Abstract
Background
Cerium dioxide (CeO2) nanoparticles have potential therapeutic applications and are widely used for industrial purposes. However, the effects of these nanoparticles on primary human cells are largely unknown. The ability of nanoparticles to exacerbate pre-existing inflammatory disorders is not well documented for engineered nanoparticles, and is certainly lacking for CeO2 nanoparticles. We investigated the inflammation-modulating effects of CeO2 nanoparticles at noncytotoxic concentrations in human peripheral blood monocytes.
Methods
CD14+ cells were isolated from peripheral blood samples of human volunteers. Cells were exposed to either 0.5 or 1 μg/mL of CeO2 nanoparticles over a period of 24 or 48 hours with or without lipopolysaccharide (10 ng/mL) prestimulation. Modulation of the inflammatory response was studied by measuring secreted tumor necrosis factor-alpha, interleukin-1beta, macrophage chemotactic protein-1, interferon-gamma, and interferon gamma-induced protein 10.
Results
CeO2 nanoparticle suspensions were thoroughly characterized using dynamic light scattering analysis (194 nm hydrodynamic diameter), zeta potential analysis (−14 mV), and transmission electron microscopy (irregular-shaped particles). Transmission electron microscopy of CD14+ cells exposed to CeO2 nanoparticles revealed that these nanoparticles were efficiently internalized by monocytes and were found either in vesicles or free in the cytoplasm. However, no significant differences in secreted cytokine profiles were observed between CeO2 nanoparticle-treated cells and control cells at noncytotoxic doses. No significant effects of CeO2 nanoparticle exposure subsequent to lipopolysaccharide priming was observed on cytokine secretion. Moreover, no significant difference in lipopolysaccharide-induced cytokine production was observed after exposure to CeO2 nanoparticles followed by lipopolysaccharide exposure.
Conclusion
CeO2 nanoparticles at noncytotoxic concentrations neither modulate pre-existing inflammation nor prime for subsequent exposure to lipopolysaccharides in human monocytes from healthy subjects.
Introduction
Nanomedicine is expected to benefit from cerium dioxide (CeO2) nanoparticle use in antioxidant therapy,Citation1 neuroprotection,Citation2 radioprotection,Citation3 and ocular protection.Citation4 Apart from these nanomedicinal uses, various industrial applications of CeO2 nanoparticles include catalysis,Citation5 ultraviolet absorbance,Citation6,Citation7 oxygen sensing,Citation8 solar and fuel cells,Citation9 and polishing (for glasses, lenses, television tubes, fuel cells, and precision optics).Citation10 Moreover, CeO2 nanoparticles have significant environmental health significance due to their widespread use as a diesel fuel additive. Indeed, it has been documented that addition of CeO2 to diesel decreases fuel consumption by 5%–8% and emission of combustion- derived nanoparticles and unburned hydrocarbons by up to 15%.Citation11,Citation12 However, the accompanying release of CeO2 nanoparticles into the environment could exert unexpected health effects.Citation13 For this reason, the Organization for Economic Cooperation and Development has included CeO2 nanoparticles in the priority list of nanomaterials requiring urgent evaluation.Citation14
Most human diseases are associated with local or systemic inflammatory responses. Moreover, exposure to environmental proinflammatory agents is ubiquitous; for example, we are all exposed to bacterial lipopolysaccharides, either through ingestion (contaminated food or water) or inhalation (house dust, particulate matter, diesel exhaust particles). Furthermore, many epidemiological and experimental studies have shown that individuals with pre-existing inflammatory conditions are more prone to the adverse effects of environmental injury.Citation15–Citation17 Indeed, aggravation of pre-existing inflammation has been documented after exposure to particulate air pollution and various types of nanoparticles.Citation18–Citation21
This study was designed to investigate the inflammation-modulating effects of CeO2 nanoparticles in human peripheral blood monocytes at noncytotoxic exposure concentrations. The proposed uses of CeO2 nanoparticles in nanomedicine make peripheral blood monocytes important target cells at the portal of entry of nanoparticles into the human body. These cells are an essential link between the adaptive and innate immune responses because they develop into various forms of antigen-presenting cells (macrophages, dendritic cells). We show here that noncytotoxic exposures to CeO2 nanoparticles do not prime or aggravate pre-existing lipopolysaccharide-induced inflammation.
Materials and methods
Study subjects and isolation of cells
This study was approved by the National Institute of Environmental Health Sciences institutional review board. Adult human volunteers without any history of a chronic medical condition (hepatitis B, hepatitis C, or human immuno-deficiency virus) and currently not taking any type of medication were recruited to the National Institute of Environmental Health Sciences Clinical Research Unit. The demographics of the study population are shown in . Recruited volunteers underwent phlebotomy, and up to 300 mL of whole blood were withdrawn from an antecubital vein into citrated tubes. Mononuclear fraction was isolated using gradient centrifugation, and CD14+ cells were purified using magnetic beads according to the manufacturer’s recommendations (Miltenyi Biotec, Boston, MA). By this method, 95%–99% viable pure human monocytes were obtained, confirmed by flow cytometry and cytospin preparations.
Table 1 Population demographics
Cells and culture conditions
After isolation, the cells were seeded in 24-well cell culture plates (400,000 cells per well) in x-vivo™ cell culture medium (Lonza, Walkersville, MD) supplemented with 1% human serum and antibiotics (1% solution of penicillin 100 μg/mL and streptomycin 100 μg/mL; Invitrogen Carlsbad, CA) and incubated at 37°C, 5% CO2, and 95% relative humidity for 2 hours (to allow sufficient time for attachment of cells). After cell attachment, the cell culture medium was aspirated and the cells were washed thoroughly with fresh medium to remove unattached cells. The cells were then incubated in fresh prewarmed medium containing the desired doses of nanoparticles or lipopolysaccharides for the different time intervals (24 or 48 hours).
Nanoparticles
CeO2 nanoparticles were obtained from Meliorum Technologies (Rochester, NY) and characterized in the Center for Environmental Implications of Nanotechnology, University of California. Studied characteristics included shape/diameter (transmission electron microscopy), crystal structure (X-ray diffraction analysis), surface area (Brunauer–Emmitt–Teller method), suspension behavior, hydrodynamic diameter, and size distribution (dynamic light scattering), zeta potential (ZetaSizer Nano; Malvern Instruments, Westborough, MA), purity (thermogravimetric analysis), and bacterial endotoxins (limulus amebocyte lysate assay). Dynamic light scattering analysis of the CeO2 nanoparticle suspensions (at 1 μg/mL) in cell culture medium was done to determine the size distribution. A nanoparticle stock solution (1 mg/mL) was prepared in water and stored at 4°C in a refrigerator. All exposure suspensions were freshly prepared from this stock solution after sonication at three pulses of 20 seconds at 235 W each with a 5-second pause using a Mesonix S 4000 cup horn sonicator (Qsonica LLC, Newtown, CT). After sonication, the particles were suspended in cell culture medium and used to expose cells within 5 minutes after vortexing.
These are the best known commercially available CeO2 nanoparticles which have been widely explored in various fields (toxicology, biology, nanotechnology) and have an excellent publication record. From an environmental perspective, study of these particles is very valid because the particles and their aggregates lie within the range of respirable particles (less than 3 μm) which can deposit in the alveolar regions of the lungs.
Experimental design
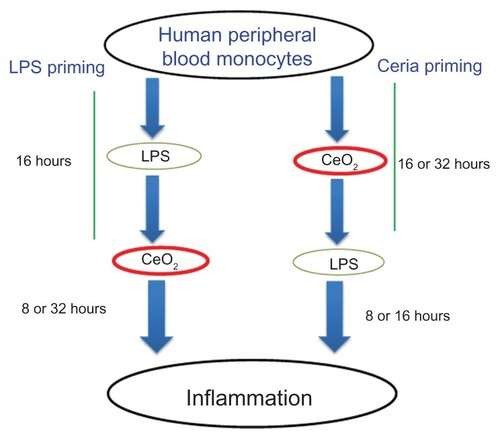
A low effective dose of lipopolysaccharides (Escherichia coli O111:B4, 10 ng/mL) was used to induce an inflammatory response in the cells. The total duration of the experiments was fixed to either 24 or 48 hours and the ability of the nanoparticles to modulate pre-existing inflammation or to prime for subsequent inflammation was assessed. A graphical description of the protocols is given in . To assess the ability of the nanoparticles to modulate pre-existing inflammation, the cells were incubated with lipopolysaccharides for 16 hours and then exposed to CeO2 nanoparticles for 8 hours (24-hour protocol) or 32 hours (48-hour protocol). On the other hand, to assess the ability of the nanoparticles to prime for subsequent exposure to inflammatory agents, the cells were exposed to CeO2 nanoparticles for 16 hours and then exposed to lipopolysaccharides for 8 hours or 32 hours (for the 24-hour and 48-hour protocols, respectively).
Transmission electron microscopy for nanoparticle-cell interaction
Cells were grown in two-chamber cell culture slides and treated with 0.5 or 1 μg/mL CeO2 nanoparticles for 24 hours. The cells were fixed in 3% glutaraldehyde and processed for transmission electron microscopic analysis. Thin sections (60–90 nm) were cut and placed on Formvar copper grids then stained with uranyl acetate and lead citrate. After staining, sections were examined on a FEI Tecnai 110 kV microscope at 80 kV and digital photomicrographs were taken.
Toxicity analysis
A propidium iodide incorporation assay was performed to evaluate membrane integrity and cytotoxicity. Briefly, cells were trypsinated after 24-hour or 48-hour exposures using trypsin-EDTA. The action of trypsin was inhibited using 10% fetal bovine serum, and the cells were centrifuged at 960 rpm for 6 minutes. Cells were resuspended in 500 μL of warm cell culture medium containing 2.5 μg/mL of propidium iodide. Analysis was performed on a FACSAria II (BD Biosciences, Franklin Lakes, NJ) instrument at 488 nm excitation and 610 nm emission wavelengths. After elimination of cellular debris, at least 10,000 cells were analyzed to determine the percentage of propidium iodide-positive cells.
Measurement of cytokines
At the end of the desired incubation time, the supernatants were recovered, centrifuged at 10,000 g for 15 minutes at 4°C, and stored at −80°C till further analysis. The concentration of tumor necrosis factor alpha (TNF-α) was evaluated using a commercially available human enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendations. The concentrations of interleukin-1beta (IL-1β), macrophage chemotactic protein-1, IP-10, and interferon-gamma (INF-γ) were determined using the BD Bioplex assay system (BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard error of the mean and were analyzed by analysis of variance, followed by Tukey’s test using GraphPad (GraphPad Prism 4.01, GraphPad Software Inc, San Diego, CA). A level of P < 0.05 (two-tailed) was considered to be statistically significant.
Results
Nanoparticle characteristics
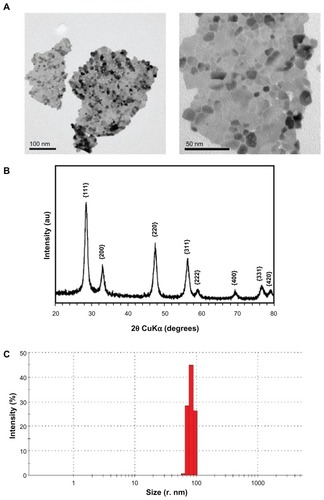
The nanoparticle characteristics are presented in and . Transmission electron microscopic analysis revealed that the CeO2 nanoparticles were irregular in shape and tended to aggregate (). The X-ray diffraction analysis pattern is shown in , demonstrating that the particles are highly crystalline and all peaks could be indexed to cubic fluorite CeO2. Dynamic light scattering analysis revealed that the CeO2 nanoparticles (1 μg/mL) suspended as a single (96 nm size) population in x-vivo cell culture media supplemented with 1% heat inactivated human serum. Further, these particles had −13 mV zeta potentials in the same suspension.
Table 2 Particle characteristics
Figure 2 Physicochemical characterization of CeO2 nanoparticles. (A) Transmission electron microscopic images of nanoparticle suspensions (low and high magnification). (B) X-ray diffraction analysis pattern of CeO2 nanoparticles. (C) Dynamic light scattering analysis of CeO2 nanoparticles suspension (1 μg/mL) in ex-vivo cell culture medium performed using Malvern Zetasizer Nano.
CeO2 nanoparticles are internalized by human monocytes
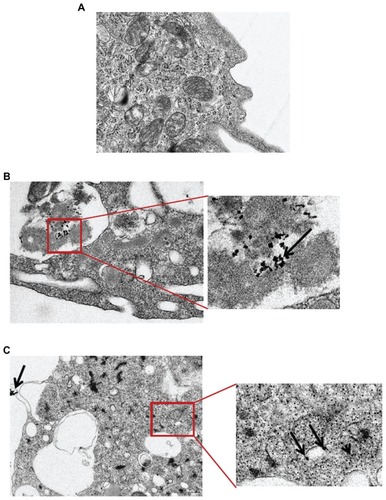
Transmission electron microscopic analysis of nanoparticle-cell interactions indicated that the CeO2 nanoparticles were taken up by human monocytes either in vesicles/phagosomes (mixed with the other debris) or were free in the cytoplasm ().
CeO2 nanoparticles induce cytotoxicity
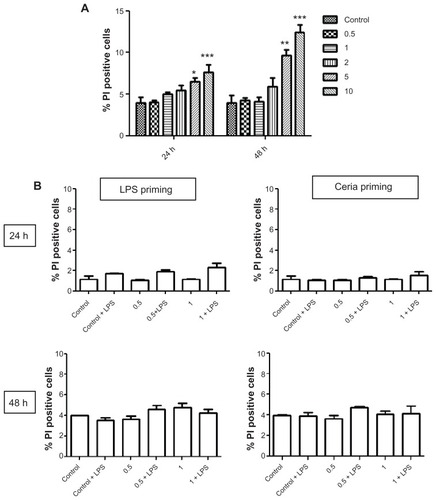
The cytotoxic potential of CeO2 nanoparticles was tested using propidium iodide staining (). shows the cytotoxic response after exposure to different doses of CeO2 nanoparticles. We demonstrated that CeO2 nanoparticles induce a cytotoxic response at doses >1 μg/mL (). We therefore decided to use only the lower doses (≤1 μg/mL) for our inflammatory response experiments. We confirmed that addition of 10 ng/mL lipopolysaccharides to these doses of CeO2 did not alter cytotoxicity ().
Figure 4 Evaluation of human monocyte cytotoxicity after exposure to CeO2 nanoparticles. (A) Cells were treated with different doses of nanoparticles (0.5–10 μg/mL) for 24 or 48 hours, stained with propidium iodide for membrane integrity, and analyzed using flow cytometry. (B) Cells were exposed to noncytotoxic doses of CeO2 nanoparticles either in the presence or absence of lipopolysaccharides for 24 or 48 hours as shown in , stained with propidium iodide and analyzed using flow cytometry.
Notes: Data are presented as the mean ± standard error of the mean and analyzed by analysis of variance, followed by Tukey’s post hoc test. n = 5; *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations: PI, propidium iodide; LPS, lipopolysaccharides.
CeO2 nanoparticles do not modulate inflammatory response to lipopolysaccharides
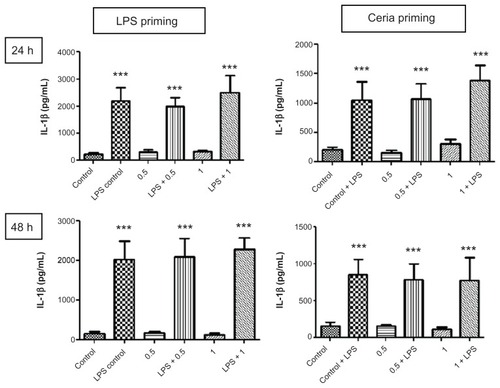
The ability of CeO2 nanoparticles to modulate or prime the inflammatory response to lipopolysaccharides was assessed using the protocol described in . As presented in , we did not find any significant difference in the production of TNF-α, a proinflammatory cytokine, after CeO2 nanoparticle exposure. A similar pattern was observed for IL-1β production (). IFN-γ and IP-10 also showed a similar trend, and the results are presented in . Macrophage chemotactic protein-1 production was increased by lipopolysaccharide exposure only at 24 hours and this increase was no longer detected at 48 hours.
Table 3A Cytokine concentrations without priming
Table 3B Cytokine concentrations after priming
Figure 5 Evaluation of inflammation modulating ability of CeO2 nanoparticles in human monocytes. Cells were treated according to the protocol presented in and the amount of TNF-α in cell culture supernatants was analyzed by enzyme-linked immunosorbent assay according to the manufacturer’s recommendations.
Notes: Data are presented as the mean ± standard error of the mean and analyzed by analysis of variance, followed by Tukey’s post hoc test. n = 5–10; ***P < 0.001.
Abbreviations: LPS, lipopolysaccharides; TNF-α, tumor necrosis factor-alpha.
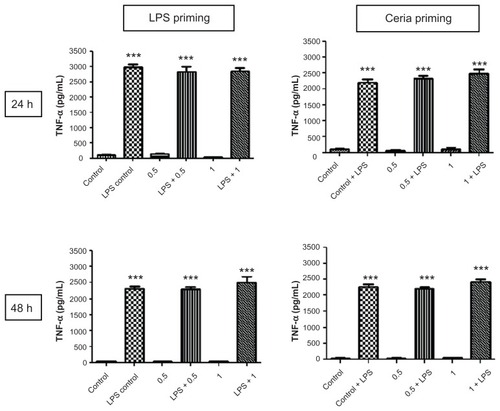
Figure 6 Evaluation of inflammation-modulating ability of CeO2 nanoparticles in human monocytes. Cells were treated according to the protocol presented in and the amount of IL-1β in cell culture supernatants was analyzed by the Bioplex assay according to the manufacturer’s recommendations.
Notes: Data are presented as the mean ± standard error of the mean and analyzed by analysis of variance, followed by Tukey’s post hoc test. n = 5–10; ***P < 0.001.
Abbreviations: IL-1β, tumor necrosis factor-alpha; LPS, lipopolysaccharides.
Discussion
Nanotechnology has shown promising potential to improve the quality of everyday life and has led to the production of a variety of novel materials for industrial, consumer product, and medicinal applications. However, there is a lack of adequate data about the effects of these nanomaterials on human health and the environment. In particular, the effects of these novel materials on susceptible populations (with pre-existing health issues) are rarely addressed. Evaluation of such effects becomes even more pertinent when considering the proposed use of nanomaterials in the medical sector. This experimentation aimed at: elucidating the inflammatory potential of CeO2 nanoparticles; evaluating the possibility of aggravation of a pre-existing inflammatory response after exposure to CeO2 nanoparticles; and exploration of the ability of CeO2 nanoparticles to prime for subsequent exposure to an inflammatory agent. Our results indicate that CeO2 nanoparticles do not significantly change the lipopolysaccharide-induced inflammatory responses of peripheral blood monocytes at noncytotoxic doses. Moreover, CeO2 nanoparticles did not prime human monocytes for subsequent exposure to lipopolysaccharides.
Controversy exists in the published literature about the inflammatory effects of CeO2 nanoparticles. Hirst et al reported anti-inflammatory effects by demonstrating reduction of inducible nitric oxide expression in J774A.1 macrophagesCitation22 and Niu et al reported suppression of inflammatory mediators (macrophage chemotactic protein-1, IL-6, and TNF-α) production by CeO2 nanoparticles in a murine cardiomyopathy model.Citation23 Moreover, CeO2 nanoparticles have been reported to reduce oxidative signaling and cell death induced by cigarette smoke, diesel exhaust, and hydrogen peroxide.Citation24–Citation26 In contrast, other in vitro and in vivo experiments suggest that CeO2 nanoparticles produce inflammation, reactive oxygen species, lipid peroxidation, liver and lung damage, acute and chronic fibrotic effects, and altered macrophage phenotypes.Citation27–Citation31 Variation in target species/ cell type, experimental design (exposure concentration and duration) and nanoparticle characteristics (shape, size, purity, agglomeration, and surface modifications) could be possible reasons for these differences and make cross-study comparisons difficult. Further, CeO2 nanoparticles can be prepared by different methods that lead to differences in relative proportions of Ce3+/Ce4+ ions (one of the reasons proposed for CeO2 nanoparticle-induced reactive oxygen species scavenging).Citation32 Nevertheless, we did not observe any significant differences in cytokine release of human immune cells after exposure to noncytotoxic doses of CeO2 nanoparticles.
The present study is unique in the sense that it addresses the question of pre-existing inflammatory conditions in human cells which are likely to exist in the event of therapeutic application of CeO2 nanoparticles. Indeed, it has already been suggested that during the development of therapeutic nanomaterials, their biocompatibility should also be evaluated in the presence of other agonists such as lipopolysaccharides. Citation33 Moreover, studies in mice and in cell lines may not accurately predict nanoparticle-elicited responses in primary human cells. Lastly, CeO2 nanoparticle-induced protective effects have been reported in oxidative stress-dependent processes, but no attempt has been made to explore the possibility of similar effects on oxidative stress-independent mechanisms (such as lipopolysaccharide-induced inflammatory responses). Our study addresses these gaps in our knowledge. Our data suggest that pre-existing inflammation does not seem to alter the response to CeO2 nanoparticles significantly. On the other hand, we did not find any beneficial effect of CeO2 nanoparticles on lipopolysaccharide-induced cytokine release, suggesting that the previously reported antioxidant effects of CeO2 nanoparticles in macrophages may be limited in their scope of action, and do not extend to a general downregulation of the inflammatory response.
We did find CeO2 nanoparticle internalization by human monocytes, either in the form of vesicles or free in cytoplasm. These results are in agreement with previous studies reporting endocytosis of CeO2 nanoparticles into cells.Citation34,Citation35 These results indicate that CeO2 nanoparticles have good biocompatibility at the tested concentrations and their presence inside the cells did not influence the production of the cytokines studied.
We and others have previously shown that nanoparticles can adsorb cytokines and other biologically significant proteins (for example, enzymes) onto their surfaces and thus may interfere with accurate assessment of their inflammatory potential.Citation36–Citation38 We checked the possibility of TNF-α binding by incubating the nanoparticles with a known concentration of TNF-α for 24 or 48 hours and reanalyzing the concentrations of cytokines by enzyme-linked immunosorbent assay. We did not find any difference between untreated and nanoparticle- treated samples, indicating that CeO2 nanoparticles did not adsorb cytokines.
Our study does have certain limitations. These include use of lipopolysaccharide priming (which can skew the inflammatory responses towards Th1) and use of Th1 cytokines only to assess inflammation. However, this does not limit the scope of our study because lipopolysaccharide exposure is well described and one of the best environmentally relevant models available in which to conduct inflammation modulation studies. More in vivo studies are in progress to study Th1/Th2 polarization of responses and to elaborate the possibilities of modulation of allergic lung inflammation after exposure to CeO2 nanoparticles.
Conclusion
Overall, our results suggest that, under noncytotoxic exposure conditions, CeO2 nanoparticles neither modulate nor prime for a lipopolysaccharide-induced inflammatory response in human peripheral blood monocytes. Our results emphasize the need to evaluate the effects of nanomaterials in the presence of agonists (such as lipopolysaccharides) which are expected to occur in real-life conditions. In the future, further studies on primary human cells focusing on susceptible populations (with pre-existing diseases) are warranted for identification of the realistic hazards of nanomaterials.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences. We gratefully acknowledge all volunteers who participated in this study. Shyamal Peddada is gratefully acknowledged for excellent statistical advice. We also thank Nicole Edwards and Gina Musselwhite for support in patient recruitment, and Erika Gutierrez, Connie Cummings, and Deloris Sutton for their technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- KorsvikCPatilSSealSSelfWTSuperoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticlesChem Commun (Camb)2007101056105817325804
- DasMPatilSBhargavaNAuto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neuronsBiomaterials200728101918192517222903
- TarnuzzerRWColonJPatilSSealSVacancy engineered ceria nanostructures for protection from radiation-induced cellular damageNano Lett20055122573257716351218
- ChenJPatilSSealSMcGinnisJFRare earth nanoparticles prevent retinal degeneration induced by intracellular peroxidesNat Nanotechnol20061214215018654167
- YuJCZhangLLinJDirect sonochemical preparation of high-surfacearea nanoporous ceria and ceria-zirconia solid solutionsJ Colloid Interface Sci2003260124024312742056
- HeckertEGKarakotiASSealSSelfWTThe role of cerium redox state in the SOD mimetic activity of nanoceriaBiomaterials200829182705270918395249
- KhanSBFaisalMRahmanMMJamalAExploration of CeO nanoparticles as a chemi-sensor and photo-catalyst for environmental applicationsSci Total Environ2011409152987299221570707
- IzuNShinWMatsubaraIMurayamaNDevelopment of resistive oxygen sensors based on cerium oxide thick filmJournal of Electroceramics200413703706
- CormaAAtienzarPGarciaHChane-ChingJYHierarchically mesostructured doped CeO2 with potential for solar-cell useNat Mater20043639439715146175
- KosynkinVDArzgatkinaAAIvanovENThe study of process production of polishing powder based on cerium dioxideJ Alloys Compd2000303421425
- NikolaouKEmissions reduction of high and low polluting new technology vehicles equipped with a CeO2 catalytic systemSci Total Environ19992351–3717610535108
- ParkBDonaldsonKDuffinRHazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive – a case studyInhal Toxicol200820654756618444008
- CasseeFRvan BalenECSinghCExposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additiveCrit Rev Toxicol201141321322921244219
- Organisation for Economic Co-operation and DevelopmentList of manufactured nanomaterials and list of end points for phase one of the OECD testing programme2008 Available from: http://www.oecd.org/document/53/0,3746,en_2649_37015404_37760309_1_1_1_1,00.htmlAccessed January 30, 2012
- PopeCA3rdThunMJNamboodiriMMParticulate air pollution as a predictor of mortality in a prospective study of US adultsAm J Respir Crit Care Med19951513 Pt 16696747881654
- DockeryDWPopeCA3rdXuXAn association between air pollution and mortality in six US citiesN Engl J Med199332924175317598179653
- HollingsworthJWMaruokaSLiZAmbient ozone primes pulmonary innate immunity in miceJ Immunol200717974367437517878331
- HussainSVanoirbeekJALuytsKLung exposure to nanoparticles modulates an asthmatic response in a mouse modelEur Respir J201137229930920530043
- KamataHTasakaSInoueKCarbon black nanoparticles enhance bleomycin-induced lung inflammatory and fibrotic changes in miceExp Biol Med (Maywood)2011236331532421427237
- InoueKTakanoHYanagisawaREffects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in miceEnviron Health Perspect200611491325133016966083
- InoueKTakanoHKoikeEEffects of pulmonary exposure to carbon nanotubes on lung and systemic inflammation with coagulatory disturbance induced by lipopolysaccharide in miceExp Biol Med (Maywood)2008233121583159018849540
- HirstSMKarakotiASTylerRDSriranganathanNSealSReillyCMAnti-inflammatory properties of cerium oxide nanoparticlesSmall20095242848285619802857
- NiuJAzferARogersLMWangXKolattukudyPECardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathyCardiovasc Res200773354955917207782
- CelardoIDe NicolaMMandoliCPedersenJZTraversaEGhibelliLCe(3)+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticlesACS Nano2011564537454921612305
- NiuJWangKKolattukudyPECerium oxide nanoparticles inhibit oxidative stress and nuclear factor-kappaB activation in H9c2 cardiomyocytes exposed to cigarette smoke extractJ Pharmacol Exp Ther20113381536121464334
- XiaTKovochichMLiongMComparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress propertiesACS Nano20082102121213419206459
- MaJYZhaoHMercerRRCerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in ratsNanotoxicology20115331232520925443
- ChoWSDuffinRPolandCAMetal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testingEnviron Health Perspect2010118121699170620729176
- ParkEJChoiJParkYKParkKOxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cellsToxicology20082451–29010018243471
- NalabotuSKKolliMBTriestWEIntratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague- Dawley ratsInt J Nanomedicine201162327233522072870
- GojovaALeeJTJungHSGuoBBarakatAIKennedyIMEffect of cerium oxide nanoparticles on inflammation in vascular endothelial cellsInhal Toxicol200921Suppl 112313019558244
- CelardoIPedersenJZTraversaEGhibelliLPharmacological potential of cerium oxide nanoparticlesNanoscale2011341411142021369578
- SuriSSFenniriHSinghBNanotechnology-based drug delivery systemsJ Occup Med Toxicol200721618053152
- LimbachLKLiYGrassRNOxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrationsEnviron Sci Technol200539239370937616382966
- SafiMSarroujHSandreOMignetNBerretJFInteractions between sub-10-nm iron and cerium oxide nanoparticles and 3T3 fibroblasts: the role of the coating and aggregation stateNanotechnology2010211414510320234082
- HussainSBolandSBaeza-SquibanAOxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amountToxicology20092601–314214919464580
- ValSHussainSBolandSHamelRBaeza-SquibanAMaranoFCarbon black and titanium dioxide nanoparticles induce pro- inflammatory responses in bronchial epithelial cells: need for multiparametric evaluation due to adsorption artifactsInhal Toxicol200921Suppl 111512219558243
- SanfinsEDairouJHussainSCarbon black nanoparticles impair acetylation of aromatic amine carcinogens through inactivation of arylamine N-acetyltransferase enzymesACS Nano2011564504451121526848