?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study is to evaluate the efficacy of composite doxorubicinloaded micelles for enhancing doxorubicin radiosensitivity in multicellular spheroids from a non-small cell lung cancer cell line.
Methods
A novel composite doxorubicin-loaded micelle consisting of polyethylene glycolpolycaprolactone/Pluronic P105 was developed, and carrier-mediated doxorubicin accumulation and release from multicellular spheroids was evaluated. We used confocal laser scanning microscopy and flow cytometry to study the accumulation and efflux of doxorubicin from A549 multicellular spheroids. Doxorubicin radiosensitization and the combined effects of irradiation and doxorubicin on cell migration and proliferation were compared for the different doxorubicin delivery systems.
Results
Confocal laser scanning microscopy and quantitative flow cytometry studies both verified that, for equivalent doxorubicin concentrations, composite doxorubicin-loaded micelles significantly enhanced cellular doxorubicin accumulation and inhibited doxorubicin release. Colony-forming assays demonstrated that composite doxorubicin-loaded micelles are radiosensitive, as shown by significantly reduced survival of cells treated by radiation + composite micelles compared with those treated with radiation + free doxorubicin or radiation alone. The multicellular spheroid migration area and growth ability verified higher radiosensitivity for the composite micelles loaded with doxorubicin than for free doxorubicin.
Conclusion
Our composite doxorubicin-loaded micelle was demonstrated to have radiosensitization. Doxorubicin loading in the composite micelles significantly increased its cellular uptake, improved drug retention, and enhanced its antitumor effect relative to free doxorubicin, thereby providing a novel approach for treatment of cancer.
Introduction
Lung cancer is the most common primary malignant tumor in humans and is the leading cause of cancer mortality in the world.Citation1 Its occurrence is increasing in both developing and developed countries, and in the latter, lung cancer is the second most frequently occurring cancer in both men and women. However, treatment for lung cancer remains relatively ineffective, and the five-year survival rate for advanced disease is only 15%.Citation2
The 2011 National Comprehensive Cancer Network guidelines for lung cancer state that radiation has a potential role in the treatment of all stages of lung cancer, as either definitive treatment or as palliative therapy. It is recommended that radiation oncology should form part of a multidisciplinary evaluation provided for all patients who may benefit from definitive local therapy, particularly those with absolute or relative contraindications to surgery as determined by a thoracic surgeon.
Although recent years have witnessed the development of radiation physics, biology, and radiotherapy facilities, the efficacy of radiation therapy is still not satisfactory. Research on radiosensitization, with the aim of improving the sensitivity of tumor cells to radiation, has been extensive.Citation3–Citation5 The ideal radiation sensitizer should significantly increase the efficacy of radiotherapy, but have little or no adverse effect on normal tissues. Antitumor drugs, such as doxorubicin, are widely used in cancer therapy. However, side effects, such as bone marrow suppression, heart toxicity, and mucositis, limit the use of cytotoxic antitumor drugs as radiosensitizers. To reduce the toxicity of these drugs and improve their therapeutic efficacy, various polymeric micelle systems have been designed as delivery vehicles. If these carriers could improve cellular uptake and retention of the delivered compound, radiosensitization might be achieved using smaller doses of chemotherapy drugs, thus lowering the risk of side effects. New drug carrier micelles have high stability and biocompatibility, via the enhanced permeability and retention effect of drugs towards tumor cells, with enhanced efficacy and reduced toxicity, and are capable of slow drug release.Citation6–Citation10 Hydrophobic drugs can be incorporated into the micelle by both chemical conjugation and physical entrapment.Citation11
However, simple polymeric micelles may not provide a satisfactory effect. We have previously reported that the combination of polyethylene glycol (PEG), polycaprolactone (PCL), and Pluronic P105 significantly decreased cytotoxicity and intracellular accumulation of loaded doxorubicin in drug-resistant K562/ADR tumor cells.Citation12 Moreover, we have previously discovered that composite micelles consisting of two kinds of polymers (PEG-PCL and P105), had a synergistic effect as drug carriers in overcoming or reversing multidrug resistance in drug-resistant tumor cells.Citation13 We observed that doxorubicin-loaded PEG-PCL/P105 composite micelles were more cytotoxic towards K562/ADR cells than were doxorubicin-loaded PEG-PCL or P105 single micelles or doxorubicin solution.Citation13
Non-small cell lung cancer is more common than small cell lung cancer, occurring in 85% of patients with the disease. For non-small cell lung cancer, adenocarcinoma is particularly prone to metastasis, and therefore the human lung adenocarcinoma A549 cell line was selected to model non-small cell lung cancer in this study. Moreover, most studies of therapeutic efficacy in lung cancer cells have been carried out in monolayer cultures.Citation14,Citation15 However, the spheroid model is a three-dimensional cell culture system that more closely resembles in vivo tumor cell growth and is more physiologically relevant than monolayer cell growth.Citation16,Citation17 Individual tumor cells growing under these conditions face different microenvironments depending on their position inside the three-dimensional framework of the spheroid.
In this study, composite doxorubicin-loaded micelles consisting of PEG-PCL/Pluronic P105 were developed. A549 multicellular spheroids were incubated with drug-loaded micelles and irradiated. Confocal laser scanning microscopy was used to measure the intensity of doxorubicin fluorescence and the depth of doxorubicin penetration into the spheroids, and flow cytometry was used to quantitate the doxorubicin fluorescence signal intensity, because doxorubicin is a fluorescent compound and cellular doxorubicin incorporation can be measured. Colony-forming assays were used to evaluate the radiosensitivity of composite doxorubicin-loaded micelles following ionizing radiation. We also studied the combined effects of irradiation and composite doxorubicin-loaded micelle treatment on tumor cell migration and proliferation in a well defined three-dimensional spheroid culture system.
This study aimed to radiosensitize non-small cell lung cancer multicellular spheroids using doxorubicin-encapsulated micelles to achieve reduced toxicity and a higher therapeutic effect. Radiosensitization by increased intracellular accumulation and delayed drug release will facilitate screening for novel and effective radiosensitizers and development of improved radiosensitizer delivery in targeting systems.
Materials and methods
Reagents
Fibroblast growth factor and epidermal growth factor were purchased from PeprotechAsia (Rocky Hill, NJ). B27 was purchased from Invitrogen Corporation (Camarillo, CA). RPMI 1640 medium, phosphate-buffered saline, fetal bovine serum, and 0.25% trypsin were purchased from Gibco-BRL (Gaithersburg, MD). Doxorubicin hydrochloride was obtained from Zhejiang Hisun Pharmaceutical Co, Ltd. MPEGs and ɛ-caprolactone were purchased from Sigma-Aldrich (St Louis, MO). Pluronic P105 was a kind gift from the BASF Corporation (Ludwigshafen, Germany). Agarose was purchased from Bio Basic Inc (Markham, ON, Canada). MTT was from Shanghai Sangon Biological Engineering Technology and Service Co, Ltd (Shanghai, China). CH2Cl2, triethylamine, and other reagents were of analytical grade and supplied by Huadong Medical (Hangzhou, China).
Cell line spheroids
The human A549 cell line was obtained from the Cancer Institute, Second Affiliated Hospital of School of Medicine, Zhejiang University, cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 5000 U/mL penicillin, and 5 mg/mL streptomycin, and incubated at 37°C in a humidified atmosphere containing 5% CO2. Spheroid A549 cultures were formed from exponentially growing monolayers. Briefly, cells were trypsinized, and 5 × 106 cells were then seeded in 50 mL of growth medium supplemented with epidermal growth factor 20 ng/mL, basic fibroblast growth factor 10 ng/mL, and B27 (B27 and medium at a 1:50 volume ratio) and agitated using a benchtop roller (Wheaton, Millville, NJ). The medium was changed every 2–3 days.
Synthesis and characterization of PEG-PCL diblock copolymers
PEG-PCL diblock copolymers were synthesized as previously described, with minor modifications.Citation13,Citation18 Briefly, ring-opening polymerization of ɛ-caprolactone in CH2CL2 was induced using mPEG and calcium ammoniate as the macroinitiator and catalyst, respectively. Precipitates were obtained by adding cold methanol, collected by filtration, and vacuum dried at 40°C. The molecular weight of the diblock copolymer was determined by 1H nuclear magnetic resonance spectroscopy using the intensity of the terminal methoxy proton signal of mPEG at Q 3.39 ppm and the methylene proton signal of polycaprolactone at 2.31 ppm. The weight ratio of the repeated PEG-PCL units was calculated to be 7.08 from the integral values of characteristic peaks, and the molecular weight was determined to be 19,278 Da. A similar molecular weight was also obtained by gel permeation chromatography.
Preparation of composite doxorubicin-loaded micelles
As we have previously reported,Citation13 polymeric micelles containing doxorubicin were prepared according to the solvent evaporation method. Briefly, 3 mg doxorubicin was dissolved in 2 mL of CH2Cl2 in the presence of a triple molar ratio of triethylamine and stirred at 500 rpm for 4 hours at room temperature. PEG-poly(lactic-co-glycolic acid) (PLGA) and Pluronic 105 at a 1:5 ratio were then added and vortexed until completely dissolved. Following this, the organic solution containing the drug and polymer was added to 20 mL of distilled water with sonication. The mixture was stirred vigorously (10,000 rpm) for one hour, and then stirred slowly (200 rpm) overnight. Residual CH2Cl2 was removed by a rotary evaporator. The micelle solution was concentrated to 5 mL and filtered (using a 0.22 μm pore size) to eliminate polymer and doxorubicin aggregates. All procedures were carried out with protection from light.
Micelle characterization
The morphology of the micelles was observed under a Morgagni 268 D transmission electron microscope (FEI, Eindhoven, The Netherlands) and particle size was measured by dynamic light scattering (Malvern Nano ZS, Malvern, Worcestershire, UK). The unloaded drug was separated by a filtration membrane (Millipore, Bedford, MA), and analytical high-performance liquid chromatography (Agilent 1100 system, Santa Clara, CA) was used to quantify the amount of doxorubicin. Drug entrapment efficiency was calculated as the amount of doxorubicin entrapped in the micelle with respect to total micellar doxorubicin.
Cytotoxicity assay
A549 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C and in a humidified 5% CO2 atmosphere. A549 cells were seeded in 96-well plates at a density of 3500 cells/well and allowed to grow overnight. On the following day, a set concentration of the various doxorubicin formulations was added into the culture medium. After 72 hours of incubation, the cytotoxic activity of doxorubicin was then evaluated using a standard MTT assay. Blank composite micelles were also tested for cell inhibition by MTT. All experiments were performed in triplicate. The results were calculated using the following equation:
Fluorescent high-performance liquid chromatography
For evaluation of the accumulation and release of doxorubicin in A549 cells, a cell suspension containing the same concentration (2.5 μg/mL) of free doxorubicin or composite doxorubicin-loaded micelles, was seeded at a density of 1 × 106 cells/mL in 2 mL of growth medium. To measure doxorubicin accumulation, cells were incubated for 4 hours. To detect released doxorubicin, cells were incubated with doxorubicin for 4 hours, then the medium was removed and the cells were washed three times with phosphate-buffered saline. Fresh complete medium was then added and the cells were harvested one hour and 2.5 hours later. At each time point for measurement of accumulation and release of doxorubicin, the medium was removed, the cells were washed three times using ice-cold phosphate-buffered saline to remove noninternalized doxorubicin, and were subsequently resuspended in water (1 mL) and treated by probe-type ultrasonication 20 times (4°C, 200 W, active every 2 seconds for a duration of 5 seconds) to obtain the cell lysate. The lysate was centrifuged at 10,000 rpm for 5 minutes in a microcentrifuge (Hettich Zentrifugen, D-78532, Tuttlingen, Germany) to remove the cellular debris. The supernatant was analyzed by high-performance liquid chromatography (Agilent 1100) equipped with a multiwavelength fluorescence detector (excitation 488 nm and emission 570 nm). Calibration curves of known concentrations of doxorubicin in water were used for quantification. The chromatographic column used was an Agilent Zorbax Extend C18 (4.6 × 250 mm, 5 μm), and the mobile phase used was acetonitrile/0.015 M NaH2PO4 solution (pH 4.5, 30:70), with the flow rate kept at 1.0 mL per minute.
Confocal laser scanning microscopy
Three-dimensional A549 multicellular spheroids were plated into 96-well culture dishes (Corning, NY) and identical concentrations of either free doxorubicin or composite doxorubicin-loaded micelles were added to the multicellular spheroids and incubated for 4 hours. The medium was then removed, the multicellular spheroids were washed with cold phosphate-buffered saline, and fresh complete medium was then added and the intensity of doxorubicin fluorescence and penetration was examined under a confocal laser scanning microscope (Zeiss LSM 510, Zurich, Switzerland) to determine the accumulation and release of the drug. Doxorubicin excitation and emission occurred at 485 nm and 595 nm, respectively.
Flow cytometry analysis
The intensity of doxorubicin fluorescence in the multicellular spheroids was determined by flow cytometry (FACSCalibur, Becton-Dickinson, Franklin Lakes, NJ). A549 spheroids were trypsinized, blown into a single cell suspension, and seeded into 24-well culture dishes at a density of 5 × 105 cells/well. The same concentration (2.5 μg/mL) of composite doxorubicin-loaded micelles or free doxorubicin was added to the culture medium, taking the efficiency of doxorubicin loading into composite micelles into account. Cells were harvested after 2 and 3 hours to measure accumulation of doxorubicin. To detect the amount of doxorubicin released, the cells were incubated with doxorubicin for 3 hours, the medium was then removed, and the cells were washed three times with phosphate-buffered saline. Fresh complete medium was then added, and the cells were harvested 2 hours and 4 hours later. At each time point, the medium was removed, the cells were washed three times with phosphate-buffered saline, and incorporation of doxorubicin was determined by fluorescence (488 nm excitation and 585 nm emission). Data were analyzed using FlowJo7.6 software.
Cell irradiation
Cell irradiation was performed using a medical linear accelerator (Primus-M, Siemens, Germany). The radiation field, dose rate, and source-to-cell distance were 40 cm × 40 cm, 2 Gy per minute, and 100 cm, respectively.
Ionizing radiation and colony-forming assay
A549 multicellular spheroids were exposed to 2.5 μg/mL blank composite micelles, doxorubicin composite micelles (MIX), or free doxorubicin for 4 hours. The multicellular spheroids were then trypsinized, counted, and seeded into 6-well culture dishes, irradiated (at 0–6 Gy), and incubated for a further 10 days. After this time, the cell colonies were washed with cold phosphate-buffered saline, fixed with 75% methanol, and stained with 0.5% crystal violet. Colonies containing more than 50 cells were counted using a dissecting microscope and cell survival curves were generated using GraphPad Prism 5.0 (GraphPad Software Inc, La Jolla, CA) based on the multitarget/single-hit model:
and D0 was then calculated.
Cell migration
Multicellular spheroids with a diameter of approximately 250 μm were chosen for these experiments because multicellular spheroids of this size do not undergo central necrosis and contain very few hypoxic cells.Citation28 Each treatment group (sham, irradiation alone, blank composite micelle + irradiation, free doxorubicin + irradiation, and composite doxorubicin-loaded micelle + irradiation) consisted of 30 individual A549 multicellular spheroids in five independent replicate experiments. The multicellular spheroids were incubated with or without the drug for 4 hours, the drugs were then removed, the multicellular spheroids were washed twice with phosphate-buffered saline, and fresh complete medium was added. The individual multicellular spheroids were then plated into 6-well culture dishes without base-coated agarose. Under these conditions, the multicellular spheroids disassembled and released cells which migrated away from the central point. The area covered by migrated cells was measured in mm2 every 24 hours for 4 days, and the migration capability was estimated by the total area covered by the cells. The spheroid migration rate was calculated using the formula:
Proliferation assay
Multicellular spheroids with diameters of approximately 250 mm were plated into 6-well culture dishes coated with 5% agarose. Each treatment group (sham, irradiation alone, blank composite micelle + irradiation, free doxorubicin + irradiation, and composite doxorubicin-loaded micelle + irradiation) consisted of 30 individual A549 multicellular spheroids in five independent replicate experiments. To study the response to treatment, the multicellular spheroids were observed for 9 days, with monitoring of their growth every second day. Spheroid volume was calculated using the equation:
where D1 and D2 are the maximal diameters of multicellular spheroids measured in the rectangular direction. A statistical comparison of the original data was performed using an unpaired sample t-test following analysis of variance. P values <0.05 were considered to be statistically significant. The proliferation rate of the spheroids was calculated using the formula:
Results
Properties of composite doxorubicinloaded micelles
Transmission electron microscopy revealed that the composite PEG-PCL/P105 micelles were spherical, with a uniform particle size distribution of approximately 20 nm, calculated using the mean diameter (number distribution). The entrapment efficiency of doxorubicin was 56.7% ± 1.8%. The critical micelle concentration, ie, the concentration of surfactants above which micelles form and for which almost all additional surfactants are incorporated into micelles, was determined by the fluorescence intensity ratio (I338/I333) using pyrene as a fluorescent probe and calculated to be 0.98 μg/mL and 0.92 μg/mL for PEG-PCL (EG/CL 7.08) micelles and Pluronic 105 micelles, respectively.
Cytotoxicity of different doxorubicin in A549 cells
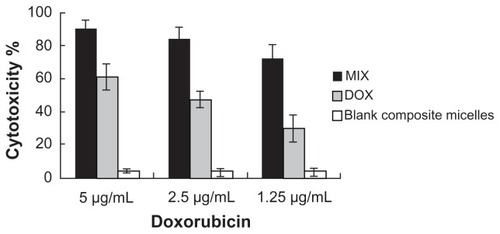
The cytotoxicity of free doxorubicin, the blank composite micelles, and the composite doxorubicin-loaded micelles in A549 cells was evaluated. As shown in , the composite doxorubicin-loaded micelles showed notable improvement of cytotoxicity compared with free doxorubicin. The blank composite micelles rarely caused cell death, indicating that any cytotoxicity was caused by doxorubicin rather than the carrier material.
More cellular uptake and sustained release of doxorubicin-loaded micelles
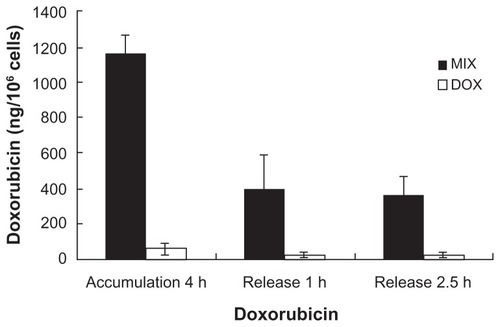
Internalization of composite doxorubicin-loaded micelles and free doxorubicin in the A549 tumor cells was examined by fluorescent high-pressure liquid chromatography. As shown in , after 4 hours of incubation, envelopment of doxorubicin in the composite micelles significantly increased cellular accumulation of the drug. After treatment with composite doxorubicin-loaded micelles or free doxorubicin for 4 hours, followed by one-hour recovery and 2.5-hour recovery periods, envelopment of doxorubicin in the composite micelles produced significantly sustained cellular release of the drug.
Figure 2 Intracellular doxorubicin in A549 cells.
Notes: Doxorubicin uptake into A549 tumor cells after incubation with composite doxorubicin-loaded micelles or free doxorubicin for 4 hours, then compounds removed for one hour and 2.5 hours. Accumulation 4 hours: A549 cells incubated in composite doxorubicin-loaded micelles or free doxorubicin for 4 hours; release one hour. A549 cells incubated in composite doxorubicin-loaded micelles or free DOX for 4 hours, then compounds removed for one hour; release 2.5 hours, A549 cells incubated in composite doxorubicin-loaded micelles or free doxorubicin for 4 hours, then compounds removed for 2.5 hours.
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles.
More cellular accumulation and slower release of doxorubicin-loaded micelles
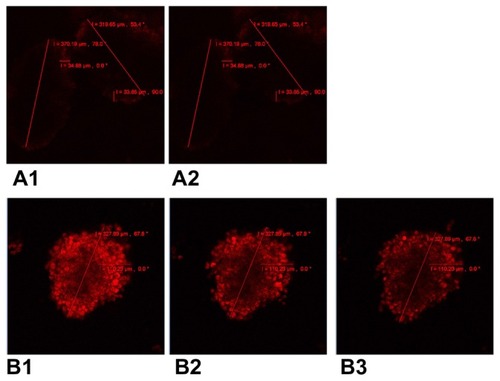
Intracellular incorporation and release of doxorubicin in A549 multicellular spheroids was measured by confocal laser scanning microscopy (). After 4 hours of incubation, A549 multicellular spheroids incubated with MIX () were more intensely fluorescent and showed deeper penetration of fluorescence than multicellular spheroids treated with free doxorubicin (), which had barely detectable levels of intracellular fluorescence. Only very weak fluorescence was observed for multicellular spheroids treated with free doxorubicin for 4 hours followed by a one-hour recovery period (). In contrast, multicellular spheroids treated with MIX were strongly fluorescent one hour after withdrawal of the drug (). Moreover, levels of fluorescence remained high at 2.5 hours ().
Figure 3 Confocal laser scanning microscopy of cellular incorporation and release of doxorubicin in A549 multicellular spheroids. (A1) A549 spheroid cells incubated in free doxorubicin for 4 hours. (A2) Free doxorubicin released from the spheroid for one hour. (B1) A549 spheroid cells incubated in composite doxorubicin-loaded micelles with the same doxorubicin densities for 4 hours. (B2) Composite doxorubicin-loaded micelles released from the spheroid for one hour. (B3) Composite doxorubicin-loaded micelles released from the spheroid for 2.5 hours.
More cellular uptake and slower release from doxorubicin-loaded micelles
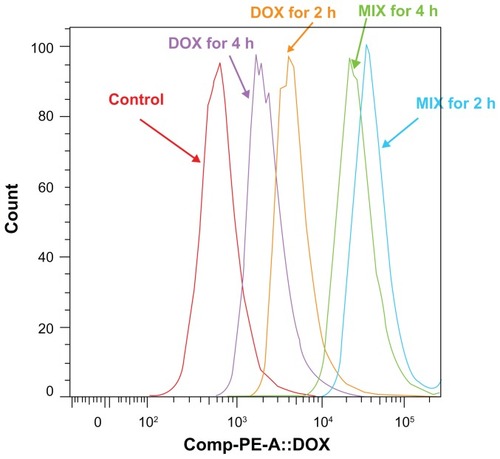
Multicellular spheroid intracellular doxorubicin uptake and release by MIX and free doxorubicin was investigated by flow cytometry. As shown in , after 2 and 3 hours, the intensity of cellular fluorescence was 14.14 and 13.61 times higher in MIX-treated multicellular spheroids than in free doxorubicin-treated multicellular spheroids for equivalent 2.5 μg/mL drug treatments. For multicellular spheroids incubated with MIX or free doxorubicin that achieved the same amount of doxorubicin incorporation after 3 hours of exposure, the intensity of cellular fluorescence in MIX-treated A549 cells at 2 and 4 hours following drug withdrawal was 9.53 and 14.93 times that of the free doxorubicin-treated cells, respectively (). Overall, the quantitative results obtained by flow cytometry are in good agreement with those obtained by confocal laser scanning microscopy ().
Figure 4 Flow cytometric histograms of A549 spheroid cells incubated with composite doxorubicin-loaded micelles or free doxorubicin with the same doxorubicin densities for 2 hours and 3 hours. DOX for 2 hours, multicellular spheroids incubated in free doxorubicin for 2 hours; DOX for 3 hours, multicellular spheroids incubated in free doxorubicin for 3 hours; MIX for 2 hours, multicellular spheroids incubated in MIX for 2 hours; MIX for 3 hours, multicellular spheroids incubated in MIX for 3 hours.
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles.
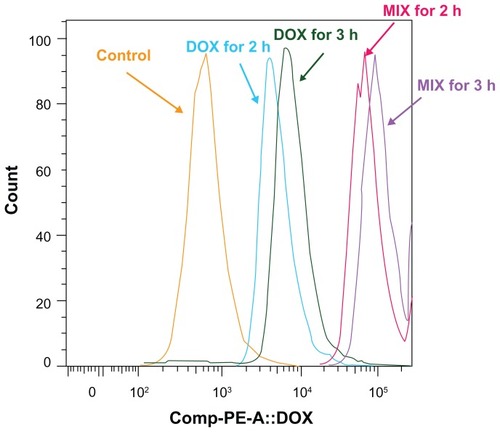
Figure 5 Flow cytometric histograms of multicellular spheroids incubated with MIX or free doxorubicin that achieved the same doxorubicin incorporation after 3 hours of exposure, at 2 and 4 hours following drug withdrawal.
Notes: DOX for 2 hours, multicellular spheroids incubated in free doxorubicin for 3 hours, then compounds removed for 2 hours; DOX for 4 hours, multicellular spheroids incubated in free doxorubicin for 3 hours, then compounds removed for 4 hours; MIX for 2 hours, multicellular spheroids incubated in MIX for 3 hours, then compounds removed for 2 hours; MIX for 4 hours, multicellular spheroids incubated in MIX for 3 hours, then compounds removed for 4 hours.
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles.
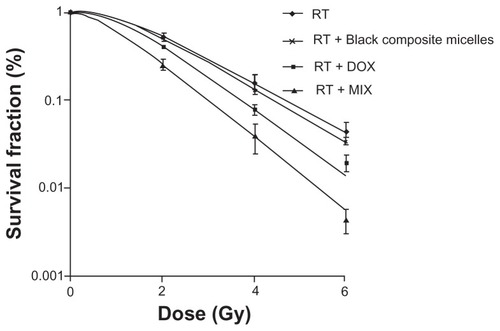
Colony formation assays
Cell survival curves are showed in . D0s was 1.466 Gy, 1.405 Gy, 1.230 Gy, and 1.020 Gy, respectively, for the groups that received irradiation only, blank composite micelles + irradiation, doxorubicin + irradiation, and MIX + irradiation, respectively. The sensitizing enhancement ratio of cells treated with equivalent amounts of free doxorubicin and doxorubicin-loaded micelles were 1.19 and 1.44, respectively (P < 0.05). There was no difference between the group receiving irradiation only and the group receiving blank composite micelles + irradiation.
Figure 6 Cell survival curves after treatments with radiation alone or combined with 2.5 μg/mL blank composite micelles (radiation alone + blank composite micelles) or doxorubicin (radiation alone + doxorubicin) or doxorubicin-loaded composite micelle (radiation alone + MIX, P < 0.05 for radiation alone + MIX versus radiation alone).
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles; RT, radiation alone; Gy, gray.
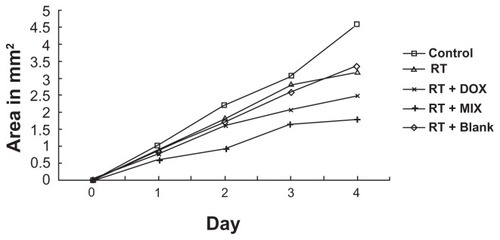
Cell migration assays
Cell migration was determined for both treated and untreated spheroids. A significant growth reduction of 30% and 28%, respectively, was seen in the irradiation only group and the blank composite micelles + irradiation group after 4 days. There was no statistically significant difference between the irradiation only group and the blank composite micelles + irradiation group. However, combined treatment with equivalent concentrations of free doxorubicin or composite doxorubicin-loaded micelles had a significant additive effect on growth reduction of 46% and 61%, respectively ().
Figure 7 Tumor cell migration from spheroids following irradiation and combination treatment (radiation alone + blank composite micelles, radiation alone + doxorubicin, radiation alone + MIX). Mean area of tumor cell migration (mm2).
Notes: Each treatment group (sham, irradiation alone, blank composite micelle + irradiation, free doxorubicin + irradiation, composite doxorubicin-loaded micelle + irradiation) consists of 30 A549 spheroids in five independent experiments. Changes at day 4 were significant (P < 0.05).
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles; RT, radiation alone.
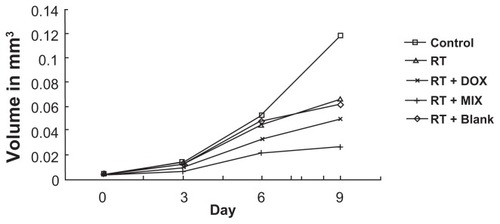
Cell proliferation assays
The proliferation capacity of spheroids treated with equivalent concentrations of doxorubicin or MIX was determined. Following irradiation alone and blank composite micelles + irradiation, the spheroids showed significant growth reduction of 44% and 47%, respectively, after 9 days. There was no difference between the irradiation only group and the blank composite micelles + irradiation group. However, following combined treatment, the spheroids showed significantly increased growth reduction of 58% (for radiation + free doxorubicin) and 77% (for radiation + MIX) after nine days ().
Figure 8 Spheroid proliferation following single mode and combinational treatment.
Notes: Each treatment group (sham, irradiation alone, blank composite micelle + irradiation, free doxorubicin + irradiation, composite doxorubicin-loaded micelle + irradiation) consists of 30 A549 spheroids in five independent experiments. Illustration of mean volume growth of spheroids following irradiation and irradiation alone + blank composite micelles, irradiation alone + doxorubicin, irradiation alone + MIX (composite doxorubicin-loaded micelles). A significant reduction in spheroid volume was observed following irradiation alone + MIX on day 9 (P < 0.05).
Abbreviations: DOX, free doxorubicin; MIX, composite doxorubicin-loaded micelles; RT, radiation alone.
Discussion
Radiotherapy is very effective for local control of tumors, but its curative potential is limited by the intrinsic radioresistance of most malignant tumor cells, which often leads to treatment failure. New strategies, such as developing radiosensitizers, are clinically important and have become an important research focus.
Doxorubicin is a tetracycline antibiotic commonly used in the treatment of cancerCitation19 and induces DNA damage by inhibition of topoisomerase II and free radical generation as an anticancer mechanism.Citation20 Doxorubicin can also bind covalently to DNA, leading to formation of doxorubicin-DNA adducts and interstrand cross-links. For these reasons, doxorubicin is the antitumor drug most widely used for enhancement of radiotherapy. The mechanism of doxorubicinmediated radiosensitization may include induction of tumor cell apoptosis, and an increase in both sublethal radiation-induced damage and DNA repair processes.
However, in addition to its low solubility in water, doxorubicin has severe side effects, including acute toxicity to normal tissue, and its therapeutic effects can be minimized by the inherent multidrug resistance of many tumor cells.Citation21 To reduce the acute toxicity of free doxorubicin and to improve its therapeutic efficacy, various polymeric micelle systems have been designed as delivery vehicles.Citation22–Citation24 Studies have shown that cellular uptake of doxorubicin-loaded micelles and free doxorubicin are very different, ie, doxorubicin micelles are transported into cells by endocytosis, whereas free doxorubicin enters cells by simple diffusion.Citation18
In this study, we prepared composite PEG-PCL and P105 micelles using the solvent evaporation method to serve as carriers for doxorubicin. This method produces a relatively small particle size distribution, due to the low molecular weight and high hydrophilicity of P105. We successfully established three-dimensional A549 multicellular spheroids that are a more physiologically relevant model of lung cancer than monolayer cells, because they resemble the architecture of in vivo tumorsCitation17,Citation25,Citation26 and can recapitulate in vivo processes which may affect drug performance, such as cell-cell contact, variations in the cell cycle, altered metabolism, and diffusion of nutrients, oxygen, or drugs.Citation17,Citation25,Citation27,Citation28 Multicellular spheroids are relatively easy to produce and maintain in culture, and treatment-related changes in spheroid growth kinetics and tumor cell outgrowth are established and reproducible endpoints.Citation17,Citation25,Citation29 As a consequence of their particular architectural characteristics, multicellular spheroids have been demonstrated to be extremely useful for testing radiotherapy protocols.Citation30
We studied the doxorubicin sensitization mechanism in a number of experiments. First, we used MTT to study the cytotoxicity of different doxorubicin formulations in A549 cells. As shown in , composite doxorubicin-loaded micelles showed notable improvement in cytotoxicity compared with free doxorubicin. Blank composite micelles rarely caused cell death, indicating that cytotoxicity was caused by doxorubicin rather than by the carrier material. Internalization of composite doxorubicin-loaded micelles and free doxorubicin into A549 tumor cells was then examined by fluorescent high-performance liquid chromatography. As shown in , after 4 hours of incubation, envelopment of doxorubicin in the composite micelles significantly increased intracellular accumulation of the drug. After treatment with composite doxorubicin-loaded micelles or free doxorubicin for 4 hours, followed by a one-hour recovery or 2.5-hour recovery period, envelopment of doxorubicin in the composite micelles sustained cellular release of the drug in a significant manner.
We also undertook a series of experiments in A549 multicellular spheroids, in which we investigated accumulation and efflux of doxorubicin by confocal laser scanning microscopy. As shown in , after 4 hours, A549 multicellular spheroids incubated with MIX showed a greater fluorescent intensity and deeper penetration than those treated with free doxorubicin, in which intracellular fluorescence was barely detectable. In contrast, high levels of fluorescence were still detectable in MIX-treated spheroids 2.5 hours after drug withdrawal, indicating that use of composite doxorubicinloaded micelles leads to improved cellular retention of doxorubicin.
By measuring the intensity of fluorescence and depth of doxorubicin penetration into multicellular spheroids, we found that treatment with doxorubicin-loaded micelles led to higher intracellular accumulation and deeper penetration than that obtained for free doxorubicin. In addition, the use of micelle carriers led to a longer doxorubicin retention time in multicellular spheroids. Our previous studyCitation13 also showed rapid efflux of free doxorubicin from K562/ADR cells. However, unlike doxorubicin in solution, uptake of composite doxorubicin-loaded PEG-PCL/P105 micelles occurs via endocytosis. Micelles enter cells slowly, avoid being pumped out, and continue to accumulate around the cell nucleus. Similar results were obtained by Shuai et al,Citation18 who reported that free doxorubicin was rapidly pumped out of cells, but that micelle carriers inhibited doxorubicin efflux and improved retention in cells.
Furthermore, quantitative flow cytometry studies verified that, for equivalent doxorubicin concentrations, composite doxorubicin-loaded micelles significantly enhanced cellular doxorubicin accumulation and inhibited doxorubicin release. Doxorubicin uptake in MIX-treated multicellular spheroids was 14.14 and 13.61 times greater than in multicellular spheroids treated with free doxorubicin after 2 hours and 3 hours, respectively. In addition, doxorubicin retention was 9.53 and 14.93 times greater in MIX-treated multicellular spheroids than in multicellular spheroids treated with an equivalent concentration of free doxorubicin 2 and 4 hours, respectively, after drug withdrawal. Overall, our quantitative results (shown in and ) agree with those obtained by confocal laser scanning microscopy (), and suggest that doxorubicin binding to the hydrophobic core of the micelle may prevent its efflux.
Colony-forming assays also demonstrated that composite doxorubicin-loaded micelles are radiosensitive, as shown by significantly reduced survival of cells treated by radiation + composite micelles compared with those treated with radiation + free doxorubicin, radiation alone, or radiation + blank composite micelles. The surviving fraction of cells treated with MIX + radiation was significantly lower than for cells subject to irradiation alone or radiation + blank composite micelles (P < 0.05). However, there was no statistically significant difference in cell death between cells treated with a combination of free doxorubicin + irradiation relative to irradiation alone or radiation + blank composite micelles.
Finally, the cellular migration area and growth capability of multicellular spheroids confirmed the increased radiosensitivity of cells treated with composite doxorubicin-loaded micelles compared with those treated with free doxorubicin. In short, we successfully increased the radiosensitivity of A549 multicellular spheroids using composite micelles loaded with doxorubicin. At equivalent drug concentrations, composite doxorubicin-loaded PEG-PCL/P105 micelles significantly enhanced the accumulation of doxorubicin in multicellular spheroids and inhibited release of doxorubicin from multicellular spheroids, thus achieving improved radiosensitivity. Preventing a burst of doxorubicin release may avoid excessive damage to normal tissue, minimizing cytotoxic effects. Therefore, this study provides a novel approach to improving radiosensitivity of tumor cells, and may achieve an improved therapeutic outcome for cancers that cannot be well controlled by traditional radiotherapy. Clinical application of this research may reduce the side effects of conventional cancer therapies, and thereby improve patient compliance.
In summary, composite doxorubicin-loaded micelles consisting of PEG-PCL/Pluronic P105 can significantly enhance doxorubicin radiosensitivity in lung cancer multicellular spheroids and provide a novel approach to lung cancer treatment. Further similar investigations should be undertaken in other tumor models. Further, combined treatment with micelle-encapsulated doxorubicin and radiation therefore warrants investigation in clinical trials as a potential anticancer strategy.
Acknowledgments
This work was supported by the Foundation of Zhejiang Educational Committee (Y200909276), the National Natural Science Foundation of China (30970863), and the National Natural Science Foundation of China (81071823).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin20106027730020610543
- CollinsLGHainesCPerkelREnckRELung cancer: diagnosis and managementAm Fam Physician200775566317225705
- ZhangXYangHGuKChenJRuiMJiangGLIn vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizerInt J Nanomedicine2011643744421499433
- YuJLiuFSunZSunMSunSThe enhancement of radiosensitivity in human esophageal carcinoma cells by thalidomide and its potential mechanismCancer Biother Radiopharm20112621922721539454
- ZhangXDGuoMLWuHYIrradiation stability and cytotoxicity of gold nanoparticles for radiotherapyInt J Nanomedicine2009416517319774115
- KohoriFYokoyamaMSakaiKOkanoTProcess design for efficient and controlled drug incorporation into polymeric micelle carrier systemsJ Control Release20027815516311772457
- RapoportNMarinALuoYPrestwichGDMuniruzzamanMDIntracellular uptake and trafficking of Pluronic micelles in drug-sensitive and MDR cells: effect on the intracellular drug localizationJ Pharm Sci20029115717011782905
- KhonkarnRMankhetkornSHenninkWEOkonogiSPEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growthEur J Pharm Biopharm20117926827521596135
- AnLWangYLiuXBlock ionomer complex micelles based on the self-assembly of poly(ethylene glycol)-block-poly(acrylic acid) and CdCl(2) for anti-tumor drug deliveryChem Pharm Bull (Tokyo)20115955956321532192
- QiuLZhangLZhengCWangRImproving physicochemical properties and doxorubicin cytotoxicity of novel polymeric micelles by poly(ɛ-caprolactone) segmentsJ Pharm Sci20111002430244221491452
- KataokaKHaradaANagasakiYBlock copolymer micelles for drug delivery: design, characterization and biological significanceAdv Drug Deliv Rev20014711313111251249
- DiaoYYHanMDingPTChenDWGaoJQDOX-loaded PEG-PLGA and Pluronic copolymer composite micelles enhances cytotoxicity and the intracellular accumulation of drug in DOX-resistant tumor cellsPharmazie20106535635820503928
- HanMDiaoYYJiangHLMolecular mechanism study of chemosensitization of doxorubicin-resistant human myelogenous leukemia cells induced by a composite polymer micelleInt J Pharm201142040441121945184
- CetintasVBKucukaslanASKosovaBCisplatin resistance induced by decreased apoptotic activity in non small cell lung cancer cell linesCell Biol Int20123626126522397496
- LeeHKimSChoiBHHyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cellsInt J Hyperthermia20112769870721992562
- LimMChuongCMRoy-BurmanPPI3K, ERK signaling in BMP7-induced epithelial-mesenchymal transition (EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional culturesHorm Cancer2011229830921948155
- FehlauerFMuenchMRichterERadesDThe inhibition of proliferation and migration of glioma spheroids exposed to temozolomide is less than additive if combined with irradiationOncol Rep20071794194517342340
- ShuaiXAiHNasongklaNKimSGaoJMicellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin deliveryJ Control Release20049841542615312997
- KievitFMWangFYFangCDoxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitroJ Control Release2011152768321277920
- MizutaniHTada-OikawaSHirakuYKojimaMKawanishiSMechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxideLife Sci2005761439145315680309
- YooHSParkTGBiodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymerControl Release2001706370
- GaoZGTianLHuJParkISBaeYHPrevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micellesJ Control Release2011152848921295088
- KimDLeeESOhKTGaoZGBaeYHDoxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pHSmall200842043205018949788
- InoueTYamashitaYNishiharaMTherapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor modelsNeuro Oncol20091115115718755917
- FehlauerFMuenchMRadesDEffects of irradiation and cisplatin on human glioma spheroids: inhibition of cell proliferation and cell migrationJ Cancer Res Clin Oncol200513172373216096850
- FehlauerFStalpersLJPanayiotidesJEffect of single dose irradiation on human glioblastoma spheroids in vitroOncol Rep20041147748514719087
- WalentaSDoetschJMueller-KlieserWKunz-SchughartLAMetabolic imaging in multicellular spheroids of oncogene-transfected fibroblastsJ Histochem Cytochem20004850952210727293
- SminiaPAckerHEikesdalHPOxygenation and response to irradiation of organotypic multicellular spheroids of human gliomaAnticancer Res2003231461146612820410
- GliemrothJZulewskiHArnoldHTerzisAJMigration, proliferation, and invasion of human glioma cells following treatment with simvastatinNeurosurg Rev20032611712412962298
- SantiniMTRainaldiGIndovinaPLMulticellular tumour spheroids in radiation biologyInt J Radiat Biol19997578779910489890