Abstract
Radiotherapy (RT) is a cancer treatment that uses high doses of radiation to kill cancer cells and shrink tumors. Although great success has been achieved on radiotherapy, there is still an intractable challenge to enhance radiation damage to tumor tissue and reduce side effects to healthy tissue. Radiosensitizers are chemicals or pharmaceutical agents that can enhance the killing effect on tumor cells by accelerating DNA damage and producing free radicals indirectly. In most cases, radiosensitizers have less effect on normal tissues. In recent years, several strategies have been exploited to develop radiosensitizers that are highly effective and have low toxicity. In this review, we first summarized the applications of radiosensitizers including small molecules, macromolecules, and nanomaterials, especially those that have been used in clinical trials. Second, the development states of radiosensitizers and the possible mechanisms to improve radiosensitizers sensibility are reviewed. Third, the challenges and prospects for clinical translation of radiosensitizers in oncotherapy are presented.
Introduction
Cancer remains one of the greatest challenges to human health. World Health Organization (WHO) reported that about 8.8 million deaths worldwide were due to cancer in 2015, and the deaths are expected to break through 13 million in 2030 according to the report by the International Agency for Research on Cancer (IARC). To reduce the deaths from cancer, several strategies have been developed in recent years to improve cancer therapy including surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, hormone therapy, stem cell transplant and precision medicine.Citation1 Among them, radiotherapy (RT) is considered as one important and effective modality to kill or control tumors since Marie Curie, the Nobel Prize winner, discovered radioactivity.Citation2 Typically, RT is a treatment modality to cancer cells by using high-energy photon radiation such as X-rays, gamma (γ)-rays, and others. RT can take effect via direct and indirect mechanisms to destroy cancer cells and tumor tissue ().
Figure 1 Schematic of the mechanism of ionizing radiation (IR) in RT. In the case of direct effect, IR directly damages the DNA, which, if unrepaired, results in cell death or permanent growth arrest. In the case of indirect effect, ROS are formed by the radiolysis of a large amount of water and oxygen, and then the ROS damage the DNA. There are many types of DNA damage, such as base change, SSB, DSB, cross-linkage with protein or with other DNA molecules.
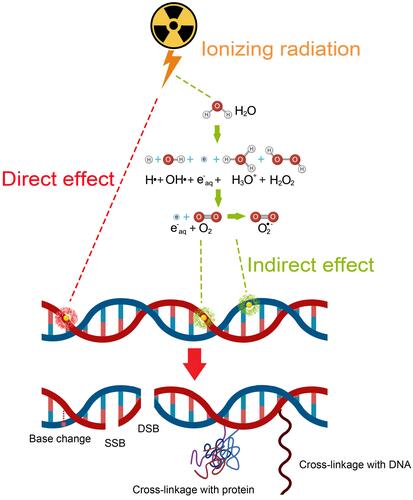
In the direct action, radiation directly induces single-strand breaks (SSB) and double-strand breaks (DSB) in DNA, resulting in the termination of cell division and proliferation, or even cell necrosis and apoptosis. In the case of indirect action, radiation induces the generation of ROS, which can induce cellular stress in, and injure biomolecules, and and ultimately alter cellular signaling pathways. Clinical studies have shown that more than half (about 70%) of patients need to receive RT, and in some cases RT is the only kind of cancer treatment.Citation3 Therefore, there is a great need to develop approaches to improve radiosensitivity.
Innovative technologies can provide alternative strategies to improve RT efficiency. For example, image-guided radiation therapy (IGRT) is the use of imaging during radiation therapy to improve the precision and accuracy of treatment delivery. IGRT can be used to treat tumors in areas of the body that move, such as the lungs. RT machines are equipped with imaging technology to allow your doctor to image the tumor before and during treatment. By comparing these images to the reference images taken during simulation, the patient’s position and/or the radiation beams may be adjusted to more precisely target the radiation dose to the tumor. To help align and target the radiation equipment, some IGRT procedures may use fiducial markers, ultrasound, MRI, X-ray images of bone structure, CT scan, 3D body surface mapping, electromagnetic transponders or colored ink tattoos on the skin.Citation4 Intensity-modulated radiation therapy (IMRT) is an advanced mode of high-precision RT that uses computer-controlled linear accelerators to deliver precise radiation doses to a malignant tumor or specific areas within the tumor.Citation5 Although the abovementioned innovative technologies greatly improve the therapeutic effect, there are still obstacles such as cancer stem cells and tumor heterogeneity making it difficult to use RT alone to cure tumors. Radiosensitizers with the ability to increase the radiosensitivity of tumor tissue and pharmacologically decrease normal tissue toxicity are expected to be an efficient way to improve RT.Citation6
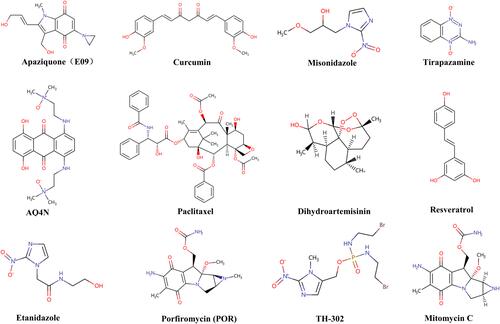
Radiosensitizers are compounds that, when combined with radiation, achieve greater tumor inactivation than would have been expected from the additive effect of each modality. G E Adams, a pioneer in the field of RT, classified radiosensitizers into five categories: (1) suppression of intracellular thiols or other endogenous radioprotective substances; (2) formation of cytotoxic substances by radiolysis of the radiosensitizer; (3) inhibitors of repair of biomolecules; (4) thymine analogs that can incorporate into DNA; and (5) oxygen mimics that have electrophilic activity.Citation7,Citation8 This classification was based on the mechanism of DNA damage and repair and indicated the direction for radiosensitizers at the early stage. However, with the continuous technological innovation, more and more materials and drugs with radiotherapy sensitization have been defined as radiosensitizers. In addition, some in-depth mechanisms for radiosensitization have also been discovered.Citation9,Citation10 According to the latest research, radiosensitizers can be classified into three categories based on their structures: small molecules (), macromolecules (), and nanomaterials ().Citation11 In the following part, the applications, the main role, and influencing factors of these three types of radiosensitizers are first summarized, especially those have currently entered clinical trials. Second, the development status and the mechanism of action of the radiosensitizer are also summarized. Third, the future development and application of the radiosensitizer was presented.
Table 1 Some Macromolecule Radiosensitizers Discussed in This Paper
Table 2 The List of Nanomaterials Used for Radiosensitization
Small Molecules
Oxygen
Hypoxia in tumor microenvironment is one of the major limitations to radiotherapy. Tumor cells in the hypoxic microenvironment are much more resistant to radiation than in the normal oxygen microenvironment.Citation12–Citation14 Oxygen enhancement ratio (OER) or oxygen enhancement effect in radiobiology refers to the enhancement of the therapeutic or detrimental effect of ionizing radiation due to the presence of oxygen. This so-called oxygen effect is most notable when cells are exposed to an ionizing radiation dose.Citation15,Citation16 Oxygen, a potent radiosensitizer, promotes free radical formation through its unique electronic configuration. As the most electrophilic cellular molecule, oxygen is easily reduced by electrons formed from the incident radiation. After oxygenated tumor irradiation, energy transfer results in the radiolysis of water with the initial formation of an ion radical that then forms the highly reactive hydroxyl radical after reaction with another water molecule. Oxygen leads to the formation of peroxide after reaction with the hydroxyl radical. Then, the peroxide results in permanent cellular and DNA damage.Citation13
Accompanied with solid tumor growth, the surrounding vasculatures are not in sufficient quantities to supply oxygen to the new cells, the cancer cell mass becomes heterogeneous gradually, and necrosis occurs following ischemia. Normally, cancer cells undergo apoptosis through the p53 pathway, while those heterogeneous cells adapt to the hypoxic environment efficiently by activation of additional signaling pathways, especially the hypoxia-inducible factor (HIF) pathway.Citation17–Citation19 Studies showed that HIF-1α was associated with vascular endothelial growth factor (VEGF) signaling pathway, glucose transport, and glycolysis pathway, which could help the tumor to build vasculature.Citation19–Citation21 Under hypoxia, the cancer cells are more aggressive and resisted radiotherapy significantly. Thus, hypoxia often occurs in most solid tumors and leads to radioresistance both through increasing free radical scavenging and changing patterns of gene expression.Citation22,Citation23
More and more research has been devoted to overcoming hypoxia problems, from using high-pressure oxygen tanks and blood substitutes that carried oxygen, to using intricate, accurate approaches that proportionated differences in partial pressure of oxygen (PO2) between tumors and healthy tissue.Citation24,Citation25 Hyperbaric oxygen is the most direct method to ameliorate hypoxia in tumor cells, while this method is inconvenient and may increase complications sometimes.Citation26,Citation27 A new radiosensitizer, Kochi oxydol-radiation therapy for unresectable carcinomas (KORTUC), is being evaluated by a Phase I/II clinical trial (NCT02757651) for the treatment of malignant tumors that contain numerous hypoxic cancer cells and/or large quantities of antioxidative enzymes.Citation28
Oxygen Mimics
Oxygen mimetics, using the chemical properties of molecular oxygen as a template, have higher electron affinity and better diffusion properties to anoxic tissue than oxygen. As oxygen mimetics can theoretically substitute for oxygen in “fixing” radiation-induced damage of DNA, making it nonrepairable and hence lethal. Therefore, oxygen mimetics are considered as “true radiosensitizers”. The most representative oxygen mimetics are nitro-containing compounds and nitric oxide (NO).Citation13
The prototype of electron-affinity radiosensitizers is nitrobenzene, and then researchers focus on nitroimidazole and its derivatives.Citation29–Citation31 Nitroimidazoles, which undergo enzymatic and radiation-induced redox reactions. These agents are intrinsic inactive, their effect becomes evident only in the presence of ionizing radiation to “fix” or stabilize DNA radical lesions in oxygen-deficient cells.Citation32 Misonidazole, a 2-nitroimidazole, is one of the earliest developed nitroimidazoles. In preclinical studies, misonidazole showed better radiosensitizing effect than 5-nitro imidazole or metronidazole (Flagyl®) in the majority of solid murine tumors.Citation33–Citation35 However, the results were unsatisfactory in clinical trials, since severe neurotoxicity was caused by misonidazole.Citation36–Citation39 Metronidazole, a 5-substituted nitroimidazole, which has less electron-affinic was proven as an inferior radiosensitizer.Citation40,Citation41 In conclusion, because of the dose-limiting toxicity at clinically tolerable doses, misonidazole and metronidazole are not the ideal candidates in radiotherapy.Citation42
In view of the issues discussed above, further efforts have been made to improve the pharmacokinetic properties of nitroimidazoles. Second-generation nitroimidazole radiosensitizers, such as etanidazole or nimorazole, are designed to increase the hydrophilicity of the reagents and thereby reduce neurotoxicity. For example, etanidazole has better hydrophilicity than misonidazole because its side chain is modified by hydroxyl.Citation43 Although etanidazole presents lower preclinical toxicity and higher efficacy, it shows no obvious benefit for head and neck cancer patients in randomized studies.Citation44 Nimorazole, a 5-nitroimidazole, is recommended for the treatment of head and neck cancers in Denmark since its beneficial effects in several clinical trials. Moreover, it has been further explored in an EORTC international trial.Citation45–Citation51 Notably, the DAHANCA 28 trial demonstrated that hyperfractionated, accelerated radiotherapy with concomitant cisplatin and nimorazole (HART-CN) for patients was feasible and yielded favorable tumor control.Citation52 Other nitro compounds have also been exploited for hypoxia radiosensitization. Dinitroazetidine, RRx-001, has been evidenced as an effective radiosensitizer with low toxicity and is now being evaluated in the NCT02871843 clinic trial.Citation53
Nitrogen oxides, in particular, NO, act as radiosensitizers through many direct and indirect mechanisms. Similar to the oxidative stress induced by oxygen, NO can “fix” or stabilize radiation-induced DNA damage through nitrosative stress pathways.Citation54 Oxidative and nitrosative stress pathways involve the generation of reactive species. For example, nitrous acid, peroxynitrite (ONOO–), and nitric acid produce cytotoxic effects through mechanisms including DNA cross-linking, protein nitrosylation, glutathione depletion, and inhibition of mitochondrial respiration.Citation55–Citation58 As an uncharged free radical, NO can diffuse across cell membranes freely and bind to soluble guanylate cyclase (sGC) to induce cyclic GMP production, thereby regulating vascular physiology.Citation59–Citation61 Researchers have reported that 5-nitroimidazoles and sanazole can release NO.Citation62,Citation63
A phase I study of non-small-cell lung cancer (NSCLC) patients suggested that NO donation increased tumor perfusion and, therefore, promoted tumor growth.Citation64 However, a phase II study of prostate cancer patients claimed that low-dose NO had no direct cytotoxic effect, but could decrease hypoxia through improving blood flow in tumor tissue.Citation65 Some anticancer drugs approved by US Food and Drug Administration (FDA), such as bevacizumab, sorafenib, and etaracizumab played their roles by blocking the VEGF pathway to some extent.Citation66 VEGF is overexpressed in anoxia environment, which leads to endothelial cell proliferation and neovascularization. In angiogenesis, there is a positive and negative feedback regulation relationship between VEGF and NO, which maintains vascular homeostasis precisely.Citation67 In addition, Liebmann et al proved that pretreatment with NO improved the survival of mice after irradiation.Citation68
Active Compounds from Chinese Herbs
In recent years, more and more researchers reported that active compounds from Chinese herbs such as curcumin,Citation69–Citation71 resveratrol,Citation72–Citation74 dihydroartemisininCitation75–Citation77 and paclitaxel,Citation78–Citation80 could enhance tumor radiotherapy sensitivity (). Curcumin is a polyphenolic active compound extracted from turmeric. Curcumin exerts anti-inflammatory effect by inhibiting the transcription factor NF-κB, which is involved in both tumorigenesis and radioresistance.Citation81 In a preclinical study, Chendil et al reported that when treated with RT and curcumin together, the human prostate cancer cell line, PC3 presented threefold fewer surviving and the mechanism was supposed to have a relationship with NF-κB.Citation82 In addition, nanocurcumin as a radiosensitizer is being evaluated by a Phase II clinical trial (NCT02724618). Other relevant research on mutant p53 Ewing’s sarcoma cells proved that radiosensitivity of curcumin was associated with other p53-response genes.Citation83
Resveratrol is an active compound extracted from grapes, knotweed, peanuts, mulberry and other plants. Tan et al proved that resveratrol enhanced the radiosensitivity in nasopharyngeal carcinoma cells by downregulating E2F1.Citation73 Liao et al found that resveratrol enhanced radiosensitivity in human NSCLC NCI-H838 cells by inhibiting NF-κB activation.Citation84 Dihydroartemisinin is a derivative of artemisinin, which can shorten the G2/M phase, while increases the G0/G1 and S phase, thereby reducing the radiation resistance.Citation85 Although the relevant clinical research has not yet been carried out, researchers have demonstrated that resveratrolCitation86–Citation89 and dihydroartemisininCitation90–Citation92 possessed radiosensitization on cancer cells in vitro.
Paclitaxel is widely known as a very good natural anticancer drug.Citation93,Citation94 As a new type of antimicrotubule drug, paclitaxel can inhibit the microtubule networks formation and prevent the tumor cells proliferation to achieve radiosensitization.Citation95 Results showed that paclitaxel could obviously enhance the radiosensitivity of inoperable patients with locally advanced esophageal cancer and improve the prognosis of patients with acceptable therapeutic effect.Citation96 A three-arm randomized Phase III trial (NCT02459457)—comparison of paclitaxel-based three regimens concurrent with radiotherapy for patients with local advanced esophageal cancer and a Phase III study (NCT01591135) of comparing paclitaxel plus 5-fluorouracil vs cisplatin plus 5-fluorouracil in chemoradiotherapy for locally advanced esophageal carcinoma are underevaluated.
Hypoxia-specific Cytotoxins
Some bioreductive agents, such as aromatic N-oxides, transition metal complexes, quinones, aliphatic N-oxides and nitro compounds, have radiosensitization effects by virtue of their preferential cytotoxicity toward hypoxic cells.Citation11 Tirapazamine (TPZ), a hypoxia-selective radiosensitizer, has shown promising results in clinical trials.Citation97,Citation98 Under hypoxic environments, TPZ can be reduced by reductase in cells to a metabolite that produces free radical and then leads to SSB, DSB, and base damage on DNA.Citation99 A Phase I clinical trial of TPZ with cisplatin and radiotherapy in small cell lung cancer showed prolonged survival of patients.Citation100 A Phase II study of TPZ with chemoradiotherapy in locally advanced head and neck cancer reported improvements in failure-free survival and response of patients.Citation101 However, further phase III trials of TPZ with chemoradiotherapy in locally advanced head and neck cancer concluded that there was no obvious improvement in patient survival.Citation102 In addition, SN30000 (previously known as CEN-209), an analog of TPZ, with more favorable diffusion property that provides greater toxicity in hypoxic cancer cells than TPZ, is currently under development by the Drug Development Office of Cancer Research UK.Citation103
AQ4N, a representative to aliphatic N-oxide, can be reduced to AQ4 by cytochrome P450 isoenzymes or nitric oxide synthase 2A.Citation104 In vivo experiments showed that combined utilization of AQ4N with radiotherapy resulted in increased antitumor efficacy, as well as negligible toxicity to normal tissue compared with radiation alone.Citation105 Positive results were also evidenced in Phase I clinical trials.Citation106 A Phase I clinical trial in glioblastoma and head and neck tumor patients proved that AQ4N could be specifically activated in hypoxic regions of solid tumors.Citation107 Unfortunately, a Phase II clinical trial of AQ4N with radiotherapy and temozolomide in glioblastoma began in 2006, was in a pending status (NCT00394628).
TH-302 (evofosafamide), a similar compound that can be reduced to bromo-isophosphoramide mustard in hypoxic conditions, has radiosensitization activity, especially in hypoxic cells.Citation108,Citation109 In preclinical models of rhabdomyosarcoma (skeletal muscle) and NSCLC, TH-302 combined with radiotherapy treatment resulted in significant tumor growth delay.Citation110 In addition, in a study in patient-derived xenograft models of pancreatic cancer, combination treatment of TH-302 and radiotherapy was more efficient than either treatment alone.Citation111 TH-302 can specifically target the hypoxic tumor cells and induce DNA damage simultaneously in adjacent tumor tissue of the hypoxic zone, and thus holds potential radiosensitization effects in solid tumor treatment.Citation112 However, on the database of US National Institutes of Health clinical trials, only one of the 26 trials listed proposed combination treatment of TH-302 with radiotherapy (NCT02598687), and it was withdrawn because two phase III trials did not meet their primary endpoint.Citation113
Mitomycin C, a quinone-based anticancer therapeutic, can be activated via DNA cross-linking. In preclinical study, mitomycin C showed only slight toxicity in hypoxic cells, which promotes the development of other hypoxia-sensitive quinones selection.Citation114 Among them, porfiromycin (POR) and apaziquone (EO9) are bioreductive prodrugs, represent the leading candidates.Citation104 Preclinical studies concluded that POR held higher hypoxic selectivity than mitomycin C.Citation115 Although preclinical trials proved POR had acceptable toxicity, the following Phase 3 trial demonstrated that POR had a poorer therapeutic effect than mitomycin C.Citation116 Preclinical studies indicated that EO9 had greater antitumor property than mitomycin C, indicating EO9 can be a ideal radiosensitizer.Citation117
Other Chemical Radiosensitizers
Other types of chemical radiosensitizers have also seen some progress and some of them are in preclinical evaluations. For example, chemicals that influence cell signaling, suppress radioprotective substances, pseudosubstrates and targeted delivery systems are exploited. With the development of research on radioresistance mechanism, it has been found that multiple signal pathways are related to radioresistance, providing more targets for radiosensitization, such as PI3K–Akt–mTOR,Citation118 Wnt,Citation119 MAPK,Citation120 MDM2Citation121 and c-MET–PI3K–Akt.Citation122 For example, BKM120, the oral PI3K inhibitor, can inhibit the activity of PI3K/Akt by targeting the PI3K-Akt pathway, thereby increasing cell apoptosis and inhibiting DNA double-strand break repair in liver cancer cells.Citation123 BEZ235, a dual PI3K–mTOR inhibitor, can improve the radiosensitivity of colorectal cancer cells.Citation124 AMG 232, a picomolar affinity piperidinone inhibitor of MDM2, can suppress tumor growth on a mouse model.Citation121
Suppression of radioprotective substances, such as glutathione (GSH), is another strategy of radiosensitization. Inhibition of GSH can prevent DNA damage repair and lead to increased damage in tumor cells, which improves the efficacy of radiotherapy in turn.Citation125 In addition, pseudosubstrates lead cells undergoing DNA synthesis unable to distinguish thymidine and its halogenated analogs efficiently. It is a new area of clinical research to use halogenated pyrimidine analogs, like bromodeoxyuridine (BrdUrd) and iododeoxyuridine (IdUrd), as potential clinical radiosensitizers.Citation126 One study demonstrated that electron affinities of 5-halogenated deoxyuridine led to enough ability to bind a radiation-produced secondary electron, thereby increasing the sensitivity of radiotherapy.Citation127
In addition, research on new indications for existing drugs provides a new paradigm for the development of radiosensitizers. For instance, papaverine, an ergot alkaloid first isolated from Papaver somniferum in 1848, has been used for treatment of vasospasm, cerebral thrombosis, pulmonary embolism and erectile dysfunction.Citation128 Denko et al identified papaverine as an inhibitor of mitochondrial complex I and proved that papaverine could increase oxygenation and enhance radiation response.Citation128 A phase I trial (NCT03824327) study on papaverine and stereotactic body radiotherapy (SBRT) for NSCLC or lung metastases is under evaluation. In summary, small-molecule chemicals as radiosensitizers initiated in the past five years under clinical trials are summarized in .
Table 3 Registered Ongoing Clinical Trials (https://Clinicaltrials.gov/) of Small-molecule Chemical Radiosensitizers
Macromolecules
Proteins and Peptides
Proteins and peptides, such as antibodies and short peptides, have high affinity with antigens and receptors overexpressed on the surface of tumor cells, making them usable as radiosensitizers.Citation129 For instance, HER3-ADC, a maytansine-based antibody-drug conjugate targeting HER3, which induces cell arrest in the G2/M phase to inhibit DNA damage repair and thereby improves radiosensitivity of HER3-positive pancreatic cancer cells.Citation130 SYM004, a epidermal growth factor receptor targeting antibody, can inhibit DNA double strand breaks repair and induces apoptosis via downregulating MAPK signaling, and thereby improves radiosensitivity in tumor cells.Citation120 Cetuximab and nimotuzumab, binding the epidermal growth factor receptor (EGFR), can increase radiation-induced apoptosis and DNA damage, and thereby improve the radiosensitivity of human epidermal-like A431 cells.Citation131 The hepatocyte growth factor (HGF)/Met signaling pathway which mediates DNA double-strand break repair is upregulated in the majority of cancers. AMG102, a monoclonal antibody against HGF, can inhibit DNA damage repair and increase radiosensitivity of glioblastoma multiforme.Citation132 In addition, proteins and peptides in serum, such as C-reactive peptide,Citation133 HSPCitation134 and paraoxonase-2Citation135 contribute to radioresistance and can be used as radiotherapy targets. ECI301, a mutant derivative of macrophage inhibitory protein-1a, can be assisted by HSP-70 and HMGB1, thereby enhancing the effect of radiotherapy.Citation134 Other proteins, like DNAzyme (DZ1)Citation136 and NKTR-214,Citation137 can also improve the effect of radiotherapy.
miRNAs
MicroRNAs (miRNAs), which encode by endogenous genes are noncoding single-stranded RNA molecules containing about 22 nucleotides. Studies have shown that some specific miRNAs can be used to improve radiotherapy efficacyCitation138,Citation139 and some miRNAs can be used as radiotherapy sensitization targets.Citation140 For example, miR-621 targets SETDB1 in hepatocellular carcinoma can be used as a tumor radiosensitizer directly.Citation141 miR-205 targets zinc finger E-box binding homeobox 1 (ZEB1) and the ubiquitin-conjugating enzyme Ubc13 to enhance the radiosensitivity of breast cancer cells.Citation142 miR-144-5p targets ATF2 to enhance radiosensitivity of NSCLC.Citation143 miR-146a-5p enhances radiosensitivity in hepatocellular carcinoma through activation of DNA repair pathway.Citation144 miR-150 modulates AKT pathway in NK/T cell lymphoma to enhance radiosensitivity.Citation145 miR-99a targets mTOR pathway to enhance the radiosensitivity of NSCLC.Citation146 miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense.Citation147 Transcriptional activation of miR-320a induces cancer cell apoptosis under ionizing radiation conditions.Citation148 However, inhibition of miR-21-5p promotes the radiation sensitivity of NSCLC.Citation149 Inhibition of miR-630 enhances radiotherapy resistance in human glioma by directly targeting CDC14A.Citation150 Furthermore, a clinical study included 55 atypical meningioma patients found in seven upregulated miRNAs (miR-4286, miR-4695-5p, miR-6732-5p, miR-6855-5p, miR-7977, miR-6765-3p, miR-6787-5p) and seven downregulated miRNAs (miR-1275, miR-30c-1-3p, miR-4449, miR-4539, miR-4684-3p, miR-6129, miR-6891-5p) in patients. Those miRNAs may induce radioresistant and radiosensitive, respectively.
siRNAs
siRNA, known as short interfering RNA or silencing RNA, is a class of double-stranded RNA, noncoding RNA molecules, typically 20–27 base pairs in length, similar to miRNA, and operating within the RNA interference (RNAi) pathway.Citation151 HuR is a protein related to radiotherapy resistance, knockdown of HuR by siRNA resulting DNA damage and enhanced radiosensitivity.Citation152 S100A4, a member of the S100 family of transcription factors, modulates various activities of malignant tumor cells through different mechanisms. A short siRNA against S100A4 enhances the radiosensitivity of human A549 cells.Citation153 NBS1 plays an important role in the radiation-induced DNA double-strand breaks reparation, siRNA targets NBS1 can increase radiation sensitivity of cancer cells.Citation154 Survivin, a member of the inhibitor of apoptosis (IAP) protein family, is overexpressed in most cancers resulting in aggressive behavior of tumor and therapy resistance. Downregulation of survivin by siRNA can enhance radiosensitivity in head and neck squamous cell carcinoma.Citation155 Therefore, numerous siRNAs can be used as radiosensitizers by silencing genes related to radioresistance.
Oligonucleotides
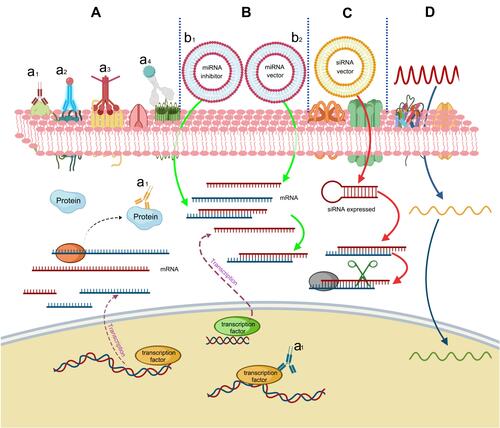
Similar to siRNAs, oligonucleotides also play important roles in gene expression regulation. Since they are easy to design and synthesize, antisense oligonucleotides have great potential to develop as radiosensitizers.Citation11 Telomerase expresses in many kinds of tumors (>85%), while the expression of telomerase is restricted in normal tissues. A study indicated that expression of telomerase could be inhibited by radiolabeled oligonucleotides, which targeted the RNA subunit of telomerase, thereby inducing DNA damage in telomerase-positive tumor cells.Citation156 In addition, the phosphorothioate-modified antisense oligonucleotides (PS-ASODN) against human telomerase reverse transcriptase were reported to promote radiotherapy effect in liver cancer.Citation157 Furthermore, Park et al reported that inhibition of cyclic AMP response element-directed transcription using decoy oligonucleotides enhanced tumor-specific radiosensitivity.Citation158 Yu et al demonstrated that antisense oligonucleotides targeted human telomerase RNA (hTR ASODN) could improve the radiosensitivity of nasopharyngeal carcinoma cells.Citation159 The radiosensitization mechanism of macromolecules was summarized in .
Figure 3 Radiosensitization mechanism of macromolecules. (A) Proteins and peptides. (a1) Direct interaction of key proteins. (a2) Loading of radioactive seeds. (a3) Radiosensitizers delivery. (a4) Conjugation with nanomaterials. (B) miRNAs can then bind with mRNAs to implement radiosensitization. (b1) Downregulation by inhibitors. (b2) Upregulation. (C) siRNAs can improve radiosensitivity by binding and degrading complementary mRNAs. (D) Oligonucleotides improve the radiosensitivity by complementary binding with DNAs.
Nanomaterials
Noble Metal nanomaterials
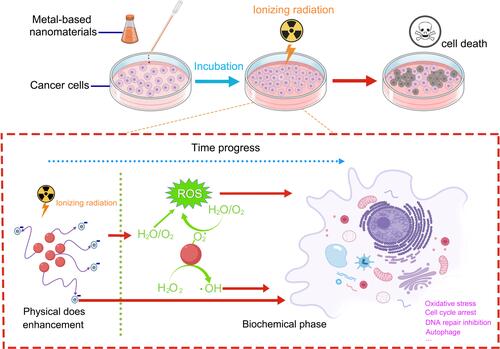
The X-ray absorption coefficient (μ) represents the relationship between the X-ray absorption phenomenon (E) and atomic number (Z), μ=ρZ4/(AE3), where ρ is the density and A is the atomic mass of the element.Citation160 Therefore, the change of atomic number (Z) causes a significant change of X-ray absorption coefficient (μ). Noble metal nanomaterials, such as gold (Au, Z=79), silver (Ag, Z=47) and platinum (Pt, Z=78) can effectively absorb X-ray energy and interact with radiation in tumor cells, and then emit photoelectrons, auger electrons, compton electrons and other secondary electrons. These secondary electrons not only interact with DNA directly, but also react with water to increase the production of ROS and further increase the sensitivity of tumor cells to radiation. This process is a physical sensitization mechanism.Citation161 Furthermore, functionalized noble metal nanomaterials promote the generation of ROS, transfer the cell cycle into a radiosensitive state, and inhibit p53 signaling pathway to induce cell autophagy and lysozyme body function disorder, thereby increasing radiotherapy sensitivity. This process is a biochemical sensitization mechanism.Citation162,Citation163
Gold nanoparticles with good chemical stability, easy preparation, controllable size and shape, easy surface functionalization, high biocompatibility, and low toxicity have proven satisfactory radiosensitizing effects in various tumors.Citation164–Citation167 Silver nanoparticles and platinum nanoparticles are also commonly used in biomedicine.Citation168,Citation169 Research found that silver nanoparticles combined with radiotherapy could enhance the radiosensitivity of human glioma cells in vitro and extended the survival time of glioma mice.Citation170,Citation171 Liu et al demonstrated that silver nanoparticles could induce apoptosis of cancer cells through G2/M phase arrest after radiation, and they suggested that silver nanoparticles could be used as a nanoradiosensitizer for hypoxic glioma radiotherapy.Citation172 Recently, Fathy reported that thymoquinone-capping silver nanoparticles represented a promising engineered nanoformulation for enhancing cancer radiosensitivity.Citation173 Li et al demonstrated that platinum nanoparticles could enhance radiosensitivity through increasing DNA damage, ROS stress, and cell cycle arrest.Citation163 They also proved that platinum nanoparticles could convert endogenic H2O2 to O2 in cancer cells, thus significantly improving radiosensitivity without apparent toxicity to animals in vivo.Citation163
Heavy Metal Nanomaterials
Similar to noble metal nanomaterials, gadolinium (Gd, Z=64), hafnium (Hf, Z=72), tantalum (Ta, Z=73), tungsten (W, Z=74), and bismuth (Bi, Z=83) are also metal elements with large atomic coefficients and have a great X-ray attenuation capability.Citation174–Citation176 Based on this, numerous studies have focused on these heavy metal nanomaterials to investigate their radiotherapy sensitization. However, they usually cause damage to healthy tissues with direct contact.Citation177 Therefore, their stable forms such as oxides, sulfides, and selenides are explored as the radiosensitizers.Citation178–Citation180
Gadolinium-based nanoparticles are usually known as magnetic resonance imaging (MRI) contrast agents. It should be noted that researchers discovered a family of gadolinium-based nanoparticles called AGuIX for combined MRI and radiosensitization.Citation181 Results showed that AGuIX could interact with X-rays and γ-rays at a certain concentration. After internalization through the enhanced permeability and retention (EPR) effect, AGuIX could be resident in the tumor for a long time before being cleared by the kidneys.Citation182 Preclinical animal experiments proved that AGuIX held obvious radiosensitization effects in several tumor models without obvious toxicity.Citation183 A Phase I clinical trial (NCT03308604) to evaluate the optimal dose of AGuIX combined with chemoradiation in patients with locally advanced cervical cancer; a Phase II clinical trial (NCT03818386) using AGuIX gadolinium-chelated polysiloxane based nanoparticles and whole brain radiotherapy in patients with multiple brain metastases; and a single-arm phase II trial (NCT04094077) aiming to evaluate the efficacy of AGuIX during fractionated stereotactic radiotherapy of brain metastasis are being evaluated.
Hafnium, in the same family as titanium and zirconium, is chemical inertness. The oxidation state of hafnium, hafnium dioxide (HfO₂), was usually used in radioactive protective coatings, biosensors, and X-ray contrast agents.Citation184,Citation185 Jayaraman et al demonstrated that HfO2 nanoparticles had excellent biocompatibility.Citation185 Researchers from France discovered that HfO₂ can be used as a radiosensitizer with low cytotoxicity.Citation186 A Phase I trial (NCT03589339) combining hafnium oxide nanoparticles (NBTXR3) with anti-PD-1 therapy in microsatellite instability-high solid malignant tumour and a Phase I–II clinical trial (NCT02805894) of NBTXR3 in prostate adenocarcinoma are under evaluation.
Tantalum is a nontoxic, biologically inert element with good biocompatibility.Citation187 Studies found that TaOx and Ta2O5 could be used as CT imaging contrast agents.Citation188–Citation190 Brown et al found Ta2O5 nanoparticles showed a radiasentizition effect on radioresistant glioma cells.Citation191 Song et al showed hollow shell tantalum oxide (HTaOx) had a large X-ray attenuation capability and could enhance radiation therapy effects by Compton scattering and Auger effect.Citation192 In addition, TaOx can be used as functional group carrier to load drugs, thereby improving tumor hypoxic environment. For example, HTaOx loaded with catalase, which reacted with H2O2 in the tumor microenvironment, then improved the oxygen content and overcame the radiotherapy tolerance of hypoxic tumor cells, thereby improving the radiotherapy effect.Citation193
Tungsten and bismuth also have significative applications in medicine.Citation194,Citation195 Hossain et al concluded that bismuth nanoparticles had stronger radiosensitizing effect than gold and platinum nanoparticles at the same physical and chemical conditions.Citation196 Yu et al found that the ultra-small semi-metallic Bi nanoparticles with LyP-1 peptide modified at 3.6 nm showed obvious radiosensitization effect.Citation197 Recently, a large number of studies shown that some nanomaterials of tungsten and bismuth had excellent photothermal absorption conversion performance and strong X-ray absorption capacity, therefore they can be used for tumor radiosensitization as well as synergistic therapy of hyperthermia and radiotherapy.Citation198–Citation201
In addition, research about several high Z metal elements combined together to further improve the radiosensitization effect were also explored. For example, SiBiGdNP chelated Bi and Gd in organosilane to improve the sensitivity of radiotherapy.Citation202 GdW10O36 contained both W and Gd to expect they had better radiotherapy sensitization effect.Citation203
Ferrite Nanomaterials
Ferrite-based nanomaterials can catalyze the generation of free radicals through Fenton’s reaction (1) and Haber–Weiss reaction (2) to enhance the effect of radiosensitization.Citation204
Fe2+ + H2O2 → Fe3+ + OH + OH−
Fe3+ + H2O2 → Fe2+ + OOH + H+ (1)
Fe3+ + O2 − → Fe2+ + O2
Fe2+ + H2O2 → Fe3+ + OH− + OH (2)
Studies proved that Fe3O4 had a dose-enhancing effect for radiotherapy, especially superparamagnetic Fe3O4 nanoparticles (SPIONS) possessing MRI imaging property had good application prospects in image-guided tumor radiotherapy.Citation205
The composition of the spinel structure ferrite is usually stated as MFe2O4, where M=Fe, Zn, Co, Mn, Ni.Citation206 Among them, ZnFe2O4, MnFe2O4, CoFe2O4 nanoparticles were widely investigated.Citation207 For example, Meidanchi et al confirmed that ZnFe2O4 nanoparticles interacted with γ-rays to produce photoelectric effect resulting in a higher release level of electron in radioresistant cells.Citation208 Studies also indicated that ZnFe2O4 nanoparticles could be used as radiosensitizers.Citation208,Citation209 Salunkhe et al demonstrated that MnFe2O4 and CoFe2O4 nanoparticles could improve the therapeutic efficacy of cancer through multimodal image-guided combination therapy.Citation210
Semiconductor Nanomaterials
Semiconductor quantum dots have unique properties, such as quantum dimension effect, surface effect, and quantum confinement effect, making them great candidates in biomedicine applications.Citation211 Until now, numerous studies focused on using semiconductor quantum dots as photosensitizers and radiosensitizers for tumor treatment have been reported.Citation212–Citation214 When the electronic energy levels are in the range of 1–5 eV, the semiconductor nanomaterials can absorb the photon energy and perform as photosensitizers, showing photocatalytic properties. When the electronic energy levels are at keV and MeV (X-rays and γ-rays), semiconductor nanomaterials can enhance absorption of high-energy photons acting as radiosensitizers and causing damage to cancer cells.Citation212 Nakayama et al synthesized a semiconductor nanomaterial PAA-TiOx to generate hydroxyl radicals under the irradiation of X-rays, which increased DNA damage and inhibited tumor growth significantly.Citation215 Morita et al clarified the radiosensitization mechanism of PAA-TiOx nanoparticles by releasing H2O2 to relieve hypoxia in tumor cells.Citation216 TiO2 nanotubes have been reported to enhance the radiosensitization effect through regulating G2/M cycle arrest and reducing DNA repair of tumor cells.Citation177 The mechanism of radiosensitization of metal-based nanomaterials is shown in .
Nonmetallic Nanomaterials
Many nonmetallic nanomaterials also possess the function of radiosensitization.Citation217 For example, C60, fullerene, has potent anticancer activities, however, the potential toxicity to normal tissues limits its further use. Therefore, nanocrystals of C60 (Nano-C60) with negligible toxicity to normal cells have been developed as a radiosensitizer.Citation218 In addition, nanodiamonds and carbon nanotubes can reduce radioresistance of tumor cells by promoting ROS generation, destroying DNA double-strands, and regulating the cell cycle.Citation219,Citation220 Selenium (Se) nanoparticles not only work as chemotherapeutic drugs, but also improve the antitumor effect of X-rays by activating ROS to induce DNA damage in cancer cells.Citation221
Nanostructured Chemicals and Drug Delivery Systems
Nano-based delivery systems are efficient approaches for drug targeted transportation, which can deliver radiosensitizers, such as chemicals, oxygen carriers, siRNAs and catalases to the tumor sites and have attracted wide interest of researchers recently.Citation222 More importantly, nanobased delivery systems can precisely deliver radioactive particles likeCitation223 Ac (releasing a-particles), 131I, and 125I to tumor sites.Citation223 With the development of nanotechnology, nanobased delivery systems have great potential for radiosensitizer delivery.
However, there is still a challenge to achieve clinical translation of nanobased delivery systems, factors like physicochemical properties of the nanoformulations, radiation sources, and indications block their clinical translation.Citation223 In addition, long circulation lifetime of nanodelivery systems may increase the risk of long-term toxicity.Citation224 Another critical factor is stability in body fluid of nanodelivery systems. Because the aggregation of nanoparticles in body fluid will influence the pharmacokinetics and the cellular response and generate serious side effects such as blocking the blood vessels.Citation222 Therefore, attention should be paid to these factors when designing the nanodelivery systems. Size is also an important factor, small size and high Z nanoparticles often hold better radiosensitizing effect than larger-size ones.Citation223 In particular, the small size nanoparticles with positive charge can bind to negative charged DNA and can be eliminated by renal clearance conveniently. In addition, functional modification of nanostructures using biocompatible materials can improve their stability and targeting.Citation225
Conclusions and Prospects
Radiosensitizers have been developed for decades from the earliest “free radical damage and fixation” strategies to gene regulation, from chemicals to biological macromolecules and nanomaterials. Although each radiosensitizer has dialectical advantages and limitations, the mechanisms of sensitization are similar. The main mechanisms include: (I) inhibiting radiation-induced repair of DNA damage, increasing the degree of DNA damage; (II) disturbing the cell cycle and organelle function to improve cytotoxicity; and (III) inhibiting the expression of radiation resistance genes or promoting the expression of radiation sensitive genes.
Although small molecules, macromolecules, and nanomaterial radiosensitizers are being developed, and some nanoradiosensitizers have been used for clinical research (), the result still cannot meet clinical translation needs. Therefore, there is an urgent need to find new targets of radiotherapy and new mechanisms of sensitization, and after that to develop more effective radiosensitizing drugs. First of all, multitarget radiosensitizers often have more obvious efficacy than single target, researchers can focus on screening multitarget radiosensitizers or drug combinations. New approaches, in particular, nanotechnology based as radiosensitizers have shown promise. Nanomaterials with low cytotoxicity, good biocompatibility, and ease of functionalization need to be explored. In addition, other technologies, such as molecular structure analysis, molecular cloning technology, and bioinformatics analysis can accelerate the development of new radiosensitizers. Moreover, development of new drug delivery systems can also improve radiosensitization efficacy. Finally, the application of artificial intelligence and machine learning in new drug discovery and clinical trials, may guide development of new radiosensitizers and optimization of existing radiosensitizers.
Table 4 Clinical Translation of Some Nanoradiosensitizers
Acknowledgment
This work was supported by Innovation Capacity Support Plan of Shaanxi Province (2018TD-002), the National Natural Science Foundation of China (No. 82000523), the Natural Science Foundation of Shaanxi province (Grant No. 2020JQ-087, 2020JQ-095), the “Young Talent Support Plan” of Xi’an Jiaotong University (YX6J001), the Fundamental Research Funds for the Central Universities (xzy012019070).
Disclosure
The authors report no conflicts of interest in this work.
References
- Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6(3–4):79–100. doi:10.1142/S2339547818300020
- Kułakowski A. The contribution of Marie Skłodowska-Curie to the development of modern oncology. Anal Bioanal Chem. 2011;400(6):1583–1586. doi:10.1007/s00216-011-4712-1
- Martin OA, Martin RF. Cancer radiotherapy: understanding the price of tumor eradication. Front Cell Dev Biol. 2020;8:261. doi:10.3389/fcell.2020.00261
- Franzone P, Fiorentino A, Barra S, et al. Image-guided radiation therapy (IGRT): practical recommendations of Italian Association of Radiation Oncology (AIRO). Radiol Med. 2016;121(12):958–965. doi:10.1007/s11547-016-0674-x
- Ge Y, Wu QJ. Knowledge-based planning for intensity-modulated radiation therapy: a review of data-driven approaches. Med Phys. 2019;46(6):2760–2775. doi:10.1002/mp.13526
- Farhood B, Goradel NH, Mortezaee K, et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin Transl Oncol. 2019;21(3):268–279. doi:10.1007/s12094-018-1934-0
- Fowler JF, Adams GE, Denekamp J. Radiosensitizers of hypoxic cells in solid tumors. Cancer Treat Rev. 1976;3(4):227–256. doi:10.1016/s0305-7372(76)80012-6
- Adams GE. Chemical radiosensitization of hypoxic cells. Br Med Bull. 1973;29(1):48–53. doi:10.1093/oxfordjournals.bmb.a070956
- Wen P, Xia J, Cao X, et al. dbCRSR: a manually curated database for regulation of cancer radiosensitivity. Database (Oxford). 2018;2018. doi:10.1093/database/bay049
- Garibaldi C, Jereczek-Fossa BA, Marvaso G, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. doi:10.3332/ecancer.2017.785
- Wang H, Mu X, He H, Zhang XD. Cancer radiosensitizers. Trends Pharmacol Sci. 2018;39(1):24–48. doi:10.1016/j.tips.2017.11.003
- Hirayama R. Mechanism of oxygen effect for photon andheavy-ion beams. Japanese Journal of Medical Physics. 2014;34(2):65–69.
- Oronsky BT, Knox SJ, Scicinski J. Six degrees of separation: the oxygen effect in the development of radiosensitizers. Transl Oncol. 2011;4(4):189–198. doi:10.1593/tlo.11166
- Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7(6):492–508. doi:10.1634/theoncologist.7-6-492
- Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309(6):C350–C360. doi:10.1152/ajpcell.00191.2015
- Richardson RB, Harper ME. Mitochondrial stress controls the radiosensitivity of the oxygen effect: implications for radiotherapy. Oncotarget. 2016;7(16):21469–21483. doi:10.18632/oncotarget.7412
- Bel Aiba RS, Dimova EY, Görlach A, Kietzmann T. The role of hypoxia inducible factor-1 in cell metabolism–a possible target in cancer therapy. Expert Opin Ther Targets. 2006;10(4):583–599. doi:10.1517/14728222.10.4.583
- Zhu H, Zhang S. Hypoxia inducible factor-1α/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J Cell Biochem. 2018;119(9):7707–7718. doi:10.1002/jcb.27120
- Verdegem D, Moens S, Stapor P, Carmeliet P. Endothelial cell metabolism: parallels and divergences with cancer cell metabolism. Cancer Metabol. 2014;2(1):19. doi:10.1186/2049-3002-2-19
- Boyle RG, Travers S. Hypoxia: targeting the tumour. Anticancer Agents Med Chem. 2006;6(4):281–286. doi:10.2174/187152006777698169
- Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16(4–5):523–530. doi:10.1016/j.semcdb.2005.03.001
- Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol. 2019;7:4. doi:10.3389/fcell.2019.00004
- Pettersen EO, Ebbesen P, Gieling RG, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem. 2015;30(5):689–721. doi:10.3109/14756366.2014.966704
- Cabrales P, Intaglietta M. Blood substitutes: evolution from noncarrying to oxygen- and gas-carrying fluids. ASAIO J. 2013;59(4):337–354. doi:10.1097/MAT.0b013e318291fbaa
- Hardavella G, Karampinis I, Frille A, Sreter K, Rousalova I. Oxygen devices and delivery systems. Breathe (Sheffield, England). 2019;15(3):e108–e116. doi:10.1183/20734735.0204-2019
- Choudhury R. Hypoxia and hyperbaric oxygen therapy: a review. Int J Gen Med. 2018;11:431–442. doi:10.2147/ijgm.S172460
- Stępień K, Ostrowski RP, Matyja E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med Oncol (Northwood, London, England). 2016;33(9):101. doi:10.1007/s12032-016-0814-0
- Ogawa Y, Kubota K, Ue H, et al. Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: a new enzyme-targeting radiosensitization treatment, Kochi oxydol-radiation therapy for unresectable carcinomas, type II (KORTUC II). Int J Oncol. 2009;34:609–618. doi:10.3892/ijo_00000186
- Chapman JD, Webb RG, Borsa J. Radiosensitization of mammalian cells by p-nitroacetophenone. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(6):561–573. doi:10.1080/09553007114550741
- Adams GE, Asquith JC, Dewey DL, Foster JL, Michael BD, Willson RL. Electron affinic sensitization. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(6):575–585. doi:10.1080/09553007114550751
- Higgins GS, O’Cathail SM, Muschel RJ, McKenna WG. Drug radiotherapy combinations: review of previous failures and reasons for future optimism. Cancer Treat Rev. 2015;41(2):105–113. doi:10.1016/j.ctrv.2014.12.012
- Spisz P, Zdrowowicz M, Makurat S, et al. Why does the type of halogen atom matter for the radiosensitizing properties of 5-halogen substituted 4-thio-2’-deoxyuridines? Molecules. 2019;24(15):2819. doi:10.3390/molecules24152819
- Brown JM. Selective radiosensitization of the hypoxic cells of mouse tumors with the nitroimidazoles metronidazole and Ro 7-0582. Radiat Res. 1975;64(3):633–647. doi:10.2307/3574253
- Dische S, Saunders MI, Lee ME, Adams GE, Flockhart IR. Clinical testing of the radiosensitizer Ro 07-0582: experience with multiple doses. Br J Cancer. 1977;35(5):567–579. doi:10.1038/bjc.1977.90
- Brown JM. Clinical trials of radiosensitizers: what should we expect? Int J Radiat Oncol Biol Phys. 1984;10(3):425–429. doi:10.1016/0360-3016(84)90063-4
- Dische S, Saunders MI, Flockhart IR, Lee ME, Anderson P. Misonidazole-a drug for trial in radiotherapy and oncology. Int J Radiat Oncol Biol Phys. 1979;5(6):851–860. doi:10.1016/0360-3016(79)90070-1
- Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi:10.1038/nrc3958
- Overgaard J, Hansen HS, Andersen AP, et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys. 1989;16(4):1065–1068. doi:10.1016/0360-3016(89)90917-6
- Urtasun RC, Chapman JD, Feldstein ML, et al. Peripheral neuropathy related to misonidazole: incidence and pathology. Br J Cancer Suppl. 1978;3:271–275.
- Wardman P. Nitroimidazoles as hypoxic cell radiosensitizers and hypoxia probes: misonidazole, myths and mistakes. Br J Radiol. 2019;92(1093):20170915. doi:10.1259/bjr.20170915
- Bonnet M, Hong CR, Wong WW, et al. Next-generation hypoxic cell radiosensitizers: nitroimidazole alkylsulfonamides. J Med Chem. 2018;61(3):1241–1254. doi:10.1021/acs.jmedchem.7b01678
- Rosenberg A, Knox S. Radiation sensitization with redox modulators: a promising approach. Int J Radiat Oncol Biol Phys. 2006;64(2):343–354. doi:10.1016/j.ijrobp.2005.10.013
- Coleman CN, Wasserman TH, Urtasun RC, et al. Final report of the phase I trial of the hypoxic cell radiosensitizer SR 2508 (etanidazole) radiation therapy oncology group 83-03. Int J Radiat Oncol Biol Phys. 1990;18(2):389–393. doi:10.1016/0360-3016(90)90105-s
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi:10.1038/nrc1367
- Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Radiother Oncol. 1998;46(2):135–146. doi:10.1016/s0167-8140(97)00220-x
- Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6(10):757–764. doi:10.1016/s1470-2045(05)70292-8
- Metwally MA, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC). Acta Oncologica (Stockholm, Sweden). 2014;53(5):654–661. doi:10.3109/0284186x.2013.864050
- Bentzen J, Toustrup K, Eriksen JG, Primdahl H, Andersen LJ, Overgaard J. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncologica (Stockholm, Sweden). 2015;54(7):1001–1007. doi:10.3109/0284186x.2014.992547
- Saksø M, Andersen E, Bentzen J, et al. A prospective, multicenter DAHANCA study of hyperfractionated, accelerated radiotherapy for head and neck squamous cell carcinoma. Acta Oncologica (Stockholm, Sweden). 2019;58(10):1495–1501. doi:10.1080/0284186x.2019.1658897
- Thomson D, Yang H, Baines H, et al. NIMRAD - a phase III trial to investigate the use of nimorazole hypoxia modification with intensity-modulated radiotherapy in head and neck cancer. Clin Oncol (Royal College of Radiologists (Great Britain)). 2014;26(6):344–347. doi:10.1016/j.clon.2014.03.003
- Toustrup K, Sørensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102(1):122–129. doi:10.1016/j.radonc.2011.09.010
- Saksø M, Jensen K, Andersen M, Hansen CR, Eriksen JG, Overgaard J. DAHANCA 28: a phase I/II feasibility study of hyperfractionated, accelerated radiotherapy with concomitant cisplatin and nimorazole (HART-CN) for patients with locally advanced, HPV/p16-negative squamous cell carcinoma of the oropharynx, hypopharynx, larynx and oral cavity. Radiother Oncol. 2020;148:65–72. doi:10.1016/j.radonc.2020.03.025
- Oronsky B, Scicinski J, Ning S, et al. RRx-001, a novel dinitroazetidine radiosensitizer. Invest New Drugs. 2016;34(3):371–377. doi:10.1007/s10637-016-0326-y
- Oronsky BT, Knox SJ, Scicinski JJ. Is Nitric oxide (NO) the last word in radiosensitization? A review. Transl Oncol. 2012;5(2):66–71. doi:10.1593/tlo.11307
- Edfeldt NB, Harwood EA, Sigurdsson ST, Hopkins PB, Reid BR. Solution structure of a nitrous acid induced DNA interstrand cross-link. Nucleic Acids Res. 2004;32(9):2785–2794. doi:10.1093/nar/gkh606
- Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9(3):157–173. doi:10.1016/j.drup.2006.05.003
- Nelson EJ, Connolly J, McArthur P. Nitric oxide and s-nitrosylation: excitotoxic and cell signaling mechanism. Biol Cell. 2003;95(1):3–8. doi:10.1016/s0248-4900(03)00004-2
- Meffert MK, Premack BA, Schulman H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron. 1994;12(6):1235–1244. doi:10.1016/0896-6273(94)90440-5
- Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122(3):216–238. doi:10.1016/j.pharmthera.2009.02.009
- Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14(9):623–641. doi:10.1038/nrd4623
- Rogers N, Seeger F, Garcin E, Roberts D, Isenberg J. Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow. Review. 2014;5(134). doi:10.3389/fphys.2014.00134
- Kondakova IV, Tcheredova VV, Zagrebelnaya GV, Cherdyntseva NV, Kagiya TV, Choinzonov EL. Production of nitric oxide by hypoxic radiosensitizer sanazole. Exp Oncol. 2004;26(4):329–333.
- Girard M, Clairmont F, Maneckjee A, Mousseau N, Dawson B, Whitehouse L. 5-Nitroimidazoles. II: unexpected reactivity of ronidazole and dimetridazole with thiols. Can J Chem. 2011;71:1349–1352. doi:10.1139/v93-174
- Ng QS, Goh V, Milner J, et al. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 2007;8(2):111–118. doi:10.1016/s1470-2045(07)70001-3
- Siemens DR, Heaton JP, Adams MA, Kawakami J, Graham CH. Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology. 2009;74(4):878–883. doi:10.1016/j.urology.2009.03.004
- Libert N, Tourtier JP, Védrine L, Chargari C, Riou B. Inhibitors of angiogenesis: new hopes for oncologists, new challenges for anesthesiologists. Anesthesiology. 2010;113(3):704–712. doi:10.1097/ALN.0b013e3181ed098d
- Pandey AK, Singhi EK, Arroyo JP, et al. Mechanisms of VEGF (Vascular Endothelial Growth Factor) inhibitor-associated hypertension and vascular disease. Hypertension (Dallas, Tex: 1979). 2018;71:e1–e8. doi:10.1161/hypertensionaha.117.10271
- Liebmann J, DeLuca AM, Coffin D, et al. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994;54(13):3365–3368.
- Schwarz K, Dobiasch S, Nguyen L, Schilling D, Combs SE. Modification of radiosensitivity by curcumin in human pancreatic cancer cell lines. Sci Rep. 2020;10(1):3815. doi:10.1038/s41598-020-60765-1
- Verma V. Relationship and interactions of curcumin with radiation therapy. World J Clin Oncol. 2016;7(3):275–283. doi:10.5306/wjco.v7.i3.275
- Li G, Wang Z, Chong T, Yang J, Li H, Chen H. Curcumin enhances the radiosensitivity of renal cancer cells by suppressing NF-κB signaling pathway. Biomed Pharmacother. 2017;94:974–981. doi:10.1016/j.biopha.2017.07.148
- da Costa Araldi IC, Bordin FPR, Cadoná FC, et al. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem Biol Interact. 2018;282:85–92. doi:10.1016/j.cbi.2018.01.013
- Tan Y, Wei X, Zhang W, et al. Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol Rep. 2017;37(3):1833–1841. doi:10.3892/or.2017.5413
- Mikami S, Ota I, Masui T, et al. Resveratrol‑induced REG III expression enhances chemo‑ and radiosensitivity in head and neck cancer in xenograft mice. Oncol Rep. 2019;42(1):436–442. doi:10.3892/or.2019.7137
- Kim SJ, Kim MS, Lee JW, et al. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. J Cancer Res Clin Oncol. 2006;132(2):129–135. doi:10.1007/s00432-005-0052-x
- Li Y, Sui H, Jiang C, et al. Dihydroartemisinin increases the sensitivity of photodynamic therapy via NF-κB/HIF-1α/VEGF pathway in esophageal cancer cell in vitro and in vivo. Cell Physiol Biochem. 2018;48:2035–2045. doi:10.1159/000492541
- Zhang L, Cheng LQ, Zhou Z, Lv LL, Liu GX. Effects of dihydroartemisinin on radiosensitivity of Raji cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2017;33:385–390. doi:10.12047/j.cjap.5565.2017.093
- Momtazi-Borojeni AA, Mosafer J, Nikfar B, et al. Curcumin in advancing treatment for gynecological cancers with developed drug- and radiotherapy-associated resistance. Rev Physiol Biochem Pharmacol. 2019;176:107–129. doi:10.1007/112_2018_11
- Chen Y, Zhu Z, Zhao W, et al. A randomized phase 3 trial comparing paclitaxel plus 5-fluorouracil versus cisplatin plus 5-fluorouracil in Chemoradiotherapy for locally advanced esophageal carcinoma-the ESO-shanghai 1 trial protocol. Radiat Oncol (London, England). 2018;13(1):33. doi:10.1186/s13014-018-0979-0
- Ren W, Sha H, Yan J, et al. Enhancement of radiotherapeutic efficacy for esophageal cancer by paclitaxel-loaded red blood cell membrane nanoparticles modified by the recombinant protein anti-EGFR-iRGD. J Biomater Appl. 2018;33(5):707–724. doi:10.1177/0885328218809019
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi:10.1016/j.ccr.2004.09.003
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23(8):1599–1607. doi:10.1038/sj.onc.1207284
- Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. Curcumin-altered p53-response genes regulate radiosensitivity in p53-mutant ewing’s sarcoma cells. Anticancer Res. 2010;30(10):4007–4015.
- Liao HF, Kuo CD, Yang YC, et al. Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. J Radiat Res. 2005;46(4):387–393. doi:10.1269/jrr.46.387
- Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through Their molecular mode of action. Int J Mol Sci. 2017;18:656. doi:10.3390/ijms18030656
- Tak JK, Lee JH, Park JW. Resveratrol and piperine enhance radiosensitivity of tumor cells. BMB Rep. 2012;45(4):242–246. doi:10.5483/bmbrep.2012.45.4.242
- Quan F, Zhao Q, Shao Y, Li H, Zhao R. Resveratrol enhances radiosensitivityof human hypopharyngeal carcinoma cell line in nudemice. Journal of Southern Medical University. 2014;34(11):1646–1649.Chinese.
- Shao Y, Quan F, Li HH, Yao XB, Zhao Q, Zhao RM. The radiosensitizing effect of resveratrol on hopypharyngeal carcinoma cell line FADU and its effect on the cell cycle. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:699–703.
- Fang Y, DeMarco VG, Nicholl MB. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci. 2012;103(6):1090–1098. doi:10.1111/j.1349-7006.2012.02272.x
- Luo J, Chen X, Chen G, et al. Dihydroartemisinin induces radiosensitivity in cervical cancer cells by modulating cell cycle progression. Saudi Med J. 2013;34(3):254–260.
- Zuo ZJ, Wang ST, Jiang LX, et al. Effect of dihydroartemisinin combined irradiation on the apoptosis of human lung cancer GLC-82 cells and its mechanism study. Chin J Integr Med. 2014;34(10):1220–1224.
- Zhang ZS, Wang J, Shen YB, et al. Dihydroartemisinin increases temozolomide efficacy in glioma cells by inducing autophagy. Oncol Lett. 2015;10(1):379–383. doi:10.3892/ol.2015.3183
- Barbuti A, Chen Z-S. Paclitaxel through the ages of anticancer therapy: exploring its role in chemoresistance and radiation therapy. Cancers. 2015;7:2360–2371. doi:10.3390/cancers7040897
- von Eiff D, Bozorgmehr F, Chung I, et al. Paclitaxel for treatment of advanced small cell lung cancer (SCLC): a retrospective study of 185 patients. J Thorac Dis. 2020;12(3):782–793. doi:10.21037/jtd.2019.12.74
- Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014;13(2):275–284. doi:10.1158/1535-7163.Mct-13-0791
- Xia Y, Li YH, Chen Y, et al. A phase II trial of concurrent chemoradiotherapy with weekly paclitaxel and carboplatin in advanced oesophageal carcinoma. Int J Clin Oncol. 2018;23(3):458–465. doi:10.1007/s10147-018-1240-4
- Cowen RL, Williams KJ, Chinje EC, et al. Hypoxia targeted gene therapy to increase the efficacy of tirapazamine as an adjuvant to radiotherapy: reversing tumor radioresistance and effecting cure. Cancer Res. 2004;64(4):1396–1402. doi:10.1158/0008-5472.can-03-2698
- Marcu L, Olver I. Tirapazamine: from bench to clinical trials. Curr Clin Pharmacol. 2006;1(1):71–79. doi:10.2174/157488406775268192
- Delahoussaye YM, Hay MP, Pruijn FB, Denny WA, Brown JM. Improved potency of the hypoxic cytotoxin tirapazamine by DNA-targeting. Biochem Pharmacol. 2003;65(11):1807–1815. doi:10.1016/s0006-2952(03)00199-0
- Le QT, McCoy J, Williamson S, et al. Phase I study of tirapazamine plus cisplatin/etoposide and concurrent thoracic radiotherapy in limited-stage small cell lung cancer (S0004): a Southwest Oncology Group study. Clin Cancer Res. 2004;10(16):5418–5424. doi:10.1158/1078-0432.Ccr-04-0436
- Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol. 2005;23:79–87. doi:10.1200/jco.2005.01.072
- Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28(18):2989–2995. doi:10.1200/jco.2009.27.4449
- Wang J, Guise C, Dachs G, et al. Identification of one-electron reductases that activate both the hypoxia prodrug SN30000 and diagnostic probe EF5. Biochem Pharmacol. 2014;91(4):436–446. doi:10.1016/j.bcp.2014.08.003
- Mistry IN, Thomas M, Calder EDD, Conway SJ, Hammond EM. Clinical advances of hypoxia-activated prodrugs in combination with radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98(5):1183–1196. doi:10.1016/j.ijrobp.2017.03.024
- Patterson LH, McKeown SR, Ruparelia K, et al. Enhancement of chemotherapy and radiotherapy of murine tumours by AQ4N, a bioreductively activated anti-tumour agent. Br J Cancer. 2000;82(12):1984–1990. doi:10.1054/bjoc.2000.1163
- Steward WP, Middleton M, Benghiat A, et al. The use of pharmacokinetic and pharmacodynamic end points to determine the dose of AQ4N, a novel hypoxic cell cytotoxin, given with fractionated radiotherapy in a phase I study. Ann Oncol. 2007;18(6):1098–1103. doi:10.1093/annonc/mdm120
- Albertella MR, Loadman PM, Jones PH, et al. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res. 2008;14(4):1096–1104. doi:10.1158/1078-0432.Ccr-07-4020
- Hong CR, Dickson BD, Jaiswal JK, et al. Cellular pharmacology of evofosfamide (TH-302): a critical re-evaluation of its bystander effects. Biochem Pharmacol. 2018;156:265–280. doi:10.1016/j.bcp.2018.08.027
- Takakusagi Y, Kishimoto S, Naz S, et al. Radiotherapy synergizes with the hypoxia-activated prodrug evofosfamide: in vitro and in vivo studies. Antioxid Redox Signal. 2018;28:131–140. doi:10.1089/ars.2017.7106
- Peeters SG, Zegers CM, Biemans R, et al. TH-302 in combination with radiotherapy enhances the therapeutic outcome and is associated with pretreatment [18F]HX4 hypoxia PET imaging. Clin Cancer Res. 2015;21:2984–2992. doi:10.1158/1078-0432.Ccr-15-0018
- Lohse I, Rasowski J, Cao P, et al. Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302. Oncotarget. 2016;7(23):33571–33580. doi:10.18632/oncotarget.9654
- Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35:S224–s243. doi:10.1016/j.semcancer.2015.01.001
- Larue RT, Van De Voorde L, Berbée M, et al. A Phase 1 ‘window-of-opportunity’ trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC Cancer. 2016;16:644. doi:10.1186/s12885-016-2709-z
- Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Distinct roles of cytochrome P450 reductase in mitomycin C redox cycling and cytotoxicity. Mol Cancer Ther. 2010;9(6):1852–1863. doi:10.1158/1535-7163.Mct-09-1098
- Rockwell S, Keyes SR, Sartorelli AC. Preclinical studies of porfiromycin as an adjunct to radiotherapy. Radiat Res. 1988;116(1):100–113. doi:10.2307/3577481
- Haffty BG, Wilson LD, Son YH, et al. Concurrent chemo-radiotherapy with mitomycin C compared with porfiromycin in squamous cell cancer of the head and neck: final results of a randomized clinical trial. Int J Radiat Oncol Biol Phys. 2005;61(1):119–128. doi:10.1016/j.ijrobp.2004.07.730
- Burd R, Lavorgna SN, Lenaz L, et al. Radiosensitization of hypoxic tumors by the bioreductive agent apaziquone (EO9, EOquin (TM)). Clin Cancer Res. 2005;9008S–9008S.
- Wang Z, Ding Y, Geng G, Zhu N. Analysis of energy efficiency retrofit schemes for heating, ventilating and air-conditioning systems in existing office buildings based on the modified bin method. Energy Convers Manage. 2014;77:233–242. doi:10.1016/j.enconman.2013.09.037
- Suwala AK, Kahlert UD, Maciaczyk J. Abstract 2515: pharmacological WNT-inhibition acts synergistically with chemo- and radiotherapy by overcoming treatment-resistance in glioma stem cells. Cancer Res. 2016;76(14Supplement):2515. doi:10.1158/1538-7445
- Saker J, Huang S, Park L, Pedersen M, Kragh M, Harari P. Abstract 1027: EGFR targeting antibody SYM004 causes radiosensitization in tumor cells expressing wild-type K-Ras via modulation of MAPK signaling. Cancer Res. 2013;73(8Supplement):1027. doi:10.1158/1538-7445
- Prabakaran PJ, Javaid AM, Swick AD, et al. Radiosensitization of adenoid cystic carcinoma with MDM2 inhibition. Clin Cancer Res. 2017;23(20):6044–6053. doi:10.1158/1078-0432.Ccr-17-0969
- Zhuang HM, Miao XL, Zhao ZQ, Zhang L. Application of nano calcium phosphate biomaterials and bone tissue engineering on in exercise-induced injury. Adv Mater Res. 2014;951:109–112. doi:10.4028/www.scientific.net/AMR.951.109
- Liu WL, Gao M, Tzen KY, et al. Targeting phosphatidylinositide3-Kinase/Akt pathway by BKM120 for radiosensitization in hepatocellular carcinoma. Oncotarget. 2014;5(11):3662–3672. doi:10.18632/oncotarget.1978
- Chen Y-H, Wei M-F, Wang C-W, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor is an effective radiosensitizer for colorectal cancer. Cancer Lett. 2015;357(2):582–590. doi:10.1016/j.canlet.2014.12.015
- Jayakumar S, Patwardhan RS, Pal D, Sharma D, Sandur SK. Dimethoxycurcumin, a metabolically stable analogue of curcumin enhances the radiosensitivity of cancer cells: possible involvement of ROS and thioredoxin reductase. Biochem Biophys Res Commun. 2016;478(1):446–454. doi:10.1016/j.bbrc.2016.06.144
- Kinsella TJ, Dobson PP, Mitchell JB, Fornace AJ. Enhancement of X ray induced DNA damage by pre-treatment with halogenated pyrimidine analogs. Int J Radiat Oncol. 1987;13(5):733–739. doi:10.1016/0360-3016(87)90292-6
- Wang S, Zhao P, Zhang C, Bu Y. Mechanisms responsible for high energy radiation induced damage to single-stranded DNA modified by radiosensitizing 5-halogenated deoxyuridines. J Phys Chem B. 2016;120:2649–2657. doi:10.1021/acs.jpcb.5b11432
- Benej M, Hong X, Vibhute S, et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc Natl Acad Sci. 2018;115(42):10756–10761. doi:10.1073/pnas.1808945115
- Lhuillier C, Rudqvist N-P, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019;11(1):40. doi:10.1186/s13073-019-0653-7
- Bourillon L, Bourgier C, Gaborit N, et al. An auristatin-based antibody-drug conjugate targeting HER3 enhances the radiation response in pancreatic cancer. Int J Cancer. 2019;145(7):1838–1851. doi:10.1002/ijc.32273
- González JE, Barquinero JF, Lee M, García O, Casaco A. Radiosensitization induced by the anti-epidermal growth factor receptor monoclonal antibodies cetuximab and nimotuzumab in A431 cells. Cancer Biol Ther. 2012;13(2):71–76. doi:10.4161/cbt.13.2.18439
- Buchanan IM, Scott T, Tandle AT, et al. Radiosensitization of glioma cells by modulation of Met signalling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2011;15(9):1999–2006. doi:10.1111/j.1582-4934.2010.01122.x
- Nieder C, Mannsåker B, Dalhaug A, Pawinski A, Haukland E. Palliative radiotherapy in cancer patients with increased serum c-reactive protein level. Article. In Vivo (Brooklyn). 2016;30(5):581–586.
- Kanegasaki S, Matsushima K, Shiraishi K, Nakagawa K, Tsuchiya T. Macrophage inflammatory protein derivative ECI301 enhances the alarmin-associated abscopal benefits of tumor radiotherapy. Cancer Res. 2014;74:5070–5078. doi:10.1158/0008-5472
- Krüger M, Amort J, Wilgenbus P, et al. The anti-apoptotic PON2 protein is Wnt-catenin-regulated and correlates with radiotherapy resistance in OSCC patients. Oncotarget. 2016;7(32).
- Cao Y, Yang L, Jiang W, et al. Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther. 2014;22(2):371–377. doi:10.1038/mt.2013.257
- Walker JM, Rolig AS, Charych DH, et al. NKTR-214 immunotherapy synergizes with radiotherapy to stimulate systemic CD8+ T cell responses capable of curing multi-focal cancer. Immunother Cancer. 2020;8(1). doi:10.1136/jitc-2019-000464
- Gandellini P, Rancati T, Valdagni R, Zaffaroni N. miRNAs in tumor radiation response: bystanders or participants? Trends Mol Med. 2014;20(9):529–539. doi:10.1016/j.molmed.2014.07.004
- de Jong MC, Ten Hoeve JJ, Grénman R, et al. Pretreatment microRNA expression impacting on epithelial-to-mesenchymal transition predicts intrinsic radiosensitivity in head and neck cancer cell lines and patients. Clin Cancer Res. 2015;21:5630–5638. doi:10.1158/1078-0432
- Huang A, Ono Y. Estimation of wrist flexion angle from muscle thickness changes measured by a flexible ultrasonic sensor. IEEE. 2016;188–191.
- Shao Y, Song X, Jiang W, et al. MicroRNA-621 acts as a tumor radiosensitizer by directly targeting SETDB1 in hepatocellular carcinoma. Mol Ther. 2019;27(2):355–364. doi:10.1016/j.ymthe.2018.11.005
- Zhang P, Wang L, Rodriguez-Aguayo C, et al. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat Commun. 2014;5:5671. doi:10.1038/ncomms6671
- Song L, Peng L, Hua S, et al. miR-144-5p enhances the radiosensitivity of non-small-cell lung cancer cells via targeting ATF2. Biomed Res Int. 2018;2018:5109497. doi:10.1155/2018/5109497
- Luo J, Si ZZ, Li T, et al. MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3-induced activation of the DNA repair pathway. Am J Physiol Cell Physiol. 2019;316(3):C299–c311. doi:10.1152/ajpcell.00189.2018
- Wu SJ, Chen J, Wu B, Wang YJ, Guo KY. MicroRNA-150 enhances radiosensitivity by inhibiting the AKT pathway in NK/T cell lymphoma. J Exp Clin Cancer Res. 2018;37(1):18. doi:10.1186/s13046-017-0639-5
- Yin H, Ma J, Chen L, et al. MiR-99a enhances the radiation sensitivity of non-small cell lung cancer by targeting mTOR. Cell Physiol Biochem. 2018;46(2):471–481. doi:10.1159/000488615
- Pajic M, Froio D, Daly S, et al. miR-139-5p Modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense. Cancer Res. 2018;78(2):501–515. doi:10.1158/0008-5472.Can-16-3105
- Hu Z, Tie Y, Lv G, Zhu J, Fu H, Zheng X. Transcriptional activation of miR-320a by ATF2, ELK1 and YY1 induces cancer cell apoptosis under ionizing radiation conditions. Int J Oncol. 2018;53(4):1691–1702. doi:10.3892/ijo.2018.4497
- Song Y, Zuo Y, Qian XL, et al. Inhibition of microRNA-21-5p promotes the radiation sensitivity of non-small cell lung cancer through HMSH2. Cell Physiol Biochem. 2017;43(3):1258–1272. doi:10.1159/000481839
- Zhang L, Wang C, Xue Z-X. Inhibition of miR-630 enhances the cell resistance to radiation by directly targeting CDC14A in human glioma. Am J Transl Res. 2017;9(3):1255–1265.
- Gu J, Li Y, Zeng J, et al. Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Sci Rep. 2017;7(1):7546. doi:10.1038/s41598-017-07973-4
- Mehta M, Basalingappa K, Griffith JN, et al. HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy. Oncotarget. 2016;7(40):64820–64835. doi:10.18632/oncotarget.11706
- Qi R, Qiao T, Zhuang X. Small interfering RNA targeting S100A4 sensitizes non-small-cell lung cancer cells (A549) to radiation treatment. Onco Targets Ther. 2016;9:3753–3762. doi:10.2147/ott.S106557
- Ohnishi K, Scuric Z, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. siRNA targeting NBS1 or XIAP increases radiation sensitivity of human cancer cells independent of TP53 status. Radiat Res. 2006;166(3):454–462. doi:10.1667/rr3606.1
- Khan Z, Khan AA, Prasad GBKS, Khan N, Tiwari RP, Bisen PS. Growth inhibition and chemo-radiosensitization of head and neck squamous cell carcinoma (HNSCC) by survivin-siRNA lentivirus. Radiother Oncol. 2016;118(2):359–368. doi:10.1016/j.radonc.2015.12.007
- Jackson MR, Bavelaar BM, Waghorn PA, et al. Radiolabeled oligonucleotides targeting the RNA subunit of telomerase inhibit telomerase and induce DNA damage in telomerase-positive cancer cells. Cancer Res. 2019;79(18):4627–4637. doi:10.1158/0008-5472
- Cao F, Ju X, Chen D, et al. Phosphorothioate‑modified antisense oligonucleotides against human telomerase reverse transcriptase sensitize cancer cells to radiotherapy. Mol Med Rep. 2017;16(2):2089–2094. doi:10.3892/mmr.2017.6778
- Park SI, Park SJ, Lee J, et al. Inhibition of cyclic AMP response element-directed transcription by decoy oligonucleotides enhances tumor-specific radiosensitivity. Biochem Biophys Res Commun. 2016;469(3):363–369. doi:10.1016/j.bbrc.2015.11.122
- Yu C, Yu Y, Xu Z, et al. Antisense oligonucleotides targeting human telomerase mRNA increases the radiosensitivity of nasopharyngeal carcinoma cells. Mol Med Rep. 2015;11(4):2825–2830. doi:10.3892/mmr.2014.3105
- Hernández-Rivera M, Kumar I, Cho SY, et al. High-performance hybrid bismuth–carbon nanotube based contrast agent for X-ray CT imaging. ACS Appl Mater Interfaces. 2017;9:5709–5716. doi:10.1021/acsami.6b12768
- Haume K, Rosa S, Grellet S, et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7(1):8. doi:10.1186/s12645-016-0021-x
- Ma N, Liu P, He N, Gu N, Wu F-G, Chen Z. Action of gold nanospikes-based nanoradiosensitizers: cellular internalization, radiotherapy, and autophagy. ACS Appl Mater Interfaces. 2017;9:31526–31542. doi:10.1021/acsami.7b09599
- Li Y, Yun K-H, Lee H, Goh S-H, Suh Y-G, Choi Y. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials. 2019;197:12–19. doi:10.1016/j.biomaterials.2019.01.004
- Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115(19):10410–10488. doi:10.1021/acs.chemrev.5b00193
- Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C. Radiosensitization by gold nanoparticles: will they ever make it to the clinic? Radiother Oncol. 2017;124(3):344–356. doi:10.1016/j.radonc.2017.07.007
- Zhang XD, Luo Z, Chen J, et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci Rep. 2015;5:8669. doi:10.1038/srep08669
- Luo D, Wang X, Zeng S, Ramamurthy G, Burda C, Basilion JP. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: does size matter for targeted particles? Chem Sci. 2019;10(35):8119–8128. doi:10.1039/c9sc02290b
- Mathur P, Jha S, Ramteke S, Jain NK. Pharmaceutical aspects of silver nanoparticles. Artif Cells, Nanomed Biotechnol. 2018;46(sup1):115–126. doi:10.1080/21691401.2017.1414825
- Li S, Porcel E, Remita H, et al. Platinum nanoparticles: an exquisite tool to overcome radioresistance. Cancer Nanotechnol. 2017;8(1):4. doi:10.1186/s12645-017-0028-y
- Pinel S, Thomas N, Boura C, Barberi-Heyob M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv Drug Deliv Rev. 2019;138:344–357. doi:10.1016/j.addr.2018.10.013
- Zhao J, Liu P, Ma J, et al. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer as1411 for glioma irradiation therapy. Int J Nanomedicine. 2019;14:9483–9496. doi:10.2147/IJN.S224160
- Liu Z, Tan H, Zhang X, et al. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):S922–s930. doi:10.1080/21691401.2018.1518912
- Fathy MM. Biosynthesis of silver nanoparticles using thymoquinone and evaluation of their radio-sensitizing activity. BioNanoScience. 2020;10(1):260–266. doi:10.1007/s12668-019-00702-3
- Rajaee A, Wang S, Zhao L, et al. Multifunction bismuth gadolinium oxide nanoparticles as radiosensitizer in radiation therapy and imaging. Phys Med Biol. 2019;64(19):195007. doi:10.1088/1361-6560/ab2154
- Fält T, Söderberg M, Wassélius J, Leander P. Material decomposition in dual-energy computed tomography separates high-Z elements from iodine, identifying potential contrast media tailored for dual contrast medium examinations. J Comput Assist Tomogr. 2015;39(6):975–980. doi:10.1097/rct.0000000000000298
- Chen X, Song J, Chen X, Yang H. X-ray-activated nanosystems for theranostic applications. Chem Soc Rev. 2019;48(11):3073–3101. doi:10.1039/c8cs00921j
- Liu Y, Zhang P, Li F, et al. Metal-based nano enhancers for future radiotherapy: radiosensitizing and synergistic effects on tumor cells. Theranostics. 2018;8(7):1824–1849. doi:10.7150/thno.22172
- Mardare AI, Mardare CC, Kollender JP, Huber S, Hassel AW. Basic properties mapping of anodic oxides in the hafnium-niobium-tantalum ternary system. Sci Technol Adv Mater. 2018;19(1):554–568. doi:10.1080/14686996.2018.1498703
- Taupin F, Flaender M, Delorme R, et al. Gadolinium nanoparticles and contrast agent as radiation sensitizers. Phys Med Biol. 2015;60(11):4449–4464. doi:10.1088/0031-9155/60/11/4449
- Shahbazi M-A, Faghfouri L, Ferreira MPA, et al. The versatile biomedical applications of bismuth-based nanoparticles and composites: therapeutic, diagnostic, biosensing, and regenerative properties. Chem Soc Rev. 2020;49(4):1253–1321. doi:10.1039/C9CS00283A
- Le Duc G, Roux S, Paruta-Tuarez A, et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014;5(1):4. doi:10.1186/s12645-014-0004-8
- Hu P, Fu Z, Liu G, et al. Gadolinium-based nanoparticles for theranostic MRI-guided radiosensitization in hepatocellular carcinoma. Front Bioeng Biotechnol. 2019;7:368. doi:10.3389/fbioe.2019.00368
- Detappe A, Lux F, Tillement O. Pushing radiation therapy limitations with theranostic nanoparticles. Nanomedicine (London, England). 2016;11(9):997–999. doi:10.2217/nnm.16.38
- McGinnity TL, Dominguez O, Curtis TE, Nallathamby PD, Hoffman AJ, Roeder RK. Hafnia (HfO2) nanoparticles as an X-ray contrast agent and mid-infrared biosensor. Nanoscale. 2016;8(28):13627–13637. doi:10.1039/C6NR03217F
- Jayaraman V, Bhavesh G, Chinnathambi S, Ganesan S, Aruna P. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater Express. 2014. doi:10.1166/mex.2014.1190
- Bonvalot S, Le Pechoux C, De Baere T, et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2016;23:908–917. doi:10.1158/1078-0432
- Prasad K, Bazaka O, Chua M, et al. Metallic biomaterials: current challenges and opportunities. Materials (Basel). 2017;10(8):884. doi:10.3390/ma10080884
- Jin Y, Ma X, Zhang S, et al. A tantalum oxide-based core/shell nanoparticle for triple-modality image-guided chemo-thermal synergetic therapy of esophageal carcinoma. Cancer Lett. 2017;397:61–71. doi:10.1016/j.canlet.2017.03.030
- Rathnayake S, Mongan J, Torres AS, et al. In vivo comparison of tantalum, tungsten, and bismuth enteric contrast agents to complement intravenous iodine for double-contrast dual-energy CT of the bowel. Contrast Media Mol Imaging. 2016;11(4):254–261. doi:10.1002/cmmi.1687
- Lu Y-C, Yang C-X, Yan X-P. Radiopaque tantalum oxide coated persistent luminescent nanoparticles as multimodal probes for in vivo near-infrared luminescence and computed tomography bioimaging. Nanoscale. 2015;7(42):17929–17937. doi:10.1039/C5NR05623C
- Brown R, Tehei M, Oktaria S, et al. High-Z nanostructured ceramics in radiotherapy: first evidence of ta2O5-induced dose enhancement on radioresistant cancer cells in an MV photon field. Part Part Syst Charact. 2014;31(4):500–505. doi:10.1002/ppsc.201300276
- Song G, Chao Y, Chen Y, et al. All-in-one theranostic nanoplatform based on hollow TaOx for chelator-free labeling imaging, drug delivery, and synergistically enhanced radiotherapy. Adv Funct Mater. 2016;26(45):8243–8254. doi:10.1002/adfm.201603845
- Song G, Chen Y, Liang C, et al. Catalase-loaded TaOx nanoshells as bio-nanoreactors combining high-Z element and enzyme delivery for enhancing radiotherapy. Adv Mater. 2016;28(33):7143–7148. doi:10.1002/adma.201602111
- Bulatov A, Moskvin L, Rodinkov O, Ermakov S. Department of analytical chemistry of saint petersburg state university celebrates one hundred and fifty years anniversary: international year of the periodic table of chemical elements. Talanta. 2020;206:119759. doi:10.1016/j.talanta.2019.03.081
- Chellan P, Sadler PJ. The elements of life and medicines. Philos Trans a Math Phys Eng Sci. 2015;373(2037). doi:10.1098/rsta.2014.0182
- Hossain M, Su M. Nanoparticle location and material-dependent dose enhancement in X-ray radiation therapy. J Phys Chem C. 2012;116(43):23047–23052. doi:10.1021/jp306543q
- Yu X, Li A, Zhao C, Yang K, Chen X, Li W. Ultrasmall semimetal nanoparticles of bismuth for dual-modal computed tomography/photoacoustic imaging and synergistic thermoradiotherapy. ACS Nano. 2017;11(4):3990–4001. doi:10.1021/acsnano.7b00476
- Qiu J, Xiao Q, Zheng X, et al. Single W18O49 nanowires: a multifunctional nanoplatform for computed tomography imaging and photothermal/photodynamic/radiation synergistic cancer therapy. Nano Res. 2015;8(11):3580–3590. doi:10.1007/s12274-015-0858-z
- Gong L, Yan L, Zhou R, Xie J, Wu W, Gu Z. Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy. J Mater Chem B. 2017;5(10):1873–1895. doi:10.1039/C7TB00195A
- Cheng X, Yong Y, Dai Y, et al. Enhanced radiotherapy using bismuth sulfide nanoagents combined with photo-thermal treatment. Theranostics. 2017;7(17):4087–4098. doi:10.7150/thno.20548
- Feng L, Yang D, Gai S, et al. Single bismuth tungstate nanosheets for simultaneous chemo-, photothermal, and photodynamic therapies mediated by near-infrared light. Chem Eng J. 2018;351:1147–1158. doi:10.1016/j.cej.2018.06.170
- Detappe A, Thomas E, Tibbitt MW, et al. Ultrasmall silica-based bismuth gadolinium nanoparticles for dual magnetic resonance–computed tomography image guided radiation therapy. Nano Lett. 2017;17(3):1733–1740. doi:10.1021/acs.nanolett.6b05055
- Yong Y, Zhou L, Zhang S, et al. Gadolinium polytungstate nanoclusters: a new theranostic with ultrasmall size and versatile properties for dual-modal MR/CT imaging and photothermal therapy/radiotherapy of cancer. NPG Asia Mater. 2016;8(5):e273–e273. doi:10.1038/am.2016.63
- Ma P, Xiao H, Yu C, et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017;17(2):928–937. doi:10.1021/acs.nanolett.6b04269
- Vallabani NVS, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech. 2018;8(6):279. doi:10.1007/s13205-018-1286-z
- Tang GD, Li ZZ, Ma L, et al. Three models of magnetic ordering in typical magnetic materials. Phys Rep. 2018;758:1–56. doi:10.1016/j.physrep.2018.06.009
- Nica V, Caro C, Páez-Muñoz JM, Leal MP, Garcia-Martin ML. Bi-magnetic core-shell CoFe(2)O(4)@MnFe(2)O(4) nanoparticles for in vivo theranostics. Nanomaterials (Basel, Switzerland). 2020;10(5). doi:10.3390/nano10050907
- Meidanchi A, Akhavan O, Khoei S, Shokri A, Hajikarimi Z, Khansari N. ZnFe2O4 nanoparticles as radiosensitizers in radiotherapy of human prostate cancer cells. Mater Sci Eng C Mater Biol Appl. 2015;46:394–399. doi:10.1016/j.msec.2014.10.062
- Hidayatullah M, Nurhasanah I, Budi WS. ZnFe2O4nanoparticles for potential application in radiosensitization. J Phys Conf Ser. 2016;694:012028. doi:10.1088/1742-6596/694/1/012028
- Salunkhe A, Khot V, Patil SI, et al. Magneto-chemotherapy with high-magnetic-moment iron oxide nanoparticles for cancer theranostics. ACS Appl Bio Mater. 2020;3(4):2305–2313. doi:10.1021/acsabm.0c00077
- Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel). 2011;11(12):11736–11751. doi:10.3390/s111211736
- Juzenas P, Chen W, Sun Y-P, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008;60(15):1600–1614. doi:10.1016/j.addr.2008.08.004
- Cline B, Delahunty I, Xie J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1541. doi:10.1002/wnan.1541
- Yang W, Read PW, Mi J, et al. Semiconductor nanoparticles as energy mediators for photosensitizer-enhanced radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(3):633–635. doi:10.1016/j.ijrobp.2008.06.1916
- Nakayama M, Sasaki R, Ogino C, et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiat Oncol. 2016;11(1):91. doi:10.1186/s13014-016-0666-y
- Morita K, Miyazaki S, Numako C, et al. Characterization of titanium dioxide nanoparticles modified with polyacrylic acid and H2O2 for use as a novel radiosensitizer. Free Radic Res. 2016;50(12):1319–1328. doi:10.1080/10715762.2016.1241879
- Jin J, Zhao Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: recent advances and future challenges. J Nanobiotechnology. 2020;18(1):75. doi:10.1186/s12951-020-00629-y
- Sun H, Wang X, Zhai S. The rational design and biological mechanisms of nanoradiosensitizers. Nanomaterials (Basel, Switzerland). 2020;10(3):504. doi:10.3390/nano10030504
- Martel A. Recent progress in biomedical applications of nanodiamonds. Nanosci Nanotechnol. 2018;8:11–24. doi:10.5923/j.nn.20180801.03
- Jia Y, Weng Z, Wang C, et al. Increased chemosensitivity and radiosensitivity of human breast cancer cell lines treated with novel functionalized single-walled carbon nanotubes. Oncol Lett. 2017;13(1):206–214. doi:10.3892/ol.2016.5402
- Chan L, He L, Zhou B, et al. Cancer-targeted selenium nanoparticles sensitize cancer cells to continuous γ radiation to achieve synergetic chemo-radiotherapy. Chem Asian J. 2017;12(23):3053–3060. doi:10.1002/asia.201701227
- Bromma K, Cicon L, Beckham W, Chithrani DB. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci Rep. 2020;10(1):12096. doi:10.1038/s41598-020-68994-0
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
- Chenthamara D, Subramaniam S, Ramakrishnan SG, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):20. doi:10.1186/s40824-019-0166-x
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330