Abstract
Regional chemotherapy was first used for lung cancer 30 years ago. Since then, new methods of drug delivery and pharmaceuticals have been investigated in vitro, and in animals and humans. An extensive review of drug delivery systems, pharmaceuticals, patient monitoring, methods of enhancing inhaled drug deposition, safety and efficacy, and also additional applications of inhaled chemotherapy and its advantages and disadvantages are presented. Regional chemotherapy to the lung parenchyma for lung cancer is feasible and efficient. Safety depends on the chemotherapy agent delivered to the lungs and is dose-dependent and time-dependent. Further evaluation is needed to provide data regarding early lung cancer stages, and whether regional chemotherapy can be used as neoadjuvant or adjuvant treatment. Finally, inhaled chemotherapy could one day be administered at home with fewer systemic adverse effects.
Keywords:
Introduction
Lung cancer is responsible for 23% of total cancer deaths.Citation1 Cancer survival tends to be poorer due to an often advanced stage at diagnosis. Only a minority of patients are eligible for curative surgical treatment. Until now, although new biomarkers have been under development for early diagnosis of lung cancer, early detection of lung cancer has not been achieved. Nonsmall cell lung cancer is the most common type of lung cancer worldwide, and various clinical studies are currently assessing new chemotherapeutic combinations.Citation2–Citation4 Although targeted and tailored therapies have been introduced for patients according to individual biological tumor characteristics, overall survival rates have failed to demonstrate the expected progression-free survival or overall survival.Citation5,Citation6 Moreover, acquired resistance to cytotoxic agents has been observed, mainly involving the apoptotic mechanism in nonsmall cell lung cancer cell lines.Citation7 In small cell lung cancer, five-year survival remains less than 10%, despite use of various drug combinations.Citation8,Citation9 In addition, acquired resistance has been observed in small cell lung cancer.Citation10 Therefore, novel therapies are in great demand. The drug concentration reached in solid tumors is a key parameter for successful treatment and, until now, drug concentration at the tumor site has been found to be low after systemic chemotherapy.Citation11,Citation12
Nevertheless, drugs already being used for systemic administration have been successfully administered regionally in various types of cancer.Citation13–Citation23 The concept of local drug delivery is proposed as a method for delivering high drug concentrations to the target site while preventing exposure of vital organs to toxic drug concentrations in the systemic circulation. In this way, systemic side effects are minimized. The respiratory system has a large surface area, thin alveolar epithelium, rapid absorption, lack of first-pass metabolism, high bioavailability, and the capacity to absorb large quantities of drug, making it an optimal route of drug administration.Citation24 Aerosol therapy has also been evaluated, and is being used for several other conditions and purposes, such as diabetes mellitus, gene therapy, and vaccination.Citation25–Citation29 Regarding aerosol chemotherapy administered for lung cancer, a number of drugs have been investigated in vitro, in animal models, and in human trials.Citation30–Citation64 Several aspects of this treatment modality have been addressed due to the necessity for trials to be conducted, but several parameters remain to be clarified and expanded. The areas of this treatment modality that need to be properly addressed are summarized under the headings: prompt inhalation device; lung airway microenvironment; appropriate molecule-chemotherapy selection; deposition evaluation; protection measures; and disease evaluation. In the current review, these topics will be addressed based on the published literature, and all prior knowledge on the subject will be presented.
We performed an electronic search of the PubMed, Google Scholar, Medscape, and Scopus databases using combinations of the following keywords: “aerolized chemotherapy”, “inhaled chemotherapy in lung cancer”, “nanoparticles”, “aerosol devices”, “encapsulation”, “inhaled doxorubicin”, “inhaled carboplatin”, “inhaled cisplatin”, “inhaled paclitaxel”, “inhaled docetaxel”, and “inhaled 5-fluororacil”. All types of articles (randomized controlled trials, clinical trials, observational cohort studies, review articles, case reports) were included. Selected references from identified articles were searched for further relevant papers.
Lung anatomy and microenvironment
Airway geometry and humidity
Human lungs have a large (>100 m2), thin (0.1–0.2 μm), and highly vascular epithelial surface area for absorption. Progressive branching and narrowing of the airways encourages impaction of particles. The lung has a relative humidity of approximately 99.5%. Drug particles are known to be hygroscopic and to grow or shrink in size in high humidity. The increase in particle size above the initial size should affect the amount of drug deposited and, particularly, distribution of the aerosolized drug within the lung.Citation65,Citation66 Further drug absorption could occur via the lymphatic pathway.Citation67,Citation68
Bronchial circulation
The lungs receive the entire cardiac output and represent the most richly perfused organ in the body. However, only the alveolar region is supplied by the pulmonary circulation. Blood flow to the larger airways (trachea, bronchi) is via the systemic circulation, and these airways receive approximately 1% of cardiac output.Citation69 The endobronchial circulation is recirculated to the peripheral airways and lung parenchyma via the bronchial veins and right atrium. Bronchial blood flow is augmented in diseases such as bronchiectasis, from 1% to as much as 30% of cardiac output.Citation24 Theoretically, inhaled drugs that are absorbed into the circulation from the tracheobronchial regions can be redistributed downstream and peripherally into otherwise poorly accessible areas of the lung, which may aid in drug effectiveness.Citation70
Lung clearance mechanisms
Drug particles deposited in the conducting airways are largely removed by mucociliary clearance. The airway epithelial goblet cells and submucosal glands secrete mucus, forming a two-layer mucus blanket over the ciliated epithelium, ie, a low-viscosity sol layer covered by a high-viscosity gel layer. Insoluble particles are trapped in the gel layer and are moved toward the pharynx (and ultimately to the gastrointestinal tract) by the upward movement of mucus generated via metachronous beating of the cilia.
In the normal lung, the rate of mucus activity varies depending on the airway area and is determined by the number of ciliated cells and their beat frequency. For normal mucociliary clearance to occur, the airway epithelial cells and ciliary structure and activity must remain intact. Further, the depth and chemical composition of the sol layer should be optimal and, finally, the rheology of the mucus must also remain within the physiological range. Mucociliary clearance is impaired in lung diseases such as immotile cilia syndrome, bronchiectasis, cystic fibrosis, and asthma.Citation71 Lipophilic molecules pass easily through the airway epithelium via passive transport. Hydrophilic molecules cross via extracellular pathways and exocytosis.Citation72 Particles are absorbed from the submucosal region into the systemic circulation, bronchial circulation, or lymphatic system. Drugs deposited in the alveolar region may be phagocytosed and cleared by alveolar macrophages or absorbed into the pulmonary circulation. Alveolar macrophages are the predominant phagocytic cells for lung defense against inhaled microorganisms, particles, and other toxic agents. There are approximately five to seven alveolar macrophages per alveolus in the lungs of healthy nonsmokers.Citation73 Macrophages phagocytose insoluble particles that are deposited in the alveolar region and are either cleared by the lymphatic system or moved into the ciliated airways along currents in alveolar fluid and then cleared via the mucociliary escalator.Citation74 This process can take weeks to months to complete.Citation75 Moreover, enzymes are still present in the lungs, so particles can be enzymatically degraded intracellularly (from alveolar macrophages) and/or extracellularly by membrane-associated proteases and peptidases (both epithelial and endothelial).Citation76
Lung disease
Bronchoconstriction, inflammation, and airway narrowing alter lung deposition. Respiratory diseases, such as cystic fibrosis and bronchiectasis, change the architecture of the lung. Alterations in bifurcation angles, turbulent flow, and obstruction of the airways due to mucus accumulation modify the deposition and distribution patterns of aerosols. A decrease in the cross-sectional area of the lung caused by obstruction increases air velocities and turbulence in regions where the airflow is usually laminar. Airway obstruction diverts inspired air to unobstructed airways and, thus, remarkably little drug is deposited in obstructed areas. Often the obstructed areas are those that need to be reached in order to achieve the optimal therapeutic effect of a drug.Citation71,Citation77–Citation80 Inhaled insulin was investigated as to whether it could be administered during an exacerbation and how this situation altered the dosage. It was observed that the drug could be administered and was tolerated by patients, but close glucose monitoring was required because release of the drug into the systemic circulation was insufficiently controlled.Citation29
Methods enhancing lung deposition
It has been confirmed by plethysmography that addition of 5%–7% CO2 into the inhalation system enhances the depth of absorption and drug quantity inhaled in every breath by reducing the respiratory rate and increasing the tidal volume by 180%. The subject is forced to breathe slowly and deeply. Nevertheless, it has been observed that if the mixture is enriched with a concentration higher than 7%, adverse effects are observed, with sleepiness, confusion, and severe dizziness being the most common.Citation81–Citation83
Tumor size
Tumor size affects the distribution and deposition of the inhaled compound. In previously published studies, the mass median diameter was required to be ≤3–5 cm upon diagnosis, otherwise patients were excluded from trials. Citation29,Citation40,Citation52,Citation53,Citation84,Citation85 Anticancer drugs penetrate normal tissues by both diffusion and convection,Citation86 with the net flow of fluid from blood vessels balanced by resorption into the lymphatic circulation. Nevertheless, tumors caused by unstructured neoangiogenesis lack functional lymphatics,Citation87,Citation88 which can lead to increased levels of interstitial fluid pressure in tumors,Citation89–Citation91 which in turn reduces convection and inhibits distribution of macromolecules.Citation92,Citation93 It has been previously demonstrated that some physicochemical properties of drugs, ie, shape, charge, molecular weight, and aqueous solubility, determine the rate of diffusion through tissue.Citation86 The penetration of a drug is also dependent on its deconstruction, which functions to remove free drug, thereby inhibiting further permeation.Citation86 Water- soluble drugs distribute most readily in the extracellular matrix and thus diffuse efficiently around and between cells. In contrast, lipid-soluble drugs penetrate lipid membranes, and so can be transported through cells.
Physical properties of drug formulations
Physical properties that have a significant role on the particle size of the inhaled suspension are viscosity ionic strength, osmolarity, and pH. If the values for pH and osmolarity in particular are not in the normal range, bronchoconstriction, coughing, and irritation of the lung mucosa is induced.Citation94,Citation95
Drug delivery systems
Optimal particle size
The inhaled drug formulation should consist of a specific particle size, in the range of 1–3 μm, to achieve substantial alveolar deposition.Citation24 Inhaled molecules of this size becomes trapped in the alveoli and taken up in vesicles by alveolar epithelial cells, so that they can be carried across and released on the opposite side in the narrow interstitial fluid compartment between the epithelial cells. Molecules are then taken up within vesicles by the endothelial cells, transported across the width of these cells, and released into the alveolar capillary bloodstream. This process of particle migration into, across, and out of a cell is known as transcytosis. Other drug formulations given via inhalation, such as corticosteroids and anticholinergics, do not have to be less than 2–3 μm in size, because they act on the larger branches of the bronchial tubes.Citation96
Pressurized metered dose inhalers
The pressurized metered dose inhalers (MDIs) use propellants, such as chlorofluorocarbons, which have been recently replaced by hydrofluoroalkanes.Citation97 The aerosol is emitted through a nozzle at a high velocity of >30 msec). Nevertheless, only 10%–20% of the aerosol emitted from pressurized MDIs is deposited on the lung parenchyma.Citation98 The main reasons for this can be summarized as inspiratory flow rate and lack of hand-mouth coordination,Citation99–Citation101 and the impact of high velocity particles and large particle size (50%–80%) on the oropharynx.Citation102 In order to maximize the effectiveness of drug absorption from a pressurized MDI, the patient has to breathe slowly by decreasing respiratory frequency and increasing tidal volume. The inhaled volume is increased and the aerosol penetrates deeply into the lung parenchyma.Citation103,Citation104 To overcome the problem of actuation-inhalation, coordination breath-actuated pressurized MDIs were introduced to the market.Citation105 Nevertheless, improved peripheral deposition was observed if patients did not hold their breath on completion of inhalation in comparison with pressurized MDIs that are not breath-actuated.Citation106 In addition, different spacer tubes, valved holding chambers, and mouth piece extensions were developed to reduce deposition in the oropharynx by decreasing particle size and slowing the velocity of the aerosol, and to produce a finer aerosol of smaller mass median aerodynamic diameter.Citation107
Dry powder inhalers
Dry powder inhalers were designed to overcome poor actuation-inhalation coordination. There are two basic types on the market, ie, multidose (containing multiple doses) and single-dose capsule dry powder inhalers. Several differences in lung deposition have been observed between the various dry powder inhalers. Approximately 12%–40% of the emitted dose is delivered to the lungs, and about 20%–25% is retained within the device.Citation108–Citation110 The reduced drug deposition has been attributed to inefficient disaggregation of ultrafine drug particles from coarser carrier lactose particles or drug pellets. Factors affecting disaggregation are high humidity, slow inhalation flow rate, and rapid and large deviations in temperature.Citation111 Therefore, dry powder inhalers have to be stored in a cool, dry place. In addition, an exhalation maneuver is required before inhalation, because there is the possibility for a patient to exhale into the inhaler nozzle and disperse the dry powder. Pulmonary drug administration is enhanced for the dry powder inhalers because of fast inhalation.Citation112 This occurs due to different internal resistance to airflow and a range differentiation from low to high resistance.Citation113,Citation114 Failure to use the device properly is a common error and therefore a dose is not delivered promptly.Citation115 Dry powder inhalers with high resistance provide increased deposition to the lung parenchyma,Citation113,Citation116 but the clinical significance of this remains to be clarified. Finally, recent developments, principally in overcoming forced inhalation effort, have produced active dry powder inhalers. This is achieved either by adding a battery-driven propeller that aids the dispersion of the powder or by using compressed air to aerosolize the powder and convert it to an aerosol in a holding chamber where its respiration is independent of the respiratory capability of the patient. Dry powder inhalers that are currently on the market are breath-actuated and still depend on the inhalation flow rate of the patient to achieve maximum drug dose inhalation.Citation117
Nebulizers
Nebulizers have been used for many years to treat various respiratory diseases. They work by inhalation through a face mask, and can be used in respiratory distress by the elderly and children younger than 2 years of age. In addition, they can deliver large quantities of solutions and suspensions as small droplets with remarkably little patient coordination required. There are certain parameters of the aerolized solution affecting their efficiency, ie, pH, viscosity, ionic strength, osmolarity, and surface tension. High drug concentration, extremely low pH, and hyperosmolarity or hypo-osmolarity reduce drug output and provoke bronchoconstriction, coughing, and irritation.Citation94,Citation95 Other additional significant factors can be summarized as the design of the nebulizer chamber, primary drug fill in the reservoir cup, tapping of the nebulizer chamber during nebulization, time taken to nebulize a solution, gas flow and compressor characteristics, and residual volume.Citation118 Until recently, there were two basic types of nebulizers, ie, jet nebulizers and ultrasonic nebulizers. Jet nebulizers take advantage of the energy provided by compressed gas flow and distribute the liquid substance in the reservoir cup into a fine mist. Jet nebulizers are widely used, but are rather inadequate (50% loss when continuously operated and only 10% deposited to the lungs) in comparison with the newer devices described below.Citation119 Their performance is largely dependent on the compressor used.Citation118,Citation120 Newly introduced to the market are the breath-enhanced jet nebulizers and the dosimetric nebulizers. The former delivers drug faster than the conventional jet nebulizers and the latter are breath-actuated, so generate aerosol only by inhalation. They are computer-controlled, and although they contribute by saving aerosol (up to 60%), they remain extremely expensive in comparison with conventional jet nebulizers.Citation121 The ultrasonic nebulizers use a piezoelectric crystal that vibrates at a high frequency (1–3 mHz) to produce a mist of liquid in the nebulizer. The higher the frequency used, the smaller the droplets produced. Ultrasonic nebulizers nebulize solutions faster than jet nebulizers, but are not suitable for suspensions. In addition, the piezoelectric crystal can heat and inactivate protein-based drugs.Citation122 The latest nebulizers introduced onto the market are the vibrating mesh nebulizers, which are divided either into active or passive systems. Their advantages over the previous systems are that they are very efficient, quiet, and portable, and have an extremely low residual volume to prevent drug waste. Moreover, some models provide feedback to the patient regarding dose delivery and patient adherence. Nevertheless, there are a number of disadvantages, in that they are expensive, and need maintenance and cleaning to prevent colonization by pathogens, buildup of deposits, and blockage of the apertures. Finally, although vibrating mesh nebulizers are highly efficient overall, their performance varies according to the drug solution used and, therefore, licensing specific drugs with specific nebulizers is essential. This has no significant clinical importance when bronchodilators are delivered, given that they have a wide therapeutic index, but it is necessary when delivering drug solutions containing liposomes or proteins.Citation123–Citation126 Facemasks are used for patients with acute respiratory distress and in uncooperative individuals. The facemask is not just a feature connecting the nebulizer to the patient, it also has to prevent face and ocular irritation. Citation127–Citation130 A mouthpiece is also used, and new mouthpiece designs are currently on the market which enable inhalation by breath actuation, incorporate drug-saving technology, and are environment friendly, protecting medical staff from having to dispose of unwanted solutions.Citation29,Citation30 Nevertheless, the mouthpiece is not indicated for acute respiratory distress conditions. Kleinstreuer et alCitation84,Citation131 have presented data regarding the optimal combination of particle size, particle release position, and inhalation waveform that may deliver inhaled drug aerosols efficiently to the desired areas. Micron-sized particles follow trackable trajectories in human lung airways under steady laminar flow conditions. Therefore, the mouth inlet plays a crucial role, because it can affect the dispersion and deposition of the aerosol by backtracking.
Soft mist inhalers
Currently there is only one drug system of this kind available, which is a mechanical achievement of outstanding value. It uses the energy of a spring to force the solution through an extremely fine nozzle system.Citation132,Citation133 It produces a fine aerosol with relatively high lung deposition.Citation134–Citation136
Inhaled particle carriers and strategies
Liposomes
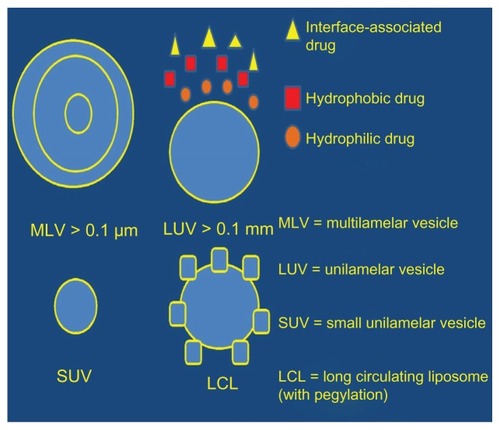
Liposomes have a variety of properties that can be summarized as sustained release with reduced toxicity and less irritation to the lung parenchyma, the possibility to manipulate release and targeting, and improved stability.Citation137 The amount of drug dose carried by the liposomes, and their release rate and deposition in the lung parenchyma depends on lipid composition, size, charge, drug/lipid ratio, and method of delivery.Citation138–Citation140 Liposomes are produced from phospholipids, which carry either no charge or a net negative/positive charge.Citation111,Citation141 Their structure consists of an aqueous volume entrapped by a synthetic lipid single layer or bilayer with or without cholesterol. They are capable of encapsulating either hydrophilic or lipophilic formulations.Citation142,Citation143 However, formulations of intermediate solubility are poorly retained by liposomes, so they are manipulated to achieve a higher degree of retention.Citation144 Liposomes are prepared for inhalation either in liquid or dry powder form.Citation145 During nebulization, an amount of the formulation is lost and hence a manipulation of the lipid composition, and size and operating conditions are necessary to minimize the loss.Citation146–Citation149 The dry powder liposome formulations are produced by lyophilization followed by milling or by spray-drying.Citation150,Citation151 The sustained-release capability of liposomes has been observed in several studies using a variety of drugs as aerosol treatment for the lung.Citation29,Citation50,Citation152 To enhance the sustained-release properties of liposomes further, a polymer surface coating, such as polyethylene glycol (PEG), was developed. This addition, provided a “stealth” shield to the molecule to bypass the body’s defense mechanismsCitation144,Citation153,Citation154 ().
Table 1 Efficiency enhancement mechanisms
Microparticles
Microparticles are produced from naturally occurring or synthetic polymers, and their size range is between 0.1 and 500 μm. They are physically and chemically more stable than liposomes, so are capable of higher drug loading. This property makes them an ideal carrier system for proteins and peptides.Citation155,Citation156 In order to encapsulate a drug, a number of factors, including heat, pH, oxygen, solvents, moisture, and mechanical stresses, must be assessed. Preparation for aerosol delivery can be undertaken using spray-drying, emulsion-solvent evaporation, phase separation, emulsion-solvent diffusion, and supercritical fluid technology.Citation157–Citation163 Moreover, manipulation of the following parameters will determine drug release: concentration, size, solubility, nature of micromolecular drug, molecular weight, porosity, tortuosity, and uniformity of the polymer. A coating is added to improve the time release characteristics further, and 1,2-dipalmitoylphosphatidylcholine is also added to poly(DL-lactide-co-glycolide) microspheres to decrease uptake by macrophages.Citation160 When chitosan and hydroxypropylcellulose are added to the particles, their time residence in the lung parenchyma is increased.Citation163 It has been widely agreed that the optimal geometric diameter for lung delivery is 1–3 μm, but these particles tend to aggregateCitation164 and are cleared by alveolar macrophages.Citation165 Therefore, large porous particles were developed with a geometric diameter of >5 μm, an aerodiameter of <5 μm, and a low density of <0.1 mg/mL.Citation166 When aerolized, large porous particles deposit homogeneously on the cell surface and, when observed by microscopy, appear nontoxic.Citation161 Further development of this molecule has led to “Trojan” particles,Citation165 which have the ability to escape both phagocytic and mucociliary clearance in the respiratory system. They are prepared from nanoparticles, which eventually assemble into a microparticle of low density (<0.1 mg/mL). These particles need to be assessed with a drug load, but published data suggest that they can be aerosolized from dry powder.Citation157 It has been shown previously that a single cancer cell can ingest one or multiple microparticles. The ingested microparticles are arranged in such a way as to reduce the space occupied inside the cell, and the same occurs with macrophagesCitation44,Citation167 (, ).
Carbohydrates
There are currently three carbohydrate formulations approved by the US Food and Drug Administration, ie, lactose (a-lactose monohydrate), glucose, and mannitol (polyol). These carriers contribute to drug flow and dispersability, and also act as stability enhancers. In a recent study, several carriers such as mannitol, sorbitol, maltitol, and xylitol, were assessed and it was concluded that mannitol is the best candidate for dry powder inhaler formulation, given that the others showed limited dispersability.Citation168 Techniques used to produce a respirable formulation are: supercritical fluid technology, spray-freeze drying, freeze-drying, and lyophilizing followed by milling/jet milling or spray-drying.Citation169–Citation173 Moreover, it has been observed that lactose enhances the uptake of polylysine into airway cells, and this has been shown to be a method of increasing intracellular localization of proteins and peptides.Citation174 Two approaches have been developed to improve delivery efficiency and increase drug dispersibility and the respirable fraction. The first approach was mixing fine lactose particles (about 5 μm in diameter) with coarse lactose to improve disaggregation, as well as the fine particle fraction.Citation175 The second approach was to add a ternary component, such as L-leucine, to the formulation.Citation111 Finally, cyclodextrins, which are cyclic oligosaccharides, have proven to be useful excipients in the respiratory distribution of small molecules.Citation176 Until recently, their use for protein/peptide delivery was limited due to the need to address penetration enhancement. However, in a recent study, the use of dimethyl-β-cyclodextrin presented increased bioavailability, with increasing concentrations of cyclodextrinCitation169 ().
Pegylation
PEG added to proteins enables sustained release on the site of deposition. This is achieved by bypassing the defense mechanisms of the respiratory tract, by decreasing degradation of the formulation, and prolonging the half-life in the lungs.Citation144,Citation154,Citation177 In addition, PEG has been demonstrated to be a safe carrier for inhalational agentsCitation178 ().
Biodegradable polymers
Polylactic acid has sustained release properties, but is not suitable for pulmonary drug delivery due to its prolonged biological half-life. An oligomer of lactic acid, with a shorter half-life (6–8 days), can be used for drug delivery. The mucoadhesive polymer, hydroxypropyl cellulose, is released over approximately 24 hours and bypasses mucociliary clearance. However, the toxicity profile of hydroxypropyl cellulose has not been establishedCitation111,Citation179 ().
Bioadhesives
Bioadhesives are used to prolong the connection between the carrier-drug and the surface cell in the airway.Citation180,Citation181 A number of multivalent binding agents have been incorporated in drug-carrier systems, including lectins, peptides, antibodies, octa-arginine, heparin, heparin sulfate, and antibodiesCitation182,Citation183 ().
Cell targeting
Cell targeting has been the focus of increasing interest in recent years, both from the prognostic and therapeutic points of view. Gene therapy has been widely investigated in cell-selective targeting.Citation184 Alveolar macrophages are an attractive vehicle by which to deliver a chemotherapeutic agent to the lymph nodes through the lymphatic circulation. Liposomes and microspheres are generally engulfed by alveolar macrophages. Several receptors are overexpressed, such as epidermal growth factor and folic acid, which can be exploited to target specific cells in cancer therapy.Citation185,Citation186 Low-density lipoprotein has been used for receptor assimilationCitation187 ().
Intracellular targeting
Intracellular targeting is an additional strategy to improve the efficiency of a drug. The general concept is to create a potent drug that would reach the proper surface area, but intracellular targeting is essential to take regional therapy a step further.Citation188,Citation189 In this regard, most chemotherapy regimens interact within the reproductive cell cycle, so this targeting strategy could be further pursued.Citation190 There are three parameters that are investigated concerning the cell microenvironment and drug-formulation interactions, ie, intracellular trafficking, endosomal release, and nuclear localization ().
Drug transporters
ATP-binding cassette transporters
ABC transporters are a large family (50 members) of transmembrane proteins, which act as an ATP-dependent efflux system exporting molecules from the cytoplasm to the surrounding cellular environment. There are seven subfamilies, from A to G. ABC transporters prevent accumulation of xenobiotics, so they serve as a defense mechanism in lung tissue.Citation191 P-glycoprotein, multidrug-resistant proteins, and the breast cancer resistance protein are known to play a role in multidrug resistance, a characteristic observed during the expulsion of chemotherapeutic agents from cancer cells.Citation192 P-glycoprotein has been extensively studied in the lung. It decreases oral drug absorption, prevents drug entry in the central nervous system, and is responsible for many drug-drug interactions.Citation193 The transporter is localized based on immunohistochemistry techniques on the apical membrane of the bronchial and bronchiolar epithelium,Citation194–Citation197 in the endothelial cells of the bronchial capillaries,Citation198 and in alveolar macrophages.Citation195,Citation196 Expression of P-glycoprotein in smokers with lung disease versus people with normal lungs has not been adequately investigated. P-glycoprotein and immunohistochemical multidrug-resistant protein analyses are a useful tool for predicting a patient’s response to chemotherapy. Citation199 In one study, mRNA levels in the lung tissue of smokers, nonsmokers, and exsmokers were not found to be significantly different.Citation194 Several studies have demonstrated directly or indirectly that underlying disease plays a role in regulation of the P-glycoprotein transporter. In addition, pharmaceuticals administered for lung or other disease can upregulate the P-glycoprotein transporter. In cystic fibrosis, due to changes induced by the disease, it has been observed that the P-glycoprotein transporter is upregulated.Citation200,Citation201 Moreover, it has been reported that toxins released from microorganisms infecting patients with cystic fibrosis also inhibit P-glycoprotein.Citation202 In patients with chronic obstructive pulmonary disease (COPD), there are no significant data indicating modification of P-glycoprotein between the disease stages,Citation203,Citation204 and no relevant data exist for asthma patients. Corticosteroids administered by the inhaled, oral, and intraperitoneal routes upregulate the P-glycoprotein transporter.Citation205–Citation207 A particularly good example of the importance of transporters in inhaled chemotherapy is the inhibition of P-glycoprotein by lipid nanocapsules, which is a crucial mechanism of resistance for paclitaxel.Citation208 There are nine multidrug proteins (MRPs). In normal lung tissue, MRP 1 and MRP 5 have been found to be highly expressed. MRP 6 and MRP 7 are moderately expressed, and MRPs 2, 3, 4, 8, and 9 are either low or undetectable.Citation209,Citation210 MRP 1 and MRP 2 are found in the bronchial and bronchiolar epithelium.Citation195,Citation211,Citation212 MRP 1 is also found in alveolar macrophages.Citation195,Citation211 MRP 1 expression and levels are altered in patients with COPD.Citation203,Citation211 It has been previously shown that smoking downregulates the transporter, and the transporter has a protective role against cell damage.Citation203,Citation213 Ipratropium, N-acetylcysteine, and budesonide stimulate MRP 1 efflux and activity.Citation214 Formoterol in combination with budesonide reduces transporter activity, but formoterol on its own does not have an effect on the transporter.Citation214 In a study of inhaled doxorubicin, MRP 1 and MRP 2 were overexpressed.Citation51 This information is crucial, because most lung cancer patients are also diagnosed with COPD. Breast cancer resistance proteins were first isolated from breast cancer cell lines. In a recent study, they were found to be highly expressed in human lung tissue using gene microarraysCitation215 ().
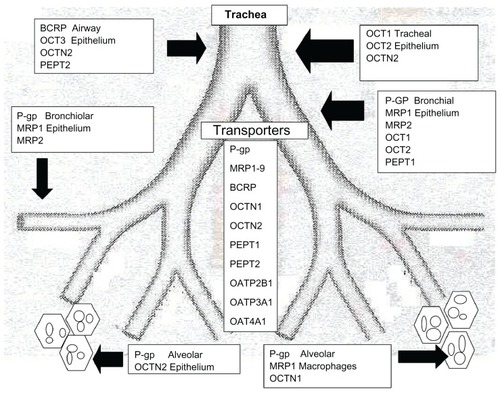
Figure 2 Transporters and their position where they are most highly expressed.
Abbreviations: P-gp, P-glycoprotein; BCRP, breast cancer resistance protein; MRP, multidrug resistance-associated proteins; PEPT, peptide transporters; OCT, organic cation transporters; OCTN, organic cation transporters electroneutral; OAT, organic anion transporters; OATP, organic anion transporting proteins.
Organic cation transporters
Organic cation transporters belong to the greatest facilitator family and comprise five types of carriers, ie, electrogenic OCT 1, OCT 2, and OCT 3, and electroneutral OCTN 1 and OCTN 2. OCT 1–3 are found in the trachea, smooth muscles of the airway, and ciliated bronchial cells, but there are contradictory data in terms of their expression. OCT N1 is expressed in the tracheal epithelium and alveolar macrophages, whereas OCT N2 is expressed in the alveolar epithelium and airway epithelium.Citation215–Citation218 Published data for animal and in vitro cell lines implicate upregulation or downregulation of OCT transporters upon induced inflammation or drug interactions related to asthma and/or COPD. Nevertheless, these are not clearly associated with a human modelCitation217–Citation220 ().
Peptide transporters
Peptide transporters are part of the proton-coupled oligo-peptide transporter group. The two main transporters are PEPT 1 and PEPT 2, which contribute to the high bioavailability of peptide-like molecules. These peptides can affect the absorption and distribution of several inhaled antibiotic and antiviral drugs.Citation221 PEPT 2 and more recently PEPT 1 were detected first in the airway epithelium and then in the bronchial epithelium.Citation222,Citation223 These two transporters have also been found in animals and cell lines.Citation224,Citation225 However, how they interact with drug formulations and their activity in respiratory diseasesCitation226 has not been fully investigated.
Organic anion transporters
There are six members identified, ie, OAT 1–4, URAT 1, and OAT 5, which are mostly found in the kidneys.Citation227 Gene microarrays have confirmed their absence in human and murine lungs, but OAT 2 was highly expressed at these sites.Citation215 In addition, OAT 4 mRNA was highly expressed in the bronchial cell lines Calu-3 and 16HBE14o-.Citation224
Organic anion transporting polypeptides
There are 11 human organic anion transporting polypeptides (OATPs), which are divided into six families.Citation228 Their actual tissue distribution has not been fully investigated.Citation228 OATP 2B1, OATP 3A1, OATP 4C1, and OATP 4A1 expression has been found in human lungs, animals, and cell lines.Citation215,Citation224,Citation229
Inhalation studies
There is a large amount of published data regarding aerosol delivery of chemotherapy in cancer cell cultures, animal models, and Phase I/II human studies (). These studies are best commented on in terms of the chemotherapeutic agent delivered to the lung parenchyma with additional individual parameters. The first chemotherapeutic agent, investigated almost 30 years ago, was 5-fluorouracil (5-FU).Citation31 Tatsumura et alCitation32 presented data for patients treated with inhaled 5-FU and underwent surgery immediately afterwards, who had higher drug concentrations in the tumor than in the surrounding tissues. In addition, high 5-FU concentrations were found for up to 4 hours after administration in the main bronchus and in the lymph nodes around the main bronchus.Citation32 This observation was confirmed in another study using 5-FU.Citation60 Moreover, additional formulations of 5-FU with lipid-coated nanoparticles or difluoromethylornithine, an important enzyme in cell proliferation, were devised to achieve sustained drug release and enhance anticancer properties.Citation60,Citation230,Citation231 In these studies, previously presented for their inhalable system carriers with 5-FU, a step was made forward in using inhaled chemotherapy as an adjuvant treatment. Studies using taxanes either with liposome carriers, nanoparticles, polymeric micelles, or lipid nanocapsules provided evidence of an increased therapeutic index by prolonging regional action in the lung. Further, 5%–7% CO2 has been used to enhance aerosolized drug deposition.Citation50,Citation82 However, the data are controversial regarding the safety of taxanes at the lung parenchyma. The data indicate that the mononuclear phagocyte system attacks the colloidal drug, so a combination of pegylated lipid nanocapsules is needed to prolong regional action. In addition, further studies will establish their efficacy in human subjects, after proper alterations/additions to the drug formulation, such as nanoparticlesCitation232 or nanospheres,Citation233 and when linked to human albumin.Citation45,Citation50,Citation59,Citation234,Citation235 Moreover, adverse effects, mainly neurotoxicity, was found to be dose-dependent, but also associated with increased tumor burden regression,Citation50 and addition of cyclosporine A to the paclitaxel aerosol was found to enhance the anticancer effect of the treatment.Citation55 It was observed that addition of cyclosporine A reversed the resistance of cancer cells to paclitaxel.Citation55 When a taxane compound was compared with doxorubicin, it was noticed that adverse effects on the lung parenchyma were observed for the doxorubicin group, indicating that taxanes are safer in comparison with doxorubicin regarding the lung region.Citation34 In addition, severe cardiotoxicity was seen in the doxorubicin group.Citation34,Citation47 Otterson et alCitation37,Citation52 created a protocol for inhaled chemotherapy in human subjects, covering all aspects of this treatment modality. Two Phase I and Phase I/II studies demonstrated the adverse effects of aerosol treatment, such as a metallic taste, mild bronchospasm, and moderate reduction of pulmonary function tests. Therefore, bronchodilators were administered before every session and patients rinsed their mouth with water afterwards. It was observed that a significant drawback was the timing of administration of the drug formulation (60 minutes). Aerosol deposition was evaluated by radiolabeling, and remission of pulmonary function tests was observed after every chemotherapy session. In addition, other basic characteristics of this protocol proposal for inhalation chemotherapy were addressed, such as inclusion criteria. It is essential to highlight tumor size, which must not be more than 5 cm in mass median diameter, because this parameter is crucial for drug deposition.Citation84,Citation131 These issues are analyzed further in the safety section. In another study using nanoparticles with doxorubicin, it was observed that macrophages clear the formulation, so smaller nanoparticles need to be developed.Citation47 Platinum analogs have also been investigated, and the findings were similar to those for inhaled doxorubicin. Moderate bronchospasm after aerosol inhalation, cough, fever (three days in some cases), and a metallic taste were observed. Bronchodilators were administered before every session. Pulmonary function tests were also performed according to the American Thoracic Society/European Respiratory Society guidelinesCitation53,Citation56,Citation236,Citation237 along with a high resolution CT scan of the thorax.Citation30,Citation53 Mild reduction in pulmonary function tests was observed immediately after the chemotherapy session, but regressed until the next cycle.
Table 2 Published studies with inhaled chemotherapy regimens and study investigated parameters
In a study by Tseng et alCitation45 a biotinylated epidermal growth factor-modified gelatin nanoparticle carrier was investigated, and found to enhance the anticancer activity of the formulation. In addition, it paved the way for targeted inhaled chemotherapy. Cancer cells with epidermal growth factor overexpression show increased uptake of this formulation. An additional benefit is reduced nephrotoxicity, because more cisplatin remains regionally in the lung cancer cells, and fewer carriers with cisplatin are delivered to the systemic circulation. Anderson et alCitation49 administered a novel nonhydrolyzable ether-linked acetic acid analog of vitamin E (a-TEA) with intraperitoneal administration of cisplatin. This treatment is mentioned due to the positive effect observed in reducing lung metastasis to the lungs.Citation49,Citation238,Citation239 The a-TEA could be used as a molecule additional to the aerosol formulation, in order to augment apoptosis of cancer cells and to decrease cancer cell proliferation.Citation49,Citation57,Citation239 Again, safety issues are discussed in the safety section. The anticancer effect of 9-nitro-camptothecin as an aerosol has been established.Citation56,Citation57,Citation240 Several formulations using carrier systems have been used to deliver sustained-release 9-nitro-camptothecin. Feasibility and effectiveness was established, and in addition to encapsulation with carriers, pegylation added a “stealth” property, as previously mentioned. The macrophages did not recognize the formulation and so did not attack, but cancer cells ingested the molecules due to the cationic charge (PEG technology).Citation44 However, the polystyrene microparticles used in this study cannot be used in human subjects because they cannot be eliminated from the body. Therefore, an initiative to develop biocompatible aggregated nanogel particles has been started using additional PEG technology.Citation44 In a study by Verschraegen et alCitation56 aerosol therapy was taken to a level never previously achieved. After effectiveness and safety had been observed, patients were educated to receive their therapy at home. This study provides evidence that, if safety and effectiveness of such a system is confirmed, patients can receive any chemotherapy agent without having the side effects that are usually observed with these agents.
Gemcitabine, a well known chemotherapeutic agent, has been evaluated in dogs and baboons as an aerosol formulation. The formulation was radiolabeled and blood samples were collected until 360 minutes.Citation43,Citation241,Citation242 The efficacy of this treatment modality on lung metastasis due to osteosarcoma was investigated, along with Fas/FasL expression. The Fas ligand is a type II transmembrane protein that belongs to the tumor necrosis factor family. Fas expression in metastatic foci was increased compared with that in lung metastases before treatment, and at even higher levels than in the primary tumor. The results of these studies indicate that aerosolized gemcitabine treatment is effective against metastatic osteosarcoma lesions.Citation61 Gemcitabine has been administered to patients with lung cancer, either as an aerosol or instilled into the lung parenchyma, and the data have demonstrated efficacy.Citation243,Citation244 The safety of gemcitabine in humans is discussed in the safety section.
Safety and protection measures
Both patients and medical staff should be considered concerning safety (). The question that needs to be answered is whether interstitial lung disease is induced due to inhalation chemotherapy. Several agents have been observed to induce this kind of damage after intravenous administration.Citation245–Citation251 On high resolution CT, a ground-glass pattern, linear opacities, interlobular or intralobular thickening, and alveolar shadows were observed. Different histopathological appearances are also observed. Determining the background of pulmonary infiltrates in patients who develop pulmonary severe involvement while receiving chemotherapeutic agents can be problematic. There are several factors that could produce this type of appearance, including pulmonary edema, alveolar hemorrhage, involvement of background disease, and radiation. Moreover, a confirmatory biopsy or bronchoalveolar lavage is often not possible to undertake due to respiratory distress or severity of the underlying condition. Because several chemotherapeutic agents may induce interstitial lung disease by intravenous administration, a through safety evaluation has had to be performed for the aerolized formulations. Regarding the safety of medical personnel, the aerolized studies were performed with certain protection measures, including special plastic cages for animals.Citation49,Citation57 Other measures for protection were the design of the delivery system or method. In a number of studies, the drug was delivered through a special nose-only chamberCitation51 and using an intracorporeal catheter.Citation41 An evaluation of whether these systems had sufficient environment safety was performed in several cases and the measures were adequate.Citation34
Evaluation of pulmonary toxicity was observed by post mortem histological examination and radiological investigations, ie, x-ray, magnetic resonance image, bioluminescent image, and V/Q scan.Citation41,Citation43,Citation47,Citation51 The most severe side effects were cough, weight loss, neurotoxicity, cardiotoxicity, alveolar interstitial pattern (radiological findings), moderate fibrosis (histopathological findings), and death as a result of pulmonary edema.Citation34,Citation41,Citation43,Citation50,Citation54 One death was reported in a Phase I study by Otterson et alCitation52 after doxorubicin aerosol administration, but autopsy revealed focal hyaline membrane deposition and obstructive pneumonia due to tumor burden. Staphylococcus and Acinetobacter baumannii were isolated from blood cultures, two bacteria commonly found in intensive care units, where the patient had been hospitalized due to severe respiratory distress. Death was attributed to disease progression. Selting et alCitation41 demonstrated clearly that when administered repeatedly to a specific part of the respiratory airway, platinum analogs moderate the fibrosis and an alveolar interstitial pattern occurs. Nevertheless, these findings are dose-associated and depend on the time interval of treatment. When bronchospasm occurred and there was a drop in pulmonary function tests, additional steroid treatment reversed these adverse effects.Citation34,Citation37,Citation52 These lesions did not appear using liposomal paclitaxel in studies which included histopathological evaluation.Citation55 In a study by Tseng et alCitation45 less nephrotoxicity was observed with biotinylated epidermal growth factor-modified gelatin nanoparticle carriers with a platinum analog, in comparison with a free circulating platinum analog. Regarding human subjects, studies used either a mouthpieceCitation30 or a facial maskCitation42,Citation53,Citation56 under a high efficiency particulate air system; the drug formulation which escaped (if any) was evaluated, and no toxic effects were found. In one study, the drug delivery system and formulation were evaluated and found to be efficient and safe enough that the patients were instructed to administer the drug formulation at home.Citation56 Methods of evaluating the pulmonary parenchyma and respiratory capacity in human subjects have included pulmonary function tests with forced expiratory volume in one second (FEV1), forced vital capacity, carbon monoxide diffusing capacity (DLCO), the 6-minute walking test, and high resolution CT or CT scan.Citation30,Citation37,Citation52,Citation53,Citation56 The patients included in these studies did not have any known collagen disease in order to be certain whether interstitial disease findings, if observed, were due to the inhaled compound. The adverse effects most commonly observed were coughing, mild bronchoconstriction, fever, nausea, pharyngitis, thickening of the bronchial wall (high resolution CT finding),Citation53 focal hyaline membrane deposition (post mortem finding),Citation52 and reduction in pulmonary function tests that responded to corticosteroids or resolved after termination of treatment.Citation30,Citation37,Citation53 In a study by Garbuzenko et alCitation51 alveolar hemorrhage and bronchial accumulation of chronic inflammatory cells were observed in histopathological specimens after aerosol administration of liposomal doxorubicin. Moreover, large bronchi were surrounded by aggregates of chronic inflammatory cells, including lymphocytes, macrophages, and plasma cells.Citation51 In three studies, a mild reduction of FEV1, forced vital capacity, and DLCO was observed after aerosol administration and therefore bronchodilators and inhaled corticosteroids were administered before every treatment.Citation30,Citation37,Citation52,Citation53 Gemcitabine in an aerosol formulation did not induce fibrotic lesions in the lung parenchyma and does not contain any chemical ingredients incompatible with aerosol delivery.Citation43,Citation62 Nevertheless, in an animal model, death from pulmonary edema occurred after aerosol administration of gemcitabine.Citation43 Studies with 9-nitro-20(S)-camptothecin did not report fibrotic lesions in the lung parenchyma, although a reversible reduction in pulmonary function tests and mild adverse effects from the aerosol compound were observed (bronchial irritation, sore throat, pharyngitis).Citation56 In studies performed in cancer cell lines, administration of the aerosol was conducted inside hoods, so medical personnel were safe from the toxic compounds. Assessment of efficacy was made by observing the decrease in the population of cancer cells.Citation58,Citation59
Conclusion
Inhaled chemotherapy is a feasible treatment modality. Nevertheless, the pulmonary side effects of this treatment have to be assessed further. Until now, inhaled chemotherapy was administered for advanced lung cancer and, therefore, although some chemotherapeutic agents provided immediate evidence of dose/time-related toxicity, others did not due to the limited duration of administration. Early-stage lung cancer studies did not administer inhaled chemotherapy for more than one session.Citation31,Citation32 Therefore, studies involving early-stage lung cancer are needed to provide time/dose-related safety data. Moreover, studies providing doublet inhaled chemotherapeutic agents need to be performed because doublet chemotherapy is the cornerstone of treatment for lung cancer. However, these trials should not be performed until sufficient data regarding the safety profile in the lung parenchyma are available. Regarding efficacy, regional studies have demonstrated positive results, but there are limitations to use of drug locoregionally according to tumor size, penetration of the drug at the tumor site, the chemical characteristics of the drug, and its biological effects. Regional therapy does not necessarily mean that higher drug levels reach the tumor. However, this concept contradicts the fact that, due to blood circulation in the lungCitation52,Citation53 and the lymphatic circulation, drug concentrations have been found in the systemic circulation and surgically resected lymph nodes.Citation31,Citation32,Citation84,Citation131,Citation230,Citation231,Citation238 In addition, a high percentage of lymph nodes in the aerosol treatment groups did not show any metastasis in several studies, so it is possible that this route of administration destroys tumor cells and prevents them from trafficking from the primary subcutaneous tumor to the lungs and lymph nodes via the lymphatic system.Citation31,Citation32,Citation49,Citation57 Nevertheless, in many of these studies, an additional intravenous chemotherapy regimen was administered, so clear conclusions cannot be drawn. Nanoparticles have the ability to evade macrophages and transport them into lung tissues other than the alveolus and into the general circulation.Citation47 However, macrophages are a defense mechanism that is also responsible for clearing the nanoparticles and, thus, reduce the anticancer effect of inhaled chemotherapy. Furthermore, an evaluation has to be made of whether inhaled chemotherapy is appropriate for early-stage lung cancer, or as neoadjuvant or adjuvant treatment.Citation57,Citation60,Citation61,Citation230,Citation231
Because lesions are of smaller size, drug deposition is not prevented by the large mass median diameter of the tumor.Citation131 Current drug delivery systems have demonstrated an ability to achieve sustained release locoregionally, but further improvement is welcomed.Citation40,Citation48,Citation252 Nevertheless, the timing of the drug administration has to be shortened.Citation30,Citation48 Additional modifications were made to the molecules, to include targeted therapies, such as the epidermal growth factor receptor.Citation45 In another study by Garbuzenko et alCitation51 additional pump and nonpump suppressors were added to inhaled doxorubicin. This concept was based on the observation that there are two main mechanisms responsible for cancer cell chemotherapy resistance, ie, pump and nonpump.Citation253–Citation255 Pump resistance is caused by membrane efflux pumps that decrease the anticancer drug concentration inside cells. Nonpump resistance is primarily attributed to the activation of antiapoptotic cellular defense, and Bcl-2 is also a key parameter in this defense. Similar to MRP 1, expression of Bcl-2 protein increases significantly after treatment with anticancer drugs.Citation253,Citation255 a-TEA could be used as a molecule additional to the aerosol formulation to augment apoptosis and decrease cancer cell proliferation.Citation49,Citation57,Citation239 Nebulizers have also demonstrated efficiency for delivery and deposition of inhaled chemotherapeutic regimens.Citation30,Citation37,Citation52,Citation53 Additional modifications have been shown to improve deposition further by modifying respiratory rate and tidal volume.Citation82 Delivery systems have been evaluated for safety regarding environmental release of toxins and it has been found that certain systems have the ability to deliver their entire drug cargo to patients, without any loss to the environment.Citation30,Citation42 Drug transporter gene expression appears to be high in the lungs.Citation215 However, interaction of these genes with inhaled drug formulations, any alterations due to underlying respiratory disease, and their role in drug deposition are not fully explored.Citation226 The transporters are less likely to influence absorption of inhaled drugs than they are to exert an effect in the gastrointestinal tract; nevertheless, efflux pumps can be exploited to prolong drug retention in situ.Citation226 Transporter properties can be fully exploited if a formulation is designed for distribution to specific sites in the respiratory tract where they are highly expressed in combination with the appropriate drug carrier. In addition to the transporters, several other carriers have been investigated in combination with chemotherapy agents, either in order to make feasible their administration to the lung parenchyma for toxicity reasons, or to add a sustained-release capability to the lung formulation.Citation34,Citation39,Citation40,Citation47–Citation51,Citation53,Citation55–Citation60 Transporters could be used in addition as a prognostic factor.Citation199 Moreover, regarding carriers, liposomes would appear to require dosing three or four times a day and would be expected to be well tolerated within the lung following repeated dosing. Microspheres and lipid-coated nanoparticles could be given once or twice daily, depending on particle residence time at the site of action. However, issues of particle accumulation within the alveoli may be encountered with repeated dosing. Due to the composition of lipid-coated nanoparticles in comparison with microspheres, there would be less of a concern with toxicity using lipid-coated nanoparticles.Citation231 The optimal formulation for the clinician would be once-a-day delivery of a chemotherapy regimen, with the additional effect of pegylation for the “stealth” properties previously mentioned.
Finally, the next step in delivering inhaled chemotherapy at home has already been made after proper patient education, and the concept is intriguing.Citation56 Monitoring of patients can be done using peak flow measurements at home between restaging, and addition of a combination of inhaled bronchodilators and corticosteroids before inhaled chemotherapy could help prevent adverse effects, such as bronchoconstriction. Addition of N-acetylcysteine could be used as a protective measure.Citation93,Citation214 Finally, inhaled chemotherapy regimens when administered alone demonstrate fewer systemic cytotoxic effects, making the concept of safe inhaled chemotherapy the next challenge in the treatment of lung cancer. Nevertheless, the safety and efficacy of such formulations have yet to be fully and completely evaluated ().
Table 3 Summary of inhaled chemotherapy in lung cancer
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- LangerCJHarrisJHorwitzEMPhase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911J Clin Oncol200725304800480517947728
- CurranWJJrSchillerJHWolkinACComisRLAddressing the current challenges of non-small-cell lung cancer clinical trial accrualClin Lung Cancer20089422222618650170
- SomerRAShermanELangerCJRestrictive eligibility limits access to newer therapies in non-small-cell lung cancer: the implications of Eastern Cooperative Oncology Group 4599Clin Lung Cancer20089210210518501096
- NovelloSLongoMLevraMGToward therapies tailored to patient characteristicsJ Thorac Oncol20072Suppl 5S384117457231
- SubramanianJWaqarSNGovindanRTargeted therapy in lung cancer: lessons learned from past experiencesJ Thorac Oncol2011611 Suppl 4S1786178822005530
- Garcia SarDAguadoLMontes BayonMRelationships between cisplatin-induced adducts and DNA strand-breaks, mutation and recombination in vivo in somatic cells of Drosophila melanogaster, under different conditions of nucleotide excision repairMutat Res20127411–2818822108251
- HosomiYShibuyaMNihoSPhase II study of topotecan with cisplatin in Japanese patients with small cell lung cancerAnticancer Res201131103449345621965760
- ShepherdFACrowleyJVan HouttePThe International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancerJ Thorac Oncol20072121067107718090577
- HunterTBManimalaNJLuddyKACatlinTAntoniaSJPaclitaxel and TRAIL synergize to kill paclitaxel-resistant small cell lung cancer cells through a caspase-independent mechanism mediated through AIFAnticancer Res201131103193320421965726
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- MinchintonAITannockIFDrug penetration in solid tumoursNat Rev Cancer20066858359216862189
- KemenyNENiedzwieckiDHollisDRHepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481)J Clin Oncol20062491395140316505413
- ArmstrongDKBundyBWenzelLIntraperitoneal cisplatin and paclitaxel in ovarian cancerN Engl J Med20063541344316394300
- GuerinCOliviAWeingartJDLawsonHCBremHRecent advances in brain tumor therapy: local intracerebral drug delivery by polymersInvest New Drugs2004221273714707492
- RobinsonWRCoberlyCBeyerJLewisABallardCOffice-based intraperitoneal chemotherapy for ovarian cancerJ Oncol Pract20084522522820856699
- HofstraLSBosAMde VriesEGA phase I and pharmacokinetic study of intraperitoneal topotecanBr J Cancer200185111627163311742479
- OsakiTHanagiriTNakanishiRYoshinoITagaSYasumotoKBronchial arterial infusion is an effective therapeutic modality for centrally located early-stage lung cancer: results of a pilot studyChest199911551424142810334163
- MaischBRisticADPankuweitSNeubauerAMollRNeoplastic pericardial effusion. Efficacy and safety of intrapericardial treatment with cisplatinEur Heart J200223201625163112323163
- MarkmanMClearySPfeifleCHowellSBCisplatin administered by the intracavitary route as treatment for malignant mesotheliomaCancer198658118213708543
- RuschVWFiglinRGodwinDPiantadosiSIntrapleural cisplatin and cytarabine in the management of malignant pleural effusions: a Lung Cancer Study Group trialJ Clin Oncol1991923133191988578
- GoldbergEPHadbaARAlmondBAMarottaJSIntratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug deliveryJ Pharm Pharmacol200254215918011848280
- DuvillardCRomanetPCosmidisABeaudouinNChauffertBPhase 2 study of intratumoral cisplatin and epinephrine treatment for locally recurrent head and neck tumorsAnn Otol Rhinol Laryngol20041133 Pt 122923315053208
- LabirisNRDolovichMBPulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medicationsBr J Clin Pharmacol200356658859914616418
- LaubeBLThe expanding role of aerosols in systemic drug delivery, gene therapy, and vaccinationRespir Care20055091161117616122400
- BakkerEMVolpiSSaloniniEImproved treatment response to dornase alfa in cystic fibrosis patients using controlled inhalationEur Respir J20113861328133521737560
- BiltonDRobinsonPCooperPInhaled dry powder mannitol in cystic fibrosis: an efficacy and safety studyEur Respir J20113851071108021478216
- YuKNMinai-TehraniAChangSHAerosol delivery of small hairpin osteopontin blocks pulmonary metastasis of breast cancer in micePLoS One2010512e1562321203518
- ZarogoulidisPPapanasNKouliatsisGSpyratosDZarogoulidisKMaltezosEInhaled insulin: too soon to be forgotten?J Aerosol Med Pulm Drug Deliv201124521322321689020
- ZarogoulidisPEleftheriadouESapardanisIFeasibility and effectiveness of inhaled carboplatin in NSCLC patientsInvest New Drugs July 82011 Epub ahead of print
- TatsumuraTYamamotoKMurakamiATsudaMSugiyamaSNew chemotherapeutic method for the treatment of tracheal and bronchial cancers – nebulization chemotherapyGan No Rinsho1983297765770 Japanese6308308
- TatsumuraTKoyamaSTsujimotoMKitagawaMKagamimoriSFurther study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinicalBr J Cancer1993686114611498260366
- GautamAKoshkinaNPaclitaxel (Taxol) and taxoid derivates for lung cancer treatment: potential for aerosol deliveryCurr Cancer Drug Targets20033428729612871059
- HersheyAEKurzmanIDForrestLJInhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a modelClin Cancer Res1999592653265910499645
- KoshkinaNVGolunskiERobertsLEGilbertBEKnightVCyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in miceJ Aerosol Med200417171415120008
- MinchinRFJohnstonMRAikenMABoydMRPharmacokinetics of doxorubicin in isolated lung of dogs and humans perfused in vivoJ Pharmacol Exp Ther198422911931986707934
- OttersonGAVillalona-CaleroMAHicksWPhase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancerClin Cancer Res20101682466247320371682
- GaviniEManuntaLGiuaSAchenzaGGiunchediPSpray-dried poly(D,L-lactide) microspheres containing carboplatin for veterinary use: in vitro and in vivo studiesAAPS Pharm Sci Tech200561E108114
- KhannaCVailDMTargeting the lung: preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancersCurr Cancer Drug Targets20033426527312871057
- GagnadouxFHureauxJVecellioLAerosolized chemotherapyJ Aerosol Med Pulm Drug Deliv2008211617018518832
- SeltingKWaldrepJCReineroCFeasibility and safety of targeted cisplatin delivery to a select lung lobe in dogs via the AeroProbe intracorporeal nebulization catheterJ Aerosol Med Pulm Drug Deliv200821325526818759657
- WittgenBPKunstPWPerkinsWRLeeJKPostmusPEAssessing a system to capture stray aerosol during inhalation of nebulized liposomal cisplatinJ Aerosol Med200619338539117034313
- GagnadouxFPapeALLemarieEAerosol delivery of chemotherapy in an orthotopic model of lung cancerEur Respir J200526465766116204597
- ChaoPDeshmukhMKutscherHLPulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer modelAnticancer Drugs2010211657619966540
- TsengCLSuWYYenKCYangKCLinFHThe use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalationBiomaterials200930203476348519345990
- SchafferMWRoySSMukherjeeSOngDEDasSKUptake of all-trans retinoic acid-containing aerosol by inhalation to lungs in a guinea pig model system – a pilot studyExp Lung Res2010361059360121043991
- RoaWHAzarmiSAl-HallakMHFinlayWHMaglioccoAMLobenbergRInhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse modelJ Control Release20111501495521059378
- HureauxJLagarceFGagnadouxFLipid nanocapsules: readyt-o- use nanovectors for the aerosol delivery of paclitaxelEur J Pharm Biopharm200973223924619560538
- AndersonKLawsonKASimmons-MenchacaMSunLSandersBGKlineKAlpha-TEA plus cisplatin reduces human cisplatin-resistant ovarian cancer cell tumor burden and metastasisExp Biol Med (Maywood)2004229111169117615564444
- KoshkinaNVWaldrepJCRobertsLEGolunskiEMeltonSKnightVPaclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma modelClin Cancer Res20017103258326211595722
- GarbuzenkoOBSaadMPozharovVPReuhlKRMainelisGMinkoTInhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistanceProc Natl Acad Sci U S A201010723107371074220498076
- OttersonGAVillalona-CaleroMASharmaSPhase I study of inhaled doxorubicin for patients with metastatic tumors to the lungsClin Cancer Res20071341246125217317836
- WittgenBPKunstPWvan der BornKPhase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lungClin Cancer Res20071382414242117438100
- WattenbergLWWiedmannTSEstensenRDChemoprevention of cancer of the upper respiratory tract of the Syrian golden hamster by aerosol administration of difluoromethylornithine and 5-fluorouracilCancer Res20046472347234915059884
- KnightVKoshkinaNVGolunskiERobertsLEGilbertBECyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in miceTrans Am Clin Climatol Assoc200411539540417060982
- VerschraegenCFGilbertBELoyerEClinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignanciesClin Cancer Res20041072319232615073107
- LawsonKAAndersonKSnyderRMNovel vitamin E analogue and 9-nitro-camptothecin administered as liposome aerosols decrease syngeneic mouse mammary tumor burden and inhibit metastasisCancer Chemother Pharmacol200454542143115197487
- El-GendyNBerklandCCombination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomerationPharm Res20092671752176319415471
- AzarmiSTaoXChenHFormulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particlesInt J Pharm20063191–215516116713150
- HitzmanCJWattenbergLWWiedmannTSPharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticlesJ Pharm Sci20069561196121116639722
- RodriguezCOJrCrabbsTAWilsonDWAerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogsJ Aerosol Med Pulm Drug Deliv201023419720619803732
- KoshkinaNVKleinermanESAerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastasesInt J Cancer2005116345846315800950
- KoshkinaNVGilbertBEWaldrepJCSeryshevAKnightVDistribution of camptothecin after delivery as a liposome aerosol or following intramuscular injection in miceCancer Chemother Pharmacol199944318719210453719
- SharmaSWhiteDImondiARPlackeMEVailDMKrisMGDevelopment of inhalational agents for oncologic useJ Clin Oncol20011961839184711251016
- SwiftDLAerosols and humidity therapy. Generation and respiratory deposition of therapeutic aerosolsAm Rev Respir Dis19801225 Pt 271777458052
- PhippsPRGondaIAndersonSDBaileyDBautovichGRegional deposition of saline aerosols of different tonicities in normal and asthmatic subjectsEur Respir J199478147414827957833
- ShinoharaHDistribution of lymphatic stomata on the pleural surface of the thoracic cavity and the surface topography of the pleural mesothelium in the golden hamsterAnat Rec1997249116239294645
- Lai-FookSJMechanical factors in lung liquid distributionAnnu Rev Physiol1993551551798466171
- NunnJFNunn’s Applied Respiratory Physiology4th edOxford, UKButterworth-Heineman1993
- DeffebachMECharanNBLakshminarayanSButlerJThe bronchial circulation. Small, but a vital attribute of the lungAm Rev Respir Dis198713524634813544986
- HoutmeyersEGosselinkRGayan-RamirezGDecramerMRegulation of mucociliary clearance in health and diseaseEur Respir J19991351177118810414423
- SummersQAInhaled drugs and the lungClin Exp Allergy19912132592681863889
- StoneKCMercerRRGehrPStockstillBCrapoJDAllometric relationships of cell numbers and size in the mammalian lungAm J Respir Cell Mol Biol1992622352431540387
- FolkessonHGMatthayMAWestromBRKimKJKarlssonBWHastingsRHAlveolar epithelial clearance of proteinJ Appl Physiol1996805143114458727524
- MartonenTBMathematical model for the selective deposition of inhaled pharmaceuticalsJ Pharm Sci19938212119111998308694
- SuarezSHickeyAJDrug properties affecting aerosol behaviorRespir Care200045665266610894458
- IlowiteJSGorvoyJDSmaldoneGCQuantitative deposition of aerosolized gentamicin in cystic fibrosisAm Rev Respir Dis19871366144514493688646
- MessinaMSSmaldoneGCEvaluation of quantitative aerosol techniques for use in bronchoprovocation studiesJ Allergy Clin Immunol19857522522573968336
- DolovichMBKillianDWolffRKObminskiGNewhouseMTPulmonary aerosol deposition in chronic bronchitis: intermittent positive pressure breathing versus quiet breathingAm Rev Respir Dis19771153397402320924
- DolovichMBSanchisJRossmanCNewhouseMTAerosol penetrance: a sensitive index of peripheral airways obstructionJ Appl Physiol1976403468471931865
- DavisJNStaggDInterrelationships of the volume and time components of individual breaths in resting manJ Physiol197524524814981142186
- KoshkinaNVKnightVGilbertBEGolunskiERobertsLWaldrepJCImproved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: pharmacokinetic studiesCancer Chemother Pharmacol200147545145611391862
- StegenKNeujensACrombezGHermansDVan de WoestijneKPVan den BerghONegative affect, respiratory reactivity, and somatic complaints in a CO2 enriched air inhalation paradigmBiol Psychol1998491–21091229792488
- KleinstreuerCZhangZLiZModeling airflow and particle transport/deposition in pulmonary airwaysRespir Physiol Neurobiol20081631–312813818674643
- ZhangZKleinstreuerCKimCSAirflow and nanoparticle deposition in a 16-generation tracheobronchial airway modelAnn Biomed Eng200836122095211018850271
- CrankJThe Mathematics of Diffusion2nd edOxford, UKClarendon Press1975
- LeuAJBerkDALymboussakiAAlitaloKJainRKAbsence of functional lymphatics within a murine sarcoma: a molecular and functional evaluationCancer Res200060164324432710969769
- JainRKMunnLLFukumuraDDissecting tumour pathophysiology using intravital microscopyNat Rev Cancer20022426627612001988
- JainRKDelivery of molecular and cellular medicine to solid tumorsAdv Drug Deliv Rev1997262–3719010837535
- MilosevicMFFylesAWWongRInterstitial fluid pressure in cervical carcinoma: within tumor heterogeneity, and relation to oxygen tensionCancer19988212241824269635535
- HeldinCHRubinKPietrasKOstmanAHigh interstitial fluid pressure – an obstacle in cancer therapyNat Rev Cancer200441080681315510161
- JainRKBarriers to drug delivery in solid tumorsSci Am1994271158658066425
- JainRKTransport of molecules in the tumor interstitium: a reviewCancer Res19874712303930513555767
- WeberAMorlinGCohenMWilliams-WarrenJRamseyBSmithAEffect of nebulizer type and antibiotic concentration on device performancePediatr Pulmonol19972342492609141110
- EschenbacherWLBousheyHASheppardDAlteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough aloneAm Rev Respir Dis198412922112156696320
- JonasDEWinesRCMDelMonteMDrug Class Review: Controller Medications for Asthma: Final Update 1 ReportPortland, OROregon Health & Science University2011
- DolovichMBRamsdaleEHReplacing CFC aerosols with powdersCMAJ19901421010362337836
- NewmanSPTherapeutic inhalation agents and devices. Effectiveness in asthma and bronchitisPostgrad Med19847651942036435107
- BennettWDSmaldoneGCHuman variation in the peripheral air-space deposition of inhaled particlesJ Appl Physiol1987624160316103597231
- DolovichMRyanGNewhouseMTAerosol penetration into the lung; influence on airway responsesChest198180Suppl 68348367307620
- CromptonGKProblems patients have using pressurized aerosol inhalersEur J Respir Dis Suppl19821191011046954081
- NewmanSPPaviaDMorenFSheahanNFClarkeSWDeposition of pressurised aerosols in the human respiratory tractThorax198136152557292382
- NewmanSPPaviaDGarlandNClarkeSWEffects of various inhalation modes on the deposition of radioactive pressurized aerosolsEur J Respir Dis Suppl198211957656807705
- PaviaDThomsonMLClarkeSWShannonHSEffect of lung function and mode of inhalation on penetration of aerosol into the human lungThorax1977322194197867333
- NewmanSPWeiszAWTalaeeNClarkeSWImprovement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler techniqueThorax199146107127161750017
- ChapmanKRLoveLBrubakerHA comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjectsChest19931045133213378222783
- DolovichMRationale for spacer use in childrenPediatr Pulmonol Suppl1997161841859443266
- PedersenSInhalers and nebulizers: which to choose and whyRespir Med199690269778730324
- DolovichMNew propellant-free technologies under investigationJ Aerosol Med199912Suppl 1S91710623341
- NewmanSPMorenFTrofastETalaeeNClarkeSWDeposition and clinical efficacy of terbutaline sulphate from Turbuhaler, a new multi-dose powder inhalerEur Respir J1989232472522731602
- LabirisNRDolovichMBPulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medicationsBr J Clin Pharmacol200356660061214616419
- BorgstromLBondessonEMorenFTrofastENewmanSPLung deposition of budesonide inhaled via Turbuhaler: a comparison with terbutaline sulphate in normal subjectsEur Respir J19947169738143834
- ClarkARHollingworthAMThe relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers – implications for in vitro testingJ Aerosol MedSummer19936299110
- ChrystynHIs inhalation rate important for a dry powder inhaler? Using the In-Check Dial to identify these ratesRespir Med200397218118712587970
- LavoriniFMagnanADubusJCEffect of incorrect use of dry powder inhalers on management of patients with asthma and COPDRespir Med2008102459360418083019
- AmaniAYorkPChrystynHClarkBJDoDQDetermination of factors controlling the particle size in nanoemulsions using artificial neural networksEur J Pharm Sci2008351–2425118617002
- DolovichMBAhrensRCHessDRDevice selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and ImmunologyChest2005127133537115654001
- KendrickAHSmithECWilsonRSSelecting and using nebuliser equipmentThorax199752Suppl 2S921019155861
- BoeJDennisJHO’DriscollBREuropean Respiratory Society Guidelines on the use of nebulizersEur Respir J200118122824211510796
- SmithECDenyerJKendrickAHComparison of twenty three nebulizer/compressor combinations for domiciliary useEur Respir J199587121412217589407
- BrandPBeckmannHMaas EnriquezMPeripheral deposition of alpha1-protease inhibitor using commercial inhalation devicesEur Respir J200322226326712952258
- NivenRWIpAYMittelmanSPrestrelskiSJArakawaTSome factors associated with the ultrasonic nebulization of proteinsPharm Res199512153597724488
- WagnerAVorauer-UhlKKatingerHNebulization of liposomal rh-Cu/Zn-SOD with a novel vibrating membrane nebulizerJ Liposome Res200616211312516753966
- ElhissiAMKarnamKKDanesh-AzariMRGillHSTaylorKMFormulations generated from ethanol-based proliposomes for delivery via medical nebulizersJ Pharm Pharmacol200658788789416805947
- KleemannESchmehlTGesslerTBakowskyUKisselTSeegerWIloprost-containing liposomes for aerosol application in pulmonary arterial hypertension: formulation aspects and stabilityPharm Res200724227728717211729
- JohnsonJCWaldrepJCGuoJDhandRAerosol delivery of recombinant human DNase I: in vitro comparison of a vibrating-mesh nebulizer with a jet nebulizerRespir Care200853121703170819025706
- SmaldoneGCBergENikanderKVariation in pediatric aerosol delivery: importance of facemaskJ Aerosol Med200518335436316181009
- HarrisKWSmaldoneGCFacial and ocular deposition of nebulized budesonide: effects of face mask designChest2008133248248818071018
- SmaldoneGCSangwanSShahAFacemask design, facial deposition, and delivered dose of nebulized aerosolsJ Aerosol Med.200720Suppl 1S667517411408
- ErzingerSSchueeppKGBrooks-WildhaberJDevadasonSGWildhaberJHFacemasks and aerosol delivery in vivoJ Aerosol Med200720Suppl 1S788317411409
- KleinstreuerCZhangZDonohueJFTargeted drug-aerosol delivery in the human respiratory systemAnnu Rev Biomed Eng20081019522018412536
- DunneAO’HaraTDevaneJA new approach to modelling the relationship between in vitro and in vivo drug dissolution/absorptionStat Med199918141865187610407257
- ZierenbergBOptimizing the in vitro performance of RespimatJ Aerosol Med199912Suppl 1S192410623337
- NewmanSPSteedKPReaderSJHooperGZierenbergBEfficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizerJ Pharm Sci19968599609648877887
- NewmanSPBrownJSteedKPReaderSJKladdersHLung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devicesChest199811349579639554631
- BatemanEDTashkinDSiafakasNA one-year trial of tiotropium Respimat plus usual therapy in COPD patientsRespir Med2010104101460147220620037
- StormGCrommelinDJColloidal systems for tumor targetingHybridoma19971611191259085138
- SuarezSGonzalez-RothiRJSchreierHHochhausGEffect of dose and release rate on pulmonary targeting of liposomal triamcinolone acetonide phosphatePharm Res19981534614659563078
- FieldingRMAbraRMFactors affecting the release rate of terbutaline from liposome formulations after intratracheal instillation in the guinea pigPharm Res1992922202231553346
- CryanSACarrier-based strategies for targeting protein and peptide drugs to the lungsAAPS J200571E204116146340
- Gonzalez-RothiRJSuarezSHochhausGPulmonary targeting of liposomal triamcinolone acetonide phosphatePharm Res19961311169917038956337
- Gonzalez-RothiRJStraubLCacaceJLSchreierHLiposomes and pulmonary alveolar macrophages: functional and morphologic interactionsExp Lung Res19911746877051657589
- LasicDDLiposomes in Gene DeliveryBoca Raton, FLCRC Press1997
- AllenTMLiposomal drug formulations. Rationale for development and what we can expect for the futureDrugs19985657477569829150
- SchreierHGagneLBockTPhysicochemical properties and in vitro toxicity of cationic liposome cDNA complexesPharm Acta Helv19977242152239372644
- NivenRWSchreierHNebulization of liposomes. I. Effects of lipid compositionPharm Res1990711112711332293210
- DesaiTRHancockREFinlayWHA facile method of delivery of liposomes by nebulizationJ Control Release2002841–2697812399169
- NivenRWSpeerMSchreierHNebulization of liposomes. II. The effects of size and modeling of solute release profilesPharm Res1991822172212023870
- NivenRWCarvajalTMSchreierHNebulization of liposomes. III. The effects of operating conditions and local environmentPharm Res1992945155201495897
- JoshiMMisraADry powder inhalation of liposomal ketotifen fumarate: formulation and characterizationInt J Pharm20012231–2152711451628
- Skalko-BasnetNPavelicZBecirevic-LacanMLiposomes containing drug and cyclodextrin prepared by the one-step spray-drying methodDrug Dev Ind Pharm200026121279128411147128
- OmriABeaulacCBouhajibMMontplaisirSSharkawiMLagaceJPulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosaAntimicrob Agents Chemother1994385109010958067743
- WoodleMCCollinsLRSponslerEKossovskyNPapahadjopoulosDMartinFJSterically stabilized liposomes. Reduction in electrophoretic mobility but not electrostatic surface potentialBiophys J19926149029101581503
- DeolPKhullerGKLung specific stealth liposomes: stability, biodistribution and toxicity of liposomal antitubercular drugs in miceBiochim Biophys Acta199713342–31611729101710
- HutchinsonFGFurrBJBiodegradable polymers for controlled release of peptides and proteinsHoriz Biochem Biophys198991111292656473
- EhrhardtCFiegelJFuchsSDrug absorption by the respiratory mucosa: cell culture models and particulate drug carriersJ Aerosol Med200215213113912184863
- KawashimaYYamamotoHTakeuchiHFujiokaSHinoTPulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effectJ Control Release1999621–227928710518661
- ChengYSYazzieDGaoJMuggliDEtterJRosenthalGJParticle characteristics and lung deposition patterns in a human airway replica of a dry powder formulation of polylactic acid produced using super-critical fluid technologyJ Aerosol Med2003161657312737686
- DhimanNKhullerGKProtective efficacy of mycobacterial 71-kDa cell wall associated protein using poly (DL-lactide-co-glycolide) microparticles as carrier vehiclesFEMS Immunol Med Microbiol199821119289657317
- EvoraCSorianoIRogersRAShakesheffKNHanesJLangerRRelating the phagocytosis of microparticles by alveolar macrophages to surface chemistry: the effect of 1,2-dipalmitoylphosphatidylcholineJ Control Release1998512–31431529685911
- FiegelJEhrhardtCSchaeferUFLehrCMHanesJLarge porous particle impingement on lung epithelial cell monolayers – toward improved particle characterization in the lungPharm Res200320578879612751635
- BittnerBKisselTUltrasonic atomization for spray drying: a versatile technique for the preparation of protein loaded biodegradable microspheresJ Microencapsul199916332534110340218
- TakeuchiHYamamotoHKawashimaYMucoadhesive nanoparticulate systems for peptide drug deliveryAdv Drug Deliv Rev2001471395411251244
- LangerRDrug delivery and targetingNature1998392Suppl 66795109579855
- TsapisNBennettDJacksonBWeitzDAEdwardsDATrojan particles: large porous carriers of nanoparticles for drug deliveryProc Natl Acad Sci U S A20029919120011200512200546
- EdwardsDAHanesJCaponettiGLarge porous particles for pulmonary drug deliveryScience19972765320186818719188534
- CannonGJSwansonJAThe macrophage capacity for phagocytosisJ Cell Sci1992101Pt 49079131527185
- SteckelHBolzenNAlternative sugars as potential carriers for dry powder inhalationsInt J Pharm20042701–229730614726144
- KobayashiSKondoSJuniKPulmonary delivery of salmon calcitonin dry powders containing absorption enhancers in ratsPharm Res199613180838668684
- WintersMAKnutsonBLDebenedettiPGPrecipitation of proteins in supercritical carbon dioxideJ Pharm Sci19968565865948773954
- SellersSPClarkGSSieversRECarpenterJFDry powders of stable protein formulations from aqueous solutions prepared using supercritical CO(2)-assisted aerosolizationJ Pharm Sci200190678579711357179
- HickeyWAFactors influencing the distortion of sex ratio in Aedes aegyptiJ Med Entomol1970767277355503471
- MaaYFNguyenPASweeneyTShireSJHsuCCProtein inhalation powders: spray drying vs spray freeze dryingPharm Res199916224925410100310
- KlinkDTChaoSGlickMCScanlinTFNuclear translocation of lactosylated poly-L-lysine/cDNA complex in cystic fibrosis airway epithelial cellsMol Ther20013683184111407896
- KawashimaYSeriganoTHinoTYamamotoHTakeuchiHA new powder design method to improve inhalation efficiency of pranlukast hydrate dry powder aerosols by surface modification with hydroxypropylmethylcellulose phthalate nanospheresPharm Res19981511174817529833998
- RajewskiRAStellaVJPharmaceutical applications of cyclodextrins. 2. In vivo drug deliveryJ Pharm Sci19968511114211698923319
- WoodleMCScariaPGaneshSSterically stabilized polyplex: ligand-mediated activityJ Control Release2001741–330931111489511
- KlonneDRDoddDELoscoPETroupCMTylerTRTwo-week aerosol inhalation study on polyethylene glycol (PEG) 3350 in F-344 ratsDrug Chem Toxicol198912139482714207
- ZhangLZhuWSongLEffects of hydroxylpropyl-beta-cyclodextrin on in vitro insulin stabilityInt J Mol Sci20091052031204019564937
- BruckAAbu-DahabRBorchardGSchaferUFLehrCMLectin- functionalized liposomes for pulmonary drug delivery: interaction with human alveolar epithelial cellsJ Drug Target20019424125111697028
- Abu-DahabRSchaferUFLehrCMLectin-functionalized liposomes for pulmonary drug delivery: effect of nebulization on stability and bioadhesionEur J Pharm Sci2001141374611457648
- YiSMHarsonREZabnerJWelshMJLectin binding and endocytosis at the apical surface of human airway epitheliaGene Ther20018241826183211821935
- KlossAHenkleinPSieleDThe cell-penetrating peptide octa-arginine is a potent inhibitor of proteasome activitiesEur J Pharm Biopharm200972121922519027853
- StrayerMSGuttentagSHBallardPLTargeting type II and Clara cells for adenovirus-mediated gene transfer using the surfactant protein B promoterAm J Respir Cell Mol Biol19981811119448040
- GorenDHorowitzATTzemachDTarshishMZalipskySGabizonANuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pumpClin Cancer Res2000651949195710815920
- CristianoRJRothJAEpidermal growth factor mediated DNA delivery into lung cancer cells via the epidermal growth factor receptorCancer Gene Ther1996314108785711
- LundbergMWikstromSJohanssonMCell surface adherence and endocytosis of protein transduction domainsMol Ther20038114315012842437
- DerossiDCalvetSTrembleauABrunissenAChassaingGProchiantzACell internalization of the third helix of the Antennapedia homeodomain is receptor-independentJ Biol Chem19962713018188181938663410
- KurtenRCSorting motifs in receptor traffickingAdv Drug Deliv Rev200355111405141914597138
- HasegawaSHirashimaNNakanishiMMicrotubule involvement in the intracellular dynamics for gene transfection mediated by cationic liposomesGene Ther20018211669167311895006
- LeslieEMDeeleyRGColeSPMultidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defenseToxicol Appl Pharmacol2005204321623715845415
- SharomFJABC multidrug transporters: structure, function and role in chemoresistancePharmacogenomics20089110512718154452
- ZhangLStrongJMQiuWLeskoLJHuangSMScientific perspectives on drug transporters and their role in drug interactionsMol Pharm200631626916686370
- Lechapt-ZalcmanEHurbainILacaveRMDR1-Pgp 170 expression in human bronchusEur Respir J1997108183718439272928
- SchefferGLPijnenborgACSmitEFMultidrug resistance related molecules in human and murine lungJ Clin Pathol200255533233911986335
- van der ValkPvan KalkenCKKetelaarsHDistribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein moleculeAnn Oncol19901156641706611
- Cordon-CardoCO’BrienJPBocciaJCasalsDBertinoJRMelamedMRExpression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissuesJ Histochem Cytochem1990389127712871974900
- SchinkelAHJonkerJWMammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overviewAdv Drug Deliv Rev200355132912535572
- HsiaTCLinCCWangJJHoSTKaoARelationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expressionLung2002180317317912177731
- LallemandJYStovenVAnnereauJPInduction by antitumoral drugs of proteins that functionally complement CFTR: a novel therapy for cystic fibrosis?Lancet199735090797117129291908
- NaumannNSiratskaOGahrMRosen-WolffAP-glycoprotein expression increases ATP release in respiratory cystic fibrosis cellsJ Cyst Fibros20054315716815964250
- YeSMacEachranDPHamiltonJWO’TooleGAStantonBAChemotoxicity of doxorubicin and surface expression of P-glycoprotein (MDR1) is regulated by the Pseudomonas aeruginosa toxin CifAm J Physiol Cell Physiol20082953C80781818650266
- van der DeenMMarksHWillemseBWDiminished expression of multidrug resistance-associated protein 1 (MRP1) in bronchial epithelium of COPD patientsVirchows Arch2006449668268817072643
- BlokzijlHVander BorghtSBokLIDecreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levelsInflamm Bowel Dis200713671072017262809
- HenrikssonGNorlanderTZhengXStiernaPWestrinKMExpression of P-glycoprotein 170 in nasal mucosa may be increased with topical steroidsAm J Rhinol19971143173219292183
- DemeuleMJodoinJBeaulieuEBrossardMBeliveauRDexamethasone modulation of multidrug transporters in normal tissuesFEBS Lett19994422–32082149929003
- Dinis-OliveiraRJDuarteJARemiaoFSanchez-NavarroABastosMLCarvalhoFSingle high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated ratsToxicology20062271–2738516956706
- SparreboomAvan AsperenJMayerULimited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestineProc Natl Acad Sci U S A1997945203120359050899
- LangmannTMauererRZahnAReal-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissuesClin Chem200349223023812560344
- KoolMde HaasMSchefferGLAnalysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell linesCancer Res19975716353735479270026
- FlensMJZamanGJvan der ValkPTissue distribution of the multidrug resistance proteinAm J Pathol19961484123712478644864
- BrechotJMHurbainIFajacADatyNBernaudinJFDifferent pattern of MRP localization in ciliated and basal cells from human bronchial epitheliumJ Histochem Cytochem19984645135179524197
- van der DeenMde VriesEGVissermanHCigarette smoke extract affects functional activity of MRP1 in bronchial epithelial cellsJ Biochem Mol Toxicol200721524325117912704
- van der DeenMHomanSTimmer-BosschaHEffect of COPD treatments on MRP1-mediated transport in bronchial epithelial cellsInt J Chron Obstruct Pulmon Dis20083346947518990976
- BleasbyKCastleJCRobertsCJExpression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug dispositionXenobiotica20063610–1196398817118916
- HorvathGMendesESSchmidNThe effect of corticosteroids on the disposal of long-acting beta2-agonists by airway smooth muscle cellsJ Allergy Clin Immunol200712051103110917920670
- LipsKSVolkCSchmittBMPolyspecific cation transporters mediate luminal release of acetylcholine from bronchial epitheliumAm J Respir Cell Mol Biol2005331798815817714
- HorvathGSchmidNFragosoMAEpithelial organic cation transporters ensure pH-dependent drug absorption in the airwayAm J Respir Cell Mol Biol2007361536016917073
- KummerWLipsKSPfeilUThe epithelial cholinergic system of the airwaysHistochem Cell Biol2008130221923418566825
- KummerWWiegandSAkinciSRole of acetylcholine and muscarinic receptors in serotonin-induced bronchoconstriction in the mouseJ Mol Neurosci2006301–2676817192631
- GronebergDAFischerAChungKFDanielHMolecular mechanisms of pulmonary peptidomimetic drug and peptide transportAm J Respir Cell Mol Biol200430325126014969997
- SondergaardHBBrodinBNielsenCUhPEPT1 is responsible for uptake and transport of Gly-Sar in the human bronchial airway epithelial cell-line Calu-3Pflugers Arch2008456361162218094991
- GronebergDAEynottPRDoringFDistribution and function of the peptide transporter PEPT2 in normal and cystic fibrosis human lungThorax2002571556011809991
- EndterSFrancombeDEhrhardtCGumbletonMRT-PCR analysis of ABC, SLC and SLCO drug transporters in human lung epithelial cell modelsJ Pharm Pharmacol200961558359119405996
- SaitoHTeradaTOkudaMSasakiSInuiKMolecular cloning and tissue distribution of rat peptide transporter PEPT2Biochim Biophys Acta1996128021731778639691
- BosquillonCDrug transporters in the lung – do they play a role in the biopharmaceutics of inhaled drugs?J Pharm Sci20109952240225519950388
- MiyazakiHSekineTEndouHThe multispecific organic anion transporter family: properties and pharmacological significanceTrends Pharmacol Sci2004251265466215530644
- HagenbuchBGuiCXenobiotic transporters of the human organic anion transporting polypeptides (OATP) familyXenobiotica2008387–877880118668430
- AdachiHSuzukiTAbeMMolecular characterization of human and rat organic anion transporter OATP-DAm J Physiol Renal Physiol20032856F1188119714631946
- HitzmanCJElmquistWFWiedmannTSDevelopment of a respirable, sustained release microcarrier for 5-fluorouracil II: In vitro and in vivo optimization of lipid coated nanoparticlesJ Pharm Sci20069551127114316570303
- HitzmanCJElmquistWFWattenbergLWWiedmannTSDevelopment of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticlesJ Pharm Sci20069551114112616570302
- LiangHFChenCTChenSCPaclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancerBiomaterials20062792051205916307794
- MusumeciTVicariLVenturaCAGulisanoMPignatelloRPuglisiGLyoprotected nanosphere formulations for paclitaxel controlled deliveryJ Nanosci Nanotechnol200669–103118312517048526
- KhalidMNSimardPHoarauDDragomirALerouxJCLong circulating poly(ethylene glycol)-decorated lipid nanocapsules deliver docetaxel to solid tumorsPharm Res200623475275816550475
- StinchcombeTENanoparticle albumin-bound paclitaxel: a novel Cremphor-EL-free formulation of paclitaxelNanomedicine (Lond)20072441542317716129
- AggarwalANAgarwalRThe new ATS/ERS guidelines for assessing the spirometric severity of restrictive lung disease differ from previous standardsRespirology200712575976217875068
- KreiderMEGrippiMAImpact of the new ATS/ERS pulmonary function test interpretation guidelinesRespir Med2007101112336234217686622
- LawsonKAAndersonKMenchacaMNovel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasisMol Cancer Ther20032543744412748305
- KnightVAnticancer effect of an alpha-TEA liposome aerosolExp Biol Med (Maywood)2005230529115855295
- KnightVKoshkinaNVWaldrepJCGiovanellaBCGilbertBEAnticancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude miceCancer Chemother Pharmacol199944317718610453718
- GagnadouxFLeblondVVecellioLGemcitabine aerosol: in vitro antitumor activity and deposition imaging for preclinical safety assessment in baboonsCancer Chemother Pharmacol200658223724416328414
- GagnadouxFLe PapeAUrbanTSafety of pulmonary administration of gemcitabine in ratsJ Aerosol Med200518219820615966774
- LemarieEVecellioLHureauxJAerosolized gemcitabine in patients with carcinoma of the lung: feasibility and safety studyJ Aerosol Med Pulm Drug Deliv201124626127021793717
- MinRLiTDuJZhangYGuoJLuWLPulmonary gemcitabine delivery for treating lung cancer: pharmacokinetics and acute lung injury aspects in animalsCan J Physiol Pharmacol200886528829818432290
- CamusPKudohSEbinaMInterstitial lung disease associated with drug therapyBr J Cancer200491Suppl 2S182315340374
- CamusPInterstitial lung disease in patients with non-small-cell lung cancer: causes, mechanisms and managementBr J Cancer200491Suppl 2S1215340371
- CamusPFantonABonniaudPCamusCFoucherPInterstitial lung disease induced by drugs and radiationRespiration200471430132615316202
- MeradMLe CesneABaldeyrouPMesurolleBLe ChevalierTDocetaxel and interstitial pulmonary injuryAnn Oncol1997821911949093730
- PavlakisNBellDRMillwardMJLeviJAFatal pulmonary toxicity resulting from treatment with gemcitabineCancer19978022862919217042
- RabinowitsGHerchenhornDRabinowitsMWeatgeDTorresWFatal pulmonary toxicity in a patient treated with gefitinib for non-small cell lung cancer after previous hemolytic-uremic syndrome due to gemcitabineAnticancer Drugs200314866566814501391
- SuzukiHAoshibaKYokohoriNNagaiAEpidermal growth factor receptor tyrosine kinase inhibition augments a murine model of pulmonary fibrosisCancer Res200363165054505912941834
- HajosFStarkBHenslerSPrasslRMosgoellerWInhalable liposomal formulation for vasoactive intestinal peptideInt J Pharm20083571–228629418328650
- PakunluRIWangYTsaoWPozharovVCookTJMinkoTEnhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery systemCancer Res200464176214622415342407
- PakunluRICookTJMinkoTSimultaneous modulation of multidrug resistance and antiapoptotic cellular defense by MDR1 and BCL-2 targeted antisense oligonucleotides enhances the anticancer efficacy of doxorubicinPharm Res200320335135912669953
- PakunluRIWangYSaadMKhandareJJStarovoytovVMinkoTIn vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drugJ Control Release2006114215316216889867