated epithelia containing M cells in swine Peyer’s patchesImmunol Lett2005981838915790512
- ChowWLLeeYKFree fucose is a danger signal to human intestinal epithelial cellsBr J Nutr200899344945417697405
- LiangYHasturkHElliotJToll-like receptor 2 induces mucosal homing receptor expression and IgA production by human B cellsClin Immunol20111381334020947433
- HeBXuWSantiniPAIntestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRILImmunity200726681282617570691
- IwasakiAKelsallBLLocalization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokineJ Exp Med200019181381139410770804
- ZhaoXSatoADela CruzCSCCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cellsJ Immunol200317162797280312960300
- CookDNProsserDMForsterRCCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissueImmunity200012549550310843382
- JaniPHalbertGWLangridgeJFlorenceATThe uptake and translocation of latex nanospheres and microspheres after oral administration to ratsJ Pharm Pharmacol19894128098122576440
- PronBBoumailaCJaubertFDendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the hostCell Microbiol20013533134011298655
- MaignienTShakwehMCalvoPetRole of gut macrophages in mice orally contaminated with scrapie or BSEInt J Pharm2005298229330415964722
- GeiserMBaumannMCruz-OriveLMIm HofVWaberUGehrPThe effect of particle inhalation on macrophage number and phagocytic activity in the intrapulmonary conducting airways of hamstersAm J Respir Cell Mol Biol1994105946038003338
- MajorASCuffCFEnhanced mucosal and systemic immune responses to intestinal reovirus infection in beta2-microglobulin-deficient miceJ Virol199771578257899223466
- StoegerTReinhardCTakenakaSInstillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in miceEnviron Health Perspect2006114332833316507453
- ZhoFNeutraMRAntigen delivery to mucosa-associated lymphoid tissues using liposomesas a carrierBiosci Rep200222235536912428910
- BosNAJiangHQCebraJJT cell control of the gut IgA response against commensal bacteriaGut200148676276411358892