Abstract
Background and methods
Despite continuous efforts, the increasing prevalence of resistance among pathogenic bacteria to common antibiotics has become one of the most significant concerns in modern medicine. Nanostructured materials are used in many fields, including biological sciences and medicine. While some bismuth derivatives has been used in medicine to treat vomiting, nausea, diarrhea, and stomach pain, the biocidal activity of zerovalent bismuth nanoparticles has not yet been studied. The objective of this investigation was to analyze the antimicrobial activity of bismuth nanoparticles against oral bacteria and their antibiofilm capabilities.
Results
Our results showed that stable colloidal bismuth nanoparticles had 69% antimicrobial activity against Streptococcus mutans growth and achieved complete inhibition of biofilm formation. These results are similar to those obtained with chlorhexidine, the most commonly used oral antiseptic agent. The minimal inhibitory concentration of bismuth nanoparticles that interfered with S. mutans growth was 0.5 mM.
Conclusion
These results suggest that zerovalent bismuth nanoparticles could be an interesting antimicrobial agent to be incorporated into an oral antiseptic preparation.
Introduction
Bacteria in nature do not grow in nutrient-rich medium nor in individual form. These microorganisms live in association within communities containing other microorganisms in a cooperative form, known as biofilm. Biofilms can form on all kinds of surfaces and interfaces, including the human body.Citation1 The most common biofilm is dental plaque in the oral cavity, with Streptococcus mutans being the main etiological agent of dental caries worldwide.Citation2,Citation3 S. mutans has also been identified in cases of endocarditis, where it colonizes the endocardium and cardiac valves, probably due to an ability to adhere to solid surfaces and form a biofilm.Citation4
Despite continuous efforts on the part of the pharmaceutical industry, increasing resistance of microorganisms to common antibiotics has become an important issue in current medicine.Citation5 The absence of new alternatives to treat multidrug-resistant pathogenic bacteria efficiently is a real problem, and there is an urgent need to synthesize new broad-spectrum drugs to fight antimicrobial resistance.
Bismuth is a metallic element of the VA group, together with nitrogen, phosphorus, antimony, and arsenic. Its oxidation numbers are +3 and +5. It is found in the same proportions as silver in the Earth’s crust, and it occupies the 73rd place in abundance. Typically, it is found as bismuthinite (bismuth sulfide), bismite (bismuth oxide), and bismuthite (bismuth carbonate).Citation6 Mexico is the second most important producer of bismuth worldwide after China. Bismuth is used in the manufacture of pharmaceutical products, cosmetics, catalysts, pigments, electronics, and alloys. In medicine, bismuth subsalicylate has been used as an antidiarrheal agent to treat nausea, vomiting, and stomach pain.Citation7
Recently, zerovalent bismuth nanoparticles have attracted interest because of their potential application in electronic devices and magnetic sensors.Citation8,Citation9 Nanoparticles have an increased surface area and therefore have increased interaction with biological targets. However, the potential for use of zerovalent bismuth nanoparticles in medicine is currently unknown. In this work, we present early evidence of the inhibitory antimicrobial effect of bismuth nanoparticles against growth of S. mutans and its capability to form a biofilm. The biocidal activity of bismuth nanoparticles was very similar to that obtained with chlorhexidine, a commonly used oral antiseptic.
Materials and methods
Synthesis of zerovalent bismuth nanoparticles
The following chemical reagents and methods were used for the synthesis of zerovalent bismuth nanoclusters: bismuth nitrate pentahydrate [Bi(NO3)3.5H2O, 98%, Sigma, St Louis, MO], sodium citrate dihydrate [Na3(C6H5O7) · 2H2O, 99%, Sigma], sodium borohydride (NaBH4, 99%, Sigma), dimethyl sulfoxide (Baker, 99.02%, Avantor, Phillipsburg, NJ), methanol (99.8%, Merck, Whitehouse Station, NJ), argon (99.99%, Praxair Inc, Biddeford, ME), and 4 Å molecular sieves (Linde, Tulsa, OK). All reagents were used as received. For a typical preparation of stable colloids, zerovalent bismuth nanoparticles were synthesized by reduction of bismuth nitrate Bi(NO3)3 · 5H2O and were then stabilized by sodium citrate Na3(C6H5O7) · 2H2O. Specifically, 0.0148 g of Na3(C6H5O7) · 2H2O were dissolved in 100 μL of water and 24 mL of dimethyl sulfoxide was added. Next, 0.0242 g of Bi(NO3)3 · 5H2O were added to the mixture. The mixture was fizzed with argon for 15 minutes, and 1 mL of sodium borohydride in methanol 0.2 M NaBH4 was added as reducing agent. The final concentrations of each Na3(C6H5O7) · 2H2O and Bi(NO3)3 · 5H2O were 2 × 10−3 M.
Characterization of zerovalent bismuth nanoparticles
The size, distribution, and morphology of the zerovalent bismuth nanoparticles were determined by high-resolution transmission electron microscopy (TEM) using a JEM 2010 FasTem microscope equipped with Digital Micrograph 1.2 software and a high angle annular dark field detector as well as energy-dispersive and GIF spectrophotometers, at a voltage of 200 kV. Conventional high-resolution TEM images were obtained in a Scherzer defocused condition. A drop of the colloidal nanoparticles was deposited onto 200 mesh copper grids coated with a carbon/collodion layer. The bismuth rhombohedral phase identification was obtained by x-ray diffraction patterns, recorded on a Bruker D-8 Advance diffractometer using Cu Kα radiation (20 mA, 40 kV, λ = 1.5418 Å).
Antimicrobial activity against S. mutans
The antimicrobial effect of the bismuth nanoparticles on growth of S. mutans (strain AU130, ATCC 700611, Manassas, VA) was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Biotium, Hayward, CA),Citation10,Citation11 following the instructions of the manufacturer. S. mutans was grown in trypticase soy broth (BD Difco, Sparks MD) at 37°C overnight in aerobic conditions. The bacteria were counted using a Neubauer chamber, and 1 × 104 cells were inoculated in 100 μL of trypticase soy broth medium in a 96-well polystyrene plate. Three wells with only trypticase soy broth medium were used as controls for growth of S. mutans. Chlorhexidine 0.12% (Ultradent Products, South Jordan, UT) was used as a positive antimicrobial control. We used 2 mM of zerovalent bismuth nanoparticles to interfere with bacterial growth. The 96-well plate was incubated at 37°C overnight. Next, 10 μL of MTT was added to each well, and the plate was protected against light and incubated at 37°C for 2 hours. Next, 200 μL of dimethyl sulfoxide was added to dissolve the reduced MTT. The amount of live cells was determined using a microplate absorbance reader (Biorad, Philadelphia, PA) at 595 nm. The experiment was repeated three times, and the measured optical density was analyzed by descriptive statistics.
Antibiofilm activity
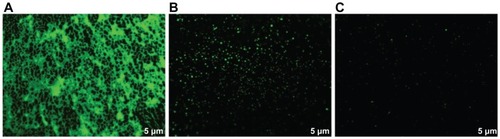
The antibiofilm activity of the bismuth nanoparticles was determined by fluorescence microscopy, following the methodology described above. To observe the biofilm, SYTO 9 green dye (Invitrogen, Carlsbad, CA) was added at a final concentration of 20 μM.Citation12,Citation13 The 96-well plate was incubated for 30 minutes at room temperature and protected against light. The S. mutans biofilm was visualized using a Carl Zeiss Z1 Axio Inverter microscope (Thornwood, NY) at 485 nm.
Determination of MIC for zerovalent bismuth nanoparticles
The minimal inhibitory concentration (MIC) was determined as previously described.Citation14 Briefly, it was obtained by dilution of a 5 tube in the McFarland scale with 1 × 109 colony-forming units. S. mutans was grown in trypticase soy broth agar and incubated at 37°C for 24 hours. One colony was inoculated in 5 mL of trypticase soy broth medium and incubated at 37°C for 24 hours. The bacteria count was determined with a Neubauer chamber. Tubes with a final concentration of 1 × 106 colony-forming units were obtained by dilution of a 5 tube in the McFarland scale. The bismuth nanoparticle solution was diluted to final concentrations of 2, 1.5, 1, 0.5, 0.25, and 0.125 mM. Next, 1 mL of zerovalent bismuth nanoparticles was mixed as a bacterial suspension and incubated at 37 °C for 18 hours. The MIC was determined from the presence or absence of turbidity in the different tubes containing the nanoparticles. Chlorhexidine 0.12% was used as a positive control for inhibition.
Results
Synthesis of zerovalent bismuth nanoparticles
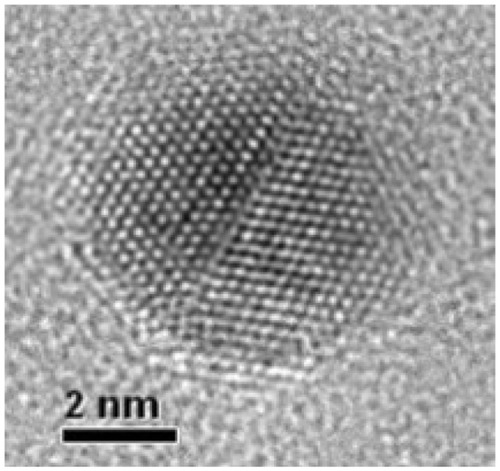
The colloidal dispersions of zerovalent bismuth nanoparticles prepared from 2 × 10−4 M Bi(NO3)3 · 5H2O, 4 × 10−4 M Na3(C6H5O7) · 2H2O, and 4 × 10−4 M NaBH4 in dimethyl sulfoxide showed a narrow size distribution and a spherical form, with an average size of 3.3 ± 0.97 nm (see for a typical particle). This mean size is the smallest reported in the literature.
Figure 1 High-resolution transmission electron microscopic image of an isolated zerovalent bismuth nanoparticle.
Notes: The colloidal sample to obtain this image was prepared a few minutes before the microscopy session. Final concentrations of the chemical reagents are 2 × 10−3 M Bi(NO3)3 · 5H2O, 4 × 10−4 M Na3(C6H5O7) · 2H2O, and 4 × 10−4 M NaBH4 in dimethyl sulfoxide.
Antimicrobial activity against S. mutans
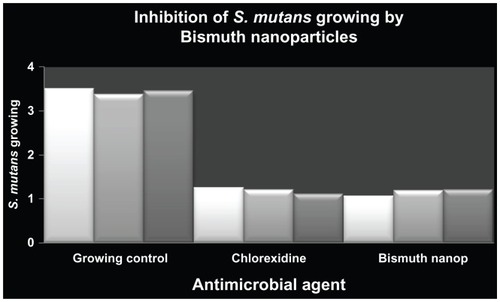
To explore the possible antimicrobial activity of zerovalent bismuth nanoparticles, their effect on S. mutans growth was determined. The results show that these nanoparticles reduced the number of bacteria by 69%, in comparison with bacteria grown in medium alone (). Similarly, treatment with 0.12% chlorhexidine (inhibition control) achieved a 63% reduction in the number of bacteria when compared with nontreated cells (). To rule out any antimicrobial effect of dimethyl sulfoxide, it was added to the S. mutans cultures and no antimicrobial effect was detected after overnight incubation under our experimental conditions (data not shown). It is important to emphasize that zerovalent bismuth nanoparticles were stable to temperatures lower than 50 °C following their characterization. Because all experiments were performed at 37 °C, no additional controls were used to determine the stability of zerovalent bismuth nanoparticles.
Figure 2 Antimicrobial activity of zerovalent bismuth nanoparticles against Streptococcus mutans growth.
Notes: The y axis shows the optical density units of S. mutans growth. S. mutans culture without any inhibitor was used as growing control and chlorhexidine 0.12% as a positive inhibition control. Zerovalent bismuth nanoparticles were used at a final concentration of 2 mM.
Inhibitory effect on biofilm
In the previous experiment, we measured the antimicrobial activity of zerovalent bismuth nanoparticles. In order to analyze for possible biofilm inhibition of S. mutans by bismuth nanoclusters, the antibiofilm activity of the nanoparticles was determined by fluorescence microscopy. The results show complete inhibition of biofilm formation by chlorhexidine () and zerovalent bismuth nanoparticles (), compared with controls (). The results did not change when the zerovalent bismuth nanoparticles were added at different post-inoculation times. We tested the inhibitory activity on biofilm at 6 and 18 hours post-inoculation, and obtained similar results (data not shown). These data indicate that zerovalent bismuth nanoparticles had antibiofilm activity which was as effective as that of chlorhexidine.
Determination of MIC
In order to characterize the antimicrobial activity of the bismuth nanoclusters, we determined the MIC of the zerovalent bismuth nanoparticles. The result obtained was 0.5 mM. This result is important in terms of knowing the minimal effective quantity of zerovalent bismuth nanoparticles required to inhibit S. mutans growth.
Discussion
Nanotechnology is a new discipline with many applications in fields like biological sciences and medicine. Nanomaterials are applied as coating materials, as well as in treatment and diagnosis.Citation15 Nanoparticles of titanium, silver, diamond, iron oxide, carbon nanotubes, and biodegradable polymers have been studied for their use in diagnosis and treatment. Nanoparticles with antimicrobial activity have been reported, including ones containing silver, copper oxide, and selenium.Citation16–Citation18 The advantages of nanoparticles are their high surface-to-volume ratios, their quantum confinement, and their nanoscale sizes, which allow more active sites to interact with biological systems, including bacteria. This is the most important difference between nanoparticles and typical antimicrobial agents, and could minimize the risk of developing antimicrobial resistance.
The mechanism of antimicrobial activity for nanoparticles is not completely understood, and their precise mechanism of action against bacteria remains to be fully elucidated. Several studies have shown that a positive charge on the metal ion is critical for antimicrobial activity, allowing for electrostatic attraction between the negative charge on the bacterial cell membrane and the positive charge on the nanoparticle.Citation19 It has been reported that silver nanoparticles can damage DNA, alter gene expression, and affect membrane-bound respiratory enzymes.Citation20–Citation22
Here we present early evidence of the antimicrobial activity of zerovalent bismuth nanoparticles. Their efficacy in inhibiting S. mutans growth was comparable with that of chlorhexidine. The MIC of zerovalent bismuth nanoparticles for bacterial growth inhibition was 0.5 mM, which should be taken into account if they are to be incorporated into a mouthwash. Our results indicate that these nanoparticles as antimicrobial agents are as good as chlorhexidine, which is the most commonly used oral antiseptic agent. Previously it has been reported that silver, copper oxide, and selenium nanoparticles have antimicrobial activity.Citation16–Citation18 In fact, silver nanoparticles have been used to prevent biofilm formation on surfaces for both biomedical and more general use.Citation23
In order to determine if zerovalent bismuth nanoparticles have the potential to interfere with S. mutans biofilm formation, we studied the antibiofilm activity of these nanoparticles. Surprisingly, they completely prevented biofilm formation. This effect was unexpected, given that zerovalent bismuth nanoparticles would only reduce cell growth and not completely inhibit it. We hypothesize that, since 69% of cells were inactivated by these nanoparticles, cell survival was not sufficient to form a biofilm. Survival bacteria were probably stressed due to the presence of zerovalent bismuth nanoparticles and it is possible that they were lost during washing out of the excess dye. In the presence of chlorhexidine and zerovalent bismuth nanoparticles, we only observed cellular debris on a dark background, comprising mainly DNA from dead bacteria with accumulation of dye. Morphologically, these dye accumulations differ from bacterial biofilm.
In this work, we focused on the effectiveness of zerovalent bismuth nanoparticles in inhibiting growth of S. mutans. Overall, the experimental data suggest that these nanoparticles could be an interesting alternative to combat bacterial infections underlying biofilms. The properties of these nanoparticles could be used in oral health, supporting the antimicrobial activity of oral antiseptics. Further experiments will be necessary to determine the possible toxicity of such nanoparticles in human fibroblast culture and to analyze their potential use in humans. In conclusion, we report that bismuth nanoparticles have antimicrobial activity against growth of S. mutans, as well as antibiofilm activity.
Acknowledgments
The authors wish to thank P Santiago-Jacinto and L Rendon from the Institute of Physics of National Autonomous University of Mexico (IF-UNAM) for obtaining TEM images. D Velasco-Arias and R Hernandez-Delgadillo wish to thank CONACyT for a scholarship. D Diaz and C Cabral-Romero also wish to thank CONACyT for financing the projects 132094 and 141616. This work was also supported by further grants from PROMEP-SEP (103.5/11/6627) and PAICYT-UANL-2011 to CCR.
Disclosure
The authors report no conflicts of interest in this work.
References
- CostertonJWOverview of microbial biofilmsJ Ind Microbiol19951531371408519468
- CostertonJWIntroduction to biofilmInt J Antimicrob Agents1999113–421722110394973
- JenkinsonHFAdherence and accumulation of oral streptococciTrends Microbiol1994262092128087454
- GuntherothWGHow important are dental procedures as a cause of infective endocarditis?Am J Cardiol19845477978016486031
- FalagasMEFragoulisKNKarydisIA comparative study on the cost of new antibiotics and drugs of other therapeutic categoriesPloS One20061e1117183637
- KirkREOthmerDFEncyclopedia of Chemical Technology3rd edNew York, NYChichester, Wiley1980
- Figueroa-QuintanillaDSalazar-LindoESackRBA controlled trial of bismuth subsalicylate in infants with acute watery diarrheal diseaseN Engl J Med199332823165316588487823
- ZhangZYuKBaiDZhuZSynthesis and electrochemical sensing toward heavy metals of bunch-like bismuth nanostructuresNanoscale Res Lett20095239840220672072
- ChenRSoMHYangJDengFCheCMSunHFabrication of bismuth subcarbonate nanotube arrays from bismuth citrateChem Commun (Camb)2006212265226716718324
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- LiuYPetersonDAKimuraHSchubertDMechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reductionJ Neurochem19976925815939231715
- YueHEastmanPSWangBBAn evaluation of the performance of cDNA microarrays for detecting changes in global mRNA expressionNucleic Acids Res2001298E4111292855
- FreyTNucleic acid dyes for detection of apoptosis in live cellsCytometry19952132652748582249
- AndrewsJMDetermination of minimum inhibitory concentrationsJ Antimicrob Chemother200148Suppl 151611420333
- ColvinVLThe potential environmental impact of engineered nanomaterialsNat Biotechnol200321101166117014520401
- SondiISalopek-SondiBSilver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteriaJ Colloid Interface Sci2004275117718215158396
- RenGHuDChengEWVargas-ReusMAReipPAllakerRPCharacterisation of copper oxide nanoparticles for antimicrobial applicationsInt J Antimicrob Agents200933658759019195845
- TranPAWebsterTJSelenium nanoparticles inhibit Staphylococcus aureus growthInt J Nanomedicine201161553155821845045
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomedicine2007319510117379174
- FengQLWuJChenGQCuiFZKimTNKimJOA mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureusJ Biomed Mater Res200052466266811033548
- YamanakaMHaraKKudoJBactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysisAppl Environ Microbiol200571117589759316269810
- BraggPDRainnieDJThe effect of silver ions on the respiratory chain of Escherichia coliCan J Microbiol19742068838894151872
- SambhyVMacBrideMMPetersonBRSenASilver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materialsJ Am Chem Soc2006128309798980816866536