Abstract
A simple, cost-effective, and environmentally friendly approach to the aqueous-phase synthesis of silver (Ag) nanoparticles was demonstrated using silver nitrate (AgNO3) and freshly extracted egg white. The bio-conjugates were characterized by UV-visible spectroscopy, transmission electron microscopy, Fourier transform infrared spectrometry, and dynamic light scattering. These results indicated that biomolecule-coated Ag nanoparticles are predominantly spherical in shape with an average size of 20 nm. The proteins of egg white, which have different functional groups, played important roles in reducing Ag+ and maintaining product attributes such as stability and dispersity. In vitro cytotoxicity assays showed that these Ag-protein bio-conjugates showed good biocompatibility with mouse fibroblast cell lines 3T3. Furthermore, X-ray irradiation tests on 231 tumor cells suggested that the biocompatible Ag-protein bio-conjugates enhanced the efficacy of irradiation, and thus may be promising candidates for use during cancer radiation therapy.
Introduction
A key aim in X-ray irradiation-aided cancer therapy is to achieve a dose that avoids damage to healthy tissues and organs while maintaining an efficient therapy outcome. Citation1 Nanobiotechnology may address this issue by improving the effectiveness and selectivity of X-ray irradiation therapy.
In recent years, bio-conjugated nanomaterials have shown significant potential for application in biological/biomedical fields through their use in luminescence tagging, labeling, drug delivery, and imaging.Citation2–Citation5 To fabricate an available bio-conjugate for such applications, it is important to select an appropriate synergism between the nanoparticle surface and biological molecules. Noble metal nanoparticles have attracted extensive attention, particularly in the biomedical field, due to their intriguing physical-chemical properties.
Many studies have examined the controlled synthesis of noble metal nanomaterials of various sizes and morphologies through the use of biomolecules as templates.Citation6,Citation7 Extracts from plants such as tea, aloe vera, apple, and pimiento have been successfully used to synthesize gold (Au) and silver (Ag) nanoparticles.Citation8–Citation12 Naturally occurring small molecules such as vitamins B2, C, and E have been used to prepare Au, platinum, and Ag nanomaterials.Citation13–Citation15 Proteins and peptides such as apoferritin, bovine serum albumin (BSA), lysozyme, and tryptophan-based peptides have also been used in the synthesis of Au and Ag nanoparticles.Citation16–Citation20
Compared with traditional chemical syntheses, biomolecule-assisted syntheses of noble metal nanomaterials have a number of advantages.Citation7,Citation21 Since biomolecule- assisted syntheses are carried out at room temperature and under aqueous conditions, energy input is reduced and the solvents or agents used are nontoxic – factors that minimize environmental damage and enhance human health. The structural diversity of biomolecules results in nanoparticles with a wide range of sizes, shapes, and polymorphisms that determine their physical-chemical properties. As is well known, biomolecules can carry on slow kinetics to self-reduce metal precursors and develop stable coating layers to avoid particle aggregation. In particular, proteins with different functional groups as capping shells will facilitate post-surface modification for further biomedical applications.
Egg white or albumen is the liquid that surrounds the yolk of an egg. There are approximately 40 types of protein present in a chicken’s egg white, all of which are beneficial to the human body. Besides their high nutritional quality, egg white proteins (ovalbumin) also have various functional properties such as gelling, foaming, emulsification, heat setting, and binding adhesion.Citation22 Another advantage is their solubility in water and tendency to associate with metal ions in solution. Metal ions such as Mn2+/Mn3+, Fe2+/Fe3+, Cu2+, Zn2+, and Ni2+ have been combined with egg white to obtain novel nanomaterials with interesting properties.Citation23–Citation28
However, to date, it appears that the use of egg white as a template for the synthesis of noble metal nanoparticles has not been investigated, let alone these nanoparticles’ application in cancer therapy. In the current paper, we report a simple, nontoxic, and eco-friendly green pathway to prepare stable spherical and spheroidal Ag-protein bio-conjugates by using egg white as a stabilizer and reductant.
Materials and Methods
Materials
Fresh eggs were bought from a local supermarket. Silver nitrate (AgNO3, analytical grade) was purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, People’s Republic of China). Purified water (18.2 mΩ-cm) was made using a Purelab Classic DI MK2 system (Veolia Water, Paris, France). Mouse fibroblast 3T3 cell line was kindly provided by Dr Jianbing Liu (School of Medicine, Shanghai Jiaotong University, People’s Republic of China). Human breast adenocarcinoma cell line was kindly donated by Dr Jianming Luo (Fudan University Shanghai Cancer Center, People’s Republic of China).
Synthesis of Ag nanoparticles in the presence of egg white
In a typical procedure, 1.0 mL egg white extract was fully dissolved in 97.0 mL water with a strong magnetic stirrer for 30 minutes. The cloudy white solution was filtered through two layers of gauze and a clear solution was obtained. Then, 2.0 mL 10.0 mM AgNO3 solution was added rapidly under vigorous stirring, bringing the total reaction volume to 100.0 mL. The reaction was carried out at room temperature for 72 hours, and it was observed that the solution gradually changed from white to yellow within this time. The collected solution was centrifuged at 15,000 rpm for 15 minutes then washed with purified water. This was repeated twice, and the final samples were dried under vacuum for further characterization.
Characterization
UV-vis spectra were measured using a Shimadzu UV-2450 UV-visible spectrophotometer (Shimadzu Scientific Instruments, Kyoto, Japan). Infrared spectra in the range of 400–4000 cm−1 were recorded using a Nicolet 870 Fourier-transform infrared (FTIR) spectrometer (Thermo Nicolet, Madison, WI). A small quantity of the sample was blended with dry potassium bromide for analysis. The size of the particles in the resulting mixtures was analyzed using a Beckman Coulter DELSA Nano C-Nano Particle Size Analyzer (Beckman Coulter Inc, Brea, CA). Transmission electron microscopy (TEM) measurements were performed at an accelerated voltage of 120 kV (JEM-2010; JEOL Ltd, Tokyo, Japan).
In vitro cytotoxicity assay
Cell Counting Kit-8 (CCK-8; Dojindo Lab, Kumamoto, Japan) was employed in this experiment to evaluate the cytotoxicity of the Ag-protein bio-conjugates.
One hundred microliters of 3T3 cell suspension was dispersed in a 96-well plate, giving a concentration of 5000 cells/well. The plate was pre-incubated for 24 hours in a humidified incubator (37°C, 5% CO2), after which 10 μL of various concentrations of Ag-protein bio-conjugates were added into the culture media in the plate. After the plate was incubated for a further 24 hours, 10 μL of CCK-8 reagent was added to each well of the plate. The plate was then incubated for another 4 hours and then the plate’s absorbance at 450 nm was measured using a microplate reader.
X-ray irradiation enhancement test
Ionizing radiation treatments were carried out using a standard radio-oncology linear accelerator (Siemens Oncor Avant-Garde, Siemens Medical Solutions, Los Angeles, CA) with a 6 meV beam irradiator at a dose rate of 1.0 Gy min−1. A sample of 231 cells was irradiated with different concentrations of Ag-protein bio-conjugates at room temperature, after which these cells were incubated for another 48 hours. Cell viability tests were determined by CCK-8. The same experiment was also performed with radio-resistant 231 cells.
Results and discussion
The general process for the synthesis of nanomaterials in egg white extract involves the electrostatic complexation of Ag ions with oppositely charged proteins, followed by the foam generation and subsequent in situ chemical reaction.Citation18 Biomolecules with carboxyl, hydroxyl, and amine functional groups have the potential to reduce metal ion and cap the newly formed nanoparticles,Citation29–Citation31 which has allowed Au and Ag nanoplates to be obtained in green algal solutions. Similarly, various types of proteins with a large number of hydroxyl groups in egg white may be expected to attach to the different faces of Ag nanocrystals, leading to isotropic growth and the subsequent formation of stable spherical Ag nanoparticles.
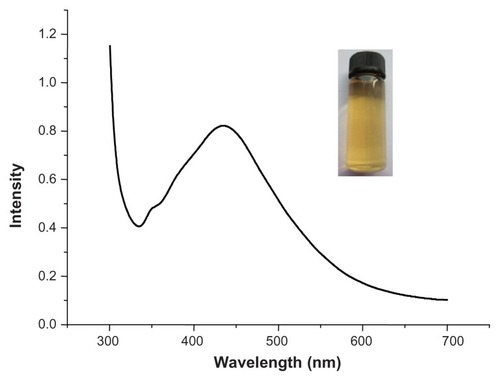
Ag nanoparticles were first characterized using a UVvis spectrophotometer. As shown in , the surface plasmon resonance peak occurred at 425 nm, indicating the formation of spherical Ag nanoparticles (reddish-yellow color, inset). Additionally, the peak at 325 nm is ascribed to the protein characteristic.
Figure 1 UV-vis spectrum of purified Ag egg-white nanoparticles.
Note: Inset shows a typical optical image of the resulting product.
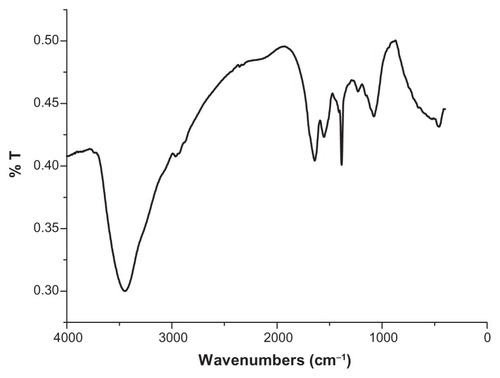
FTIR has become an important tool in understanding the involvement of functional groups in interactions between metal particles and biomolecules.Citation32 In the current study, FTIR measurements were performed to identify the biomolecules responsible for capping and stabilizing the Ag nanoparticles. As is shown in , the spectra record was carried out in the range of 400–4000 cm−1. The very strong peak at 3442 cm−1 was assigned as –OH stretch of the proteins in the egg white extract. A peak was observed around 2950 cm−1 that could be assigned to the C–H stretching vibrations of methyl, methylene, and methoxy groups. The peak located at 1643 cm−1 was assigned to the C=O stretching in carboxyl or C=N bending in the amide group. The absorption band at 1539 cm−1 was characteristic of amide II (N=H) bonds of proteins. The peak at 1072 cm−1 was assigned to the stretch of the C–O bond.
Figure 2 Fourier-transform infrared spectrum recorded from the powder of Ag nanoparticles synthesized through the use of an egg-white template.
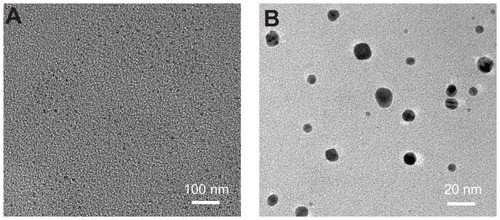
TEM and dynamic light scattering (DLS) were used to evaluate the size, morphology, and hydrodynamic size of the Ag nanoparticles. As can be seen in , the nanoparticles are homogeneous and spherical with an average diameter of 12 nm. It was observed that the edges of the particles were lighter than the centers (), showing that biomolecules (such as proteins in egg white) coated the surface of the Ag nanoparticles. This is consistent with the FTIR results.
Figure 3 Transmission electron microscopy images of Ag nanoparticles at different magnifications, (A) the lower image and (B) the higher image.
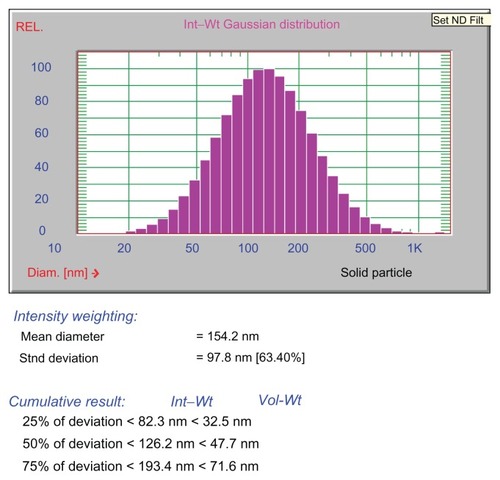
Given the importance of the hydrodynamic volume of nanoparticles for their biomedical implications, a DLS experiment was performed to study the polydispersity of the Ag nanoparticles in aqueous solution. The particle size distribution was fitted by a Gaussian curve as shown in . It was ascertained that the particles were 154.2 nm in diameter, larger than was indicated by TEM. This was attributed to the fact that the DLS is sensitive to the size of the entire protein-nanoparticle ensemble as well as the water associated with this, while only the metallic crystal lattice is visible under the electron beam in bright-field TEM observations.Citation33 Both the TEM and DLS exhibited a narrow particle size distribution, a finding which has important biomedical implications.
Figure 4 Size distribution of the egg white and Ag ensemble in aqueous solution according to dynamic light scattering measurements.
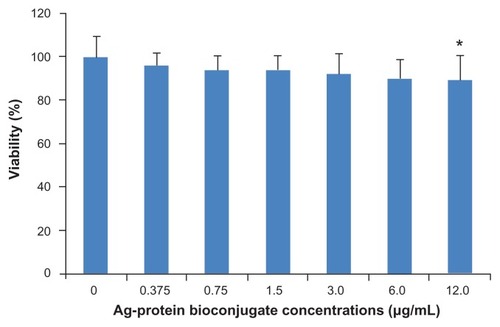
Potential biomedical applications of nanoparticles also depend greatly on their biocompatibility. Therefore, the cytotoxicity of Ag-protein bio-conjugates was examined under in vitro conditions in the mouse fibroblast cell line 3T3. This was assessed in terms of the effect of Ag-protein bio-conjugates on cell viability, determined by CCK-8 assay. After treatment with Ag-protein bio-conjugates for 24 hours, it was demonstrated that viability remains above 80% when the Ag-protein bio-conjugate concentration remained below 12 μg/mL (). It has been reported that some Ag nanoparticles produce significant toxicity in cell culture media,Citation34 while protein-coated Ag nanoparticles show minimal or no toxicity in a 3T3 cell culture, which is consistent with the results of cinnamon phytochemical-coated Au nanoparticles.Citation35 A possible explanation is that both of these are naturally occurring biomolecules that have evolved and have been proven safe in the human body.Citation36,Citation37 They supply a nontoxic coating for Ag nanoparticles, which provides biocompatibility for in vivo administrations. The lack of any noticeable toxicity of Ag-protein bio-conjugates thus presents new opportunities for safe delivery and applications of such nanopharmacuticals in molecular therapy.
Figure 5 Cell viability of mouse fibroblast 3T3 cells after 24 hours’ incubation with increasing amounts of Ag-protein bio-conjugates showing nontoxic profiles.
Notes: Means ± standard deviation; n = 5; * P < 0.05.
To further explore their potential for biomedical application, an experiment was performed to assess the effects of Ag-protein bio-conjugates on 231 tumor cells when these were treated with X-ray irradiation. Radiotherapy is widely used in the clinical treatment of cancer and it is hoped that the rapid development of nanomedicine will allow nanomaterials to be combined with radiation therapy to produce a novel and effective strategy for the treatment of this disease.Citation38 Besides preserving the advantages of standard radiotherapy, such as high penetration, the presence of nanoparticles can enhance radiotherapy’s cancer-killing effect. However, this is dependent on nanoparticles becoming concentrated in cancerous regions and interacting strongly with the irradiating beam.
Although the detailed mechanism remains largely unexplored, it is clear that the presence of strongly absorbing elements in the cells can increase the production of photoelectrons or free radicals and lead to the damage of organelles and/or nuclei. It has been noted that Au nanoparticles can enhance the efficacy of radiation therapy on a murine squamous cell carcinoma,Citation39 while glucose-capped Au nanoparticles can enhance radiation sensitivity in radiation-resistant human prostate cancer cells through regulation of the cell cycle.Citation40 Other researchers have found that polyethylene glycol-modified Au nanoparticles accumulate at tumor sites and enhance the response of CT26 cells to X-ray irradiationCitation1 and that fetal bovine serum-modified Ag nanoparticles increased the sensitivity of glioma cells to ionizing radiation (IR) treatment.Citation41
The current study employed Ag-protein bio-conjugates as novel radiosensitizers. The Ag-protein bio-conjugates were applied to human breast adenocarcinoma 231 cells at different concentrations (0 μg/mL, 0.375 μg/mL, 0.75 μg/mL, 1.5 μg/mL, 3 μg/mL, 6 μg/mL, and 12 μg/mL) and 4 Gy X-ray irradiation was applied. shows the number of changes and morphology of these changes to cancer cells following 24 hours’ incubation. It is clear that the cell numbers decrease as nanoparticle concentration increases, and morphological changes are also observed with the addition of Ag-protein bio-conjugates. The spindles of treated cells become spherical in the presence of Ag nanoparticles and X-ray (, in contrast to the control, ). It was concluded that the combined use of nanoparticles and X-ray treatment caused significant damage to the cells’ cycle.
Figure 6 Optical pictures of 231 colonies. A–H are images of 4 Gy X-ray-irradiated 231 cells with the addition of Ag-protein bio-conjugate concentrations at 0, 0.375, 0.75, 1.5, 3.0, 6.0, and 12.0 μg/mL respectively. A′–H′ are images of 10 Gy X-ray-irradiated 231 cells (note that these cells were 4 Gy X-ray resistant) with the addition of Ag-protein bio-conjugate concentrations at 0, 0.375, 0.75, 1.5, 3.0, 6.0, and 12.0 μg/mL respectively.
Note: H and H′ are the crystal violet-stained results corresponding G and G′, respectively.
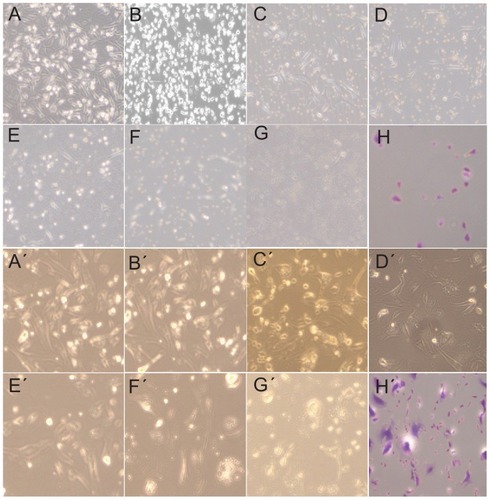
Since some solid tumors are radio resistant, 4 Gy-resistant 231 cells were selected and the irradiation dose was increased to 10 Gy to determine whether Ag-protein bio-conjugates are also available to radio-resistant cells. As is shown in , the cell nuclei are larger than the corresponding cells exposed to 4 Gy. This change in the nucleus could retard its division and lead to death.
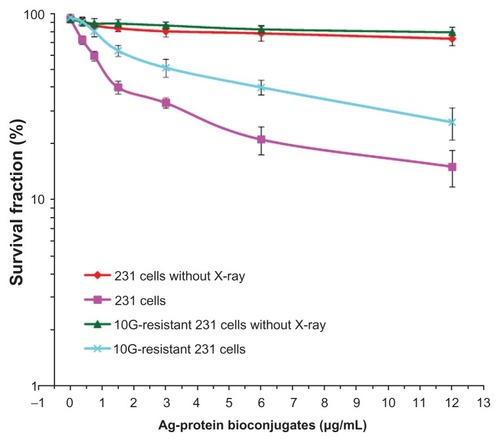
A detailed study of cell survival is shown in . These results will be discussed based on normal 231 cells and X-ray irradiated 231 cells with different Ag-protein bio-conjugates concentrations. In the case of cells that received no X-ray irradiation, the survival of normal cells and the irradiated group remain very similar as Ag-protein bio-conjugates increase, indicating that the Ag-protein bio-conjugates possess good biocompatibility within the experimental concentrations. When cells are exposed to a certain dosage of X-ray irradiation, the survival of both the normal and the irradiated cell groups begin to decrease as the Ag-protein bio-conjugate concentration increases. It was noted that the survival of the normal cells decreased more quickly than those that were irradiated, indicating that X-ray treatment kills more normal 231 cells when these have been treated with Ag-protein bio-conjugates. Both the irradiation dosage and Ag-proteins bio-conjugate concentration affected cell survival. These results suggest that Ag-protein bio-conjugates may function as a radio-therapeutic sensitizer in anticancer therapy.Citation42
Figure 7 Survival curves of different strains of 231 cells at different Ag-protein bio-conjugate concentrations, contrasting those with/without X-ray irradiation. Clearly, the combined used of both Ag-protein bio-conjugates and X-ray did enhance the irradiation to cancer cells.
Notes: Means ± standard deviation; n = 3.
The practical clinical application of treatments such as this will ultimately depend on in vivo biological absorption, distribution, and metabolism of Ag-protein bio-conjugates. The researchers behind the current study will report on the mechanism behind the interactions between Ag-protein bio-conjugates and cancer cells in the near future. This work will include detail across molecular, organ, and animal levels.
Conclusion
The current study has illustrated that egg white may be used as an active template for the spontaneous reduction of Ag ions and the consequent “one-pot” formation of protein-conjugated Ag nanoparticles. This synthesis method is very simple, cost-effective, and environmentally friendly, and the nanoparticles produced were shown to be biocompatible through in vitro cell arrays. Further work is needed to investigate the mechanism of metal-ion uptake and bioreduction by egg white. This study also showed that Ag-protein bio-conjugates can strongly enhance the cell damage induced by X-ray irradiation, which suggests that protein-conjugated Ag nanoparticles are a promising candidate for the development of green nanomaterials for biomedical and health applications.Citation43
Acknowledgments
The work was supported by Shanghai Science and Technology Commission (074119642), and the Shanghai Health Bureau Foundation (JGQN 08001).
Disclosure
The authors declare no conflicts of interest in this work.
References
- LiuCJWangCHChienCCEnhanced x-ray irradiation-induced cancer cell damage by gold nanoparticles treated by a new synthesis method of polyethylene glycol modificationNanotechnology2008192929510421730596
- WestJLHalasNJEngineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeuticsAnnu Rev Biomed Eng2003528529214527314
- MedintzILUyedaHTGoldmanERMattoussiHQuantum dot bio-conjugates for imaging, labelling and sensingNat Mater20054643544615928695
- JainPKHuangXHEl-SayedIHEl-SayedMANoble metals on the nano-scale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicineAcc Chem Res200841121578158618447366
- YangDPCuiDXAdvances and prospects of gold nanorodsChem Asian J20083122010202218956474
- FanTXChowSKZhangDBiomorphic mineralization: from biology to materialsProg Mater Sci2009545542659
- DickersonMBSandhageKHNaikRRProtein- and peptide-directed syntheses of inorganic materialsChem Rev2008108114935497818973389
- NadagoudaMNVarmaRSGreen synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extractGreen Chem200810859862
- ChandaNNuneSKShuklaRGreen nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticlesJ Mater Chem200919192912292020161162
- ChandranSPChaudharyMPasrichaRAhmadASastryMSynthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extractBiotechnol Prog200622257758316599579
- SharmaJTaiYImaeTBiomodulation approach for gold nanoparticles: synthesis of anisotropic to luminescent particlesChem Asian J201051707319937865
- LiSShenYXieAGreen synthesis of silver nanoparticles using Capsicum annuum L. extractGreen Chem20079852858
- NadagoudaMNVarmaRSGreen and controlled synthesis of gold and platinum nanomaterials using vitamin B-2: density-assisted self-assembly of nanospheres, wires and rodsGreen Chem20068516518
- YangDPChenSHHuangPBacteria-template synthesized silver microspheres with hollow and porous structures as excellent SERS substrateGreen Chem20101220382042
- ZhangLShenYHXieAJLiSJinBZhangQOne-step synthesis of monodisperse silver nanoparticles beneath vitamin E Langmuir monolayersJ Phys Chem B2006110136615662016570962
- KeyesJDHiltonRJFarrerJWattRKFerritin as a photocatalyst and scaffold for gold nanoparticle synthesisJ Nanopart Res201113625632575
- XieJPZhengYGYingJYProtein-directed synthesis of highly fluorescent gold nanoclustersJ Amer Chem Soc2009131388888919123810
- SinghAVBandgarBMKastureMPrasadBLSastryMSynthesis of gold, silver and their alloy nanoparticles using bovine serum albumin as foaming and stabilizing agentJ Mater Chem20051551155121
- EbyDMSchaeublinNMFarringtonKEHussainSMJohnsonGRLysozyme catalyzes the formation of antimicrobial silver nanoparticlesACS Nano20093498499419344124
- SiSMandalTKTryptophan-based peptides to synthesize gold and silver nanoparticles: a mechanistic and kinetic studyChem Eur J200713113160316817245786
- HuangPBaoLYangDPProtein-directed solution-phase green synthesis of BSA-conjugated M(x)Se(y) (M = Ag, Cd, Pb, Cu) nanomaterialsChem Asian J2011651156116221341374
- MineYRecent advances in egg protein functionality in the food systemWorlds Poult Sci J2002583139
- GabalMAStructural and magnetic properties of nano-sized Cu-Cr ferrites prepared through a simple method using egg whiteMater Lett2010641718871890
- SenthilSMJayaprakashRSinghVNMehtaBRGovindarajGSynthesis and magnetic properties of nanosized cobalt substituted nickel ferrites (Ni1-xCoxFe2O4) using egg white (ovalbumin) by thermal evaporationNano Res20084107116
- SenthilSMJayaprakashRMurthySRPhaniARSinghVNGovindarajGEffect of annealing on dielectric property in Ni1-x CoxFe2O4 nanoparticles synthesized using albumen (egg white)Nano Res20096205213
- DurmusZBaykalAKavasHDirekçiMToprakMSOvalbumin mediated synthesis of Mn3O4Polyhedron2009281121192122
- NourooziFFarzanehFSynthesis and characterization of brush-like ZnO nanorods using albumen as biotemplateJ Braz Chem Soc2011223484488
- MaensiriSMasingboonCLaokulPEgg white synthesis and photoluminescence of platelike clusters of CeO2 nanoparticlesCryst Growth Des200775950955
- XieJPLeeJYWangDICSynthesis of single-crystalline gold nanoplates in aqueous solutions through biomineralization by serum albumin proteinJ Phys Chem C2007111281022610232
- XieJPLeeJYWangDICTingYPSilver nanoplates: from biological to biomimetic synthesisACS Nano20071542943919206664
- XieJPLeeJYWangDICTingYPIdentification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutionsSmall20073467268217299827
- PrathnaTCChandrasekaranNRaichurAMMukherjeeABiomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle sizeColloid Surf B Biointerfaces2011821152159
- GuerraJBurtJLFerrerDAMejíaSJosé-YacamanMInfluence of morphology in the catalytic activity of bio-conjugated platinum nanostructuresJ Nanopart Res201113417231735
- HussainSMHessKLGearhartJMGeissKTSchlagerJJIn vitro toxicity of nanoparticles in BRL 3A rat liver cellsToxicol In Vitro200519797598316125895
- ChandaNShuklaRZambreAAn effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cellsPharm Res201128227929120872051
- ZhangHYYangDPTangGYMultipotent antioxidants: from screening to designDrug Discov Today20061115–1674975416846803
- YangDPJiHFTangGYRenWZhangHYHow many drugs are catecholicsMolecules200712487888417851440
- ChenWZhangJUsing nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatmentJ Nanosci Nanotechnol2006641159116616736782
- HainfeldJFDilmanianFAZhongZSlatkinDNKalef-EzraJASmilowitzHMGold nanoparticles enhance the radiation therapy of a murine squamous cell carcinomaPhys Med Bio201055113045305920463371
- RoaWZhangXJGuoLHGold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycleNanotechnology2009203737510119706948
- XuRMaJSunXCAg nanoparticles sensitize IR-induced killing of cancer cellsCell Res20091981031103419621033
- HuangPYangDPZhangCLProtein-directed one-pot synthesis of Ag microspheres with good biocompatibility and enhancement of radiation effects on gastric cancer cellsNanoscale2011393623362621842073
- IravaniSGreen synthesis of metal nanoparticles using plantsGreen Chem20111326382650