Abstract
Recent studies reflect the importance of using naturally occurring biopolymers as three-dimensional corneal keratocyte scaffolds and suggest that the porous structure of gelatin materials may play an important role in controlling nutrient uptake. In the current study, the authors further consider the application of carbodiimide cross-linked porous gelatin as an alternative to collagen for corneal stromal tissue engineering. The authors developed corneal keratocyte scaffolds by nanoscale modification of porous gelatin materials with chondroitin sulfate (CS) using carbodiimide chemistry. Scanning electron microscopy/energy dispersive X-ray spectroscopy and Fourier transform infrared spectroscopy showed that the amount of covalently incorporated polysaccharide was significantly increased when the CS concentration was increased from 0% to 1.25% (w/v). In addition, as demonstrated by dimethylmethylene blue assays, the CS content in these samples was in the range of 0.078–0.149 nmol per 10 mg scaffold. When compared with their counterparts without CS treatment, various CS-modified porous gelatin membranes exhibited higher levels of water content, light transmittance, and amount of permeated nutrients but possessed lower Young’s modulus and resistance against protease digestion. The hydrophilic and mechanical properties of scaffolds modified with 0.25% CS were comparable with those of native corneas. The samples from this group were biocompatible with the rabbit corneal keratocytes and showed enhanced proliferative and biosynthetic capacity of cultured cells. In summary, the authors found that the nanoscale-level modification has influence on the characteristics and cell-material interactions of CS-containing gelatin hydrogels. Porous membranes with a CS content of 0.112 ± 0.003 nmol per 10 mg scaffold may hold potential for use in corneal stromal tissue engineering.
Introduction
Penetrating keratoplasty is the standard treatment for restoration of vision in patients with Stevens-Johnson syndrome, chemical/thermal burns, ocular cicatricial pemphigoid, corneal ulcers, pseudophakic bullous keratopathy, and Fuchs’ endothelial dystrophy. However, the donor shortage has led to the expansion of research in the field of corneal regenerative medicine.Citation1 The stroma, which consists of keratocytes and extracellular matrix (ECM) components such as collagen and glycosaminoglycans (GAGs), is the major structural element of the cornea. Therefore, collagenous biomaterials have been extensively investigated for tissue engineering of corneal stroma.Citation2–Citation4 These studies reflect the importance of using naturally occurring biopolymers as three-dimensional corneal keratocyte scaffolds.
Gelatin is obtained by the thermal, chemical, or physical denaturation of collagen. As a protein-based biomaterial, gelatin is inexpensive and has no antigenicity under physiological conditions. Using the anterior chamber of a rabbit eye as a model, the authors have demonstrated the ocular biocompatibility of gelatin hydrogels.Citation5 Because of their bio-adhesiveness and biodegradability, gelatin carriers are found to temporarily hold the tissue-engineered corneal endothelial cell sheets in the proper position for graft-host integration.Citation6,Citation7 More recently, the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) cross-linked porous gelatin discs have been designed to improve aqueous humor circulation and minimize adverse biological effect.Citation8 The results suggest that the porous structure of gelatin materials may play an important role in controlling nutrient uptake. In this study, the authors further consider the application of carbodiimide cross-linked porous gelatin as an alternative to collagen for corneal stromal tissue engineering.
GAG is known to possess many biological activities such as interaction with growth factors and regulation of cell proliferation and differentiation. It has been documented that the corneal stroma contains on average GAG 0.0103 g per cm3.Citation9 A study from Liu et alCitation10 demonstrated that the decrease in the refractive index of EDC cross-linked collagen hydrogels is presumably due to the absence of GAG chains. Additionally, GAGs are thought to influence corneal hydration through interactions with collagen matrices.Citation11 Although GAG is able to enhance hydrodynamic characteristics, the water sorption may simultaneously decrease the mechanical stability of biomaterial scaffolds. It is highly desirable to introduce the optimal amount of GAG into cross-linked porous gelatin membranes.
Chondroitin sulfate (CS) is a linear anionic polysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-galactosamine. It is one of the two major classes of GAG side chains in the corneal stroma and it is attached to serine residues on the core protein. Citation12 In essence, CS has a molecular mass of 20–60 kDa and contains free carboxylic acid groups available for carbodiimide-mediated modification.Citation13 Hence, in this study, to fabricate the CS-modified porous gelatin scaffolds, the EDC was used to activate the free carboxyl groups of CS molecules for subsequent reaction with the remaining free amino groups of cross-linked gelatin molecules. The method based on covalent binding of polysaccharide to gelatin may offer the advantage of extending the in vivo residence time of CS to allow for better modulation of bioactivity of corneal keratocytes over that based on blending. To the authors’ knowledge, the influence of nanoscale modification of gelatin with CS on the characteristics of corneal keratocyte scaffolds is yet to be investigated. Various CS-modified gelatin membranes were characterized by scanning electron microscopy (SEM), energy dispersive X-ray (EDS) and Fourier transform infrared (FTIR) spectroscopy, and dimethylmethylene blue (DMMB) assays. Then water content and light transmission measurements, mechanical and in vitro degradation tests, and glucose permeation and in vitro biocompatibility studies were performed to examine the relationship between CS levels and scaffold functionality. Cell-material interaction was also analyzed by monitoring the cell proliferation and morphology, as well as ECM production.
Materials and methods
Materials
Gelatin (type A, from porcine skin, 300 Bloom), chondroitin-4-sulfate (type A, from bovine trachea), EDC, ninhydrin reagent, collagenase (type I Clostridium histolyticum, EC 3.4.24.3), Ehrlich’s aldehyde reagent, and glutaraldehyde were purchased from Sigma-Aldrich (St Louis, MO). N-hydroxysuccinimide (NHS) was supplied by Acros Organics (Geel, Belgium). Deionized water used was purified with a Milli-Q® system (Millipore, Bedford, MA). DMMB was purchased from Serva Feinbiochemica GmbH (Heidelberg, Germany). Balanced salt solution (BSS, pH 7.4) was obtained from Alcon Laboratories (Fort Worth, TX). Phosphate-buffered saline (PBS, pH 7.4) was acquired from Biochrom (Berlin, Germany). Dispase type II was obtained from Roche Diagnostics (Indianapolis, IN). Medium 199, gentamicin, 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium (DMEM/F12), trypsin-ethylenediaminetetraacetic acid, and TRIzol® reagent were purchased from Gibco-BRL (Grand Island, NY). Fetal bovine serum (FBS) and the antibiotic/antimycotic (A/A) solution (penicillin 10,000 U/mL, streptomycin 10 mg/mL, and amphotericin B 25 μg/mL) were obtained from Biological Industries (Kibbutz Beit Haemek, Israel). All the other chemicals were of reagent grade and were used as received without further purification.
Preparation of cross-linked porous gelatin scaffolds
The cross-linked porous gelatin membranes were fabricated according to a previously published method.Citation8 In brief, an aqueous solution of 10% (w/v) gelatin was obtained by dissolution of gelatin powder in deionized water at 40°C. The resulting solution was then poured into a polystyrene planar mold (5 × 5 cm2, 1.5 cm depth), subjected to freezing at −80°C for 24 hours, and lyophilized at −55°C for 2 days. All the prepared gelatin sheets were cross-linked by directly immersing the samples in an ethanol-water mixture (8:2, v/v; pH 4.75) containing EDC 50 mmol/L and NHS 10 mmol/L. The cross-linking reaction was allowed to proceed at 25°C for 1.5–96 hours, and the treated samples were washed thoroughly with deionized water to remove excess EDC and urea by-product. Using a 7 mm diameter corneal trephine device, the gelatin sheets were cut out to obtain porous membrane scaffolds (approximately 100 μm in thickness).
The ninhydrin assay was used to determine the amount of free amino groups of the chemically cross-linked gelatin samples.Citation14 Following reaction with ninhydrin, the optical absorbance of the test solution was recorded with an ultraviolet-visible spectrophotometer (Evolution 300; Thermo Scientific, Waltham, MA) at 570 nm, using glycine at various known concentrations as standard. The amount of free amino groups in the material before (Ci) and after (Cf) cross-linking is proportional to the optical absorbance of the solution. The extent of cross-linking of the gelatin scaffolds was calculated as cross-linking index (%) = ((Ci – Cf)/Ci) × 100. Results were averaged on five independent runs.
Modification of porous gelatin scaffolds with CS
After EDC/NHS cross-linking at 25°C for 12 hours, the porous gelatin membrane scaffolds (10 mg) were again immersed in an ethanol-water mixture (8:2, v/v; pH 4.75) containing EDC 10 mmol/L, NHS 2 mmol/L, and 0%–1.25% (w/v) CS. The reaction proceeded for 24 hours under gentle shaking. To remove ionically bound CS, the treated samples were further washed with sodium chloride 500 mmol/L, followed by washing with deionized water. The porous gelatin scaffolds were divided into four groups, depending on the amount of CS used (0%, 0.05%, 0.25%, or 1.25% (w/v)), and were labeled accordingly: GCS000, GCS005, GCS025, and GCS125.
Scaffold characterizations
The samples were observed under a scanning electron microscope (S-3000 N; Hitachi, Tokyo, Japan) and analyzed with an energy dispersive X-ray spectroscope (Oxford Instruments, Concord, MA).Citation15 Small pieces of the membrane scaffolds were cut off and mounted onto stubs using double-sided adhesive tape. The cross-section morphology of the samples was studied using a scanning electron microscope with an accelerating voltage of 20 kV. Twenty different pores were randomly selected, and the average pore diameters were calculated. Results were averaged on three independent runs. The elemental distribution was determined using X-ray elemental mapping. The solvent displacement method was used for porosity measurements. Each gelatin scaffold was first dried to a constant weight (Wi) in vacuo. The test samples were immersed in absolute ethanol overnight, blotted with tissue paper to remove excess ethanol on the surface, and weighed (Wf) immediately. The porosity (%) was calculated as ((Wf – Wi)/Vρ) × 100, where V is the volume of the hydrogel scaffold and ρ is the density of absolute ethanol. Results were averaged on five independent runs.
The FTIR spectroscopy of various samples was performed using a FT-730 ATR-FTIR spectrophotometer (Horiba, Kyoto, Japan) according to a previously published method.Citation16 The spectra were recorded between 3700 and 900 cm−1, with a resolution of 8 cm−1. The data were analyzed using FTIR spectrum software (Horiba) to obtain quantitative peak information. The results were the average of three independent experiments.
The CS content of various modified gelatin scaffolds was determined by DMMB assay. After hydrolysis of membranes with hydrogen chloride 6 N at 105°C for 6 hours, the samples were mixed with DMMB reagent solution (sodium chloride 40 mmol/L; glycine 40 mmol/L ; DMMB 46 μmol/L, pH 3.0). The absorbance was read at 525 nm, using a spectrophotometer (Multiskan® Spectrum microplate; Thermo Labsystems, Vantaa, Finland), and referenced to a standard curve of CS over a range of concentrations from 0.01 to 2.5 nmol/mL. All experiments were conducted in quadruplicate.
Water content measurements
For water content measurements, the samples were first dried to a constant weight (Wi) in vacuo and were then immersed in BSS at 34°C (physiological temperature of the cornea) with reciprocal shaking (50 rpm) in a thermostatically controlled water bath. After 4 hours, the swollen membrane scaffolds were weighed (Ws), and the equilibrium water content (%) of the test sample was defined by ((Ws – Wi)/Ws) × 100, as described previously.Citation17 Results were averaged on six independent runs.
Light transmission measurements
After equilibrium swelling was reached, each sample was placed in a cell in the middle of the custom-built holder. The optical transmittance of the membranes was measured using the ultraviolet-visible spectrophotometer, operating in a spectral range of 400–700 nm.Citation18 During spectrometry of scaffolds, the reference sample is the holder only, and the test sample consists of the holder with the membrane in place.
Mechanical tests
The uniaxial tensile tests were used to characterize the mechanical properties of various membrane scaffolds.Citation19 Each sample was immersed in BSS for 4 hours to reach the fully swollen state. Subsequently, wet membranes under pressure were cut with a suitable mold to prepare dumbbell-shaped specimens. The gauge length of the specimens was 10 mm and the width was 5 mm. All measurements were performed at 25°C and a relative humidity of 50% using an Instron universal testing machine (Mini 44; Instron, Canton, MA). The crosshead speed was set at 1 mm per minute. Young’s modulus was determined from load-displacement curves. Results were averaged on eight independent runs.
In vitro degradation tests
To measure the extent of degradation, each sample was first dried to a constant weight (Wi) in vacuo and was then immersed in 1 mL of BSS containing collagenase 12 μg at 34°C. Degradation medium was replaced weekly with fresh buffer solution containing the same concentration of enzyme. At predetermined time intervals, the degraded membranes were collected and further dried in vacuo. The dry weight of scaffolds after degradation (Wd) was determined and the percentage of weight remaining was calculated as (Wd/Wi) × 100. Results were averaged on five independent runs.
Glucose permeation studies
Glucose permeation studies were performed at 34°C using a horizontal glass diffusion cell (PermeGear, Inc, Hellertown, PA), having two stirred chambers with sampling ports. The donor chamber was filled with a 50 mg/mL glucose solution in BSS (3 mL) and the receptor chamber was filled with BSS (3 mL). After immersion in BSS until equilibrium swelling, the membranes were placed between the two chambers. During the measurements, all solutions were stirred continuously to provide uniform solute distribution and to reduce the boundary layer of glucose. After 12 hours, the receptor chamber was sampled and analyzed using a glucose assay kit (Sigma-Aldrich).Citation8 Photometric readings at 540 nm were measured with a spectrophotometer (Thermo Scientific) and compared with a standard curve of known glucose concentrations. Results were averaged on five independent runs.
Isolation and culture of rabbit corneal keratocytes
Twelve adult male New Zealand white rabbits (National Laboratory Animal Breeding and Research Center, Taipei, Taiwan, Republic of China) weighing 2.5–3.0 kg were used for this study. All animal procedures were approved by the Institutional Review Board and were performed in accordance with the ARVO (Association for Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research. For the isolation of stromal keratocytes, each cornea was exposed to Medium 199 and 50 μg/mL of gentamicin. Under a dissecting microscope (Leica, Wetzlar, Germany), Descemet’s membrane with the attached endothelium was aseptically stripped from the stroma.Citation20 The remaining stroma with epithelium was incubated in 5 mg/mL of dispase II at 4°C overnight.Citation21 Loose epithelial sheets were removed, and stromal discs were cut into small pieces and digested using 4 mg/mL of collagenase I for 24 hours at 37°C. The keratocytes were mechanically dissociated into single cells, and a cell pellet was collected via centrifugation (1000 rpm, 25°C, 5 minutes). Thereafter, rabbit corneal keratocytes (RCKs) were resuspended and maintained in regular culture medium consisting of DMEM/ F12, 10% FBS, and 1% A/A solution. Cultures were incubated in a humidified atmosphere of 5% carbon dioxide (CO2) at 37°C. The medium was changed every other day. Confluent cell layers were subcultured by treating with trypsin-ethylenediaminetetraacetic acid for 2 minutes and seeded at a 1:4 split ratio. Only third-passage RCKs were used for this study.
In vitro biocompatibility studies
The procedure for the in vitro biocompatibility evaluation of the membrane scaffolds conducted was adapted from the ISO10993-5 standard test method.Citation22 The material samples were sterilized in a 70% ethanol solution overnight and were thoroughly rinsed in sterilized PBS. A single extract of the test article was prepared using regular growth medium containing DMEM/F12, 10% FBS, and 1% A/A solution. The extracts were obtained by incubation of the sterilized materials with culture medium at 37°C for 24 hours, with an extraction ratio of 0.2 g/mL. Each test extract was then placed onto RCK cultures with a seeding density of 5 × 104 cells/well, and maintained at 37°C in the presence of 5% CO2 for 48 hours. The cells in regular growth medium without contacting material samples served as control groups.
Cell morphology was observed by phase contrast microscopy (Nikon Instruments, Inc, Melville, NY). Proinflammatory cytokine interleukin-6 (IL-6) expression was detected at messenger RNA levels.Citation23 Total RNA was isolated from cells with TRIzol reagent according to the manufacturer’s guidelines. Reverse transcription of the extracted RNA (1 μg) was performed using ImProm-II (Promega, Madison, WI) and Oligo(dT)Citation15 Primer (Promega). The primers used to amplify the rabbit IL-6 complementary DNA (cDNA) were 5′-AAGAAAACACCAGGGTCAGCAT-3′ (sense) and 5′-CTTGAGGGTGGCTTCTTCATTC-3′ (antisense); those used to amplify the internal control cDNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were 5′-TTGCCCTCAATGACCACTTTG-3′ (sense) and 5′-TTACTCCTTGGAGGCCATGTG-3′ (antisense). Quantitative real-time reverse transcription polymerase chain reaction was performed on a Light Cycler® instrument (Roche Diagnostics) according to the manufacturer’s instructions and with Light Cycler FastStart® DNA Master SYBR® Green I reagent (Roche Diagnostics). Each sample was determined in triplicate and the results for IL-6 were normalized to the level of GAPDH messenger RNA.
Cell culture studies
After attachment of RCKs (3.5 × 104 cells/scaffold) onto the sterilized membrane scaffolds, the samples were washed three times with PBS to remove nonattached cells. The cell-polymer constructs were immediately transferred to a new culture well, and the medium was replaced with fresh growth medium. After 1–5 days of incubation at 37°C, the proliferation of the cells was evaluated using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (methane-thiosulfonate) (Promega), in which the methanethiosulfonate tetrazolium compound is bioreduced by cells to form a water-soluble colored formazan.Citation24 The amount of colored product is proportional to the number of metabolically active cells. The combined methanethiosulfonate/ phenazine methosulfate (20:1) reagent (100 μL) was added to each well of the 24-well plate, and incubated for 3 hours at 37°C in a CO2 incubator. The data of absorbance readings at 490 nm were measured using the Multiskan® Spectrum microplate spectrophotometer (Thermo Labsystems), and referenced to a standard curve of absorbance versus cell number as determined by hemacytometer. All experiments were carried out in quadruplicate.
After RCK cultivation on various scaffolds for 1, 3, and 5 days, the supernatants were respectively collected for analyses of collagen and GAG contents. Blank experiments (culture medium exposed to the cell-free scaffolds) were conducted simultaneously for correction of results. The amount of hydroxyproline, which is an amino acid marker of collagen, was determined with some modifications of the protocol used for quantification of hydroxyproline in aqueous humor.Citation8 Briefly, after the addition of Ehrlich’s aldehyde reagent to each sample, the absorbance was read at 550 nm by using a spectrophotometer (Thermo Labsystems) and was compared with a standard calibration curve to quantify the amount of hydroxyproline. Colorimetric assay with DMMB reagent was used to determine the GAG content, as described earlier. All experiments were conducted in triplicate.
Additionally, the constructs prepared from 5 days’ cultivation of the RCKs on GCS000 and GCS025 samples were further processed for SEM observations.Citation25 Evaluations of cell-free scaffolds were conducted simultaneously for comparison. In brief, the samples were fixed with 2% glutaraldehyde in 0.1 mol/L of cacodylic acid buffer (pH 7.4), post fixed in 1% osmium tetroxide, and dehydrated in a graded series of ethanol solutions. The dried specimens were then sputter coated with gold before examination under scanning electron microscope.
Quantitative real-time reverse transcription polymerase chain reaction studies were performed according to the method described for the in vitro biocompatibility studies. The sequences of the primer pairs for rabbit keratocan were 5′-CTCACGTGGCTTTGATGTGT-3′’ (sense) and 5′-GACCTTTGTGAGGCGATTGT-3′ (antisense); the primers used to amplify the rabbit biglycan cDNA were 5′-CGGGAGCTTCATCTAGACAACA-3′ (sense) and 5′-TCGTTGACACCCACCTTGGT-3′ (antisense). Each sample was determined in triplicate, and the gene expression results were normalized to the expression of GAPDH.
Statistics
Results were expressed as mean plus or minus standard deviation. Comparative studies of means were performed using one-way analysis of variance, and P < 0.05 was considered statistically significant.
Results and discussion
Preparation of cross-linked porous gelatin scaffolds
Freeze-drying is a widely used method to prepare porous gelatin hydrogel scaffolds.Citation26,Citation27 In order to enhance the mechanical and degradation-resistant properties of gelatin materials, the porous hydrogel scaffolds were further cross-linked with EDC/NHS (molar ratio of EDC to NHS, 5:1).Citation18 The cross-linking index of gelatin hydrogels as a function of treatment time is shown in Figure S1. After reaction with cross-linkers for a short period of time (ie, 1.5 hours), the samples had a cross-linking index of 19.4% ± 1.2%. The cross-linking degrees continued to increase, reaching 75.4% ± 1.8% by 12 hours of treatment, probably because of the gradual formation of new cross-links. Although the number of free amino groups available for cross-linking has been reported to be 30 per 1000, compared with carboxylic acid groups at 120 per 1000, the number of the consumed carboxylic acid groups is simply 16 per 1000 during carbodiimide cross-linking of collagenous biomaterials.Citation28 In the present work, the plateau level was maintained even for long cross-linking times (ie, 96 hours), implying that the NHS-activated carboxylic acid groups of glutamic or aspartic acid residues have already completely reacted with free amino groups of lysine residues to generate amide bonds in gelatin. Therefore, the remaining free amino groups of cross-linked gelatin molecules are subsequently available to react with free carboxyl groups of CS molecules.
Characterizations of CS-modified porous gelatin scaffolds
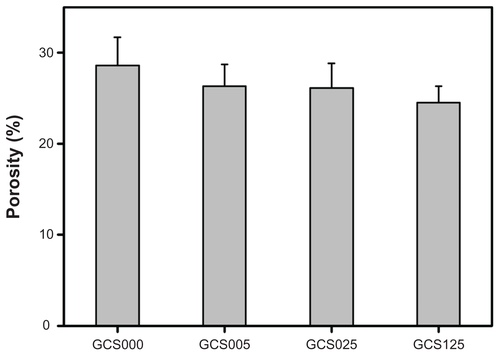
Previously, the authors have shown that the pore size and porosity of freeze-dried gelatin hydrogels can be controlled by the heat transfer rate on the nucleation and growth of ice crystals during the freezing process at different temperature levels.Citation8 The increase in freezing temperature may lead to the enlarged pore structure of the membranes. In this study, to fabricate the scaffolds with adequate pore size for RCK growth, the gelatin solutions were subjected to freezing at −80°C for 24 hours. shows the cross-sectional SEM images of various CS-modified gelatin scaffolds. In all groups, the samples presented a porous three-dimensional structure with thin pore walls. Additionally, similar pore size of around 50 μm and porosity of around 25% were found (Figures S2 and S3), indicating that the modification with 0%–1.25% (w/v) CS does not markedly change the porous structure of EDC/NHS cross-linked gelatin scaffolds. Since the dimension of each corneal stromal keratocyte is 24–35 μm long and 11–14 μm wide,Citation29 the results suggest that the porous gelatin scaffolds can provide sufficient space to support cell cultivation.
Figure 1 Scanning electron microscopy/energy dispersive X-ray spectroscopy (EDS) mapping of various porous gelatin scaffolds modified with chondroitin-4-sulfate (GCS000, GCS005, GCS025, and GCS125): (A) scanning electron microscopy images, (B) EDS mapping of sulfur, and (C) EDS spectrogram.
Notes: Scale bars are 50 μm; scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
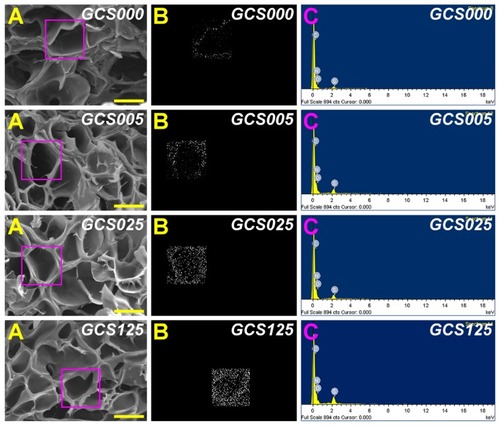
The energy dispersive X-ray microanalysis mapping technique was employed to investigate the sulfur distribution of the gelatin scaffolds before and after modification with CS. The region enclosed by a square on the SEM images shows the area analyzed with EDS spectra (). The white dots represent the detected sulfur element and the black area indicates the absence of a specific element. Gelatin is a naturally occurring biopolymer that preserves a unique amino acid composition.Citation30 As shown in , the carbon, oxygen, and nitrogen peaks in the EDS spectrogram were noted for all samples. In the GCS000 groups, the S signals were detected because the methionine of gelatin is a sulfur-containing amino acid. Moreover, in the other groups, the porous scaffolds had stronger sulfur peak intensities, suggesting the successful incorporation of CS into the gelatin materials. Although EDS is a semiquantitative characterization tool and its spectrum simply provides a measure of specific elements in the selected area, the present results demonstrate that after modification with CS of high concentration, more white dots appear ().
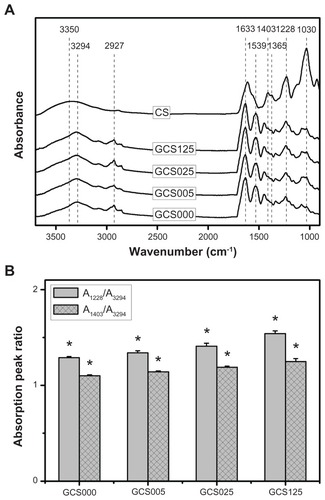
Representative spectra for the CS and various CS-modified gelatin samples in the wavenumber range of 3700–900 cm−1 are shown in . In the GCS000 groups, the EDC/ NHS-treated gelatin materials had several characteristic absorption bands at 3294 cm−1 (N–H stretching vibration), 2927 cm−1 (C–H stretching vibration), 1633 cm−1 (amide I, C=O stretching vibration), 1539 cm−1 (amide II, N–H bending vibration), and 1228 cm−1 (amide III, N–H bending vibration). For the CS samples, the spectra exhibited peaks at 3350 cm−1 (–OH stretching vibration), 1030 cm−1 (C–O–C stretching vibration attributed to the saccharide structure), and 1228 cm−1 (S=O stretching vibration attributed to the negatively charged SO42− groups of CS molecules). In addition, the bands at 1403 and 1365 cm−1 were due to the coupling of the C–O stretching vibration and O–H variable-angle vibration, indicating the existence of the free carboxyl group.Citation31 Although the samples GCS005, GCS025, and GCS125 showed a similar pattern of spectra, the peak intensities were dependent on the CS concentration (). In this study, the increased intensity ratios of A1228/A3294 and A1403/A3294 are indicative of increased CS signals. When compared with their counterparts treated with 0.05% CS, the gelatin scaffolds modified with 1.25% CS revealed higher intensity S=O stretching bands. Despite the possibility that formation of amide bonds between gelatin and CS may involve the consumption of free carboxyl groups in the polysaccharide, the peak intensity at 1403 cm−1 slightly increased with an increase in the feed amount of CS for covalent attachment to gelatin. These findings confirm the presence of varying levels of CS in the porous gelatin scaffolds and further support the observations shown in .
Figure 2 (A) Fourier transform infrared spectroscopy spectra of chondroitin-4-sulfate (CS) and the gelatin samples modified with varying concentrations of CS (0%–1.25% (w/v)); (B) the absorption peak ratios of the S=O stretching to N–H stretching bands (A1228/A3294) and C–O stretching to N–H stretching bands (A1403/A3294) for the gelatin samples modified with varying concentrations of CS (GCS000, GCS005, GCS025, and GCS125).
Notes: Values are mean plus or minus standard deviation (n = 3); *P < 0.05 versus all groups (compared only within A1228/A3294 or A1403/A3294 groups); scaffold groups labeled according to CS concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Abbreviation: A/A, absorbance/absorbance.
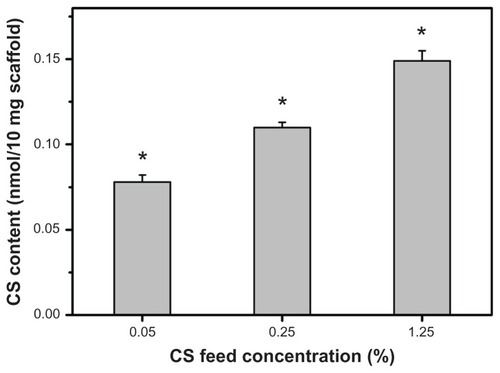
During the carbodiimide-mediated modification of gelatin with CS, the formation of amide bonds between the free amino and carboxyl groups of the polypeptide chain may occur because the gelatin is an amphiphilic polyelectrolyte. To avoid the undesirable protein self-cross-linking, in this study, the porous gelatin scaffolds were treated with EDC/NHS for 12 hours prior to reaction with CS for a further 24 hours. The results of ninhydrin assays demonstrated that the number of free amino groups of gelatin does not change following repeated exposure to EDC and NHS in the absence of CS (data not shown). In contrast, under the same coupling conditions in the presence of CS, the number of free amino groups decreased, indicating that the NHS-activated carboxyl groups of mobile CS molecules are able to react with the remaining free amino groups of cross-linked gelatin molecules. The CS content of porous gelatin scaffolds as a function of CS concentration is shown in . After modification, the samples from GCS005, GCS025, and GCS125 groups had the CS content of 0.078 ± 0.004, 0.112 ± 0.003, and 0.149 ± 0.006 nmol per 10 mg scaffold, respectively. The values revealed significant differences between these three groups (P < 0.05). Given that the generation of amide bonds between gelatin and CS is affected by the collision frequency of these biomacromolecules, the higher concentration may lead to the covalent incorporation of larger amounts of CS into the porous gelatin scaffolds.
Water content measurements
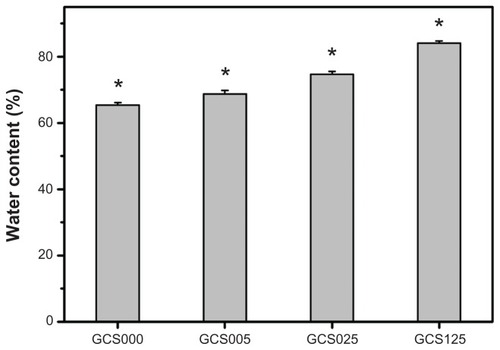
Gelatin is known to exhibit rapid dissolution in aqueous environments. In this study, carbodiimide chemistry was applied to treat gelatin matrices to circumvent the constraint. After incubation in BSS at 34°C for 4 hours, the EDC/NHS cross-linked porous gelatin scaffolds from GCS000 groups had an equilibrium water content of 65.3% ± 0.8% (). It has been documented that the water content of normal cornea is around 76%.Citation32 When the hydration levels of cornea exceed 83%, epithelial and/or endothelial damage may occur.Citation33 Hence, the scaffold material should be modified by introduction of an optimal amount of CS into the gelatin. The water content in the GCS025 (74.6% ± 0.9%) groups was significantly higher than that of the GCS005 (68.7% ± 1.1%) groups and significantly lower than that of the GCS125 (84.1% ± 0.6%) groups (P < 0.05).
Figure 4 Equilibrium water content of various porous gelatin scaffolds modified with concentrations of chondroitin-4-sulfate (GCS000, GCS005, GCS025, and GCS125).
Notes: Values are mean plus or minus standard deviation (n = 6); *P < 0.05 versus all groups; scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Light transmission measurements
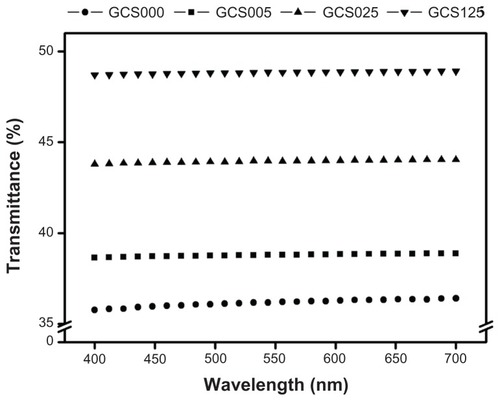
The cornea is the transparent circular part of the front of the eyeball that covers the pupil, iris, and anterior chamber.Citation1 shows the light transmission through various scaffolds, in the visible light range of 400–700 nm. The sample GCS000 had an average transmittance of 36.1%, which was lower than that reported for human cornea (around 60%).Citation34 Teoh et alCitation35 have demonstrated that the porous structure in ultra high-molecular-weight polyethylene film causes light scattering and reduces the light transmission. This may account for the poor transparency of porous gelatin membranes without CS modification. In contrast, the transmittance values in the GCS025 and GCS125 groups increased to 43.9% and 48.8%, respectively, suggesting the role of CS in the regulation of material optical properties. Results of the current study support a report by Vrana et alCitation36 showing that the addition of GAG substantially improves the transparency of collagen films.
Figure 5 Optical transmittance of various porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125) modified with concentrations of chondroitin-4-sulfate.
Notes: Scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Mechanical tests
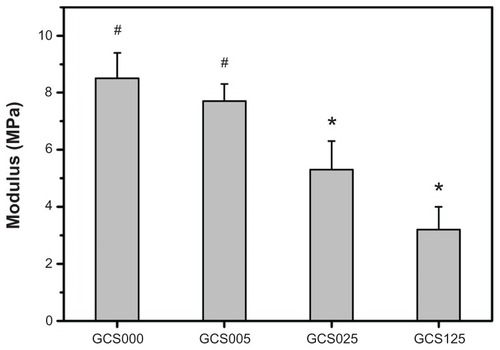
The mechanical properties of various CS-modified gelatin scaffolds are shown in . The Young’s modulus was significantly lower in the GCS025 (5.3 ± 1.0 MPa) groups than in the GCS005 (7.7 ± 0.6 MPa) and GCS000 (8.5 ± 0.9 MPa) groups (P < 0.05). The authors’ findings suggest that the samples with a CS content ≥0.112 ± 0.003 nmol per 10 mg scaffold exhibit poor mechanical stability. One possible explanation is that the presence of absorbed water has a profound influence on the tensile properties of hydrophilic biomacromolecules.Citation19 Although the treatment of gelatin with 1.25% CS significantly enhances the hydrophilicity and decreases the structural strength, the Young’s modulus of these porous scaffolds corresponds to the range of the measured normal corneal tissue values (ie, 0.3–7 MPa).Citation37 The authors have also attempted to perform microscale modification of porous gelatin scaffolds with CS. However, when the CS content is around 1 μmol per 10 mg scaffold, the water content is 93.3% ± 0.9% (far above 76%) and the Young’s modulus is 0.21 ± 0.06 MPa (slightly below 0.3 MPa), indicating that the hydrophilic and mechanical properties of the samples are much different from those of native corneas. Therefore, the present study proposes nanoscale modification of gelatin with CS for potential use as tissue-engineered corneal keratocyte scaffolds.
Figure 6 Young’s modulus of various porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125) modified with concentrations of chondroitin-4-sulfate.
Notes: Values are mean plus or minus standard deviation (n = 8); *P < 0.05 versus all groups; #P < 0.05 versus GCS025 and GCS125 groups; scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
In vitro degradation tests
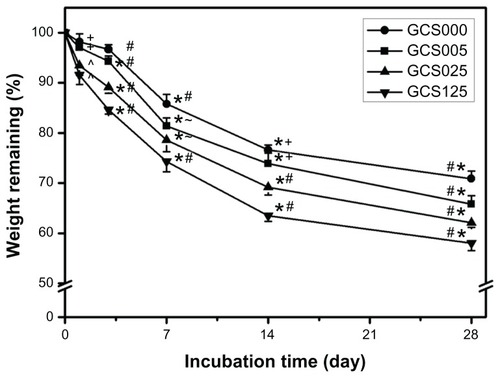
Given that the formation of cross-links can increase the resistance against protease digestion, the in vitro degradation rate of gelatin materials strongly depends on their cross-linking degree.Citation16 The percentage of weight remaining for various CS-modified gelatin scaffolds as a function of incubation time is presented in . In the GCS000 groups, approximately 20% of weight loss was observed during the period from 1 to 14 days, and the residual membranes slowly degraded with time. A similar trend was also noted for the other three groups. In particular, at each time point, the measured remaining weight showed a significant difference between the GCS005 and GCS125 groups (P < 0.05), suggesting that the incorporation of larger CS amounts may accelerate the cleavage of peptide bonds within the triple-helical structure of gelatin. As mentioned, the samples with more CS content had higher water content, thus possibly increasing the access of enzyme to the active sites of the polymer chains and promoting exogenous collagenolytic degradation of scaffold matrices.
Figure 7 Time course of the percentage of weight remaining for various chondroitin-4-sulfate-modified porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125) after incubation at 34°C in a balanced salt solution containing collagenase.
Notes: An asterisk indicates statistically significant differences (*P < 0.05; n = 5) for the mean value of weight remaining compared with value at previous time point; #P < 0.05 versus all groups; +P < 0.05 versus GCS025 and GCS125 groups; ^P < 0.05 versus GCS000 and GCS005 groups; –P < 0.05 versus GCS000 and GCS125 groups (compared only within each time point group); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Glucose permeation studies
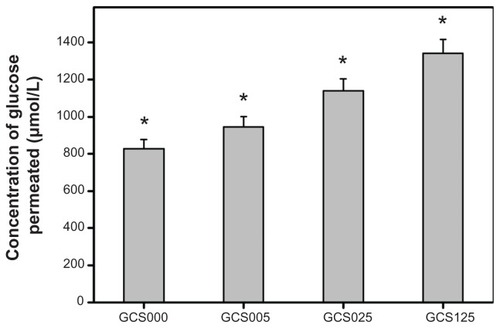
The concentration of glucose permeated through various CS-modified gelatin scaffolds at 34°C for 12 hours is shown in . The glucose concentration in the GCS000, GCS005, GCS025, and GCS125 groups was 828.3 ± 49.4, 946.1 ± 55.6, 1140.0 ± 65.0, and 1341.7 ± 76.1 μmol/L, respectively. There were significant differences among these groups (P < 0.05). The present data indicate that an increase in the amount of permeated nutrients is observed with increasing CS concentration. The EDC/NHS cross-linked gelatin hydrogels are macromolecular networks able to absorb large amounts of water without dissolution. In the range 0.1–0.001 mol/L of ionic strength, the pKa of the carboxyl group of CS increases from 3.5 to 5.7.Citation38 As a result, a stronger electrostatic repulsive force existing between carboxylate ions contributes to the expansion of the biopolymer network structure of sample GCS125, thereby detecting the higher glucose concentration in the receptor chamber under physiological buffer conditions.
Figure 8 Concentration of glucose permeated through various chondroitin-4-sulfate-modified porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125) at 34°C.
Notes: Values are mean plus or minus standard deviation (n = 5); *P < 0.05 versus all groups; scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
In vitro biocompatibility studies
Representative light microscopic images of RCK cultures photographed after 2 days are shown in . The keratocytes exposed to serum in vitro are known to exhibit a fibroblastic morphology. In this study, all the examined cells in the control groups showed this characteristic feature. Except for the GCS125 groups, the CS-modified gelatin materials of all groups demonstrated similar culture results. The RCKs also appeared healthy and actively proliferated. After the exposure to the sample GCS125, the cells are wider/ flatter and more cuboidal than the control cells.
Figure 9 (A) Phase contrast micrographs of rabbit corneal keratocyte cultures incubated for 2 days at 37°C with extract medium conditioned with various chondroitin-4-sulfate-modified porous gelatin scaffolds (control, GCS000, GCS005, GCS025, and GCS125); (B) gene expression of interleukin-6 (IL-6) in rabbit corneal keratocytes incubated with extract medium conditioned with various chondroitin-4-sulfate-modified porous gelatin scaffolds for 2 days, measured by real-time reverse transcription polymerase chain reaction.
Notes: Scale bars, 100 μm; glyceraldehyde-3-phosphate dehydrogenase was used for normalization; data in the experimental groups are percentages relative to that of control groups (without materials); values are mean plus or minus standard deviation (n = 3); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Abbreviation: mRNA, messenger RNA.
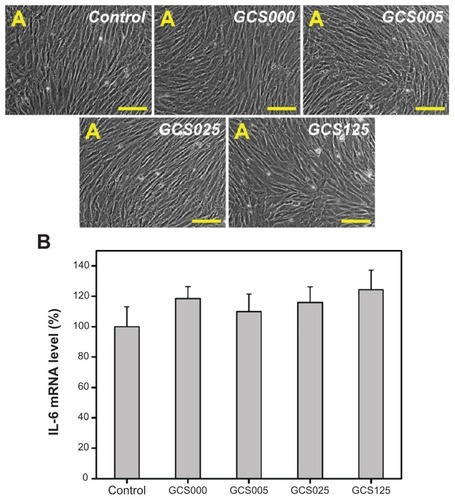
The proinflammatory gene expression of RCKs exposed to various CS-modified gelatin samples for 2 days is shown in . It was found that the IL-6 expression levels of cells did not differ significantly between the control and experimental groups treated with concentrations of CS ranging from 0% to 1.25%. The present results indicate that the incubation of RCKs with extract medium conditioned with the test samples has little effect on inflammation. According to the authors’ recent reports, the carbodiimide cross-linked gelatin hydrogel has excellent compatibility toward corneal endothelial cells,Citation8 iris pigment epithelial cells,Citation39 and retinal pigment epithelial cells.Citation14 Moreover, GAGs are nonimmunogenic and biodegradable to nontoxic oligosaccharides.Citation40 In this study, the RCK cultures contacting with CS-modified gelatin materials does not upregulate their expression of IL-6, suggesting good in vitro biocompatibility of the scaffolds.
Cell culture studies
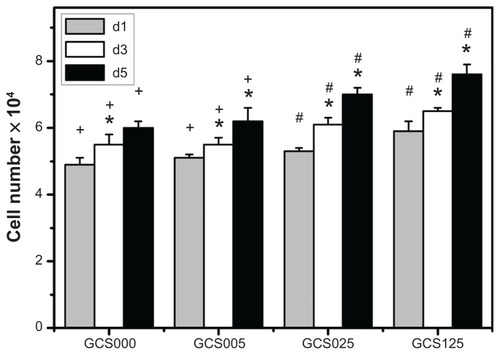
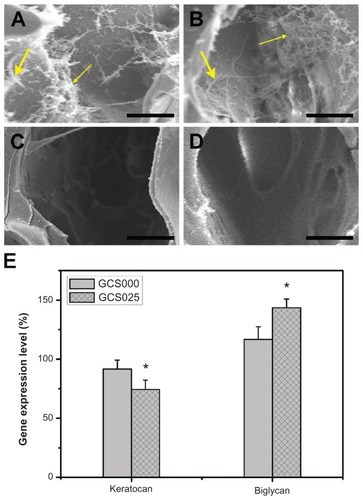
The results of quantitative analysis for RCK proliferation within various scaffolds are shown in . After 1 day of culture, the total cell number in GCS000, GCS005, and GCS025 groups reached about 5 × 104, compared with the initial adherent cell number (ie, 3.5 × 104). It has been reported that the doubling time for RCKs is 18 hours.Citation41 In the current study, the cultured cells from porous three-dimensional scaffolds displayed a slower proliferation rate. For all the CS-modified gelatin samples, the cell proliferation was significantly increased in a time-dependent manner (P < 0.05). Both the gelatin membranes from GCS000 and GCS005 groups did not affect cell proliferation at each time point. During the period of 5 days, the total cell number in GCS000 groups ranged from 5 × 104 to 6 × 104. However, the RCKs on the scaffolds modified with higher CS into the porous gelatin scaffolds can effectively expand the biosynthetic capacity of cultured RCKs. In addition to the measurement of the collagen and GAGs in the medium supernatants, the ECM production of RCKs was examined by the direct observation of the cell-biopolymer constructs after 5 days of cultivation (). SEM images showed that the cells of the GCS000 groups synthesized and deposited their own ECM components. In contrast, the cultures of the GCS025 groups had more abundant ECM. The cell-free scaffolds from both groups did not contain newly deposited ECM (). It has been documented that the biochemical composition of the biomaterial scaffolds plays an important role in the regulation of metabolic parameters of the cells.Citation45 Meier and HayCitation46 reported that the CS proteoglycan had a stimulatory effect on GAG production by corneal epithelial cells.Citation46 A study from van Susante et alCitation47 showed that the collagen-CS scaffold contained significantly more newly synthesized GAGs than in the absence of CS after CS concentration (ie, GCS025 and GCS125 groups) showed significantly higher cell numbers (P < 0.05). Investigators have performed studies on the biological effects of CS, but the results are inconsistent. Ida et alCitation42 demonstrated that the epidermal growth factor–mediated proliferation of neural stem/progenitor cells was not stimulated by CS. In contrast, Westergren-Thorsson et alCitation43 reported that while the l-iduronic acid–containing galactosaminoglycans such as dermatan sulfate could inhibit the human lung fibroblast proliferation in vitro, the galactosaminoglycan not containing l-iduronic acid (ie, CS) effectively stimulated the proliferation of these cells. Zou et alCitation44 also demonstrated that continuous supplementation of the culture medium with CS led to a dose-dependent increase in the number of palatal fibroblasts after a 7-day incubation period. The results of the current study indicate that the gelatin materials from GCS025 and GCS125 groups can markedly enhance the proliferative capacity of RCKs.
Figure 10 Total cell number on various chondroitin-4-sulfate-modified porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125). after rabbit corneal keratocyte seeding for 1, 3, and 5 days (d1, d3, and d5, respectively).
Notes: An asterisk indicates statistically significant differences (*P < 0.05; n = 4) for the mean value of total cell number compared with value at previous time point; #P < 0.05 versus all groups; +P < 0.05 versus GCS025 and GCS125 groups (compared only within each time point group); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
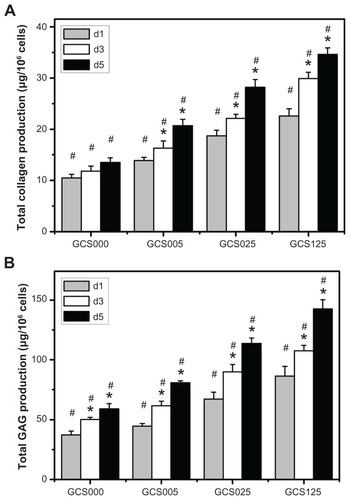
The total collagen and GAG contents after RCK growth within various scaffolds over the 5-day culture are shown in , respectively). The level of ECM secretion was elevated in the culture medium with increasing incubation time. At day 5, the amount of total collagen in the GCS000 groups was 13.5 ± 0.9 μg/106 cells, which was significantly lower than those of the GCS005 (20.7 ± 1.2 μg/106 cells), GCS025 (28.2 ± 1.5 μg/106 cells), and GCS125 (34.6 ± 1.3 μg/106 cells) groups (P < 0.05). A similar trend was found for the effect of material composition on the variation in GAG secretion. These findings suggest that the covalent incorporation of larger amounts of 14 days of chondrocyte culture. Results of the present study are compatible with these earlier observations and indicate that the differences in the CS content of porous gelatin scaffolds may have a profound influence on the ECM production capacity of RCKs.
Figure 11 Extracellular matrix production capacity of rabbit corneal keratocytes at days 1, 3, and 5 (d1, d3, and d5, respectively) after plating on various chondroitin-4-sulfate-modified porous gelatin scaffolds (GCS000, GCS005, GCS025, and GCS125): (A) collagen content and (B) glycosaminoglycan (GAG) content.
Notes: An asterisk indicates statistically significant differences (*P < 0.05; n = 3) for the mean value of extracellular matrix content compared with value at previous time point; #P < 0.05 versus all groups (compared only within each time point group); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
shows the gene expression profiles for two phenotypic markers (keratocan and biglycan). The detected expression level for each gene in the tissue culture polystyrene groups was defined as 100%. After cultivation for 5 days, the RCKs on GCS025 samples showed significantly lower keratocan levels and higher biglycan levels than those of GCS000 groups (P < 0.05), indicating that the cell phenotype is not adequately maintained by the incorporation of CS into the gelatin materials. Vrana et alCitation48 have previously reported the development of a reconstructed cornea from collagen-CS foams and human cell cultures. The results of their study indicate that even though the keratocytes lose a phenotypic marker, these cells do not differentiate into myofibroblasts during culture within the foams.
Figure 12 Scanning electron microscopy images of (A and B) the constructs prepared from 5 days’ cultivation of the rabbit corneal keratocytes on scaffold samples and (C and D) the cell-free scaffolds (large arrow indicates cell, fine arrow indicates extracellular matrix). Groups are labeled according to chondroitin-4-sulfate concentration used: (A and C) GCS000 (0% (w/v)) and (B and D) GCS025 (0.25% (w/v)). (E) Gene expression level of keratocan and biglycan in rabbit corneal keratocytes grown on tissue culture polystyrene and scaffold samples GCS000 and GCS025 for 5 days by real-time reverse transcription polymerase chain reaction.
Notes: Scale bars, 20 μm; glyceraldehyde-3-phosphate dehydrogenase was used for normalization; data in the experimental groups are percentages relative to that of tissue culture polystyrene groups; an asterisk indicates statistically significant differences (*P < 0.05; n = 3) as compared with the GCS000 groups.
Conclusion
Three-dimensional biomaterial scaffolds have been designed to provide temporary support for cell growth. In this study, nanoscale modification of gelatin with CS was performed to control characteristics in tissue-engineered corneal keratocyte scaffolds. When compared with their counterparts without CS modification, the porous gelatin membranes treated with 0.05%–1.25% CS had higher levels of water content, light transmittance, and amount of permeated nutrients, but had lower Young’s modulus and resistance against protease digestion. The hydrophilic and mechanical properties of GCS025 samples were comparable with those of native corneas. Additionally, the scaffolds modified with 0.25% CS exhibited excellent compatibility toward RCKs and demonstrated enhanced proliferative and biosynthetic capacity of cultured cells. The findings suggest that the gelatin materials with the CS content of 0.112 ± 0.003 nmol per 10 mg scaffold may hold potential for use in corneal stromal tissue engineering. The validity of the CS-modified porous gelatin scaffolds requires further biological testing, which is currently underway.
Acknowledgments
This work was supported by grant NSC99-2221-E-182-008 from the National Science Council of the Republic of China and grant CMRPD190431 from Chang Gung Memorial Hospital.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary figures
Figure S1 Cross-linking index of porous gelatin scaffolds treated with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride/N-hydroxysuccinimide as a function of cross-linking time.
Notes: An asterisk indicates statistically significant differences (*P < 0.05; n = 5) for the mean value of cross-linking index compared with the value at the previous time point.
Abbreviations: EDC, 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride; NHS, N-hydroxysuccinimide.
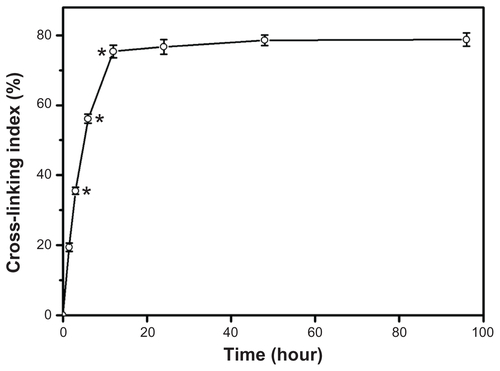
Figure S2 Pore size of various porous gelatin scaffolds modified with chondroitin-4-sulfate.
Notes: Values are mean plus or minus standard deviation (n = 3); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Abbreviation: CS, chondroitin-4-sulfate.
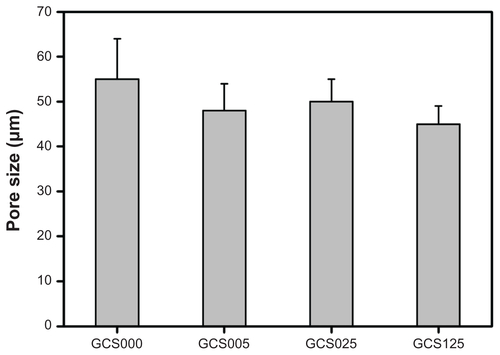
Figure S3 Porosity of various porous gelatin scaffolds modified with chondroitin-4-sulfate.
Notes: Values are mean plus or minus standard deviation (n = 5); scaffold groups labeled according to chondroitin-4-sulfate concentration used (0%, 0.05%, 0.25%, or 1.25% (w/v)): GCS000, GCS005, GCS025, and GCS125.
Abbreviation: CS, chondroitin-4-sulfate.
References
- LaiJYHsiueGHFunctional biomedical polymers for corneal regenerative medicineReact Funct Polym2007671112841291
- MinamiYSugiharaHOonoSReconstruction of cornea in three-dimensional collagen gel matrix cultureInvest Ophthalmol Vis Sci1993347231623247685009
- GriffithMOsborneRMungerRFunctional human corneal equivalents constructed from cell linesScience199928654472169217210591651
- VranaNEBuillesNKocakHEDC/NHS cross-linked collagen foams as scaffolds for artificial corneal stromaJ Biomater Sci Polym Ed200718121527154517988518
- LuPLLaiJYTabataYHsiueGHA methodology based on the “anterior chamber of rabbit eyes” model for noninvasively determining the biocompatibility of biomaterials in an immune privileged siteJ Biomed Mater Res A200886110811617941023
- HsiueGHLaiJYChenKHHsuWMA novel strategy for corneal endothelial reconstruction with a bioengineered cell sheetTransplantation200681347347616477237
- LaiJYChenKHHsiueGHTissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterialsTransplantation200784101222123218049106
- LaiJYLiYTFunctional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriersBiomacromolecules20101151387139720355704
- LevickJRFlow through interstitium and other fibrous matricesQ J Exp Physiol19877244094373321140
- LiuYGriffithMWatskyMAProperties of porcine and recombinant human collagen matrices for optically clear tissue engineering applicationsBiomacromolecules2006761819182816768403
- FreundDEMcCallyRLFarrellRACristolSML’HernaultNLEdelhauserHFUltrastructure in anterior and posterior stroma of perfused human and rabbit corneas: relation to transparencyInvest Ophthalmol Vis Sci1995368150815237601631
- RadaJACornuetPKHassellJRRegulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteinsExp Eye Res19935666356488595806
- KuijpersAJEngbersGHMMeyvisTKLCombined gelatin-chondroitin sulfate hydrogels for controlled release of cationic antibacterial proteinsMacromolecules2000331037053713
- LaiJYLiYTEvaluation of cross-linked gelatin membranes as delivery carriers for retinal sheetsMater Sci Eng C2010305677685
- LaiJYChenKHHsuWMLeeTHLinSYMultiple elements in the deposits of opacified Hydroview intraocular lensAm J Ophthalmol200513961123112515953454
- LaiJYLiYTInfluence of cross-linker concentration on the functionality of carbodiimide cross-linked gelatin membranes for retinal sheet carriersJ Biomater Sci Polym Ed2011221–3277295
- LaiJYLuPLChenKHTabataYHsiueGHEffect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapyBiomacromolecules2006761836184416768405
- MaDHKLaiJYChengHYTsaiCCYehLKCarbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cellsBiomaterials201031256647665820541801
- LaiJYLinPKHsiueGHChengHYHuangSJLiYTLow Bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantationBiomacromolecules200910231031919063667
- LaiJYChenKHHsuWMHsiueGHLeeYHBioengineered human corneal endothelium for transplantationArch Ophthalmol2006124101441144817030712
- YoshidaSShimmuraSShimazakiJShinozakiNTsubotaKSerumfree spheroid culture of mouse corneal keratocytesInvest Ophthalmol Vis Sci20054651653165815851565
- KotzarGFreasMAbelPEvaluation of MEMS materials of construction for implantable medical devicesBiomaterials200223132737275012059024
- LaiJYThe role of Bloom index of gelatin on the interaction with retinal pigment epithelial cellsInt J Mol Sci20091083442345620111679
- LuPLLaiJYMaDHKHsiueGHCarbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: characterization and interaction with corneal endothelial cellsJ Biomater Sci Polym Ed200819111818177550
- LaiJYMaDHKChengHYOcular biocompatibility of carbodiimide cross-linked hyaluronic acid hydrogels for cell sheet delivery carriersJ Biomater Sci Polym Ed201021335937620178691
- KangHWTabataYIkadaYFabrication of porous gelatin scaffolds for tissue engineeringBiomaterials199920141339134410403052
- Van VlierbergheSCnuddeVDubruelPPorous gelatin hydrogels: 1. Cryogenic formation and structure analysisBiomacromolecules20078233133717291055
- DuanXSheardownHCross-linking of collagen with dendrimersJ Biomed Mater Res A200575351051816108047
- DoughtyMJSeabertWBergmansonJPGBlockerYA descriptive and quantitative study of the keratocytes of the corneal stroma of albino rabbits using transmission electron microscopyTissue Cell200133440842211521958
- HsiueGHLaiJYLinPKAbsorbable sandwich-like membrane for retinal-sheet transplantationJ Biomed Mater Res2002611192512001241
- SuiWHuangLWangJBoQPreparation and properties of chitosan chondroitin sulfate complex microcapsulesColloid Surf B Biointerfaces20086516973
- LeonardDWMeekKMRefractive indices of the collagen fibrils and extrafibrillar material of the corneal stromaBiophys J1997723138213879138583
- MontiDChetoniPBurgalassiSNajarroMSaettoneMFIncreased corneal hydration induced by potential ocular penetration enhancers: assessment by differential scanning calorimetry (DSC) and by desiccationInt J Pharm20022321–213914711790497
- RafatMLiFFagerholmPPEG-stabilized carbodiimide cross-linked collagen-chitosan hydrogels for corneal tissue engineeringBiomaterials200829293960397218639928
- TeohSHTangZGRamakrishnaSDevelopment of thin elastomeric composite membranes for biomedical applicationsJ Mater Sci Mater Med199910634335215348135
- VranaNEElsheikhABuillesNDamourOHasirciVEffect of human corneal keratocytes and retinal pigment epithelial cells on the mechanical properties of micropatterned collagen filmsBiomaterials200728294303431017618681
- JayasuriyaACScheinbeimJILubkinVBennettGKramerPPiezoelectric and mechanical properties in bovine corneaJ Biomed Mater Res A200366226026512888995
- Scordilis-KelleyCOsteryoungJGVoltammetric studies of counterion transport in solutions of chondroitin sulfateJ Phys Chem19961002797804
- LaiJYBiocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic useJ Mater Sci Mater Med20102161899191120238149
- MalafayaPBSilvaGAReisRLNatural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applicationsAdv Drug Deliv Rev2007594–520723317482309
- CookSDAitkenDABrownSMGrowth and characterization of rabbit corneal cells in vitroGraefes Arch Clin Exp Ophthalmol198722553513563311893
- IdaMShuoTHiranoKIdentification and functions of chondroitin sulfate in the milieu of neural stem cellsJ Biol Chem200628195982599116373347
- Westergren-ThorssonGPerssonSIsakssonAÖnnervikPOMalmströmAFranssonLAL-iduronate-rich glycosaminoglycans inhibit growth of normal fibroblasts independently of serum or added growth factorsExp Cell Res1993206193997683278
- ZouXHFoongWCCaoTBayBHOuyangHWYipGWChondroitin sulfate in palatal wound healingJ Dent Res2004831188088515505240
- NehrerSBreinanHARamappaAMatrix collagen type and pore size influence behaviour of seeded canine chondrocytesBiomaterials199718117697769177854
- MeierSHayEDStimulation of extracellular matrix synthesis in the developing cornea by glycosaminoglycansProc Natl Acad Sci U S A1974716231023134276294
- Van SusanteJLCPieperJBumaPLinkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitroBiomaterials200122172359236911511033
- VranaNEBuillesNJustinVDevelopment of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell culturesInvest Ophthalmol Vis Sci200849125325533118708614