 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
To develop mucoadhesive liposomes by anchoring the polymer chitosan-thioglycolic acid (chitosan-TGA) to the liposomal surface to target intestinal mucosal membranes.
Methods
Liposomes consisting of phosphatidylcholine (POPC) and a maleimide-functionalized lipid were incubated with chitosan-TGA, leading to the formation of a thioether bond between free SH-groups of the polymer and maleimide groups of the liposome. Uncoated and newly generated thiomer-coated liposomes were characterized according to their size, zeta potential, and morphology using photon correlation spectroscopy and transmission electron microscopy. The release behavior of calcitonin and the fluorophore/quencher-couple ANTS/DPX (8-aminonaphthalene-1,3,6-trisulfonic acid/p-xylene-bis- pyridinium bromide) from coated and uncoated liposomes, was investigated over 24 hours in simulated gastric and intestinal fluids. To test the mucoadhesive properties of thiomer-coated and uncoated liposomes in-vitro, we used freshly excised porcine small intestine.
Results
Liposomes showed a concentration-dependent increase in size – from approximately 167 nm for uncoated liposomes to 439 nm for the highest thiomer concentration used in this study. Likewise, their zeta potentials gradually increased from about −38 mV to +20 mV, clearly indicating an effective coupling of chitosan-TGA to the surface of liposomes. As a result of mucoadhesion tests, we found an almost two-fold increase in the mucoadhesion of coupled liposomes relative to uncoupled ones. With fluorescence microscopy, we saw a tight adherence of coated particles to the intestinal mucus.
Conclusion
Taken together, our current results indicate that thiomer-coated liposomes possess a high potential to be used as an oral drug-delivery system.
Introduction
The oral route of drug delivery is considered the most convenient route for patients and medics alike, since it allows for easy, painless administration, thus leading to a high patient compliance. Nevertheless, several drugs, particularly peptide and protein drugs, still have to be administered intravenously due to enzymatic degradation and/or low absorption in the gastrointestinal tract. Different delivery systems have been developed to enhance the oral bioavailability of such drugs, among them liposomes. Liposomes are lipid nanoparticles, which have already shown their potential as drug carriers to deliver sensitive drugs to specific targets.Citation1–Citation3 Although liposomes showed some problems with reproducibility when they were first developed as oral drug carriers, they seem to be promising delivery systems with a good biocompatibility and a high versatility.Citation4,Citation5 To improve the properties of liposomes for oral delivery, various strategies have been designed, such as combining liposomes with permeation enhancers, or coating them with polymers like chitosan, carbopol, eudragit, or silica.Citation6–Citation11 Polymers used for this coating procedure provide an interesting characteristic for oral drug delivery: they interact with mucosal surfaces, thereby increasing the time the delivery system is trapped at the site of drug absorption. Longer et al first showed that a delayed gastrointestinal transit, as induced by bioadhesive polymers, has a high potential of increasing the oral bioavailability of drugs.Citation12 Hence, mucoadhesive delivery systems have been developed not only for oral but also for buccal, rectal, pulmonary, ocular, or nasal drug delivery.Citation13–Citation15
In the present study, we aimed to develop a novel delivery system for the oral application of drugs, based on the coating of liposomes with a specific class of polymers called thiomers. Thiomers are prepared by immobilizing agents with thiol groups, such as L-cysteine, thioglycolic acid, 6-mercaptonicotinic acid, or 2-imminothiolane HCl, on polymeric backbones like polycarbophil, chitosan, or sodium carboxymethylcellulose.Citation16–Citation21 Their main advantage is the increase in mucoadhesion relative to unmodified polymers. Unmodified polymers interact with mucin layers via non-covalent bonds, such as ionic interactions or hydrogen bonds, whereas thiomers are thought to form disulfide bridges with cysteine-rich subdomains of mucus glycoproteins.Citation16 Furthermore, thiomers have been shown to act as permeation enhancers by temporarily opening tight junctions in a reversible manner, which could further enhance the uptake of drugs.Citation22
The objective of this study was to investigate the possibility of taking chitosan-thioglycolic acid (chitosan-TGA) as a coating material for lipid nanoparticles. Chitosan has been chosen because of its biocompatibility, biodegradability, and safe toxicity profile.Citation23 In contrast to a loose coupling of chitosan to the liposomal surface, which is mostly achieved by ionic interactions between polymer and liposomes, we covalently anchored the thiolated chitosan to the liposome.Citation24–Citation26 We then investigated the morphology of the newly generated particles, as well as the influence of the polymer coating on the size and zeta potential of the liposomes, their release behavior, and their mucoadhesive properties.
Materials and methods
Materials
Palmitoyl-oleoyl-phosphatidylcholine (POPC); 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p- maleimidomethyl) cyclohexane-carboxamide] (DOPE-MCC); and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) were purchased from Avanti Polar Lipids (Alabaster, AL). Chitosan-thioglycolic acid (chitosan-TGA) – with a molecular weight of 77 kDa and 539 ± 57 μmol SH-groups/g polymer – was synthesized and provided by Thiomatrix (Innsbruck, Austria). Fluorescein isothiocyanat (FITC)-labeled calcitonin (32 amino acids; molecular weight: 3803.29 g/mol) was synthesized and provided by piCHEM (Graz, Austria). The phosphate buffer (pH 7.4) used for mucoadhesion studies was purchased from Invitrogen (Darmstadt, Germany). All other chemicals were of reagent grade and purchased from Sigma-Aldrich (Vienna, Austria).
Preparation of liposomes
Stock solutions of POPC and DOPE-MCC were prepared in chloroform-methanol (2:1 v/v) and chloroform, respectively. Aliquots of both stock solutions were mixed to obtain a molar ratio of POPC:DOPE-MCC of 3:0.3 for the coupling of chitosan- TGA. For control liposomes, pure POPC was used. To test mucoadhesive behavior, the fluorescent phospholipid 1,2- dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) was added to both phospholipid solutions, giving a final ratio of 3.3:0.01 (lipid to label). The organic solvent was evaporated under a stream of nitrogen to obtain a lipid film, which was dried overnight in a vacuum chamber to ensure complete removal of the organic solvent. Hydration of the dry lipid film was accomplished by adding 10 mM phosphate buffer containing 150 mM NaCl, pH 7.4 (PBS), to yield a final lipid concentration of 30 mg/mL. The lipid suspension was incubated for one hour at room temperature with repeated vortexing. The resulting multilamellar vesicles were sized by freeze and thaw (six cycles), followed by extrusion through 200 nm polycarbonate membranes (Whatman Inc, Clifton, NJ) with a Mini-Extruder (Avanti Polar Lipids, Alabaster, AL).
To investigate the release from coated and uncoated liposomes, the liposomes were loaded either with anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS) and the cationic quencher p-xylene-bis-pyridinium bromide (DPX), or with FITC-labeled calcitonin. Hydration of the POPC/DOPE-MCC film was performed with 5.4 mg/mL of ANTS and 19 mg/mL of DPX in PBS, or with 200 μg/mL of FITC-calcitonin in PBS, followed by freeze and thaw and size extrusion, as described.
Coupling of chitosan-TGA
Coupling was accomplished by the formation of thioether bonds between free SH-groups of chitosan-TGA and functionalized maleimide-groups of the liposome (see ). Chitosan-TGA was synthesized according to the method described previously.Citation17 Different amounts of polymer, which were dissolved in deionized water to achieve a concentration of 3 mg/mL, were added to liposomal suspensions, which were diluted via PBS to a concentration of 10 mg/mL, to obtain molar ratios of 1:6, 1:4, 1:2, 1:1, 2:1, 4:1, and 6:1 (SH-groups:maleimide groups). The mixtures were incubated overnight under agitation at room temperature. Control liposomes were treated the same way.
Ellman’s test
A photometric assay was performed to determine the amount of free SH-groups of chitosan-TGA according to a slightly modified version of the Ellman’s method.Citation27 Briefly, the Ellman’s reagent 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was dissolved in water to a concentration of 7.5 mM and the pH was adjusted by adding NaHCO3. L-cysteine was used as standard substance and a standard curve was prepared in a concentration range from 1 to 80 μM. Samples and standard substance were diluted to 800 μl with phosphate buffer (100 mM Na-phosphate, 15 mM NaCl, 1 mM EDTA; pH 7.4) followed by the addition of 200 μl DTNB-solution. The reaction was allowed to proceed for 30 minutes at room temperature in the dark. The amount of SH-groups was determined by measuring the absorbance at 412 nm using a Hitachi U-2000 spectrophotometer (Hitachi, Tokyo, Japan).
To determine the total amount of thiol groups after coupling, samples with molar ratios of 4:1 and 6:1 (SH-groups to maleimide groups, respectively) were used. In addition to coupled liposomes, control liposomes, mixed with the same amount of polymer, were also measured. All samples were reduced prior to the Ellman’s test by adding 65 μL of freshly prepared 1% sodium borohydride (NaBH4) solution to 100 μL of liposomal suspension. After incubation for 1 hour at room temperature under argon and agitation, excess NaBH4 was removed by adding the same amount of 1 M HCl. The Ellman’s test was accomplished as described; however, before measuring, the turbid liposomes had to be removed. For this purpose, centrifugal filter units with a cutoff of 10 kDa (Millipore, Vienna, Austria) were used (swinging bucket rotor at 5000 g for 20 minutes at 4°C). The flow through (yellow-colored reaction product) was transferred to a plastic cuvette and the absorbance was measured immediately.
Particle size determination
Particle size distribution was determined by photon correlation spectroscopy using a Zetasizer 3000HSA (Malvern Instruments, Herrenberg, Germany), which operated with a 10 mW helium-neon laser at a wavelength of 632.8 nm. The scattered light was measured at an angle of 90° and the temperature was maintained at 25°C. Particle size was analyzed by calculating the auto correlation function of the detected intensity; the polydispersity index of the liposomal suspension was given by the width of the size distribution. Coupled and control liposomes were measured after diluting them to a final lipid concentration of 0.03 mg/mL with ultra-pure water (USF ELGA, High Wycombe, UK).
Determination of the zeta potential
The zeta potential of coupled and control liposomes was determined using the Zetasizer Nano ZS (Malvern Instruments) – according to the Helmholtz-Smoluchowski equation – from the mobility of the liposomes in an oscillating electric field. Samples were diluted with a buffer containing 10 mM of Tris and 2 mM of CsCl (pH 7.0) to a lipid concentration of 0.3 mg/mL, and were measured using a folded capillary cell (Malvern Instruments).
Negative-staining transmission electron microscopy
A total of 10 μL of coated or uncoated POPC/DOPE-MCC liposomes (3 mg/mL) was placed on a carbon-over-Pioloform®-coated copper grid and incubated for 1 minute. The excess sample was blotted with filter paper and immediately replaced by 10 μL of staining agent, which was allowed to settle for 2 minutes; then, it was blotted again. Ammoniummolybdate (5%), phosphotungstic acid (1%), and uranyl acetate (2%) were tried as staining agents. Visualization of the samples was performed using a Zeiss EM 902 transmission electron microscope (Carl Zeiss Microscopy, Oberkochen, Germany) at an acceleration voltage of 80 kV. Digital images were made using a Proscan Slow Scan CCD camera at 1 × 1 K resolution.
Freeze fracture transmission electron microscopy
Coated and uncoated POPC/DOPE-MCC liposomes were mixed with 30% glycerol (v/v), frozen in liquid propane, and stored in liquid nitrogen until further use. Samples were fractured in a Balzers BAF400D freeze-etching apparatus (Balzers, Liechtenstein) under vacuum, with a pressure between 1.3 * 10−4 and 1.3 * 10−5 Pa. Replicas were produced by vacuum deposition of the surface with platinum and carbon and controlled with a quartz crystal thin-film monitor. To clean the replicas, they were put into a sodium hypochlorite solution for about 3 hours and stored overnight in 50% NaOH. Before mounting them on an uncoated copper grid, replicas were washed with distilled water at least three times. Visualization of the grids was accomplished with the system already described, at an acceleration voltage of 50 kV.
Release studies using ANTS/DPX
Free ANTS/DPX was removed from ANTS/DPX-loaded liposomes by size exclusion chromatography using a Sephadex G75 column (Amersham Biosciences, Uppsala, Sweden). Subsequently, liposomes were coupled with chitosan-TGA at a 4:1 molar ratio (SH-groups:maleimide groups), or diluted with the same amount of deionized water (uncoupled liposomes). Release of ANTS/DPX from both liposomal suspensions was determined in simulated gastric fluid (SGF; 1 L contained 2 g of sodium chloride, 3.2 g of pepsin, and 7 mL of hydrochloric acid; pH 1.2) and simulated intestinal fluid (SIF; 1 L contained 6.8 g of monobasic potassium phosphate, 10 g of pancreatin, and 77 mL of 0.2 N sodium hydroxide; pH 6.8), which were prepared according to the US Pharmacopeial Convention. Samples were diluted 1:1 with one of these simulated body fluids and incubated for 24 hours. At fixed time points, 60 μL of these mixtures was withdrawn, mixed with 2 mL of PBS, and measured fluorimetrically using a SPEX FLUOROMAX-3 fluorescence spectrometer (Jobin Yvon Horiba, Longjumeau Cedex, France) at an excitation wavelength of 360 nm and an emission wavelength of 530 nm. To determine the fluorescence corresponding to a 100% release of ANTS/DPX, 10 μL of 10% Triton X-100™ was added to the cuvette before measuring.
Release studies using FITC-calcitonin
Purification and coupling of chitosan-TGA to FITC-calcitonin-loaded liposomes were performed in the same manner as for the ANTS/DPX-loaded liposomes. To determine the release of FITC-calcitonin from liposomes, a dialysis membrane was used to separate free from encapsulated peptide, as described previously by Saarinen-Savolainen et al.Citation28 Briefly, 1 mL of freshly prepared drug-loaded liposomes, with or without chitosan-TGA coating, was mixed with 500 μL of SGF or SIF, respectively. The mixture was transferred to a dialysis bag (molecular cut-off: 300 kDa), which was put into 15 mL of PBS solution. The solution outside the bag was stirred with a magnetic stirrer to ensure a continuous movement of the buffer solution and a homogenous distribution of released FITC-calcitonin. Samples of 800 μL were withdrawn at fixed time intervals from the outer compartment and replaced immediately with equal volumes of PBS buffer solution. After 24 hours of testing, 15 μL of 10% Triton X-100™ was added to the mixture inside the bag to disrupt the liposomes and release all of the entrapped peptide. This mixture was stirred for another 24 hours before determining the fluorescence of a 100% release. Samples were analyzed for FITC-calcitonin by measuring the fluorescence of 200 μL of the withdrawn solution at an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
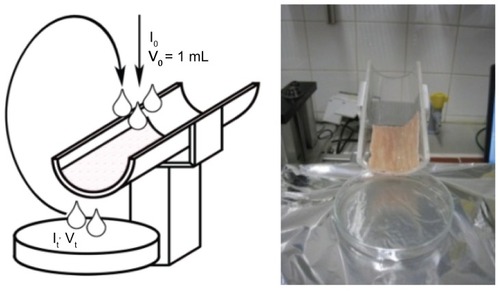
In-vitro mucoadhesion studies
For the mucoadhesion studies, we used a modified version of the falling liquid film technique previously described by Belgamwar et al.Citation29 A freshly excised porcine small intestine was supplied by Karneta (Graz, Austria); 15 cm pieces were cut from the intestine and washed with physiological saline (37°C). The intestinal tube was cut longitudinally, and a 5 × 9 cm excised sheet was placed on a semicylindrical Plexiglas® support with the mucosal side up (see ). The intestinal tissue was rinsed with 1 mL of rhodamine-labeled POPC or thiomer-coated liposomes (4:1 molar ratio of SH-groups to maleimide groups) containing a total lipid amount of either 25 or 100 μg/mL. The excess was collected in a petri dish and reapplied. This procedure was repeated ten times and the fluorescence intensity of the final residual solution (It) was measured with a fluorimeter (FluoStar Galaxy; LABTECH, Offenburg, Germany) at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. A 96-well plate (Cellstar®, Greiner Bio-One GmbH, Friedrichshafen, Germany) was used throughout the study. The mean value of at least three measurements was determined to calculate the amount of bound liposomes according to the following equation:
Figure 2 Falling Liquid Film technique to measure the mucoadhesion of coated and uncoated liposomes.
where I0 is the fluorescence of the initial solution, V0 is the volume that is applied to the tissue; Vt is the final volume of the unbound solution; and Adhesive % corresponds to the adhesive fraction of the applied liposomal suspension.
Cell viability
To make sure that the intestinal tissues used for these experiments were still viable, cell vitality assays were carried out using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolinum bromide (MTT).Citation30 Consequently, tissues were sliced into 5 mm samples after extensively washing them (n = 12). Half of the samples were boiled in water for 1 hour to inactivate the enzymes (zero value). All excidates were transferred to a 12-well plate containing 2 mL of MTT solution (2 mg/mL), and were incubated for 2 hours at 37°C, 95% air/5% CO2 in the incubator. Subsequently, the MTT was removed and the samples were washed twice with 1 mL of PBS. The samples were then minced with scissors and the formazan precipitate was extracted in 4 mL of dimethyl sulfoxide for 80 minutes on a rotating platform (80 rpm; Orbital Schuttler OD 10C; Al-Labortechnik, Amstetten, Austria). The formazan absorbance was recorded at 544 nm, with dimethyl sulfoxide as blank, on a plate reader.
Fluorescence microscopy
After the application of liposomes, the intestinal tissue was incubated for 30 minutes at 37°C/95% air/5% CO2 and fixed with 4% formalin for 3–4 hours at room temperature. Tissues were embedded in Tissue Tec OCT (Sanova, Vienna, Austria) and 10 μm sections were cut using a HM560 Cryostat (Thermo Scientific, Walldorf, Germany). To assess tissue penetration, bright field images and fluorescent images – with excitation BP 520–550 nm and emission LP 580 nm for red fluorescence – were acquired with an Olympus BX-51 microscope (Olympus, Vienna, Austria). Sections with tissue not exposed to liposomes (negative controls) were used to evaluate autofluorescence.
Cytotoxicity screening
Human colorectal adenocarcinoma cells were used for cytotoxicity testing; 2 × 104 cells/well were seeded 24 hours before treatment in 96-well plates, exposed to thiomer-coated liposomes (4:1 molar ratio of SH-groups to maleimide groups), and suspended in Dulbecco’s modified Eagle medium in concentrations of 0–1000 μg/mL. Exposures were performed at 37°C in a 95% air/5% CO2 atmosphere, and two time points (4 and 24 hours) were evaluated. Contamination with endotoxin was assessed via a PYROGENT® Ultra Gel Clot Limulus Amoebocyte Lysate Assay (LONZA, Vienna, Austria). Only low concentrations of endotoxin were detected at the highest sample concentration. Cytotoxicity was studied by formazan bioreduction (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega, Vienna, Austria); ATP content (CellTiter-Glo Luminescent Cell Viability Assay; Promega); and membrane integrity (CytoTox-ONE™ Homogeneous Membrane Integrity Assay; Promega), as previously described.Citation31
Statistical analysis
Data are presented as mean ± standard deviation. Data sets were compared using Student’s t-test and differences were considered as significant at P < 0.05.
Results and discussion
Preparation and characterization of thiomer-coated liposomes
To develop lipid nanoparticles with improved mucoadhesive properties, we explored the potential of thiolated polymers to coat the surface of preformed liposomes. For this purpose, we used low-molecular-weight chitosan, which had previously been modified by thioglycolic acid, as described by Kast et al.Citation17 Chitosan-TGA is characterized by 539 ± 57 μmol free-SH groups/g polymer and 72 ± 38 μmol disulfide bridges/g polymer. An earlier study showed that nanoparticles formulated by such thiolated chitosans revealed significantly higher mucoadhesive properties than unmodified chitosan nanoparticles.Citation19 In the present study, we covalently linked some of the free SH-groups of chitosan-TGA to maleimidegroups – provided by functionalized phospholipid molecules (DOPE-MCC) – which are part of the liposome bilayer and make approximately 10 mol% of total lipids (). As controls, liposomes without functionalized phospholipids were incubated with the same amount of polymer.
Size distribution
The size of the unmodified particles was determined to be 167 nm (n = 3) for POPC/DOPE-MCC liposomes and 195 nm (n = 3) for liposomes containing only POPC. The polydispersity index values were in the range of 0.1–0.2, displaying one homogeneous population of liposomes with a narrow size distribution. The difference in the size of POPC liposomes and POPC/DOPE-MCC liposomes is likely to be caused by the presence of the maleimide-group on the head region of the DOPE lipid. With an increasing amount of polymer, given by the molar ratio of SH-groups to maleimide-groups, the size of the POPC/DOPE-MCC liposomes gradually increased. Above a ratio of 2:1, the curve levels off and only small increases are detectable as more polymer is added (). No significant change in size was detectable for control liposomes. The polydispersity of POPC/DOPE-MCC liposomes increased after coupling to a value of ~0.5 compared with ~0.2 for control liposomes. This indicates a more heterogeneous size distribution of coupled relative to uncoupled liposomes.
Figure 3 Particle size after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).
Note: Indicated values are means ± standard deviation of at least three measurements.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- [4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.
![Figure 3 Particle size after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).Note: Indicated values are means ± standard deviation of at least three measurements.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- [4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.](/cms/asset/38560174-e5e4-4aee-8a60-dc27543baa44/dijn_a_29980_f0003_b.jpg)
To test whether the increase in particle size was caused by one polymer chain binding to more than one liposome, or by intermolecular disulfide bridges between free SH-groups of the chitosan-TGA polymer coat, we added a reducing agent before measuring particle size. As we found no differences in the size values before and after reduction, size increase caused by disulfide formation is highly unlikely.
Zeta potential
To investigate the influence of the polymer coating on the surface charge of liposomes, the zeta potential was measured. The negatively charged phosphate group of the functionalized lipid is responsible for the highly negative zeta potential of uncoated POPC/DOPE-MCC liposomes, whereas control liposomes consisting only of POPC are roughly neutrally charged (). The zeta potential of maleimide-functionalized liposomes increased from −38 mV to +20 mV, with increasing polymer concentration. The polymer itself is positively charged, and coupling of the polymer to the liposome increased the potential of the resulting particle. After the addition of the smallest amount of polymer used in this study, a rapid increase in the zeta potential was observed. Then, the zeta potential slightly increased – coinciding with the increasing amount of polymer – and became positive, reaching a plateau at a 4:1 ratio of SH-groups to maleimide-groups. This observation confirms the results obtained from the size measurements, indicating a polymer saturation concentration. Therefore, a ratio of 4:1 was chosen for mucoadhesion and drug release studies. In contrast to POPC/DOPE-MCC liposomes, no change was measurable after adding different amounts of polymer to pure POPC liposomes.
Figure 4 Zeta potential after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).
Note: Indicated values are means ± standard deviation of at least three measurements.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine- N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.
![Figure 4 Zeta potential after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).Note: Indicated values are means ± standard deviation of at least three measurements.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine- N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.](/cms/asset/55ba17b0-239c-4e8c-beba-da76863e59fb/dijn_a_29980_f0004_c.jpg)
To summarize, the change in the zeta potential from highly negative to positive values and the increase in the size of the liposomes by about 270 nm, led us to conclude that the polymer is successfully linked to the liposomal surface. This increase after coupling correlates well with the observations of other groups.Citation32–Citation35 The polymer is most likely bound to the liposomal surface by no other force than the covalent bond, since no effect of the polymer on the size or zeta potential of control liposomes could be detected.
Determination of free SH-groups
The amount of free SH-groups was measured after previous incubation of the samples with NaBH4 to reduce disulfide bridges, which may be formed between thiol groups. After coupling, we found 283 nmol free SH-groups/mg lipid, for a coupling ratio of 4:1 and about 494 nmol SH-groups/mg lipid, for a coupling ratio of 6:1 (SH-groups to maleimide groups) (see ). When applied to the small intestine, the polymer, which is bound to the surface of liposomes after coupling, is able to interact with cysteine residues of mucus glycoproteins via freely available SH-groups. Consequently, thiomer-coated liposomes become covalently attached to the intestinal mucus by disulfide bridges. This should lead to an improved mucoadhesion of the particles.Citation16
Table 1 Amount of SH-groups after coupling
For maleimide-functionalized liposomes, we found ~100 nmol SH-groups/mg lipid less than for control liposomes. This reduction in the amount of free SH-groups after coupling is a strong indication for a successful binding of chitosan- TGA to liposomes via maleimide groups. This difference of 100 nmol SH-groups/mg lipid did not change by increasing the coupling ratio, as this difference represents the amount of maleimide groups in the sample, to which free SH-groups were coupled.
Liposome morphology
To study the morphology of coated and uncoated liposomes, negative staining transmission electron microscopy was chosen. Ammonium molybdate (5%), phosphotungstic acid (1%), and uranyl acetate (2%) were tried as staining agents. Uranyl acetate was found to give the best contrast and the most homogeneous distribution of the dye and was used for all further preparations. Pictures of uncoated liposomes showed mostly spherical liposomes (170–200 nm mean size). After adding the polymer, the liposomes were apparently linked with each other (). With this technique, we were able to rule out the possibility that the increase in size of the liposomes, after adding the polymer, was caused by fusion. Nevertheless, we were not able to detect the polymer coat itself in negative-contrasted samples. Therefore, freeze fracturing was used to reveal the polymer coat (). Drying and staining steps, during which the liposomes may suffer from different pH or salt conditions, are not necessary; however, images prepared by freeze fracturing do not reflect the real size of the liposomes, since fracturing does not have to occur through the center of the particles.Citation36 The freeze fractures of the coated samples clearly revealed the presence of the polymer at the periphery of the liposomes, which is marked by arrows in . Although no association of liposomes was found in uncoated samples (), single liposomes – as well as aggregates, which involve about three to ten liposomes – were visible in the coated sample (). Henriksen et al observed equally heterogeneous aggregates after adding negatively charged liposomes to a chitosan solution using cryo-electron microscopy.Citation35 This heterogeneity is also reflected in the polydispersity index of the size measurements, which increased with increasing the amount of polymer.
Figure 5 Transmission electron micrographs with negative staining technique of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).
Notes: Magnification: 30,000×. Scale bar indicates 200 nm.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 5 Transmission electron micrographs with negative staining technique of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).Notes: Magnification: 30,000×. Scale bar indicates 200 nm.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/d2502c97-b7fd-4af7-a0e5-1827e0bf2d09/dijn_a_29980_f0005_b.jpg)
Figure 6 Transmission electron micrographs using freeze fracturing of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).
Notes: Arrows indicate the polymer coat. Magnification: 30,000×. Scale bar indicates 200 nm.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 6 Transmission electron micrographs using freeze fracturing of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).Notes: Arrows indicate the polymer coat. Magnification: 30,000×. Scale bar indicates 200 nm.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/13529686-d0d0-4b4d-b95d-3351decd6a32/dijn_a_29980_f0006_b.jpg)
Aggregates between liposomes and the polymer can be formed by different mechanisms. One possibility is that one polymer chain binds to different liposomes, thereby cross-linking single particles. This mechanism was also proposed by Mertins et al,Citation37 where they bound chitosan to preformed liposomes by ionic interactions. Another possibility would be an entanglement of polymer chains that were previously bound to different liposomes. Disulfide formation has already been ruled out, as previously stated. Neither negative staining nor freeze-fracture electron microscopy allowed us to differentiate between these scenarios. However, since aggregates are still present at very high dilutions, the first mechanism is more likely.
Drug encapsulation and release
To study drug loading and release profiles of thiomer-coated liposomes, we tested two different model drug systems. First, the fluorophore/quencher couple ANTS/DPX was used. Second, the fluorescent-labeled peptide calcitonin (FITC-calcitonin) was encapsulated with an entrapment efficiency of 21.5%, which gave a final peptide concentration of 1.4 μg/mg lipid.
The ANTS/DPX assay is usually performed to measure the influence of different agents on membrane stability.Citation38 Hence, this system seems very suitable to quantify drug release.Citation39 In principle, both the fluorophore ANTS and the quencher molecule DPX are encapsulated in liposomes during the hydration step. The molecules are in close proximity to each other and the short distance between the quencher and fluorophore leads to a quenching of the latter; consequently, the monitored fluorescence intensity is low. Upon leakage and release of ANTS/DPX, the fluorescence intensity increases in a concentration-dependent manner. With this technique, we could monitor the release profile from coupled and uncoupled liposomes at different environmental conditions. In particular, we investigated the release properties in simulated gastric and intestinal fluids. Released ANTS/DPX can be measured at fixed time points without separating the released fluorophore. In the second model drug system, we separated released from encapsulated FITC-calcitonin by dialysis before measuring the fluorescence.
The release of ANTS/DPX () corresponded well with the release of FITC-calcitonin (); albeit, the release of FITC-calcitonin in SGF was slightly higher than the release of ANTS/DPX in the same medium. Comparing the release within the first 2 hours, it was for all tested liposomes and model drugs in both SGF and SIF about 25%–30%. Even though there was no significant difference in the release profiles of model drugs within the first few hours, we observed a slightly higher release from thiomer-coated liposomes after 24 hours ( and ). To further investigate this effect, coupled and uncoupled liposomes containing either ANTS/DPX or FITC-calcitonin were kept in PBS and measured after 24 hours. For ANTS/DPX, the released fraction was 38% for coated liposomes compared with 10% for uncoated liposomes, which were just mixed with water. The release behavior of FITC-calcitonin was similar, showing 39% and 13% for coated and uncoated liposomes, respectively. In both cases, a higher release of encapsulated compounds was found for thiomer-coated liposomes in the buffer. Polymer linkage apparently induced this increased release, suggesting the occurrence of some defects in the bilayer, which could be explained by force being exerted on phospholipid head-groups by intermolecular crosslinking. These results suggest that coated liposomes are, by themselves, more leaky, due to the polymer coat. Nonetheless, they are not less stable in simulated body fluid than uncoated ones. Nevertheless, neither coated nor uncoated liposomes released the encapsulated compounds abruptly, nor were they immediately disrupted by the enzymes – a fact that is crucial for a sustained release system.
Figure 7 Release of ANTS/DPX at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).
Note: Each point represents the mean value of two different determinations.
Abbreviations: ANTS, anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid; DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl) cyclohexane-carboxamide]; DPX, p-xylene-bis-pyridinium bromide; POPC, Palmitoyl-oleoyl-phosphatidylcholine.
![Figure 7 Release of ANTS/DPX at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).Note: Each point represents the mean value of two different determinations.Abbreviations: ANTS, anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid; DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl) cyclohexane-carboxamide]; DPX, p-xylene-bis-pyridinium bromide; POPC, Palmitoyl-oleoyl-phosphatidylcholine.](/cms/asset/9a6e8ec3-42d0-421b-9c2b-718dd617606e/dijn_a_29980_f0007_b.jpg)
Figure 8 Release of FITC-calcitonin at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).
Note: Results are means ± standard deviation (n = 3).
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine.
![Figure 8 Release of FITC-calcitonin at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).Note: Results are means ± standard deviation (n = 3).Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine.](/cms/asset/b321fc51-578a-4584-af5c-f782191b0c76/dijn_a_29980_f0008_c.jpg)
In vitro mucoadhesion
To mimic physiological conditions for all experiments, the viability of the membrane, which represents a key parameter for reproducible data, was evaluated. The results revealed that the viability decreased as time increased, indicating that the mucosa has to be used immediately. The mucoadhesiveness of surface-modified liposomes was investigated using the falling liquid film technique (see ). By measuring the fluorescence of the particle solution before and after applying it to the mucus of the porcine small intestine, we could determine differences in the mucoadhesion of chitosan- TGA-coated and uncoated liposomes. Thiomer-coated liposomes showed a ~1.8-fold higher mucoadhesive effect relative to the uncoated lipsomes. The improvement ratio of coated to uncoated liposomes was not significantly different, whether 25 or 100 μg of liposomes was added to the tissue ().
Table 2 Percentage of uncoated/coated liposomes bound to the tissue after using the falling liquid film technique
To visualize the adhered liposomes, the treated tissues were viewed by fluorescence microscopy (). The results demonstrate that tissues incubated with coated liposomes clearly showed a higher concentration of rhodamine- labeled particles than tissues exposed to uncoated liposomes. Additionally, we observed a tight adherence of the coated liposomes to the surface of the intestinal mucus. The fluorescence microscopic images illustrate that the intestinal tissue seems to be affected by the procedure; via formalin fixation and paraffin embedding, we could show that the integrity of the epithelial layer is preserved (data not shown).
Figure 9 Histological sections of porcine small intestine. (A) untreated intestinal tissue (negative control), (B) tissue treated with POPC/DOPE-Liss Rhod liposomes and (C) with POPC/DOPE-Liss Rhod/DOPE-MCC liposomes coated with chitosan-TGA (molar ratio of SH-groups to maleimide-groups: 4:1).
Abbreviations: DOPE-Liss Rhod, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 9 Histological sections of porcine small intestine. (A) untreated intestinal tissue (negative control), (B) tissue treated with POPC/DOPE-Liss Rhod liposomes and (C) with POPC/DOPE-Liss Rhod/DOPE-MCC liposomes coated with chitosan-TGA (molar ratio of SH-groups to maleimide-groups: 4:1).Abbreviations: DOPE-Liss Rhod, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/b0648607-032f-453c-a5d7-6d94fe159fd6/dijn_a_29980_f0009_c.jpg)
Although various groups could show a strong electrostatic interaction between chitosan and liposomes, a covalent bond is generally considered more stable than electrostatic surface interactions. In terms of mucoadhesion, this might be advantageous, since the whole liposome-polymer complex is bound to the mucosal membrane. The observed positive zeta potential of thiomer-coated liposomes could also contribute to an improved mucoadhesion due to ionic interactions between the positively charged polymer and the negatively charged constituents of the mucus layer; that is, sulfonic and sialic acid residues.Citation40,Citation41 Moreover, thiolated polymers, such as the chitosan-TGA used for our experiments, form disulfide bridges with cysteine-rich subdomains of mucus glycoproteins.Citation5 Thus, thiomer-coated liposomes can be attached covalently to the intestinal mucus, where the encapsulated compound is sustainedly released, as was shown by our release studies. Even though electrostatically chitosan-coated liposomes show a high mucoadhesionCitation42,Citation43 it is hard to judge whether the polymer stays attached to the liposomal surface throughout the whole gastrointestinal transit.
Cytotoxicity screening
For the assessment of cytotoxicity, the metabolic functions, according to formazan bioreduction and cellular ATP content, were determined. In addition, cell membrane integrity was assessed by the release of lactate dehydrogenase. In all three assays (up to 1 mg/mL of thiomer-coated liposomes), no indication of cellular damage was seen after incubation for 4 and 24 hours. Consistent with these results, cell membrane integrity, which was verified by the absence of lactate dehydrogenate release, was maintained. Taken together, these results demonstrate the absence of cytotoxic effects in the concentration range tested.
Conclusion
A novel delivery system, based on the coating of liposomes with thiolated chitosan, has been successfully synthesized and characterized. In contrast to the common coating procedure, in which polymers are attached to liposomes by ionic interactions, we have established a covalent thioether bond between chitosan-TGA and the liposome. This covalent coupling was confirmed by size and zeta potential measurements, as well as negative-staining transmission electron microscopy images. In vitro mucoadhesion studies of thiomer-coated and uncoated liposomes showed that the residence time on porcine small intestine could be almost doubled by the addition of the polymeric layer around the liposome. Based on these investigations, we propose that coating liposomes with chitosan-TGA represents a promising strategy to create a mucoadhesive oral delivery system for sustained drug release.
Acknowledgments/Disclosure
This work was supported by the Austrian Nano-Initiative, which cofinanced this work as part of the Nano-Health Project (no 819721), financed by the Austrian Research Promotion Agency (FFG). For technical assistance with electron microscopy, we would like to thank Elisabeth Bock and Gertrud Havlicek. For assistance with mucoadhesion studies, we would like to thank Christina Judmaier and Dagmar Stratil. The authors report no conflicts of interest in this work.
References
- LasicDDPapahadjopoulosDMedical Applications of LiposomesAmsterdamElsevier Science1998
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- TorchilinVPRecent advances with liposomes as pharmaceutical carriersNat Rev Drug Discov20054214516015688077
- PatelHMStevensonRWParsonsJARymanBEUse of liposomes to aid intestinal absorption of entrapped insulin in normal and diabetic dogsBiochim Biophys Acta198271621881937046805
- ChiangCMWeinerNGastrointestinal uptake of liposomes. II. In vivo studiesInt J Pharm1987401–2143150
- ParmentierJHartmannFJFrickerGIn vitro evaluation of liposomes containing bio-enhancers for the oral delivery of macromoleculesEur J Pharm Biopharm201076339440320849953
- TakeuchiHMatsuiYSugiharaHYamamotoHKawashimaYEffectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugsInt J Pharm20053031–216017016125348
- TakeuchiHMatsuiYYamamotoHKawashimaYMucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to ratsJ Control Release2003862–323524212526820
- KarnPRVanicZPepicISkalko-BasnetNMucoadhesive liposomal delivery systems: the choice of coating materialDrug Dev Ind Pharm201137448248820961263
- DwivediNArunagirinathanMASharmaSBellareJSilica-coated liposomes for insulin deliveryJ Nanomater20102010 Article ID 652048
- WerleMTakeuchiHChitosan-aprotinin coated liposomes for oral peptide delivery: Development, characterisation and in vivo evaluationInt J Pharm20093701–2263219073243
- LongerMACh’ngHSRobinsonJRBioadhesive polymers as platforms for oral controlled drug delivery III: oral delivery of chlorothiazide using a bioadhesive polymerJ Pharm Sci19857444064113999000
- ZaruMMancaMLFaddaAMAntimisiarisSGChitosan-coated liposomes for delivery to lungs by nebulisationColloids Surf B Biointerfaces2009711889519201583
- LiNZhuangCWangMSunXNieSPanWLiposome coated with low molecular weight chitosan and its potential use in ocular drug deliveryInt J Pharm2009379113113819559775
- AminMJaafariMRTafaghodiMImpact of chitosan coating of anionic liposomes on clearance rate, mucosal and systemic immune responses following nasal administration in rabbitsColloids Surf B Biointerfaces200974122522919699067
- Bernkop-SchnurchASteiningerSSynthesis and characterisation of mucoadhesive thiolated polymersInt J Pharm2000194223924710692648
- KastCEBernkop-SchnurchAThiolated polymers – thiomers: development and in vitro evaluation of chitosan-thioglycolic acid conjugatesBiomaterials200122172345235211511031
- MillottiGSambergerCFrohlichEBernkop-SchnurchAChitosan-graft- 6-mercaptonicotinic acid: synthesis, characterization, and biocompatibilityBiomacromolecules200910113023302719821557
- Bernkop-SchnurchAWeithalerAAlbrechtKGreimelAThiomers: preparation and in vitro evaluation of a mucoadhesive nanoparticulate drug delivery systemInt J Pharm20063171768116595166
- Bernkop-SchnurchASchollerSBiebelRGDevelopment of controlled drug release systems based on thiolated polymersJ Control Release2000661394810708877
- BarthelmesJDunnhauptSHombachJBernkop-SchnurchAThiomer nanoparticles: stabilization via covalent cross-linkingDrug Deliv201118861361922111974
- Bernkop-SchnurchAKastCEGuggiDPermeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systemsJ Control Release20039329510314636716
- IllumLChitosan and its use as a pharmaceutical excipientPharm Res1998159132613319755881
- TakeuchiHThongborisuteJMatsuiYSugiharaHYamamotoHKawashimaYNovel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systemsAdv Drug Deliv Rev200557111583159416169120
- LayeCMcClementsDJWeissJFormation of biopolymer-coated liposomes by electrostatic deposition of chitosanJ Food Sci2008735N71518577008
- MadyMMDarwishMMKhalilSKhalilWMBiophysical studies on chitosan-coated liposomesEur Biophys J20093881127113319649627
- Bravo-OsunaISchmitzTBernkop-SchnurchAVauthierCPonchelGElaboration and characterization of thiolated chitosan-coated acrylic nanoparticlesInt J Pharm20063161–217017516580797
- Saarinen-SavolainenPJarvinenTTaipaleHUrttiAMethod for evaluating drug release from liposomes in sink conditionsInt J Pharm199715912733
- BelgamwarVShahVSuranaSJFormulation and evaluation of oral mucoadhesive multiparticulate system containing metoprolol tartarate: an in vitro-ex vivo characterizationCurr Drug Deliv20096111312119418963
- RobleggEFrohlichEMeindlCTeublBZaverskyMZimmerAEvaluation of a physiological in vitro system to study the transport of nanoparticles through the buccal mucosaNanotoxicology2011Epub May18
- FröhlichESambergerCKueznikTCytotoxicity of nanoparticles independent from oxidative stressJ Toxicol Sci200934436337519652459
- ZhuangJPingQSongYQiJCuiZEffects of chitosan coating on physical properties and pharmacokinetic behavior of mitoxantrone liposomesInt J Nanomedicine2010540741620957162
- MertinsOCardosoMBPohlmannARda SilveiraNPStructural evaluation of phospholipidic nanovesicles containing small amounts of chitosanJ Nanosci Nanotechnol2006682425243117037851
- MertinsOSchneiderPHPohlmannARda SilveiraNPInteraction between phospholipids bilayer and chitosan in liposomes investigated by 31P NMR spectroscopyColloids Surf B Biointerfaces201075129429919773149
- HenriksenISmistadGKarlsenJInteractions between liposomes and chitosanInt J Pharm19941013227236
- EgelhaafSUWehrliEMuellerMAdrianMSchurtenbergerPDetermination of the size distribution of lecithin liposomes: a comparative study using freeze fracture, cryoelectron microscopy and dynamic light scatteringJ Microsc19961843214228
- MertinsODimovaRBinding of chitosan to phospholipid vesicles studied with isothermal titration calorimetryLangmuir20112795506551521466162
- EllensHBentzJSzokaFCpH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contactBiochemistry1984237153215386722105
- NayarRSchroitAJGeneration of pH-sensitive liposomes: use of large unilamellar vesicles containing N-succinyldioleoylphosphatidylethanolamineBiochemistry19852421596759714084501
- HassanEEGalloJMA simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strengthPharm Res1990754914951694990
- HePDavisSSIllumLIn vitro evaluation of the mucoadhesive properties of chitosan microspheresInt J Pharm199816617588
- TakeuchiHYamamotoHNiwaTHinoTKawashimaYEnteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomesPharm Res19961368969018792429
- Filipovic-GrcicJSkalko-BasnetNJalsenjakIMucoadhesive chitosan-coated liposomes: characteristics and stabilityJ Microencapsul200118131211201339
![Figure 1 Reaction scheme for the covalent coupling of chitosan-TGA to a maleimide-functionalized phospholipid to form a stable thioether bond.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; TGA, thioglycolic acid.](/cms/asset/3c0e8b41-5526-4f4c-b283-b5d85b5f0515/dijn_a_29980_f0001_c.jpg)