?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Membranous nanostructures, such as nanovesicles and nanotubules, are an important pool of biological membranes. Recent results indicate that they constitute cell-cell communication systems and that cancer development is influenced by these systems. Nanovesicles that are pinched off from cancer cells can move within the circulation and interact with distant cells. It has been suggested and indicated by experimental evidence that nanovesicles can induce metastases from the primary tumor in this way. Therefore, it is of importance to understand better the mechanisms of membrane budding and vesiculation. Here, a theoretical description is presented concerning consistently related lateral membrane composition, orientational ordering of membrane constituents, and a stable shape of nanovesicles and nanotubules. It is shown that the character of stable nanostructures reflects the composition of the membrane and the intrinsic shape of its constituents. An extension of the fluid mosaic model of biological membranes is suggested by taking into account curvature-mediated orientational ordering of the membrane constituents on strongly anisotropically curved regions. Based on experimental data for artificial membranes, a possible antimetastatic effect of plasma constituents via mediation of attractive interaction between membranous structures is suggested. This mediated attractive interaction hypothetically suppresses nanovesiculation by causing adhesion of buds to the mother membrane and preventing them from being pinched off from the membrane.
Introduction
According to a World Health Organization report, cancer is a leading cause of death worldwide, and it was estimated that 7.6 million people died from cancer in 2008, with metastases being the major cause of death.Citation1 More than 100 distinct types of human cancer have been described, while subtypes of tumors can be found within specific organs, leading to the conclusion that cancer is a highly complex disease, both in time and space.Citation2 Due to the heterogeneity of hitherto revealed mechanisms, the hypothesis has been put forward that each tumor is unique, and the spectrum of biological changes determining tumors is highly variable.Citation2 The question arises as to whether major underlying mechanisms exist that could be addressed in diagnosis and treatment.
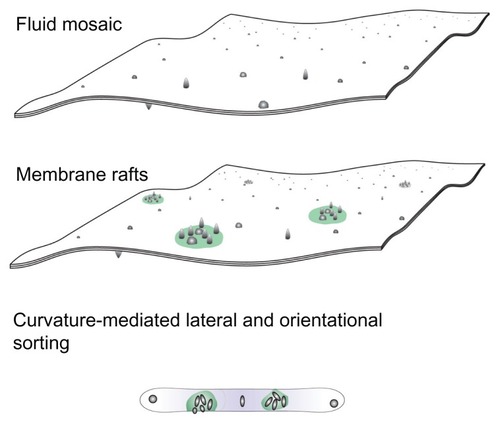
Figure 1 A schematic representation of three models of the membrane, ie, the fluid mosaic model,Citation27 the membrane raft model,Citation28–Citation30 and the curvature-mediated lateral and orientational sorting model.Citation34,Citation35
Note: Violet color indicates orientational ordering of lipid molecules in the tubular portion.
In this work, a basic (bio)physical mechanism of biological membrane configuration is explored. Lateral redistribution of membrane constituents is connected with an increase in membrane curvature. Nanosized membrane buds are formed which may elongate into nanotubules, or be pinched off from the membrane to become nanovesicles. Nanotubules and nanovesicles reflect the composition of the membrane and the interior of the mother cell. Nanotubules may become attached to adjacent cells while nanovesicles travel within the circulation. Thereby, these nanostructures convey matter and information to other cells and represent cell-cell communication systems. Nanovesicles have been found to transfer surface-bound ligands and receptors,Citation3–Citation7 prion proteins,Citation8–Citation10 genetic material including RNA,Citation11–Citation14 and infectious particlesCitation15,Citation16 between cells. It has also been suggested that in cancer they contribute to metastasis.Citation17,Citation18
Information on the presence of tumors could be obtained from cell-derived nanovesicles which are expected to be found in body fluids, such as blood, synovial fluid, ascites, pleural fluid, and cerebrospinal fluid.Citation19,Citation20 For example, it was recently demonstrated that tumors of various originCitation21 and various clinical outcomesCitation22 can be classified by their micro RNA (miRNA) profiles. miRNAs are short (about 18–25 nucleotides long) noncoding RNAs, including small interfering RNA, ribosomal RNA, transfer RNA, and small nuclear RNA.Citation23 Over 700 miRNAs have been identified in the human genome.Citation24 It has been suggested that an miRNA profile is associated with prognostic factors and disease progressionCitation22,Citation25 and that mutations in miRNA genes are frequent and may have functional importance,Citation22 by either suppressing tumors or promoting their growth and proliferation.Citation24 miRNA profiling has already been used to determine whether patients with chronic lymphocytic leukemia have slow-growing or aggressive forms of the cancer,Citation21 while plasma samples collected from patients with early (stage II) colorectal cancer could be distinguished from those of healthy gender-matched and age-matched volunteers.Citation26 Compared with current methods used to diagnose most malignancies, assessment of blood is advantageous because it is much less invasive. Nanovesicles can be considered as potentially relevant biomarkers for diagnosis, prognosis, and treatment of cancer, encouraging the study of the processes of membrane budding and nanovesiculation.
In studying cancer and its underlying mechanisms, extensive work has been devoted to chemical and biochemical methods involving specific molecules, reactions, pathways, and the binding of particles. Although substantial progress has been made, an essential unifying mechanism or mechanisms have not yet been revealed. Hitherto, the contributions of physics and biophysics cannot match those of chemistry and biochemistry, although physics essentially strives to reveal the relevant general mechanisms necessary to understand and manipulate cancer. Living creatures are commonly considered to be complex systems beyond the reach of physical methods which are effective in the description of simple systems.
However, even in highly complex living creatures, some relevant issues can be highlighted to simplify the system so that methods of theoretical physics can be applied. In this work, we focus on the curvature of the membrane connected to the redistribution of constituents, a field which is subject to the methods of statistical physics and thermodynamics. Using these methods, it is demonstrated that, to understand whether the membrane is likely to produce a nanotubule or a nanovesicle, it is enough to distinguish only one property of the diverse constituents, ie, their symmetry with respect to the axis perpendicular to the membrane, which may lead to energetically favorable ordering of membrane constituents in strongly anisotropically curved membrane regions. These theoretical predictions are supported by experimental evidence from membranous nanostructures. Further, orientational ordering of particles with an internally distributed charge provides an explanation for mediated attractive interaction between membranes which could prevent the bud being pinched off from the membrane, and is the basis of a suggested antimetastatic and anticoagulant effect of body fluid constituents.
Description of the cell membrane
Fluid mosaic model and its extension by function/curvature-related lateral inhomogeneities
The cell membrane is an important building element of the cell. Understanding the interdependence of processes which take place in cells seems impossible without understanding the features relevant to the cell membrane. Therefore, the cell membrane has been a subject of interest in many studies. After performing their thorough and inspired work, Singer and Nicolson in 1972 suggested a fluid mosaic model for the membrane,Citation27 describing it in general as a lipid bilayer with embedded proteins and other large molecules. Within the physical implementation of the fluid mosaic model, the phospholipid bilayer shows the properties of a two-dimensional laterally isotropic liquid, while proteins and other large molecules are more or less free to move laterally in the membrane. Many experiments and theoretical studies performed during the last 40 years have established the fluid mosaic model as the standard model for description of the cell membrane.
After 25 years, the fluid mosaic model was upgraded by considering lateral inhomogeneities.Citation28–Citation30 According to the upgraded model, the membrane is a two-dimensional liquid with embedded microdomains of specific composition, called membrane rafts. Membrane rafts are small (10–200 nm), relatively heterogeneous, dynamic structures with an increased concentration of cholesterol and sphingolipids.Citation31,Citation32 From a biochemical point of view, membrane rafts are structures which resist solvation by detergents at low temperatures, while from a biophysical point of view, within the raft, increased ordering takes place due to interactions between the highly saturated fatty acids of sphingolipids. Fatty acids within the rafts have limited mobility with respect to the unsaturated fatty acids in other parts of the membrane. Dynamic accumulation of specific membrane constituents in rafts regulates the spatial and temporal dependence of signalization and transport of matter, thereby forming transient but vitally important signaling platforms.Citation33
In membranes, there is an interdependence between structure and shape because the membrane constituents create the membrane geometry, ie, membrane curvature is determined by the shape of the membrane constituents and their interactions. The curvature of the raft is formed by accumulation of a specific type of constituent, and may be different from the curvature of the surrounding membrane. In other words, lateral sorting of membrane constituents may cause changes in local membrane curvature. Considering this interdependence, the fluid mosaic model was further modified, as described below.
Membrane as a composite two-dimensional surface
To make the system simple, it is considered that one of the membrane extensions (thickness) is much smaller than the other two extensions, so the membrane may be treated as a two-dimensional surface. The membrane is also viewed as being composed of a large number of particles (building units) which act one upon another.Citation35 Taking into account the above, the membrane layer is described as a surface composed of building units (molecules, groups of molecules, membrane rafts, nanodomains), which attains a shape corresponding to the minimum of its free energy.
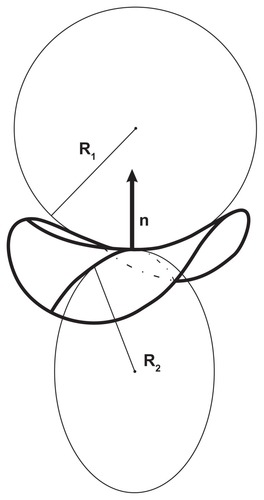
Cutting the surface at a chosen point by a plane through a normal to the surface defines a curve, known as the “normal cut”. The curvature of the normal cut is the inverse value of the radius of the circle which fits the curve at the chosen point, C = 1/R. An infinite number of possible normal cuts can be made through the normal. The cuts with the maximal and minimal curvatures, ie, C1 = 1/R1 and C2 = 1/R2, respectively, are called the principal curvatures, while the corresponding mutually perpendicular directions of the cuts are the principal directions ().
The diagonalized curvature tensor of the membrane surface at a given point is
A membrane can be imagined which would completely fit the constituent at a given point, in the sense that no energy would be needed to insert the constituent into the membrane. The curvature tensor of such a membrane is the intrinsic curvature tensor
where C1,m and C2,m are the intrinsic principal curvatures. Lying perpendicular to the membrane plane, the principal axes system of the membrane may be rotated with respect to the intrinsic principal axes system.
We now define the isotropic and anisotropic membrane constituents, whereby a constituent is called isotropic if C1,m = C2,m and anisotropic if C1,m ≠ C2,m.
Energy of a single membrane constituent
When assembled into the membrane, the constituents cannot all resume the most energetically favorable curvature (ie, intrinsic curvature). Namely, the shape corresponds to the minimal energy of the whole membrane. Also, constraints regarding the area and volume must be taken into account. Mismatch of the intrinsic and actual curvature at the site of the constituent is a source of the single constituent energy. In other words, the constituent deforms and rotates in the membrane plane for an angle ω in order to constitute the membrane.
The energy of a single constituent is given in terms of the mismatch tensor M,Citation35
where R is the rotation matrix
Using (Equation2(2) ) and (Equation4
(4) ),
so that
The energy of the constituent is given by a phenomenological expression subject to two invariants of the mismatch tensor, M, of the second order in curvatures. The trace and the determinant of the mismatch tensor are considered as fundamental invariantsCitation35
where K and K are constants.
It follows from expression (Equation6(6) ) that
Taking into account the relationship between the trigonometric functions sin2ω = 1/2[1 − cos(2ω)] and cos2ω = 1/2[1 + cos(2ω)], after some rearranging, we obtain
and
where we have introduced the mean curvature of the membrane H
and
The corresponding intrinsic quantities are
and
A membrane constituent is characterized by Hm and Ĉm. For isotropic constituents Ĉm = 0.
The trace of the mismatch tensor (Equation9(9) ) can be expressed by the invariants (traces) of the curvature tensors C and Cm. Further, it follows from EquationEquations (11)
(11) and Equation(12)
(12) that
so that the determinant of the mismatch tensor can also be expressed by invariants of the curvature tensors [(H, Ĉ) and (Hm, Ĉm), respectively],
Inserting the expressions (Equation9(9) ) and (Equation16
(16) ) into EquationEquation (7)
(7) yields the expression for the single constituent
or convenientlyCitation35
where
and
It can be seen from EquationEquation (18)(18) that the energy E depends on the angle ω multiplied by the difference between the two intrinsic curvatures (Ĉm). This means that for anisotropic building units, the orientation with respect to the coordinate system is important.
Local thermodynamic equilibrium of membrane monolayer
To ensure uniformity of the curvature field within the system which is being described by ensemble statistics, the monolayer area is imagined to be divided into small patches containing a large number of constituents. Let there be P kinds of membrane constituents in a chosen patch. All constituents of the i-th type are taken to be equal and independent. The lattice statistics approach is used, analogous to the problem of noninteracting magnetic dipoles in a magnetic field.Citation36 Here, the curvature field takes the role of the magnetic field.
Considering a subset of constituents of the i-th type (i = 1,2, ..., P) it follows from (Equation18(18) ) that the single-constituent energy attains a minimum when cos(2ωi) = 1, while the single-constituent energy attains a maximum when cos(2ωi) = −1. In the first case, the single-constituent energy is
whereas in the second case the single-constituent energy is
where
and
are the curvature deviator and the intrinsic curvature deviator, respectively. The states with ωi = 0, π and with ωi = π/2, 3π/2, respectively, are degenerate. We say that the ordering is quadrupolar.
We assume a simple model where we have Mi equivalent constituents in a patch. Each constituent is in one of the two possible states, ie, Ei, min and Ei, max, respectively (EquationEquations (21)(21) and Equation(22)
(22) , so that Ni constituents are in the state with the higher energy Ei, max while (Mi − Ni) constituents are in the state with the lower energy, Ei, min.
The partition function of a constituent of the i-th type in the lower energy state isCitation36
while the partition function of a constituent of the i-th type in the higher energy state is
where
and k is the Boltzmann constant.
Constituents in the same energy state are treated as indistinguishable. For Ni constituents of the i-th type in the state with higher (maximal) energy and (Mi −Ni) constituents in the state with lower (minimal) energy, the number of possible arrangements is Mi!/Ni!(Mi − Ni)!. To calculate the partition function, we must consider that Ni can be any number from 0 to Mi; Ni = 0 means that all the constituents are in the state with the lower energy, Ni = 1 means that one constituent is in the state with the higher energy, while Mi − 1 constituents are in the state with the lower energy, …
The canonical partition function Qi(Mi, T, C) of Mi constituents in the membrane patch is
Considering EquationEquations (25)(25) and Equation(26)
(26) ,
Using the binomial formula
where
We call the quantity di eff, the effective curvature deviator. After some rearranging, we obtain the canonical partition function of the constituents of the i-th type
Local ordering of constituents
The average number of constituents in each of the energy states representing the local ordering of the constituents is obtained using the local canonical partition function of the constituents of the i-th type, Qi. The average number of constituents with higher energy (Ei,max) is
while the average number of constituents with lower energy (Ei,min) is
An alternative definition can be used,
where
It follows from EquationEquations (35)(35) and Equation(36)
(36) that
and
It can be seen from EquationEquations (37)(37) and Equation(38)
(38) that at di,eff = 0, ie, when the principal curvatures are equal, both energy states are equally occupied (〈Ni〉/Mi = 〈Mi − Ni 〉/Mi = 1/2). The fraction of the number of constituents in the lower energy state increases with increasing di,eff to 1, while the fraction of constituents in the higher energy state decreases to 0.
Global equilibrium of a multicomponent membrane
Taking into account that there are P types of constituents which can be treated as independent, the partition function of the membrane patch is
were
and Qi is given by EquationEquation (32)(32) . The free energy of the patch is
Taking into account EquationEquations (32)(32) and Equation(41)
(41) yields
To calculate the free energy of the whole monolayer, the contributions of all the patches are integrated,
where
The integration is performed over the monolayer area A. In the equilibrium state the free energy attains its minimum
The total number of constituents of each kind in the monolayer (Mi,T, i = 1, 2, ..., P) is kept constant at minimization,
while a local constraint is applied by requiring that all sites in the patch are occupied
To perform the variation, a functional is constructed
where λ is a local Lagrange multiplier and λi, i = 1, 2, ..., P are global Lagrange multipliers. It is assumed that the average density of the constituents is uniform over the monolayer so that
where MT is the total number of sites in the monolayer. The variational problem is expressed by the Euler-Lagrange equations
and
It follows from EquationEquations (48)(48) and Equation(50)
(50) that
while expression (Equation48(48) ) and the Euler-Lagrange Equationequation (51)
(51) yield the condition (Equation47
(47) ).
To determine the parameter λ, EquationEquation (52)(52) is inserted into EquationEquation (47)
(47) . The parameter λ is included in all terms of the sum and is independent of i, so it can be expressed as
Using EquationEquations (52)(52) and Equation(53)
(53) gives
The global Lagrange multipliers λi are determined by fulfilling conditions (Equation46(46) ),
Expression (Equation54(54) ) represents the probability of finding a constituent in the state with a given curvature-dependent energy. Because the background is consistent with Boltzmann statistics (explicitly independent and indistinguishable constituents), expression (Equation54
(54) ) can be described as a modified Boltzmann distribution. The explicit independence of constituents in the derivation of the local thermodynamic equilibrium is complemented by introducing the excluded volume effect [the condition (Equation47
(47) )] which is reflected in the denominator of EquationEquation (54)
(54) .
The equilibrium free energy is obtained by inserting distribution (Equation54(54) ) into expression (Equation43
(43) ). After some rearranging, we obtain
One-component membrane. Comparison with Helfrich bending energy
If the monolayer is composed of equal constituents which occupy all sites in the lattice, it follows that
Taking into account the definition of q0 (Equation27(27) ) and the above equation gives
The free energy of the bilayer is obtained by summing the contributions of both layers. It is taken into account that the principal curvatures in the inner layer are opposite in sign to the principal curvatures of the outer layer
The area densities of the constituents m are taken to be equal and constant over both layers. The outer and inner membrane areas are regarded as equal in integration. The contributions to the free energy which turn out to be constant are omitted. We obtain
Using relationship (Equation15(15) )
EquationEquation (60)(60) givesCitation37,Citation38
We compared the expression for free energy of the one-component membrane (Equation62(62) ) with the acknowledged Helfrich local bending energy of a thin, laterally isotropic surface,Citation39,Citation40
where kc and kG are the membrane local and Gaussian bending constants, respectively. Expression (Equation62(62) ) obtained by statistical mechanics recovers the Helfrich isotropic bending energy (Equation63
(63) ) if
and
and if the constituents are isotropic (Dm = 0).
Expression (Equation62(62) ) is more general because it takes into account the possibility of in-plane orientational ordering of the constituents. The new term deriving from orientational ordering of the constituents [the third term in EquationEquation (62)
(62) ] is called the deviatoric elastic energy of the membrane, and is always negative. In other words, orientational ordering of the anisotropic constituents on the anisotropically curved membrane regions diminishes the free energy of the membrane, and therefore stabilizes shapes with anisotropically curved membrane regions. The orientational ordering of the constituents provides an additional degree of freedom which also proves relevant in other systems (eg, the electric double layer).Citation41–Citation43
For isotropic constituents, the membrane free energy can be equivalently expressed by either set of invariants of the curvature tensor C, ie, the trace and the determinant of the curvature tensor or the trace and the deviator of the curvature tensor. However, for anisotropic constituents, the orientational ordering of constituents can be described by the trace and the deviator of the curvature tensor and not by the trace and the determinant of the curvature tensor. Both cases can be described by the trace and the determinant of the mismatch tensor, M.
The variational problem for the one-component membrane considering orientational ordering of the constituents was expressed by a system of Euler equations and solved by numerical methods.Citation38 It has been shown that the energies of the equilibrium shapes were considerably affected by ordering of the constituents in strongly anisotropically curved regions, but the shapes were only slightly different from shapes calculated by minimization of the isotropic bending energy. For convenience, the shapes calculated by minimization of membrane isotropic bending can therefore also be used when calculating energies taking into account the deviatoric terms. Good agreement between the predicted and observed structuresCitation44 demonstrates that the statistical mechanical description of the membrane is relevant and useful.
Equilibrium configuration of the two-component membrane
To illustrate the interdependence between equilibrium composition and shape of the membrane, we consider a two-component membrane. Two types of membrane constituents (first and second) are distinguished. The local constraint (Equation47(47) ) is omitted and the concentration of the second type of constituents is expressed using the local conservation equation:
so the functional can be expressed by the concentration, m1
The Euler-Lagrange equation subject to m1 gives the local fraction of constituents of the first type,
Considering (Equation66(66) ), we also obtain the local concentration of constituents of the second type,
Further, it is assumed that the concentration of constituents of the first type is much smaller than the concentration of constituents of the second type,
implying that 2q10 cosh (d1,eff/2) exp(−λ1/kT)/2q20 cosh (d2,eff/2) is small.
The global constraint
gives
Taking into account the approximation for small x, ln(1 + x) ≅ x and the definitions for qi0 (EquationEquation (27)(27) and di,eff (EquationEquation (31)
(31) ) yields, for the monolayer,
The first two terms of EquationEquation (73)(73) recover the elastic energy of the membrane composed of constituents of the second (abundant) type, while the third term describes the nonlocal effect caused by distribution and orientational ordering of constituents of the first (scarce) type.
To obtain the free energy of the bilayer membrane, the two monolayer contributions are summed. It is taken into account that the curvature tensor has different signs in the opposing monolayers (EquationEquation (59)(59) ). Omitting the constant terms gives
The self-consistent solution of the variational problem based on a functional including the system free energy and relevant local and global constraints, yields equilibrium lateral and orientational distribution functions and the equilibrium free energy, all of which derive from the same principle of single-constituent energy.Citation38
In solving the variational problem,Citation38 dimensionless quantities are used, ie, h = HRs, d = DRs, hm = HmRs, and dm = DmRs, where
. The equilibrium free energy of the nanovesicle membrane bilayer enclosing dimensionless volume V = (36πV2/A3)1/2, where V is the enclosed volume, as a function of the average mean curvature of the membrane 〈h〉 = ∫ hdA/4πRs2 is shown in . Three interaction constants (ξ1/kTRs2) were considered. It can be seen that the energy increases with increasing 〈h〉 due to an increase in the isotropic bending energy. With increasing 〈h〉, the shape of the nanovesicle becomes undulated and the necks become narrow. Anisotropy of the curvature becomes high in the necks so the constituents undergo ordering. Redistribution and orientational ordering of the constituents diminish the free energy. For high enough interaction constants (ξ1/kTRs2), the free energy reaches a maximum and then decreases. In such cases, the energy dependence exhibits two local minima corresponding to the limit shapes (cylinders and spheres, respectively).
Figure 3 Free energy of a two-component nanovesicle as a function of the average mean curvature of the membrane for three interaction constants, ξ1/2kTRs2 (A) 0.001, (B) 0.020, and (C) 0.040. It was taken that ξ1 = ξ1* and ξ2 = ξ2*.
Notes: The values of other model parameters were ξ1/2kTRs2 = 0.001, h1,m = 2, d1,m = 2, h2,m = 0, d2,m = 0, M1,T = 0.1 MT, v = 0.5. Five characteristic equilibrium shapes obtained by solving the system of Euler-Lagrange equations subject to isotropic bending energy are also depicted at the corresponding 〈h〉 values.
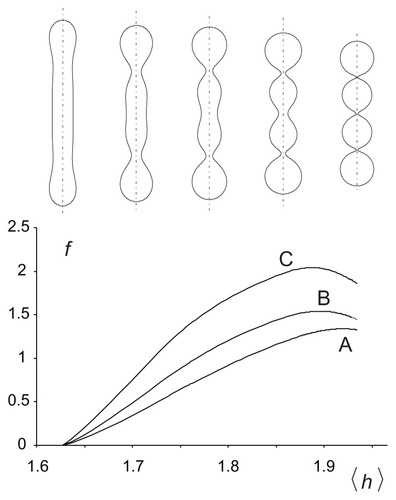
The shape corresponding to the minimum of the average mean curvature within the given class of shapes is composed of a cylinder with hemispherical caps, and the shape corresponding to the maximum of the average mean curvature is composed of quasispherical units connected by very thin necks. For the chosen parameters, the tubular limit shape corresponds to the minimum of the global energy ().
shows the mean curvature and the curvature deviator of the nanovesicle as a function of the coordinate along the long axis (A–C), ie, the average orientation of the anisotropic constituents 〈o〉 = 1/[1 + exp(−d1,eff)] which we describe as the average local number of constituents with the lowest energy of type 1 (scarce type) for the three interaction constants (ξ1/kTRs2) considered in , (D–F, J–L, P–R), the equilibrium distribution of constituents of type 1 in both membrane layers (G–I, M–O, S–U) and the respective shapes (V–X), which are also considered in . If the interaction constant (ξ1/kTRs2) is small, the constituents are uniformly distributed over both membrane layers in all shapes (S–U). There is almost no ordering of the constituents in the tubular shape (P), while weak ordering takes place in the necks (Q, R). For higher values of the interaction constant, deviations of the distributions from uniformity are small in the tubular shape (M) while they are strong in the necks (N, O), the effect being stronger if the necks are thinner. Type 1 molecules are depleted from the necks, but the ones that remain undergo substantial ordering (L, F). The values of the curvature deviator in the narrow necks may be rather high (up to 40 in panel C). Because the value of the normalized intrinsic deviator was taken as 2, constituents do not favor the necks. provides clarification of the free energy dependence depicted in .
Figure 4 Calculated equilibrium configuration of three characteristic shapes of a two-component nanovesicle. It was taken that ξ1 = ξ1* and ξ2 = ξ2*,ξ2/2kTRs2 = 0.001, h1,m = 2, d1,m = 2, h2,m = 0, d2,m = 0, M1,T = 0.1 MT, v = 0.5. (D–I)ξ1/2kTRs2 = 0.04, (J–O) ξ1/2kTRs2 = 0.02, (P–U)ξ1/2kTR s2 = 0.001. (V–X) the characteristic shapes and (A–C) the respective invariants of the curvature tensor.
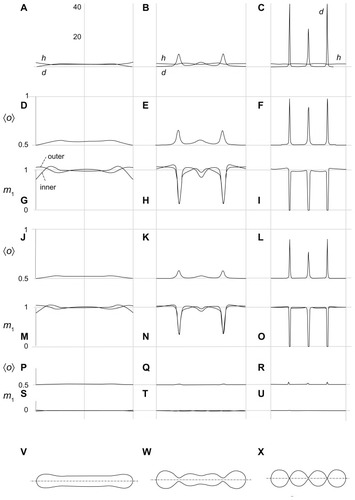
It can be seen in and that the nanotubule corresponds to the global minimum of free energy, so it could be considered as energetically the most favorable and therefore the most probable. However, if there is a process in the system that increases the average mean curvature of the membrane (such as integration of molecules into the outer membrane layer), the system may be driven towards the shape composed of spherical units.
Deviatoric elasticity may stabilize anisotropic nanostructures
Quadrupolar ordering of phospholipid molecules in a deviatoric field has been used to describe the stability of shapes with strongly anisotropically curved structures, such as nanotubular protrusions,Citation37 tubular and spherical nanovesicles of the erythrocyte membrane,Citation45 torocyte endovesicles,Citation46 narrow necks of one-component phospholipid vesicles,Citation38 two-component vesicles,Citation34,Citation47 peptidergic vesicles,Citation48 nanotubules in astrocytesCitation49 and urothelial cancer cells,Citation50–Citation52 flattened structures in Golgi bodies,Citation53 inverse hexagonal lipid phases,Citation54 and membrane pores.Citation55,Citation56 While it was previously acknowledged that membrane composition and shape are interdependent,Citation34,Citation57–Citation59 the orientational ordering model provides a unified explanation of the above feature, and has been reviewed extensively elsewhere.Citation37,Citation45,Citation60,Citation61
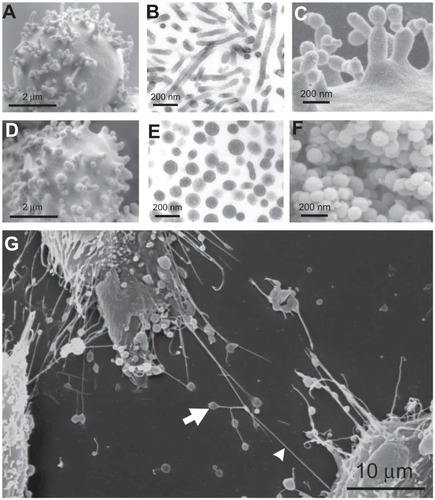
presents some of these nanostructures, with buds and nanovesicles of the erythrocyte membrane and nanotubules observed in urothelial cancer cells. Dilatations of the nanotubules are often present. These dilatations travel along the tube and discharge material when they reach the cell surface, so are called gondolae. Transport by gondolae is also observed in phospholipid vesicles.Citation62
Figure 5 Nanobuds and nanovesicles of the erythrocyte membrane and nanotubules connecting T24 cancer cells. In erythrocytes, budding and vesiculation was induced by adding a detergent. The type of detergent determines the character of the nanobuds and nanovesicles. (A) Scanning electron micrograph of echinocyte budding induced by dodecylmaltoside, (B) transmission electron micrograph of isolated tubular nanovesicles induced by dodecylmaltoside, (C) scanning electron micrograph of the budding erythrocyte membrane, (D) scanning electron micrograph of echinocyte budding induced by dodecylzwittergent, (E) transmission electron micrograph of isolated spherical nanovesicles, (F) scanning electron micrograph of isolated spherical nanovesicles, (G) nanotubules with dilatations connecting urothelial cancer cells. (A, B, D, E, and G) reproduced with permission of Schara et alCitation63 and (C and F) reproduced from Sustar et al.Citation64
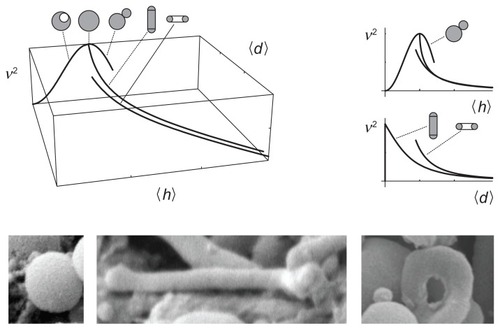
The proposed description is based on two invariants of the curvature tensor, ie, the trace (mean curvature) and the deviator. It seems natural that the average values of these two invariants would span a phase diagram of possible shapes
The third parameter which determines the shape is the relative volume, v. Therefore it is convenient to present the set of equilibrium shapes within the (v, 〈h〉, 〈d〉) coordinate system. shows a (v, 〈h〉, 〈d〉) phase diagram, with selected curves representing limit shapes composed of spheres, tubes, and tori. The predicted shapes are indeed observed in blood isolates ().
Clinical relevance of cell nanovesicles
It was reported early on that “platelet dust” existed in plasma.Citation65 It was also observed that nanosized particles called microvesicles are shed from the membranes of erythrocytes during storageCitation66–Citation68 and from membranes of other cells, including cancer cells.Citation69–Citation72 These nanoparticles were connected to coagulopathies secondary to cancer.Citation73–Citation76 It has been suggested that they may play a role in the coagulation process inside blood vessels.Citation77
Membrane asymmetry in nanovesicles is corrupted so that negatively charged phospholipid phosphatidylserine appears in the outer membrane layer, a process which is necessary to trigger formation of a blood clot. Further, the membrane of platelet-derived nanovesicles contains tissue factor, an integral membrane protein present in endothelial cells, platelets, and leukocytes. Tissue factor is the primary cellular initiator of the coagulation protease cascade, which leads to fibrin deposition and activation of platelets. Thus, the nanovesicle membranes contribute considerably to the catalytic surface needed for formation of blood clots. Aberrant tissue factor expression within the vasculature initiates life-threatening thrombosis in a number of diseases, including cancer. Also, recent studies have revealed a nonhemostatic role of tissue factor in the generation of coagulation proteases and subsequent activation of receptors on vascular cells, and this tissue factor-dependent signaling contributes to a variety of biological processes, including metastasis.Citation78–Citation80 Nanovesicles that are pinched off from cells interact with other cellsCitation81,Citation82 and thereby mediate interactions between platelets, endothelial cells, and tumor cells which can be expressed by thromboembolism in cancer.Citation83,Citation84 Further, nanovesicles were shown to stimulate proliferation of cancer cells, mRNA expression for angiogenic factors, as well as adhesion to fibrinogen and endothelial cellsCitation85 and downregulation of antitumoral immune responses in the host.Citation86
Clinical studies have shown that the concentration of nanovesicles isolated from blood in patients with a range of diseases is changed with respect to healthy subjects. For example, the concentration of nanovesicles was found to be increased in patients with lung cancer,Citation74 dermatofibroma,Citation87 dermatofibrosarcoma protuberans,Citation87 carcinoma of the oral cavity,Citation88 ovarian cancer,Citation89 and gastrointestinal cancer.Citation90,Citation91 It was recently suggested that the material isolated from blood contains both nanovesicles and residual cells, and that residual cells, mostly platelets, are the origin of the nanovesicles found in isolates as an artifact of the isolation procedure.Citation64 However, clinical studies show differences between concentrations of nanovesicles isolated from the blood of patients with cancer and from that from healthy subjects, suggesting that the properties of blood cells and plasma which determine the state of the isolate in cancer patients and in healthy subjects differ from each other.Citation64
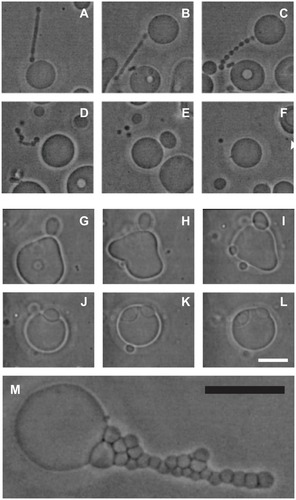
Given that nanovesicles are considered to be procoagulant and prometastatic, it could be beneficial to develop methods for suppression of nanovesiculation. To obtain an insight into the processes taking place during budding and pinching off from cells, studies were undertaken of giant phospholipid vesicles which are large enough to be observed directly by phase-contrast microscopy. illustrates the effect of the composition of the surrounding solution on budding of the giant phospholipid vesicle membrane. Budding was induced by raising the temperature of the sample. Citation92 When the tube was of sufficient length (), heating was discontinued and phosphate-buffered saline was added to the sample. The protrusion underwent beading (), substantial movement, followed by its detachment from the mother vesicle (), and finally decomposition into spherical vesicles (), which migrated away from the mother vesicle (). However, if molecules which mediate the attractive interaction between membranes (specific proteins) were present in the solution, the bud () was attracted back to the mother membrane () and remained bound to the surface of the mother vesicle ().
Figure 7 (A–F) Vesiculation of a giant phospholipid vesicle. After addition of phosphate-buffered saline to a suspension of vesicles, the tubular bud (A) exhibited undulations (B and C), detached itself from the mother vesicle (D), and decomposed into separate spherical vesicles (E), which were free to migrate away from the mother vesicle (F). (G–L) show suppression of vesiculation. When molecules which mediate attractive interaction between membranes (proteins dissolved in phosphate-buffered saline) were present in the solution, the bud (G and H) was attracted back to the mother membrane (I) where it remained bound to the surface of the mother vesicle (J–L). (M) Bead-like structures forming a long bud adhered to each other due to the mediating effect of added proteins dissolved in phosphate-buffered saline.
Notes: Bars represent 10 μm.
Reprinted from Urbanija et alCitation92 with the permission of Elsevier.
Observing this process inspired the hypothesis that molecules which mediate attractive interaction between membranes are both anticoagulant and antimetastatic.Citation44,Citation92,Citation93 Blood plasma mediates this attractive interaction,Citation94 indicating that molecules with the required properties are present in blood. Heparin (a common choice of anticoagulant prophylaxis and treatment) induces adhesion between phospholipid vesicles.Citation44,Citation93,Citation94 Heparin is known to have an antimetastatic effect in some types of cancer,Citation95 which supports the hypothesis of the anticoagulant and antimetastatic effect of plasma constituents based on suppression of nanovesiculation.Citation44
Conclusion
It is now acknowledged that cell-cell communication may take place via nanotubulesCitation96–Citation98 and nanovesicles,Citation99 and that these processes are important in cancer.Citation100 In order to manipulate membranous nanostructures, they should be better understood. Membrane properties that are the key to formation of tubules and vesicles can be subjected to the methods of theoretical physics.Citation39,Citation40,Citation101
In addition to considering membrane nanodomains (rafts) as an acknowledged extension of the fluid mosaic model of the membrane,Citation102 another major mechanism should also be acknowledged, ie, orientational ordering of membrane constituents on strongly anisotropically curved membrane regions. This mechanism provides an explanation for the stability of different types of membranous nanostructures, including nanotubules and nanovesicles, which are important in cell-cell communication and involved in cancer progression. This approach has led to prediction of transport by membranous nanotubulesCitation62 which was then found experimentally in cells.Citation96 Also, the orientational ordering of mediating molecules is the basis of short-ranged attractive interactions involving membrane surface(s).Citation103
It has been shown experimentally that there are some common properties in most biological membranes. Budding and vesiculation takes place in erythrocytes which lack a nucleus and cytoskeleton, and in cells with a nucleus and cytoskeleton. Moreover, these features can also be observed in artificial membranes composed of pure or mixed lipids, demonstrating that the phospholipid bilayer is indeed the backbone of the biological membrane. It is an essential feature of membranes that they create their own geometry, and furthermore, this geometry is the relevant field which determines their energy. The theoretical description of the system is based on the notion that the system seeks the state of lowest energy consistent with one of the most basic laws of nature, ie, it will attain the state that is the most probable.
This work focuses on a particular mechanism involved in metastasis (ie, cell-to-cell communication by nanovesicles), which does not exclude other mechanisms that were suggested previously (eg, crawling over a surface, phagocytosis, extension of pseudopodia). The experimental evidence indicates that nanovesiculation takes place in vitro and in vivo, but it is not yet certain to what extent it increases the probability of cancer spreading in vivo. It can be concluded that the stability of membranous nanostructures is a possible key mechanism of cancer progression.
Acknowledgments
The author acknowledges support from the Slovenian Research Agency (projects J3-2120 and J3-4108), EUREKA grant IMIPEB, and Novartis International AG.
Disclosure
The author reports no conflicts of interest in this work.
References
- World Health Organization Media CentreFact sheet 297220 Available from: http://www.who.int/mediacentre/factsheets/fs297/en/Accessed April 9, 2012
- GrizziFChiriva-InternatiMCancer: looking for simplicity and finding complexityCancer Cell Int20066416480511
- RatajczakJWysoczynskiMHayekFJanowska-WieczorekARatajczakMZMembrane-derived microvesicles: important and underappreciated mediators of cell to cell communicationLeukemia2006201487149516791265
- Baj-KrzyworzekaMMajkaMPraticoDPlatelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cellsExp Hematol20023045045912031651
- Janowska-WieczorekAMajkaMKijowskiJPlatelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftmentBlood2001983143314911698303
- RozmyslowiczTMajkaMKijowskiJPlatelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIVAIDS200317334212478067
- RatajczakJMiekusKKuciaMEmbryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein deliveryLeukemia20062084785616453000
- FevrierBViletteDArcherFCells release prions in association with exosomesProc Natl Acad Sci U S A20041019683968815210972
- RobertsonCBoothSABeniacDRCellular prion protein is released on exosomes from activated plateletsBlood20061073907391116434486
- VellaLJGreenwoodDLVCappaiREnrichment of prion protein in exosomes derived from ovine cerebral spinal fluidVet Immunol Immunopathol200812438539318501435
- PisetskyDSMicroparticles as biomarkers in autoimmunity: from dust bin to center stageArthritis Res Ther20091113519954508
- ReichCFPisetskyDSThe content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosisExp Cell Res200931576076819146850
- ArdoinSPShanahanJCPisetskyDSThe role of microparticles in inflammation and thrombosisScand J Immunol20076615916517635793
- Baj-KrzyworzekaMSzatanekRWeglarczykKTumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytesCancer Immunol Immunother20055580881816283305
- Pelchen-MatthewsARaposoGMarshMEndosomes, exosomes and Trojan virusesTrends Microbiol20041231031615223058
- ColtelNCombesVWassmerSCChiminiGGrauGECell vesiculation and immunopathology: implications in cerebral malariaMicrobes Infect200682305231616829152
- RatajczakMZEnhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cellsTransfusion2006461199120916836568
- Janowska-WieczorekAMarquez-CurtisLAWysoczynskiMRatajczakMZEnhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cellsTransfusion2006461199120916836568
- JunkarISustarVFrankMBlood and synovial microparticles as revealed by atomic force and scanning electron microscopeOpen Autoimmun J20091e50e58
- Mrvar-BreckoASustarVJansaVIsolated microvesicles from peripheral blood and body fluids as observed by scanning electron microscopeBlood Cells Mol Dis20104430731220199878
- LuJGetzGMiskaEAMicroRNA expression profiles classify human cancersNature200543583483815944708
- CalinGAFerracinMCimminoAA microRNA signature associated with prognosis and progression in chronic lymphocytic leukemiaN Engl J Med20053531793180116251535
- MetiasSMLianidouEYousefGMMicroRNAs in clinical oncology: at the crossroads between promises and problemsJ Clin Pathol20096277177619734473
- PatelNSauteRBody fluid micro(mi)RNA as biomarkers for human cancerJ Nucleic Acids Investig20112e1
- WhiteNMYousefGMMicroRNAs: exploring a new dimension in the pathogenesis of kidney cancerBMC Med201086520964839
- NielsenBSJorgensenSFogJUHigh levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patientsClin Exp Metastasis201028273821069438
- SingerSJNicolsonGLThe fluid mosaic model of the structure of cell membranesScience19721757207314333397
- SimonsKIkonenEFunctional rafts in cell membranesNature19973875695729177342
- BrownDALondonEFunctions of lipid rafts in biological membranesAnnu Rev Cell Dev Biol1998141111369891780
- BrownDALondonEJStructure and origin of ordered lipid domains in biological membranesJ Membr Biol19981641031149662555
- PikeLJRafts defined: a report on the keystone symposium on lipid rafts and cell functionJ Lipid Res2006471597159816645198
- SimonsKGerlMJRevitalizing membrane rafts: new tools and insightsNat Rev Mol Cell Biol20101168869920861879
- MelkonianKAOstermeyerAGChenJZRole of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylatedJ Biol Chem1999274391039179920947
- Kralj-IglicVHeinrichVSvetinaSZeksBFree energy of closed membrane with anisotropic inclusionsEur Phys J B19991058
- Kralj-IglicVRemskarMIglicADeviatoric elasticity as a mechanism describing stable shapes of nanotubesReimerAHorizons in World PhysicsHauppauge, NYNova Science Publishers2004
- HillTLAn Introduction to Statistical ThermodynamicsReading, MAAdison Wesley1986
- Kralj-IglicVIglicAGomiscekGMicrotubes and nanotubes of a phospholipid bilayer membraneJ Phys A: Math Gen20023515331549
- Kralj-IglicVBabnikBGaugerDRQuadrupolar ordering of phospholipid molecules in narrow necks of phospholipid vesiclesJ Stat Phys2006125727752
- HelfrichWElastic properties of lipid bilayers – theory and possible experimentsZ Naturforsch C1973286937034273690
- SeifertUConfigurations of fluid membranes and vesiclesAdv Phys19974613137
- MaySIglicARescicJBridging like-charged macroions through long divalent rod-like ionsJ Phys Chem B20081121685169218205341
- GongadzeEIglicADecrease of permittivity of an electrolyte solution near a charged surface due to saturation and excluded volume effectsBioelectrochemistry10.1016/j.bioelchem.2011.12.001
- GongadzeEvan RienenUIglicAGeneralized Stern models of an electric double layer considering the spatial variation of permittivity and finite size of ions in saturation regimeCell Mol Biol Lett20111657659421847663
- Kralj-IglicVSustarVHagerstrandHSuppression of membrane vesiculation: A possible anticoagulant, antimetastatic and antiinflammatory effect of heparinPiyathilakeDELiangRHHeparin: Properties, Uses and Side EffectsHauppauge, NYNova Science Publishers20112757
- Kralj-IglicVIglicAHagerstrandHPeterlinPStable tubular microexovesicles of the erythrocyte membrane induced by dimeric amphiphilesPhys Rev E20006142304234
- Bobrowska-HagerstandMKralj-IglicVIglicABialkowskaKIsomaaBHägerstrandHTorocyte membrane endovesicles induced by octaethyleneglycol dodecylether in human erythrocytesBiophys J1999773356336210585958
- IglicABabnikBBohincKOn the role of anisotropy of membrane constituents in formation of a membrane neck during budding of a multicomponent membraneJ Biomech20074057958516584736
- JorgacevskiJFosnaricMVardjanNFusion pore stability of peptidergic vesiclesMol Membr Biol201027658020334578
- GimsaUIglicAFiedlerSActin is not required for nanotubular protrusions of primary astrocytes grown on metal nanolawnMol Membr Biol20072424325517520481
- VeranicPLokarMSchutzGJDifferent types of cell-to-cell connections mediated by nanotubular structuresBiophys J2008954416442518658210
- KabasoDLokarMKralj-IglicVTemperature and cholera toxin B are factors that influence formation of membrane nanotubes in RT4 and T24 urothelial cancer cell linesInt J Nanomedicine2011649550921468353
- KabasoDGongadzeEElterPAttachment of rod-like (BAR) proteins and membrane shapeMini Rev Med Chem20111127228221428902
- IglicAFosnaricMHagerstrandHKralj-IglicVCoupling between vesicle shape and the non-homogeneous lateral distribution of membrane constituents in Golgi bodiesFEBS Lett200457491215358531
- MaresTDanielMPerutkovaSRole of phospholipid asymmetry in the stability of inverted hexagonal mesoscopic phasesJ Phys Chem B20088165751658419367813
- FosnaricMKralj-IglicVBohincKStabilization of pores in lipid bilayers by anisotropic inclusionsJ Phys Chem B20031071251912526
- KanduserMFosnaricMSentjurcMEffect of surfactant polyoxyethylene glycol (C12E8) on electroporation of cell line DC3FColloid Surfaces A2003214205217
- GozdzWTGompperGComposition-driven shape transformations of membranes of complex topologyPhys Rev Lett19988042134216
- GozdzWTGompperGShapes and shape transformations of two-component membranes of complex topologyPhys Rev E19995943054316
- ShlomovitzRGovNSRouxAMembrane-mediated interactions and the dynamics of dynamin oligomers on membrane tubesNew J Phys201113125
- IglicAKralj-IglicVEffect of anisotropic properties of membrane constituents on stable shape of membrane bilayer structureTienHTOttova-LeitmannovaAPlanar Lipid Bilayers (BLMs) and their ApplicationsAmsterdam, The NetherlandsElsevier2003143172
- IglicABabnikBGimsaUKralj-IglicVOn the role of membrane anisotropy in beading transition of undulated tubular membrane structuresJ Phys A Math Gen20053885278536
- IglicAHagerstrandHBobrowska-HagerstrandMPossible role of phospholipid nanotubes in directed transport of membrane vesiclesPhys Lett A2003310493497
- ScharaKJansaVSustarVMechanisms for the formation of membranous nanostructures in cell-to-cell communicationCell Mol Biol Lett20091463665619554268
- SuštarVBedina-ZavecAStukeljRNanoparticles isolated from blood – a reflection of vesiculability of blood cells during the isolation processInt J Nanomedicine201162737274822128248
- WolfPThe nature and significance of platelet products in human plasmaBr J Haematol1967132692886025241
- RumsbyMGTrotterJAllanDMichellRHRecovery of membrane micro-vesicles from human erythrocytes stored for transfusion: a mechanism for the erythrocyte discocyte-to-spherocyte shape transformationBiochem Soc Trans19775126128892138
- GreenwaltTJThe how and why of exocytic vesiclesTransfusion20064614315216398744
- SensPGovNForce balance and membrane shedding at the red-blood-cell surfacePhys Rev Lett20079814
- BlackPHShedding from normal and cancer-cell surfacesN Engl J Med1980303141514167001235
- TaylorDDChouINBlackPHIsolation of plasma-membrane fragments from cultured murine melanoma cellsBiochem Biophys Res Commun19831134704766870870
- Kralj-IglicVBatistaUHagerstrandHOn mechanisms of cell plasma membrane vesiculationRadiol Oncol199832119123
- TaylorDDBlackPHNeoplastic and developmental importance of plasma membrane vesiclesAm Zool198726411415
- BastidaEOrdinasAJamiesonGAIdentity of procoagulant and platelet aggregating activities in microvesicles from human glioblastoma cellsThromb Haemost198350218
- del CondeIBharwaniLDDietzenDJMicrovesicle-associated tissue factor and Trousseau’s syndromeJ Thromb Haemost20075707417239164
- RauchUAntoniakSTissue factor-positive microparticles in blood associated with coagulopathy in cancerThromb Haemost20079791017200763
- TesselaarMERomijnFPvan der LindenIKMicroparticle-associated tissue factor activity in cancer patients with and without thrombosisJ Thromb Haemost200971421142319500241
- MullerIKlockeAAlexMIntravascular tissue factor initiates coagulation via circulating microvesicles and plateletsFASEB J20001747647812514112
- MackmanNRole of tissue factor in hemostasis, thrombosis, and vascular developmentArterioscler Thromb Vasc Biol2004241015102215117736
- GarnierDMilsomCMagnusNRole of the tissue factor pathway in the biology of tumor initiating cellsThromb Res2010125Suppl 2S445020434004
- LeeTHD’AstiEMagnusNMicrovesicles as mediators of intercellular communication in cancer – the emerging science of cellular “debris”Semin Immunopathol20113345546721318413
- ProkopiMPulaGMayrUProteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell culturesBlood200911472373219369228
- BoilardENigrovicPALarabeeKPlatelets amplify inflammation in arthritis via collagen-dependent microparticle productionScience201032758058320110505
- HronGKollarsMWeberHTissue factor positive microparticles – cellular origin and association with coagulation activation in patients with colorectal cancerThromb Haemost20079711912317200778
- FurieBFurieBCCancer-associated thrombosisBlood Cells Mol Dis20063617718116490369
- Janowska-WieczorekAWysoczynskiMKijowskiJMicrovesicles derived from activated platelets induce metastasis and angiogenesis in lung cancerInt J Cancer200511375276015499615
- LimaLGChammasRMonteiroRQTumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine- dependent mannerCancer Lett200928316817519401262
- Dominguez-MalagonHdel Carmen Valdez-CarrilloMCano-ValdezMDermatofibroma and dermatofibrosarcoma protuberans: a comparative ultrastructural studyUltrastruct Pathol2006428329116971353
- KimJWWieckowskiETaylorDDFas-ligand positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytesClin Cancer Res2005111010102015709166
- AbrahamsVMStraszewskiSLKamsteegMEpithelial ovarian cancer cells secrete functional Fas ligandCancer Res2003635573558114500397
- JansaRSustarVFrankMNumber of microvesicles in peripheral blood and ability of plasma to induce adhesion between phospholipid membranes in 19 patients with gastrointestinal diseasesBlood Cells Mol Dis20084112413218387323
- BaranJBaj-KrzyworzekaMWeglarczykKCirculating tumour-derived microvesicles in plasma of gastric cancer patientsCancer Immunol Immunother20105984185020043223
- UrbanijaJTomsicNLokarMCoalescence of phospholipid membranes as a possible origin of anticoagulant effect of serum proteinsChem Phys Lipids2007150495717662972
- SustarVJansaRFrankMSuppression of membrane microvesiculation. A possible anticoagulant and anti-tumor progression effect of heparinBlood Cells Mol Dis20094222322719261492
- FrankMSodin-SemrlSRozmanBEffects of low-molecular-weight heparin on adhesion and vesiculation of phospholipid membranes – a possible mechanism for the treatment of hyper-coagulability in antiphospholipid syndromeAnn N Y Acad Sci2009117387488619758240
- SmorenburgSMHettiarachchiRJVinkRBullerHRThe effects of unfractionated heparin on survival in patients with malignancy – a systematic reviewThromb Haemost1999821600160410613641
- RustomASaffrichRMarkovicINanotubular highways for intercellular organelle transportScience20043031007101014963329
- WatkinsSCSalterRDFunctional connectivity between immune cells mediated by tunneling nanotubulesImmunity20052330931816169503
- VidulescuCClejanSO’ConnorKCVesicle traffic through intercellular bridges in DU 145 human prostate cancer cellsJ Cells Mol Med20048388396
- MauseSFWeberCMicroparticles: protagonists of a novel communication network for intercellular information exchangeCirc Res20101071047105721030722
- van DoormaalFFKleinjanADi NisioMCell-derived microvesicles and cancerNeth J Med20096726627319687520
- CanhamPBThe minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cellJ Theor Biol19702661815411112
- MrowczynskaLSalzerUIglicAHgerstrandHCurvature factor and membrane solubilisation, with particular reference to membrane raftsCell Biol Int20113599199521438858
- GongadzeEKabasoDBauerSAdhesion of osteoblasts to a nanorough titanium implant surfaceInt J Nanomedicine201161801181621931478