Abstract
Chemoprevention that impedes one or more steps in carcinogenesis, via long-term administration of naturally occurring or synthetic compounds, is widely considered to be a crucial strategy for cancer control. Selenium (Se) has chemopreventive effects, but its application is limited due to a low therapeutic index as shown in numerous animal experiments. In contrast to Se, which was known for its toxicity prior to the discovery of its beneficial effects, the natural compound epigallocatechin-3-gallate (EGCG) was originally considered to be nontoxic. Due to its preventive effects on many types of cancer in various animal models, EGCG has been regarded as a prime example of a promising chemopreventive agent without major toxicity concerns. However, very recently, evidence has accumulated showing that efficacious doses of EGCG used in health promotion may not be far from its toxic dose level. Therefore, both Se and EGCG need to be modified by novel pharmaceutical technologies to attain enhanced efficacy and/or reduced toxicity. Nanotechnology may be one of these technologies. In support of this hypothesis, the characteristics of polylactic acid and polyethylene glycol-encapsulated nano-EGCG and elemental Se nanoparticles dispersed by bovine serum albumin are reviewed in this article. Encapsulation of EGCG to form nano-EGCG leads to its enhanced stability in plasma and remarkably superior chemopreventive effects, with more than tenfold dose advantages in inducing apoptosis and inhibition of both angiogenesis and tumor growth. Se at nanoparticle size (“Nano-Se”), compared with Se compounds commonly used in dietary supplements, has significantly lower toxicity, without compromising its ability to upregulate selenoenzymes at nutritional levels and induce phase II enzymes at supranutritional levels.
Chemoprevention
Chemoprevention is defined as the use of compounds to inhibit the development of cancer, either by blocking the DNA damage that initiates carcinogenesis or by arresting or reversing the progression of premalignant cells in which such damage has already occurred.Citation1 The expanded definition of chemoprevention is: through the use of natural or synthetic substances, to reverse, suppress, and prevent either the initial phase of carcinogenesis or the progression of neoplastic cells to cancer.Citation2 Among many diverse chemopreventive agents, epigallocatechin-3-gallate (EGCG) and various forms of selenium (Se) have been extensively investigated.Citation3,Citation4
EGCG
Tea is one of the most widely consumed beverages worldwide. Green tea contains large quantities of biologically active catechins, which have been identified as important dietary factors for health promotion over the past two decades.Citation5 Among the six kinds of catechins in green tea, EGCG accounts for half or more of total catechin in green tea.Citation6
Efficacious and toxic doses of EGCG
The chemopreventive effects of EGCG have been confirmed in at least 13 human or animal organs, including the esophagus, stomach, lung, small intestine, large intestine, colon, skin, liver, bladder, prostate, pancreas, mammary glands, and oral cavity.Citation7 Most of the studies used the regimen of adding EGCG or green tea extract with EGCG as the major component to drinking water.Citation8–Citation13 As a prominent chemopreventive agent, EGCG must be amenable to prolonged ingestion at levels in excess of normal dietary intake without inducing adverse effects. Because the potential toxicity related to such a regimen has not been investigated, it remains uncertain whether the efficacious doses for chemoprevention are really as far away from the toxic doses as have been superficially inferred.
However, EGCG administration via diet or intraperitoneal (IP) injection has been reported to be associated with various adverse effects. Mice consuming a diet with 1% EGCG for 6 weeks exhibited elevated splenocyte and macrophage proinflammatory markers such as tumor necrosis factor-α, interleukin-6, interleukin-1β, and prostaglandin E2 and disturbed immune cell populations.Citation14 A single IP administration of 100 mg/kg EGCG to mice can generate hepatotoxicity, whereas 150 mg/kg results in 100% mortality within 24 hours.Citation15 Furthermore, a causal association between the consumption of green tea extract and liver damage has recently been established in humans.Citation16 Thirty-four cases of hepatitis following the consumption of green tea extract for the purpose of obesity control have been documented.Citation16 Upon liver histological examination, inflammatory reactions, cholestasis, steatosis, and necrosis were noted. A positive dechallenge was reported in 29 cases, and a positive rechallenge occurred in seven cases.Citation16 The mechanism of EGCG toxicity has been ascribed to its pro-oxidant action, because oxidative stress-associated biomarkers, including hepatic malonyldialdehyde, 4-hydroxynonenal, metallothionein, and phosphorylated histone 2AX, substantially increase in EGCG-intoxicated mice.Citation17 Tea can contain pesticide residues that might cause adverse effects, including hepatotoxicity. Therefore, some cases of tea-related hepatotoxicity may be ascribed to the presence of pesticide residues in tea. However, the published data showing that EGCG is able to cause adverse effects in experimental animals were obtained by using purified EGCG.
In addition to its cancer-preventive effect, EGCG has potential in the prevention of obesity, diabetes, and neurodegenerative diseases. Herein we listed some literature-reported beneficial doses administered through diet or the IP route to obtain a window concept of efficacious doses and toxic doses (). In the studies involving cancer, 0.5%–1% EGCG in the diet was used for 7–24 weeks;Citation18,Citation19 50–60 mg/kg EGCG was IP administered for 2–23 weeks.Citation20,Citation21 In the studies involving type 2 diabetes mellitus and obesity, 0.32%–1% EGCG in the diet was used for 4–16 weeks.Citation22–Citation25 In the studies involving liver and brain protection, 50–75 mg/kg EGCG was IP administered for 1–56 days.Citation14,Citation26,Citation27 Based on the evidence that both 1% EGCG in the diet for 6 weeks of administration and a single IP injection of 100 mg/kg EGCG have adverse effects on mice, it seems obvious that the efficacious doses of EGCG including chemopreventive doses are not far from its toxic dose levels; furthermore, some efficacious doses actually overlap with the toxic doses.
Table 1 Efficacious doses and toxic doses of epigallocatechin-3-gallate in mice
Intervention time of EGCG in chemoprevention
In addition to the aforementioned dose concerns, results obtained regarding the optimal intervention time for EGCG chemoprevention are not particularly promising either. In a transgenic adenocarcinoma of the mouse prostate (TRAMP) model, which closely emulates human disease, it was found that an intervention starting at the age of 8 weeks by adding green tea polyphenol (GTP), with EGCG as the major component to drinking water at a concentration of 0.1% (w/v), generated pronounced chemopreventive efficacy.Citation28 Specifically, the GTP treatment reduced the cancer incidence in TRAMP mice from 100% to 35% and the cancer metastasis to lymph and liver to null from 95% and 65%, respectively. Without the GTP treatment, the TRAMP mice had enlarged prostate and genitourinary weight (4.6- and 8.3-fold compared with the nontransgenic mice, respectively), whereas the GTP treatment decreased prostate and genitourinary hyperplasia by 64% and 72%, respectively. The GTP treatment significantly increased median life expectancy of TRAMP mice from 42 weeks to 68 weeks. In the serum of TRAMP mice, elevated insulin-like growth factor-1 and vascular endothelial growth factor were significantly reversed by the GTP treatment. In the prostate tissue of TRAMP mice, several key proliferation-associated signaling proteins and metastasis-related proteins were substantially suppressed by the GTP treatment.Citation28,Citation29 These impressive experimental results suggest that GTP or EGCG has tremendous potential for prostate cancer prevention.
The impact of GTP intervention time on prostate cancer prevention in TRAMP mice was further investigated by the same team. Significantly, unlike the promising results obtained when GTP was initiated at the age of 8 weeks, when treatment started at 18 weeks the preventive effect was largely compromised, whereas when begun at the age of 28 weeks the preventive effect disappeared almost completely.Citation30 The median life span of TRAMP mice is 42 weeks. Given that the human median life span is 70 years, accordingly (if the effect in humans was analogous), someone starting to drink green tea at 13 years old (equivalent to the 8th week of TRAMP mice) might gain a pronounced chemopreventive effect, whereas starting at 30 years old (equivalent to the 18th week of TRAMP mice) might have only a weak effect on cancer development. Thus, the promising chemopreventive effects of GTP might be largely limited to earlier intervention for adolescents, who nowadays prefer carbonated drinks rather than tea in many parts of the world. For adults who are willing to accept chemopreventive practices, the chemopreventive efficacy of EGCG needs to be enhanced.
Encapsulated nano-EGCG for chemoprevention
Typically, but not exclusively, nanoscience investigates objects in the range of 1–100 nm.Citation31 As applied to biology, this field has led to the advent of nanomedicine, which has many facets, one of the most important of which is the nanofabrication of drugs in nanoparticle-based drug-delivery systems.Citation32–Citation34 One advantage of this technology is that drugs included in nanoformulations can be protected from the destructive action of external media.Citation35 In addition, it is now well established that drugs encapsulated in nanoparticles exhibit distinct pharmacokinetic and pharmacodynamic profiles as compared with the nonencapsulated free drugs.Citation36,Citation37
“Nanochemoprevention”, a term coined by Siddiqui and MukhtarCitation38 very recently, involves the utilization of nanotechnology to improve the pharmacokinetic and pharmacodynamic profiles of chemopreventive agents. For example, curcumin is a widely studied phytochemical with chemopreventive potential. Encapsulated nanocurcumin manifests enhanced cellular uptake and cytotoxicity in vitro, as well as superior bioavailability and anticancer activity in vivo over nonencapsulated free curcumin.Citation39–Citation41 EGCG encapsulated in lipid nanocapsules exhibited a stable status without degradation in the aqueous phase over 4 weeks, whereas free EGCG totally degraded within 4 hours.Citation42 When EGCG is encapsulated in chitosan, its bioavailability significantly increases compared with nonencapsulated free EGCG. Specifically, oral administration of chitosan-encapsulated nano-EGCG enhanced intestinal absorption by a factor of 1.8 relative to free EGCG and enhanced the plasma exposure of total EGCG by a factor of 1.5 relative to free EGCG.Citation43,Citation44 Polylactic acid (PLA) and polyethylene glycol (PEG) are biologically inert and completely biocompatible without toxicity or antigenic reactions.Citation45 EGCG can be encapsulated in PLA–PEG nanoparticlesCitation46 whose average size is 260 nm, as shown in supplementary figure S1–2 in Siddiqui et al.Citation46 The biological activities of PLA–PEG-encapsulated nano-EGCG versus nonencapsulated free EGCG have been compared in term of apoptosis induction, inhibition of angiogenesis and tumor growth, and EGCG retention in blood after IP administration, as summarized in .Citation46 Overall, PLA–PEG-encapsulated nano-EGCG, compared with the nonencapsulated free EGCG, is resistant to degradation in blood and produces remarkably superior chemopreventive effects, with an over tenfold dose advantage in inducing apoptosis and inhibiting angiogenesis and tumor growth. Together, these studies reveal that nanoparticle-mediated delivery of EGCG could serve as a basis for enhancing the bioavailability of EGCG.
Table 2 Comparison of biological activities between encapsulated nanoepigallocatechin-3-gallate (EGCG) and nonencapsulated free EGCG
Selenium
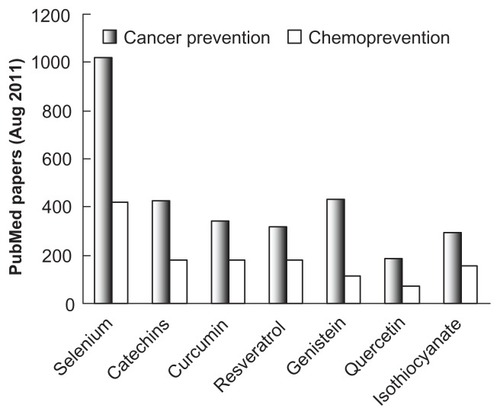
Although phytochemicals including EGCG have received considerable attention for their cancer-preventive effects, the most extensively investigated chemopreventive agent is Se, as indicated in , which depicts the search results for the number of papers in PubMed using the keywords “cancer prevention” or “chemoprevention” along with the specific chemopreventive agent.
Se-dependent selenoproteins and cancer prevention
Se is capable of exerting multiple actions on the physiological system by modifying the expression of 25 human selenoproteins, whose synthesis is dependent upon the incorporation of the 21st genetically encoded protein amino acid, selenocysteine.Citation47–Citation49 Most of the selenoproteins, such as selenoenzymes of glutathione peroxidases (GPx) and thioredoxin reductases (TrxR), take part in antioxidant defense.Citation50–Citation52 Activities of seleoenzymes are affected by Se at nutritional levels; therefore, Se deficiency leads to reduced activities of seleoenzymes.Citation53,Citation54 Transgenic mice whose selenoprotein synthesis is disrupted are predisposed to precancerous changes.Citation55,Citation56 Human epidemiological studies have found an inverse relationship between Se status and cancer risk.Citation4,Citation57 In SELECT (the Selenium and Vitamin E Cancer Prevention Trial), the participants had optimal Se status; thus, Se supplementation at nutritional level had no effect on cancer risk, whereas the participants with low Se status showed reduced cancer risk after Se supplementation at nutritional levels.Citation58,Citation59 These results suggest that Se at nutritional levels has a cancer-prevention effect via enhancing the expression of selenoproteins in those subjects with suboptimal Se status.
Se-induced phase II enzymes in cancer prevention
Phase II enzymes such as quinone reductase and glutathione S-transferase (GST) are a class of inducible enzymes that are upregulated in response to toxic insults.Citation60 Upregulation of phase II enzymes has been implicated in the detoxification of numerous oxidative and electrophilic species during xenobiotic metabolism.Citation61 The cancer-preventive effect of many chemopreventive agents, including EGCG, sulforaphane, curcumin, and resveratrol, is associated with the upregulation of phase II enzymes.Citation62–Citation66 Among various phase II enzymes, GST plays an important role in cellular protection against carcinogens by conjugating their electrophilic metabolites with GSH.Citation67,Citation68 Evidence suggests that the level of GST expression is a crucial factor in determining the sensitivity of cells to a broad spectrum of toxic chemicals; hence, the induction of GST by chemopreventive agents enables experimental animals to tolerate exposure to carcinogens.Citation69 Enhanced GST expression can limit tumor development.Citation70 Many Se compounds can oxidize thiols, consequently producing superoxide and other reactive oxygen species (ROS).Citation71,Citation72 Modest amounts of ROS promote the translocation of the transcription factor Nrf2 into the nucleus, where Nrf2 binds to the antioxidant response element in phase II enzyme genes to activate the transcription of phase II enzyme mRNAs.Citation73 Xiao and ParkinCitation74 found that 16 Se compounds were able to increase quinone reductase activity, and seven of them also increased GST activity in murine hepatoma cells. Se at supranutritional levels, which are roughly ten- to 30-fold higher than nutritional levels, is capable of inducing phase II enzymes and exhibits powerful chemopreventive effects.Citation75–Citation79 Thus, the chemopreventive effects of Se at supranutritional levels are associated with the induction of phase II enzymes.
Se-mediated cytotoxicity and cancer prevention
The ROS that originate from Se-promoted thiol oxidation, if in sufficient quantity, will result in intracellular and extracellular oxidative stress, leading to cytotoxicity.Citation80,Citation81 The cytotoxic effects of Se may partially account for their chemopreventive activity.Citation82–Citation84 However, the general therapeutic utility of this mechanism is questionable and should be approached with caution. It is known that inhibition of TrxR results in enhanced selenite cytotoxicity and that cells overexpressing TrxR1 are significantly more resistant to selenite cytotoxicity than control cells.Citation85,Citation86 TrxR1 has been shown to be upregulated in various cancer cells; thus, cancer cells are likely to be more resistant than normal cells to Se cytotoxicity. Drug-resistant tumor cells with high intracellular GSH exhibit a high degree of sensitivity to selenite cytotoxicity, whereas normal cells with high intracellular GSH would be more sensitive to Se cytotoxicity than some types of cancer cells with low intracellular GSH.Citation87,Citation88 Normal cells possess functional p53, which is mutated in most cancer cells. It has been shown that p53 can enhance the cytotoxicity of Se, suggesting that normal cells may be more sensitive to Se cytotoxicity than p53-mutated cancer cells.Citation89–Citation92 Indeed, the cytotoxic effects of both inorganic and organic Se compounds were more potent in normal hepatocytes as compared with hepatic carcinoma cells, and nontumorigenic prostate cells are highly sensitive to Se toxicity as compared with prostate cancer cells at physiologically relevant concentrations.Citation93,Citation94 For Se-induced cytotoxicity to be able to operate as a chemopreventive mechanism, Se toxicity would appear to be unavoidable. Therefore, Se-dependent selenoproteins and Se-induced phase II enzyme mechanisms, which are not associated with evoked toxicity, become more attractive to explain the cancer-preventive effects of Se, whereas the “enhanced cytotoxicity” mechanism ought to be limited to Se-sensitive cancer cells whose proliferation can be effectively suppressed by Se at safe doses.
Preparation of elemental Se nanoparticles
A decade ago, elemental Se in the redox state of zero was considered to be biologically inert.Citation95 Indeed, red elemental Se, formed in the redox system of selenite and GSH, is unstable and can further aggregate into gray or black elemental Se if there are no controlling factors in the redox system, leading to the disappearance of bioactivities.Citation96 We reported in 2001 that the presence of proteins such as bovine serum albumin (BSA) in the redox system at a tenfold excess by mass relative to Se can control the aggregation of elemental Se atoms; the resultant Se particles, referred to as Nano-Se, fall into a size distribution of 20–60 nm, with an average size of 36 nm.Citation96 Consistent with this finding, Mishra et alCitation97 demonstrated the formation of BSA-dispersed Se nanoparticles when selenourea was oxidized into elemental Se. Dobias et alCitation98 recently showed that some particular proteins, such as alcohol dehydrogenase, can specifically bind to Se nanoparticles, resulting in a narrower size distribution. In addition to protein, polysaccharides have been revealed to be effective dispersants for controlling the formation of Se nanoparticles.Citation99,Citation100 The formation of Se nanoparticles is not limited to in vitro conditions, as some strains of micro-organisms have the capacity of reducing selenite into Se nanoparticles.Citation101–Citation103
The bioactivities and toxicities of inorganic sodium selenite, organic selenomethionine (SeMet), and Se-methylselenocysteine (SeMSC) have been extensively investigated. Based on the preceding review, the optimal form of Se for nutritional supplementation and cancer prevention would be expected to have distinctly low toxicity and to possess good bioactivities in terms of upregulating selenoenzymes at nutritional levels and inducing phase II enzymes at supranutritional levels.
Comparison of bioactivities and toxicities between inorganic Se and Nano-Se
Sodium selenite has been used in livestock and humans to prevent Se-deficiency disorders. Usually, it is used as a reference Se compound in the studies of Se bioavailability and toxicity. In HepG2 cells, although selenite dose-dependently increased GPx and PHGPx activities (R2 = 0.9881 and 0.9956, respectively), there were no significant differences in elevating these selenoenzyme activities between selenite and Nano-Se at the same doses, based on total Se content.Citation96 When Nano-Se and selenite were added to a Se-deficient diet at a level of 0.1 ppm Se for Se supplementation in rats, selenite significantly increased hepatic Se by 8.8-fold and hepatic GPx activity by 48.8-fold. There were no significant differences in these biomarkers between selenite and Nano-Se.Citation96 These in vitro and in vivo results demonstrate that the bioavailability of the two Se sources is equal.
Selenite toxicity is associated with the interaction of selenite with GSH to form reactive selenotrisulfides, leading to the production of ROS.Citation80 Selenite is one order of magnitude more effective than Nano-Se in oxidizing GSH, suggesting that the cytotoxic effect of selenite but not Nano-Se may be enhanced by extracellular GSH.Citation96 Indeed, exposure of HepG2 cells to the cotreatment of nontoxic Nano-Se and GSH reveals no cytotoxicity, whereas exposure of HepG2 cells to the cotreatment of an otherwise nontoxic dose of selenite, but in the presence of GSH, produced significant cytotoxicity.Citation96 According to this evidence, it is anticipated that in tissues where extracellular GSH is elevated, enhanced cytotoxicity will be much more likely to occur for selenite than for Nano-Se. Consequently, selenite would be more toxic than Nano-Se in vivo. Indeed, the oral acute toxicity of selenite was 7.2-fold that of Nano-Se, according to the medium lethal dose (LDCitation50) values obtained from mice.Citation96 The US National Research Council recommends growth inhibition as the best indicator of Se toxicity.Citation104 The major target of Se toxicity is liver tissue.Citation105 In a short-term toxicity study, mice were orally administered saline as control, Nano-Se, and selenite at 4 mg Se/kg for 4 weeks. Body weight in the selenite group was significantly suppressed by 30%, whereas body weight in the Nano-Se group remained not significantly different from the control.Citation106 At the end of the experiments, selenite caused prominent liver injury, whereas the hepatic architecture in the Nano-Se group remained unaltered.Citation106 Furthermore, in a subchronic toxicity study in which rats were fed with diets containing 0 ppm, 2 ppm, 3 ppm, 4 ppm, and 5 ppm Se for 13 weeks, Nano-Se unequivocally manifested lower toxicity compared with either inorganic selenite or naturally occurring Se-enriched soy protein (high-Se protein) in all observed biomarkers, including growth inhibition, hematology, clinical chemistry, relative organ weights, and histopathology parameters ().Citation107
Table 3 Subchronic toxicity of selenium (Se) compounds in rats
Comparison of bioactivities and toxicities between organic Se and Nano-Se
SeMet is the predominant chemical form of Se in foodstuffs. Numerous experimental studies have suggested that SeMet has excellent bioavailability and lower toxicity, as compared with selenite.Citation108 Because Nano-Se has lower toxicity profiles compared with the high-Se protein whose Se constitution would be largely taken up by SeMet, it is warranted to make direct and comprehensive comparisons between Nano-Se and SeMet in terms of bioactivities and toxicity.Citation107 SeMet and Nano-Se were orally administered to Se-deficient mice daily for 7 days at two nutritional doses to compare bioavailability, and at a supranutritional dose to evaluate phase II enzyme induction. Although SeMet has been considered as a good Se source with excellent bioavailability, at the two tested nutritional doses, SeMet and Nano-Se equally increased tissue Se levels and the activities of GPx and TrxR.Citation109 Significant differences between the two Se sources were found at the supranutritional dose; SeMet increased Se levels more efficiently than Nano-Se in all measured tissues, including the liver, kidney, and blood.Citation109 However, the high retention of Se in the liver subjected to SeMet did not guarantee that SeMet could increase hepatic GST activity; in contrast, Nano-Se, which provided less Se to the liver compared with SeMet, generated a significant induction of hepatic GST compared with either the control group or the SeMet group.Citation109 Se sequestration in protein via the nonspecific replacement of methionine using SeMet can readily explain such a paradoxical result.Citation110 Excess substitution of methionine residues by SeMet may alter physiochemical properties of some structural proteins and reduce the accumulation of active Se species that exert anticancer actions.Citation110
Thus, the high Se accumulation deposited by SeMet at supranutritional levels cannot necessarily be considered as a merit; in contrast, it reduces the chemopreventive potential of SeMet, as evidenced by the GST induction, and increases the risk of SeMet toxicity. This interpretation is supported by the following results: (1) the acute oral toxicity of SeMet was 3.6-fold that of Nano-Se according to the LD50 values obtained from mice;Citation109 (2) following administration of a single oral dose of 10 mg Se/kg to mice, after 12 hours, Nano-Se did not significantly elevate serum liver enzymes, but SeMet significantly increased serum alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase activities by fourfold to 25-fold relative to the control;Citation109 and (3) following repetitive daily oral administration of 5 mg Se/kg/day to mice for 7 days, SeMet exhibited significantly higher toxicity than Nano-Se in terms of growth suppression and liver injury.Citation109
SeMSC is considered to be one of the most effective Se compounds for chemoprevention, but unfortunately its systemic toxicities are high as well.Citation112–Citation114 Zhang et alCitation111 have demonstrated that SeMSC and Nano-Se have equal bioavailability at nutritional doses. Although the GST induction efficacy of the two Se sources was similar at supranutritional doses, SeMSC had a greater tendency toward Se toxicity.Citation111 This is evidenced by: (1) the acute oral toxicity of SeMSC was 6.3-fold that of Nano-Se according to the LD50 values obtained from mice;Citation111 (2) following administration of a single oral dose of 10 mg Se/kg to mice, after 12 hours, serum alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase activities were all significantly higher in SeMSC-treated mice than in Nano-Se-treated mice;Citation111 and (3) following repetitive daily oral administration of 10 mg Se/kg/day to mice for 7 days, SeMSC resulted in 80% mortality, whereas Nano-Se resulted in only 10% mortality.Citation111
At supranutritional levels, Wang et alCitation109 and Zhang et alCitation111 found that SeMSC, as with SeMet, increased Se levels more efficiently than Nano-Se in all measured tissues. High Se accumulation in SeMet-treated mice can be attributed to Se sequestration via nonspecific substitution of SeMet for methionine in proteins. Obviously, such a mechanism cannot apply in the case of high Se accumulation produced by supranutritional SeMSC; however, the significant differences in tissue Se retention between SeMSC and Nano-Se may be affected by the pore size of vessels. The pore sizes of normal vessels are 2–6 nm, so the entry of SeMSC molecules at approximately 1 nm appears not to be limited, whereas the entry of Se nanoparticles with an average size of 36 nm (20–60 nm) should be affected by vessel pore sizes. To support this hypothesis, ie, to observe the impact of size on Se accumulation, we prepared two kinds of Nano-Se with different size distributions, based on the principle that, during their preparation, a higher BSA concentration generates smaller Se nanoparticles.Citation115,Citation116 As expected, we found that Se accumulation at supranutritional levels was size dependent, such that small size led to high Se retention.Citation116 It is worth noting that size-dependent Se accumulation has an important implication in explaining the low toxicity of Nano-Se. Cells may change to passive absorption of Se at near-toxic supranutritional levels after the most fundamental physiological needs of a cell for selenoenzyme synthesis have been fully met. Under such conditions, large size would constitute a barrier for Se nanoparticles to enter into cells.Citation117
Therefore, the significantly reduced Se accumulation in tissues subjected to Nano-Se at supranutritional levels as compared with SeMet or SeMSC may effectively prevent Se toxicity.Citation109,Citation111 In addition, although Se nanoparticles show reduced Se retention in normal tissues as compared with SeMet and SeMSC, Se nanoparticles may show enhanced Se permeation and retention in tumor tissues, because tumor blood vessels possess large pores with a size distribution ranging from 100 nm to 800 nm, in stark contrast to small pores of 2–6 nm in the vessels of healthy tissues.Citation118 This would confer a unique “targeting” advantage to Nano-Se, unavailable with other forms of Se. Recently, Sommer et alCitation119 demonstrated that pulsed red laser light can force cancer cells to take up cytotoxic drugs, including EGCG, via nanoscopic interfacial water layers in cells, thereby resulting in enhanced cytotoxicity. Because nanoparticles have inherent characteristics of enhanced permeation and retention in tumor tissues,Citation118 with the auxiliary effect of pulsed red laser light, the targeting advantage of Nano-Se and nano-EGCG would likely be further enhanced.
Conclusions and future prospectives
EGCG is a naturally occurring chemopreventive agent. PLA–PEG-encapsulated nano-EGCG, compared with nonencapsulated free EGCG, is resistant to degradation in blood and produces remarkably superior chemopreventive effects, with over a tenfold dose advantage in inducing apoptosis, inhibiting angiogenesis and tumor growth. So Nano-EGCG provides a paradigm for the use of nanoparticle-mediated delivery to enhance bioavailability. Se is a chemopreventive agent with a narrow margin between toxic amounts and the amounts needed for dietary requirements or therapeutic effects, ie, a low therapeutic ratio. Compared with selenite, SeMet, and SeMSC, Nano-Se has significantly lower toxicity, without compromising the important therapeutic capacities of increasing the activities of selenoenzymes and phase II enzymes. The safety margin and potential toxic effects of Se are important considerations for its role in supplementation. Therefore, Nano-Se can be considered as a novel chemoprevention agent with reduced risk of Se toxicity. Nanotechnology holds promise for chemoprevention, because anticancer nutrients fabricated at the nanometer scale exhibit drastically altered bioactivities and toxicity. Sustained exploration of the nanochemoprevention concept may lead to exciting new horizons in the discovery of novel chemopreventive agents, with an expanded window between efficacious doses and toxic doses.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31170648, to J Zhang), National Basic Research Program of China (973 Program, 2009CB930204), a grant from Anhui Agricultural University to J Zhang, a grant from the Ministry of Education of Auhui Province (2011SQRL051, to Y Wang), and the earmarked fund for China modern agro-industry technology research system.
Disclosure
No conflict of interest was reported by the authors of this article.
References
- GreenwaldPKelloffGBurch-WhitmanCKramerBSChemopreventionCA Cancer J Clin19954531497804897
- SoriaJCKimESFayetteJLantuejoulSDeutschEHongWKChemoprevention of lung cancerLancet Oncol2003465966914602246
- YangCSWangXLuGPicinichSCCancer prevention by tea: animal studies, molecular mechanisms and human relevanceNat Rev Cancer2009942943919472429
- Fairweather-TaitSJBaoYBroadleyMRSelenium in human health and diseaseAntioxid Redox Signal2011141337138320812787
- YangCSWangXGreen tea and cancer preventionNutr Cancer20106293193720924968
- NagleDGFerreiraDZhouYDEpigallocatechin-3-gallate (EGCG): chemical and biomedical perspectivesPhytochemistry2006671849185516876833
- JuJLuGLambertJDYangCSInhibition of carcinogenesis by tea constituentsSemin Cancer Biol20071739540217686632
- XuYHoCTAminSGHanCChungFLInhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidantsCancer Res199252387538791617663
- LandauJMWangZYYangGYDingWYangCSInhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green teaCarcinogenesis1998195015079525286
- MimotoJKiuraKMatsuoK(−)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J miceCarcinogenesis20002191591910783312
- LiaoJYangGYParkESInhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green teaNutr Cancer200448445315203377
- JuJHongJZhouJNInhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green teaCancer Res200565106231063116288056
- LuGLiaoJYangGReuhlKRHaoXYangCSInhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeineCancer Res200666114941150117145898
- PaeMRenZMeydaniMDietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in miceJ Nutr Biochem6162011 [Epub ahead of print.]
- GalatiGLinASultanAMO’BrienPJCellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechinsFree Radic Biol Med20064057058016458187
- MazzantiGMenniti-IppolitoFMoroPAHepatotoxicity from green tea: a review of the literature and two unpublished casesEur J Clin Pharmacol20096533134119198822
- LambertJDKennettMJSangSReuhlKRJuJYangCSHepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in miceFood Chem Toxicol20104840941619883714
- LiGXChenYKHouZPro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer growth: a comparative study in vivo and in vitroCarcinogenesis20103190291020159951
- ZhangQFuHPanJEffect of dietary polyphenon E and EGCG on lung tumorigenesis in A/J micePharm Res2010271066107120112129
- XuHLuiWTChuCYNgPSWangCCRogersMSAnti-angiogenic effects of green tea catechin on an experimental endometriosis mouse modelHum Reprod20092460861819088106
- YinPZhaoJChengSZhuQLiuZZhengguoLExperimental studies of the inhibitory effects of green tea catechin on mice large intestinal cancers induced by 1,2-dimethylhydrazineCancer Lett19947933388187052
- KlausSPultzSThone-ReinekeCWolframSEpigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidationInt J Obes (Lond)20052961562315738931
- Sae-tanSGroveKAKennettMJLambertJD(−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed miceFood Funct2011211111621779555
- BoseMLambertJDJuJReuhlKRShapsesSAYangCSThe major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed miceJ Nutr20081381677168318716169
- WolframSRaederstorffDPrellerMEpigallocatechin gallate supplementation alleviates diabetes in rodentsJ Nutr20061362512251816988119
- ParkJWHongJSLeeKSKimHYLeeJJLeeSRGreen tea polyphenol (−)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemiaJ Nutr Biochem201011038104419962294
- TipoeGLLeungTMLiongECLauTYFungMLNanjiAAEpigallocatechin- 3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in miceToxicology2010273455220438794
- GuptaSHastakKAhmadNLewinJSMukhtarHInhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenolsProc Natl Acad Sci U S A200198103501035511504910
- AdhamiVMSiddiquiIAAhmadNGuptaSMukhtarHOral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancerCancer Res2004648715812215574782
- AdhamiVMSiddiquiIASarfarazSEffective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the diseaseClin Cancer Res2009151947195319276266
- OdomTWPileniMPNanoscienceAcc Chem Res200841156519075560
- KimBYRutkaJTChanWCNanomedicineN Engl J Med20103632434244321158659
- JiangWKimBYRutkaJTChanWCAdvances and challenges of nanotechnology-based drug delivery systemsExpert Opin Drug Deliv2007462163317970665
- FarokhzadOCLangerRImpact of nanotechnology on drug deliveryACS Nano20093162019206243
- WinterhalterMLasicDDLiposome stability and formation: experimental parameters and theories on the size distributionChem Phys Lipids19936435438242841
- GrefRMinamitakeYPeracchiaMTTrubetskoyVTorchilinVLangerRBiodegradable long-circulating polymeric nanospheresScience1994263160016038128245
- NieSXingYKimGJSimonsJWNanotechnology applications in cancerAnnu Rev Biomed Eng2007925728817439359
- SiddiquiIAMukhtarHNanochemoprevention by bioactive food components: a perspectivePharm Res2010271054106020221894
- ShahaniKSwaminathanSKFreemanDBlumAMaLPanyamJInjectable sustained release microparticles of curcumin: a new concept for cancer chemopreventionCancer Res2010704443445220460537
- YallapuMMGuptaBKJaggiMChauhanSCFabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastic cancer cellsJ Colloid Interface Sci2010351192920627257
- AnandPNairHBSungBDesign of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivoBiochem Pharmacol20107933033819735646
- BarrasAMezzettiARichardAFormulation and characterization of polyphenol-loaded lipid nanocapsulesInt J Pharm200937927027719501139
- DubeANicolazzoJALarsonIChitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallateEur J Pharm Sci20104121922520600878
- DubeANicolazzoJALarsonIChitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stabilityEur J Pharm Sci20114442242621925598
- XiaoRZZengZWZhouGLWangJJLiFZWangAMRecent advances in PEG-PLA block copolymer nanoparticlesInt J Nanomedicine201051057106521170353
- SiddiquiIAAdhamiVMBharaliDJIntroducing nanochemo-prevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallateCancer Res2009691712171619223530
- LobanovAVHatfieldDLGladyshevVNEukaryotic selenoproteins and selenoproteomesBiochim Biophys Acta200917901424142819477234
- KryukovGVCastellanoSNovoselovSVCharacterization of mammalian selenoproteomesScience20033001439144312775843
- CastellanoSGladyshevVNGuigóRBerryMJSelenoDB 1.0: a database of selenoprotein genes, proteins and SECIS elementsNucleic Acids Res200836Database issueD33233818174224
- LubosELoscalzoJHandyDEGlutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunitiesAntioxid Redox Signal2011151957199721087145
- ImaiHNakagawaYBiological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cellsFree Radic Biol Med20033414516912521597
- ArnérESFocus on mammalian thioredoxin reductases – important selenoproteins with versatile functionsBiochim Biophys Acta2009179049552619364476
- BarnesKMEvensonJKRainesAMSundeRATranscript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activityJ Nutr200913919920619106321
- SundeRAThompsonKMEvensonJKThompsonBMBlood glutathione peroxidase-1 mRNA levels can be used as molecular biomarkers to determine dietary selenium requirements in ratsExp Biol Med (Maywood)20092341271127919855070
- MoustafaMECarlsonBAEl-SaadaniMASelective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing is opentenyladenosine-deficient seleno-cysteine tRNAMol Cell Biol2001213840385211340175
- Diwadkar-NavsariwalaVPrinsGSSwansonSMSelenoprotein deficiency accelerates prostate carcinogenesis in a transgenic modelProc Natl Acad Sci U S A20061038179818416690748
- BrinkmanMBuntinxFMulsEZeegersMPUse of selenium in chemoprevention of bladder cancerLancet Oncol2006776677416945772
- LippmanSMKleinEAGoodmanPJEffect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT)JAMA2009301395119066370
- ClarkLCCombsGFJrTurnbullBWEffects of Se supplementation for cancer prevention in patients with carcinoma of skin. A randomized controlled trial. Nutritional Prevention of Cancer Study GroupJAMA1996276195719638971064
- ZhuHJiaZZhangLAntioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stressExp Biol Med (Maywood)200823346347418367636
- LiskaDJThe detoxification enzyme systemsAltern Med Rev199831871989630736
- TanXLShiMTangHHanWSpivackSDCandidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cellsJ Nutr20101401404141020554899
- NaHKSurhYJModulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCGFood Chem Toxicol2008461271127818082923
- BrooksJDPatonVGVidanesGPotent induction of Phase 2 enzymes in human prostate cells by sulforaphaneCancer Epidemiol Biomarkers Prev20011094995411535546
- PiperJTSinghalSSSalamehMSTormanRTAwasthiYCAwasthiSMechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liverInt J Biochem Cell Biol1998304454569675878
- LiYCaoZZhuHUpregulation of endogenous antioxidants and Phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stressPharmacol Res20065361516169743
- TalalayPFaheyJWHoltzclawWDPresteraTZhangYChemoprotection against cancer by phase 2 enzymes inductionToxicol Lett19958283
- ColesBKettererBThe role of glutathione and glutathione transferases in chemical carcinogenesisCrit Rev Biochem Mol Biol19902547702182291
- HayesJDPulfordDJThe glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistanceCrit Rev Biochem Mol Biol1995304456008770536
- HayesJDFlanaganJUJowseyIRGlutathione transferasesAnn Rev Pharmacol Toxicol200545518815822171
- SpallholzJEShriverBJReidTWDimethyldiselenide and methylseleninic acid generates superoxide in an in vitro chemiluminesence assay in the presence of glutathione: implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteineNutr Cancer200140344111799920
- SpallholzJEPalaceVPReidTWMethioninase and selenomethionine but not Se-methylselenocysteine generates methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acidsBiochem Pharmacol20046754755415037206
- Pool-ZobelBVeeriahSBöhmerFDModulation of xenobiotic metabolising enzymes by anticarcinogens: focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesisMutat Res2005591749216083918
- XiaoHParkinKLInduction of phase II enzyme activity by various selenium compoundsNutr Cancer20065521022317044777
- IpCLiskDJModulation of phase I and phase II xenobiotic-metabolizing enzymes by selenium-enriched garlic in ratsNutr Cancer1997281841889290126
- El-SayedWMFranklinMRHepatic chemoprotective enzyme responses to 2-substituted selenazolidine-4(R)-carboxylic acidsJ Biochem Mol Toxicol20062029230117163488
- ProkopczykBRosaJGDesaiDChemoprevention of lung tumorigenesis induced by a mixture of benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by the organoselenium compound 1,4-phenylenebis(methylene)selenocyanateCancer Lett2000161354611078911
- NovoselovSVCalvisiDFLabunskyyVMSelenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic miceOncogene2005248003801116170372
- WangLBonordenMJLiGXMethyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefitCancer Prev Res (Phila)2009248449519401524
- SpallholzJEOn the nature of selenium toxicity and carcinostatic activityFree Radic Biol Med19941745647959166
- SekoYImuraNActive oxygen generation as a possible mechanism of selenium toxicityBiomed Environ Sci1997103333399315327
- ShenHMYangCFOngCNSodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cellsInt J Cancer19998182082810328239
- SinhaREl-BayoumyKApoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compoundsCurr Cancer Drug Targets20044132814965264
- ZengHCombsGFJrSelenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasionJ Nutr Biochem2008191717588734
- SeleniusMFernandesAPBrodinOBjörnstedtMRundlöfAKTreatment of lung cancer cells with cytotoxic levels of sodium selenite: effects on the thioredoxin systemBiochem Pharmacol2008752092209918405881
- MadejaZSrokaJNyströmCThe role of thioredoxin reductase activity in selenium-induced cytotoxicityBiochem Pharmacol2005691765177215935149
- CaffreyPBFrenkelGDThe development of drug resistance by tumor cells in vitro is accompanied by the development of sensitivity to seleniteCancer Lett19948159658019989
- CaffreyPBFrenkelGDSelenite cytotoxicity in drug resistant and nonresistant human ovarian tumor cellsCancer Res199252481248161511444
- ZhaoRXiangNDomannFEZhongWExpression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cellsCancer Res2006662296230416489034
- LiGXHuHJiangCSchusterTLuJDifferential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cellsInt J Cancer20071202034204317230520
- KralovaVBrigulovaKCervinkaMRudolfEAntiproliferative and cytotoxic effects of sodium selenite in human colon cancer cellsToxicol in Vitro2009231497150319602434
- SarveswaranSLiroffJZhouZNikitinAYGhoshJSelenite triggers rapid transcriptional activation of p53, and p53-mediated apoptosis in prostate cancer cells: Implication for the treatment of early-stage prostate cancerInt J Oncol2010361419142820428765
- WeillerMLattaMKresseMLucasRWendelAToxicity of nutritionally available selenium compounds in primary and transformed hepatocytesToxicology2004201213015297016
- RebschCMPennaFJ3rdCopelandPRSelenoprotein expression is regulated at multiple levels in prostate cellsCell Res20061694094817160069
- CombsGFJrGarbisuCYeeBCBioavailability of selenium accumulated by selenite-reducing bacteriaBiol Trace Elem Res1996522092258811279
- ZhangJSGaoXYZhangLDBaoYPBiological effects of a nano red elemental seleniumBiofactors200115273811673642
- MishraBHassanPAPriyadarsiniKIMohanHReactions of biological oxidants with seleourea: formation of redox active nanoseleniumJ Phys Chem B2005109127181272316852575
- DobiasJSuvorovaEIBernier-LatmaniRRole of proteins in controlling selenium nanoparticle sizeNanotechnology20112219560521430311
- ZhangYWangJZhangLCreation of highly stable selenium nanoparticles capped with hyperbranched polysaccharide in waterLangmuir201026176171762320964304
- ChenTWongYSZhengWBaiYHuangLSelenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cellsColloids Surf B Biointerfaces200867263118805679
- DebieuxCMDridgeEJMuellerCMA bacterial process for selenium nanosphere assemblyProc Natl Acad Sci U S A2011108134801348521808043
- WangTYangLZhangBLiuJExtracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensorCollids Surf B Biointerfaces20108094102
- MishraRRPrajapatiSDasJDangarTKDasNThatoiHReduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced productChemosphere2011841231123721664643
- OrskovHFlyvbjergASelenium and human healthLancet200035694294311036921
- DiskinCJTomassoCLAlperJCGlaserMLFliegelSELong-term selenium exposureArch Intern Med1979139824826454075
- ZhangJWangHPengDTaylorEWFurther insight into the impact of sodium selenite on selenoenzymes: high-dose selenite enhances hepatic thioredoxin reductase 1 activity as a consequence of liver injuryToxicol Lett200817622322918215477
- JiaXLiNChenJA subchronic toxicity study of elemental Nano-Se in Sprague-Dawley ratsLife Sci2005761989200315707881
- SchrauzerGNSelenomethionine: a review of its nutritional significance, metabolism and toxicityJ Nutr20001301653165610867031
- WangHZhangJYuHElemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in miceFree Radic Biol Med2007421524153317448899
- YangWHendricksonWACrouchRJSatowYStructure of ribonuclease H phased at 2A resolution by MAD analysis of the selenomethionyl proteinScience1990249139814052169648
- ZhangJWangXXuTElemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in miceToxicol Sci2008101223117728283
- MedinaDThompsonHGantherHIpCSe-methylselenocysteine: a new compound for chemoprevention of breast cancerNutr Cancer200140121711799917
- WhangerPDSelenocompounds in plants and animals and their biological significanceJ Am Coll Nutr20022122323212074249
- JohnsonWDMorrisseyRLKapetanovicICrowellJAMcCormickDLSubchronic oral toxicity studies of Se-methylselenocysteine, an organoselenium compound for breast cancer preventionFood Chem Toxicol2008461068107818082924
- HuangBZhangJHouJChenCFree radical scavenging efficiency of Nano-Se in vitroFree Radic Biol Med20033580581314583345
- ZhangJWangHBaoYZhangLNano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and miceLife Sci20047523724415120575
- PengDZhangJLiuQTaylorEWSize effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activityJ Inorg Biochem20071011457146317664013
- TranPAWebsterTJSelenium nanoparticles inhibit Staphylococcus aureus growthInt J Nanomedicine201161553155821845045
- SommerAPZhuDScharnweberTLaser modulated transmembrane convection: Implementation in cancer chemotherapyJ Control Release201014813113420934473