Abstract
Background
Multidrug resistance, a major impediment to successful cancer chemotherapy, is the result of overexpression of ATP-binding cassette (ABC) transporters extruding internalized drugs. Silencing of ABC transporter gene expression with small interfering RNA (siRNA) could be an attractive approach to overcome multidrug resistance of cancer, although delivery of siRNA remains a major hurdle to fully exploit the potential of siRNA-based therapeutics. Recently, we have developed pH-sensitive carbonate apatite nanoparticles to efficiently carry and transport siRNA across the cell membrane, enabling knockdown of the cyclin B1 gene and consequential induction of apoptosis in synergy with anti-cancer drugs.
Methods and results
We report that carbonate apatite-mediated delivery of the siRNAs targeting ABCG2 and ABCB1 gene transcripts in human breast cancer cells which constitutively express both of the transporter genes dose-dependently enhanced chemosensitivity to doxorubicin, paclitaxel and cisplatin, the traditionally used chemotherapeutic agents. Moreover, codelivery of two specific siRNAs targeting ABCB1 and ABCG2 transcripts resulted in a more robust increase of chemosensitivity in the cancer cells, indicating the reversal of ABC transporter-mediated multidrug resistance.
Conclusion
The delivery concept of multiple siRNAs against ABC transporter genes is highly promising for preclinical and clinical investigation in reversing the multidrug resistance phenotype of breast cancer.
Introduction
Cancer is the leading cause of deaths worldwide with breast cancer being second in the list of cancer related deaths.Citation1 Among all of the available treatment options, chemotherapy is the treatment of choice for advanced stage breast cancer that is refractory to hormonal therapy.Citation2,Citation3 Although traditional breast cancer chemotherapy regimens include combinations of cyclophosphamide, methotrexate, and 5-flurouracil,Citation4 anthracycline- and taxane-based chemotherapy is being commonly used nowadays for the treatment of breast malignancy due to its better performance in patient’s overall survival rate.Citation5–Citation7 Though breast cancer is considered to be highly chemosensitive with response rates as high as 80%,Citation8 the majority of initially chemoresponsive tumours develop resistance to once effective chemotherapeutic agents.Citation9 Therefore, a switch to other chemotherapy regimens is ineffective because of the tumour’s cross-resistance to multiple structurally and functionally unrelated chemotherapy drugs.Citation10
ABC transporter represents the key component of energy-dependent efflux transport system contributing to the development of multidrug-resistant phenotype in cancer.Citation11 ABCB1 transporter or P-glycoprotein, the first cytotoxic drug efflux pump to be identified,Citation12 functions by actively extruding drugs including anthracyclines, taxanes, and vinca alkaloids out of cancer cells, thus reducing intracellular drug concentration below the threshold level required for cell killing.Citation10,Citation11 The poor chemotherapy response in breast cancer is generally correlated to the extent of ABCB1 gene expression.Citation13–Citation16 A second type of ABC transporter discovered in a multidrug resistant breast cancer cell line is ABCG2,Citation17 though there are no conclusive data currently available to correlate its expression with chemotherapy efficacy in breast cancer.Citation18 However, judging from the ability of ABCG2 transporter in extruding doxorubicin, a very commonly used anthracycline drug,Citation19 it might be reasonable to correlate ABCG2 expression with development of clinical multidrug resistance in cancer.
Although low molecular weight pharmacologically active compounds have been developed to circumvent multidrugresistant phenotypes by either directly or indirectly modulating the ABC transporter efflux activity,Citation20 the first generation modulators failed in clinical trials because of their inherent toxicitiesCitation21 while the second generation molecules were associated with undesirable pharmacokinetic interactions.Citation22,Citation23 Thus, no drug has been clinically approved for the reversal of cancer multidrug resistance.Citation24 A new promising approach to enhance chemosensitivity without undesirable side effects, other than the structure-based drug design, is to prevent the biosynthesis of ABC transporters by selectively inhibiting the expression of ABC transporters through gene silencing technologies.Citation25
In the last decade, ribozymesCitation26–Citation28 and antisense oligonucleotidesCitation29–Citation31 were introduced to modulate cancer multidrug resistance through inhibition of ABC transporter gene expression. Very recently, small interfering RNAs (siRNAs), a double-stranded RNA of between 21–28 nucleotides that selectively degrade mRNA and thus block production of a particular protein,Citation32 have been applied in vitro for reversal of ABC transporter-mediated drug efflux by targeting both ABCB1 and ABCG2 transporters. A pioneering study using exogenous siRNA demonstrated suppression of ABCB1 expression in conjunction with reversal of doxorubicin and paclitaxel resistance in human breast cancer cells.Citation33 Reversal of multidrug-resistant ABCG2 phenotype was also investigated with a siRNA-mediated knockdown study in several human cancer cells.Citation34
Currently, we have established pH-sensitive carbonate apatite as a potential tool to efficiently deliver siRNA across the cell membrane and silence cyclin B1 gene transcript, thus inducing apoptosis of cervical cancer cells in synergy with anti-cancer drugs.Citation35,Citation36 Here, we reveal that carbonate apatite-mediated delivery of the siRNA targeting either ABCG2 or ABCB1 gene transcript in MCF-7, a human breast cancer cell line constitutively expressing ABCG2 and ABCB1 transporters, led to an increased chemosensitivity to doxorubicin, paclitaxel, and cisplatin, depending on the doses of the individual drug. Moreover, co-delivery of two specific siRNAs targeting ABCB1 and ABCG2 transcripts caused a dramatic enhancement of chemosensitivity activity in MCF-7 cells, indicating the reversal of ABC transporter-mediated multidrug resistance.
Methods and materials
Materials
Dulbecco’s modified Eagle medium (DMEM) was purchased from BioWhittaker (Walkersville, MD). DMEM powder, fetal bovine serum (FBS) and trypsin-ethylenediamine tetraacetate (trypsin-EDTA) were obtained from Gibco BRL (Carlsbad, CA). Calcium chloride dihydrate (CaCl2·2H2O), sodium bicarbonate, diammineplatinum(ll) dichloride, dimethyl sulphoxide (DMSO), doxorubicin hydrochloride, paclitaxel, and thiazolyl blue tetrazolium bromide (MTT) were from Sigma-Aldrich (St Louis, MO).
siRNA design and sequence
The siRNA sequence targeting ABCG2 (GenBank accession no NM_004827) and ABCB1 (GenBank accession no NM_000927) purchased from QIAGEN (Valencia, CA) correspond to coding regions of these genes. One target sequence (5′-CTGGTCTAATTTATTAATCTA-3′) was selected for ABCG2 (ABCG2_v1) and one (5′-GACAGAAAGCTTAGTACCAAA-3′) for ABCB1 (ABCB1_v4). The 21-nucleotide siRNAs having a 3′-dTdT extension were chosen based on recommendations made by others.Citation37,Citation38 siRNA was supplied in lyophilised form and upon delivery, the siRNA (1 nmol) was reconstituted with RNase-free water provided by manufacturer to obtain a stock solution of 20 μM.
Cell culture and seeding
Human breast cancer cell line MCF-7 was grown in 25 cm2 culture flask in DMEM, supplemented with 10% heat-inactivated FBS in a humidified atmosphere containing 5% CO2 at 37°C. Once the monolayer cells had reached 80%–90% confluency, the medium was removed and the cells were washed with phosphate-buffered saline (PBS). Trypsin-EDTA was added to detach the cells from the flask and the suspended cells were collected in a fresh medium and transferred to new 25 cm2 flasks. The cells were then sub-cultured at densities of 1.0–5.0 × 105 cells/10 mL every 3 to 4 days. Exponentially growing MCF-7 cells (log phase) were trypsinised and fresh medium was added to wash remaining cells from the bottom of the culture flask. Cell suspension was centrifuged at 10,000 rpm for 5 minutes and supernatant was discarded. Fresh medium was then added to resuspend the pellet. The cells were counted using hemocytometer and appropriate dilutions were made using culture medium to produce a cell suspension with a concentration of 5.0 × 104 cells/mL. One mL of the prepared cell suspension was subsequently added into each of the wells in 24-well plate and allowed to attach overnight at 37°C and 5% CO2 before siRNA transfection.
Generation of carbonate apatite nanoparticles containing siRNA and transfection of breast cancer cells
On the day of siRNA transfection, 100 mL of DMEM was prepared using 1.35 g of DMEM powder and 0.37 g of sodium bicarbonate. pH of the prepared DMEM solution was adjusted to 7.4 using 0.1 M hydrochloric acid. DMEM was then filtered using 0.2 a μm syringe filter in a laminar flow hood, followed by transferring 1 mL of the filtered medium into 1.5 mL microcentrifuge tubes. 3 μL of 1 M calcium chloride was then added into the microcentrifuge tubes, followed by addition of 1 or 10 nM siRNACitation35,Citation36 and incubation at 37°C for 30 minutes. After the incubation, 10% FBS was added into each microcentrifuge tube. Ten nM to 100 μM of a drug (doxorubicin, paclitaxel, or cisplatin) prepared from serial dilution using 1 mM stock solution was then added into the respective microcentrifuge tubes. Culture medium from the wells seeded one day before was aspirated and replaced with 1 mL of the prepared medium containing siRNA-loaded carbonate apatite nanoparticles and free drugs. Plates were then incubated at 37°C and 5% CO2 for 2 consecutive days.
Cell viability assessment with 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay
Following two days of siRNA transfection, the fraction of viable MCF-7 cells was determined using MTT assay as previously described.Citation39 Briefly, 50 μL of MTT (5 mg/mL in PBS) was added aseptically into each of the wells in siRNA transfected plates, followed by incubation at 37°C and 5% CO2 for 4 hours. After the incubation period, medium containing MTT was aspirated and the purple formazan crystals at the bottom of each well were dissolved by mixing with 300 μL of DMSO solution. Absorbance of the resulting formazan solution was then determined spectrophotometrically at wavelength 595 nm using a microplate reader (Dynex Technologies Inc., Chantilly, VA) with reference to 630 nm. Each experiment was performed in triplicate and the data were plotted as mean ± standard deviation (SD) of three independent experiments.
Results and Discussion
Chemosensitivity enhancement in breast cancer cells with intracellularly delivered ABCG2 transcript-targeting siRNA
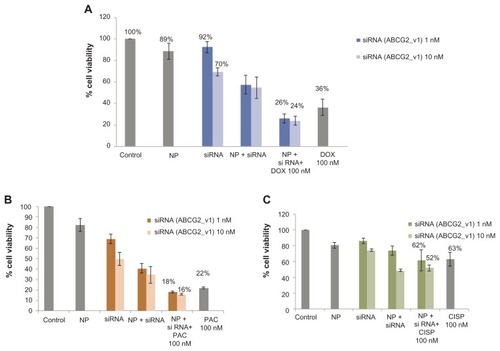
Since ABC transporter gene expression is essential in causing multidrug-resistant phenotype in breast cancer cells, knockdown of this gene might enhance sensitivity of the cancer cells towards chemotherapeutic agents, and possibly reverse cancer multidrug resistance. Therefore, MCF-7, the widely used human breast model cell line intrinsically expressing ABCG2 (alternatively called BRCP or breast cancer resistance protein),Citation17 was transfected with the target siRNA/carbonate apatite complexes in presence or absence of traditionally used chemotherapeutic agents (doxorubicin, paclitaxel, or cisplatin) for a consecutive period of 48 hours prior to cell viability assessment by MTT assay. As shown in , while the viability of the cancer cells was reduced to 36% due to the potent cytoxicity of 100 nM doxorubicin, the concerted effect of siRNA delivery and drug exposure resulted in 26% and 24% reduction in cell viability for 1 nM and 10 nM of siRNA initally used for complex formation, respectively. Thus, the combination of siRNA and doxorubicin killed a much higher number of cells than the drug and the siRNA alone, suggesting that silencing of the ABCG2 gene might have inhibited to a significant extent the efflux of doxorubicin across the membrane, and raised intracellular concentration of the drug for effective killing of the cancer cells (). ABCG2 gene knockdown similarly accelerated chemosensitivity of paclitaxel (100 nM) particularly at the higher dose of siRNA (10 nM), while demonstrating an insignificant response with cisplatin (, ). The apparent enhancement of chemosensitivity to cisplatin, as shown in , was virtually due to the silencing effect of siRNA () alone. Indeed, the decrease in viability of the cells treated with siRNA/nanoparticle complexes in comparison with those untreated or treated with nanoparticles alone indicates the role of ABC expression in delaying apoptosis of cancer cells.Citation40–Citation42
Table 1 Role of ABCG2 knockdown in enhancing cytotoxic effects of anti-cancer drugs at 100 nM
Figure 1 Effect of carbonate apatite-mediated delivery of ABCG2-targeted siRNA (ABCG2_v1) on MCF-7 cell viability in presence of traditionally used chemotherapeutic agents. Anti-ABC siRNA-carbonate apatite complexes were generated by mixing exogenously added 3 mM calcium chloride in 1 mL bicarbonate-buffered DMEM (pH 7.4), followed by addition of anti-ABCG2 siRNA (1 or 10 nM) and incubation at 37°C for 30 minutes. Supplementation of 10% FBS was followed by addition of 100 nM of doxorubicin (A) or paclitaxel (B) or cisplatin (C).
Note: Transfection of MCF-7 cells was performed with the siRNA/nanoparticle complexes in presence of the free drugs for a consecutive period of 48 hours and viability of the cells was determined using MTT assay.
Abbreviations: NP, nanoparticles; siRNA, small interfering RNA; DOX, doxorubicin; PAC, paclitaxel; CISP, cisplatin.
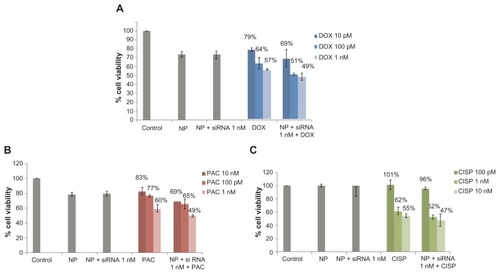
Based on the notion that anti-cancer drugs could dose-dependently influence the expression profile or susbtrate specificity of ABC transporters, a range of relatively lower concentrations of the drugs was investigated in the knockdown study of ABC transporters using 1 nM of target siRNAs. As shown in and , with drug doses ranging from 10 pM to 1 nM for doxorubicin as well as paclitaxel and 100 pM to 10 nM for cisplatin, silencing of ABCG2 gene expression resulted in significant enhancement of chemosensitivity towards doxorubicin and paclitaxel in all of the drug concentrations used, whereas a weak response to 100 pM of cisplatin was observed probably due to the poor cytotoxic effect of cisplatin at the particular concentration. The finding thus suggests that the chemosensitivity of MCF-7 breast cancer cells due to the knockdown of ABCG2 gene is virtually similar among the three drugs in respect to the maximum enhancement and dependent on the dose of the invidual drug.
Table 2 Role of ABCG2 knockdown in enhancing cytotoxic effects of anti-cancer drugs at 10 pM to 1 nM
Figure 2 Effect of carbonate apatite-mediated delivery of ABCG2-targeted siRNA (ABCG2_v1) on MCF-7 cell viability in presence of low dose of traditionally used chemotherapeutic agents. Anti-ABC siRNA-carbonate apatite complexes were generated by mixing exogenously added 3 mM calcium chloride in 1 mL bicarbonate-buffered DMEM (pH 7.4), followed by addition of anti-ABCG2 siRNA (1 nM) and incubation at 37°C for 30 minutes. Supplementation of 10% FBS was followed by addition of 10 pM, 100 pM or 1 nM of doxorubicin (A) or paclitaxel (B) or 100 pM, 1 nM, 10 nM cisplatin (C).
Note: Transfection of MCF-7 cells was performed with the siRNA/nanoparticle complexes in presence of the free drugs for a consecutive period of 48 hours and viability of the cells was determined using MTT assay.
Abbreviations: NP, nanoparticles; siRNA, small interfering RNA; DOX, doxorubicin; PAC, paclitaxel; CISP, cisplatin.
Chemosensitivity enhancement in breast cancer cells with intracellularly delivered ABCB1 transcript-targeting siRNA
The enhancement of chemosensitivity to the popular anti-cancer drugs by virtue of silencing ABCG2 gene expression triggered us to carry out a similar study in the same breast cancer cells using the siRNA targeting the gene of ABCB1, the most extensively studied ABC transporter involved in development of multi-drug resistance in various cancer cells including MCF-7.Citation13 As shown in , in synergy with 100 nM of paclitaxel, anti-ABCB1 siRNA at both 1 and 10 nM led to killing of much more cancer cells than the target siRNA (1 and 10 nM) or the drug (100 nM) alone, resulting in 14% and 33% enhancement of chemosensitivity respectively. On the contrary, the apparent enhancement of c hemosensitivity (to the same extent) towards both 100 nM of doxorubicin and cisplatin following knock-down of ABCB1 transporter () was solely due to the effect of gene silencing killing more cells than the individual drugs or the target siRNA alone (). However, when the doses of the individual drugs were reduced to 10 pM to 1 nM for doxorubicin and paclitaxel, siRNA-mediated cleavage of ABCB1 gene transcript surprisingly increased chemosensitivity towards each of the two drugs () with demonstration of significantly higher level of cytotoxicity than siRNA or drugs alone (), suggesting the broader substrate-specificity of ABCB1 transporter at lower concentrations of the individual cancer drugs. On ther other hand, the apparent increase in chemosensitivity for 100 pM to 10 nM of cisplatin was statistically insignificant, which is consistent with the fact that cisplatin is not a substrate for ABCB1.
Table 3 Role of ABCB1 knockdown in enhancing cytotoxic effects of anti-cancer drugs at 100 nM
Table 4 Role of ABCB1 knockdown in enhancing cytotoxic effects of anti-cancer drugs at 10 pM to 1 nM (doxorubicin and paclitaxel) and 100 pM to 10 nM (cisplatin)
Figure 3 Effect of carbonate apatite-mediated delivery of ABCB1-targeted siRNA (ABCB1_v4) on MCF-7 cell viability in presence of traditionally used chemotherapeutic agents. Anti-ABC siRNA-carbonate apatite complexes were generated by mixing exogenously added 3 mM calcium chloride in 1 mL bicarbonate-buffered DMEM (pH 7.4), followed by addition of anti-ABCB1 siRNA (1 or 10 nM) and incubation at 37°C for 30 minutes. Supplementation of 10% FBS was followed by addition of 100 nM of doxorubicin (A) or paclitaxel (B) or cisplatin (C).
Note: Transfection of MCF-7 cells was performed with the siRNA/nanoparticle complexes in presence of the free drugs for a consecutive period of 48 hours and viability of the cells was determined using MTT assay.
Abbreviations: NP, nanoparticles; siRNA, small interfering RNA; DOX, doxorubicin; PAC, paclitaxel; CISP, cisplatin.
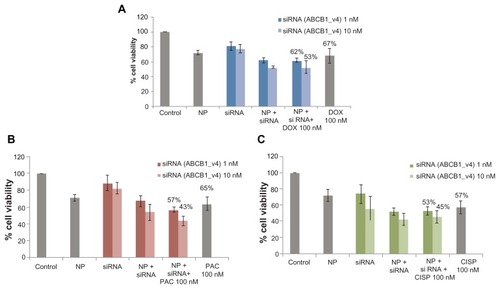
Figure 4 Effect of carbonate apatite-mediated delivery of ABCB1-targeted siRNA (ABCB1_v4) on MCF-7 cell viability in presence of low dose of traditionally used chemotherapeutic agents. Anti-ABC siRNA-carbonate apatite complexes were generated by mixing exogenously added 3 mM calcium chloride in 1 mL bicarbonate-buffered DMEM (pH 7.4), followed by addition of anti-ABCB1 siRNA (1 nM) and incubation at 37°C for 30 minutes. Supplementation of 10% FBS was followed by addition of 10 pM, 100 pM or 1 nM of doxorubicin (A) or paclitaxel (B) or 100 pM, 1 nM, 10 nM cisplatin (C).
Note: Transfection of MCF-7 cells was performed with the siRNA/nanoparticle complexes in presence of the free drugs for a consecutive period of 48 hours and viability of the cells was determined using MTT assay.
Abbreviations: NP, nanoparticles; siRNA, small interfering RNA; DOX, doxorubicin; PAC, paclitaxel; CISP, cisplatin.

Regardless of the type of siRNA (ABCG2 or ABCB1) used in this study, the enhancement of chemosensitivity after single siRNA treatment was only modest. This observation was similar to that made by other groups, where no complete reversal of cancer multidrug resistance was noted even at siRNA concentration as high as 200 nM.Citation33,Citation43 Such incomplete reversal of cancer multidrug resistance may be attributed to the inherent nature of the target protein under study. Due to its long half-life (from 16 hoursCitation44 to 72 hoursCitation45) and high cellular abundance, ABC transporter protein remains a challenging target for siRNA silencing.Citation46
Chemosensitivity enhancement in breast cancer cells with co-delivery of two siRNAs targeting ABCG2 and ABCB1 gene transcripts
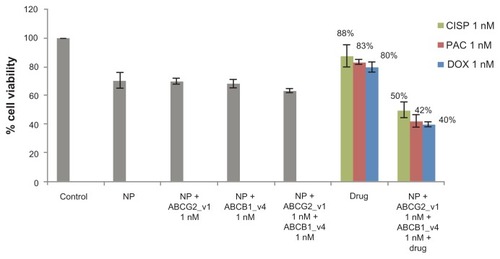
Delivery of single siRNA targeting either ABCG2 or ABCB1 transporter with carbonate apatite nanoparticles demonstrated a modest 15% to 30% enhancement in c hemosensitivity in MCF-7 cells (–). To explore whether the combined delivery of two specific siRNAs simultaneously targeting the two different transporters could further improve the chemo-sensitivity towards the anti-cancer drugs, MCF-7 cells were transfected with the siRNAs (at 1 nM each) targeting the gene transcripts of ABCG2 and ABCB1 in presence of 1 nM of the individual drugs. As shown in , nanoparticle-facilitated intracellular delivery of single siRNA targeting a particular transporter killed fewer cells than co-delivery of the two target siRNAs, suggesting that both of the transporters, which are intrinsically expressed in MCF-7 cells,Citation13,Citation17 are important for survival of the cells.Citation40–Citation42 On the other hand, simultaneous delivery of the two target siRNAs into MCF-7 cells in presence of cisplatin, paclitaxel, or doxorubicin, was found to dramatically enhance cytotoxicity with enhancement of chemosensitivity by 50%, 49% and 43%, respectively (), in comparison to the individual drugs and the siRNAs co-delivered without a drug. This suggests that both ABCG2 and ABCB1 are fully functional at a time in extruding any of those popular anti-cancer drugs from the cytoplasm of MCF-7 cells and inhibition or knockdown of a single transporter could only partly block the cellular efflux process, allowing drug efflux to continue through the other channel(s).
Table 5 Role of ABCG2 and ABCB1 knockdown in enhancing cytotoxic effects of anti-cancer drugs at 1 nM
Figure 5 Combination effect of two siRNAs targeting ABCG2 and ABCB1 (ABCG2_v1 and ABCB1_v4 respectively) co-delivered with carbonate apatite nanoparticles on MCF-7 cell viability in presence of traditionally used chemotherapeutic agents. Anti-ABC siRNAs-carbonate apatite complexes were generated by mixing exogenously added 3 mM calcium chloride in 1 mL bicarbonate-buffered DMEM (pH 7.4), followed by addition of anti-ABCG2 (1 nM) and anti-ABCB1 siRNA (1 nM) and incubation at 37°C for 30 minutes. Supplementation of 10% FBS was followed by addition of 1 nM drug (doxorubicin, paclitaxel or cisplatin).
Note: Transfection of MCF-7 cells was performed with the siRNA/nanoparticle complexes in presence of the free drugs for a consecutive period of 48 hours and viability of the cells was determined using MTT assay.
Abbreviations: NP, nanoparticles; siRNA, small interfering RNA; DOX, doxorubicin; PAC, paclitaxel; CISP, cisplatin.
Conclusion
Due to the heterogenous nature of tumours where several drug resistance mechanisms may coexist and simultaneously contribute to the development of cancer multidrug resistant-phenotype, Citation11 the use of siRNA combinations might represent a potential mechanism for circumventing this problem. Additionally, the concern about saturating the cellular RNA interference machinery by exogenous addition of two siRNAs may have been potentially overcome by combining siRNAs at the lowest possible concentration. Thus, the synergism achieved with combination of ABCG2 and ABCB1 targeting siRNAs would provide valuable insight for complete reversal of clinical cancer multidrug resistance otherwise deemed impossible to be achieved by currently available therapeutics.
Disclosure
The authors report no conflicts of interest in this work.
References
- MoulderSHortobagyiGNAdvances in the treatment of breast cancerClin Pharmacol Ther2008831263618091763
- EstevaFJValeroVPusztaiLBoehnke-MichaudLBuzdarAUHortobagyiGNChemotherapy of metastatic breast cancer: what to expect in 2001 and beyondOncologist20016213314611306725
- HortobagyiGNTreatment of breast cancerN Engl J Med1998339189749849753714
- Tubiana-HulinMHow to maximize the efficacy of taxanes in breast cancerCancer Treat Rev200531Suppl 4S3916360546
- FisherBRedmondCWickerhamDLDoxorubicin-containing regimens for the treatment of stage II breast cancer: the National Surgical Adjuvant Breast and Bowel Project experienceJ Clin Oncol1989755725822651576
- NabholtzJMFalksonCCamposDDocetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trialJ Clin Oncol2003211096897512637459
- HendersonICBerryDADemetriGDImproved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancerJ Clin Oncol200321697698312637460
- BurgerHFoekensJALookMPRNA expression of breast cancer resistance protein, lung resistance related protein, multi-drug resistance gene-1 in breast cancer: correlation with chemotherapeutic responseClin Cancer Res20039282783612576456
- ModokSMellorHRCallaghanRModulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancerCurr Opin Pharmacol20066435035416690355
- AmbudkarSVDeySHrycynaCARamachandraMPastanIGottesmanMMBiochemical, cellular, and pharmacological aspects of the multidrug transporterAnnu Rev Pharmacol Toxicol19993936139810331089
- SzakácsGPatersonJKLudwigJABooth-GentheCGottesmanMMTargeting multidrug resistance in cancerNat Rev Drug Discov201053219230
- JulianoRLLingVA surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutantsBiochem Biophys Acta19764551152162990323
- TrockBJLeonessaFClarkeRMultidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significanceJ Natl Cancer Inst197789139179319214671
- LeonessaFClarkeRATP binding cassette transporters and drug resistance in breast cancerEndocr Relat Cancer200310437312653670
- KrögerNAchterrathWHegewisch-BeckerSMrossKZanderARCurrent options in treatment of anthracycline-resistant breast cancerCancer Treat Rev199925527929110544072
- TsukamotoFShibaETaguchiTImmunohistochemical detection of P-glycoprotein in breast cancer and its significance as a prognostic factorBreast Cancer19974425926311091611
- DoyleLAYangWAbruzzoLVA multidrug resistance transporter from human MCF-7 breast cancer cellsProc Natl Acad Sci U S A1998952615665156709861027
- RobeyRWToKKPolgarOABCG2: a perspectiveAdv Drug Deliv Rev200961131319135109
- NoguchiKKatayamaKMitsuhashiJSugimotoYFunctions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapyAdv Drug Deliv Rev2009611263319111841
- WuCPCalcagnoAMAmbudkarSVReversal of ABC drug t ransportermediated multidrug resistance in cancer cells: evaluation of current strategiesCurr Mol Pharmacol2008119310519079736
- BartlettNLLumBLFisherGAPhase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistanceJ Clin Oncol19941248358428151326
- BooteDJDennisIFTwentymanPRPhase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancerJ Clin Oncol19961426106188636778
- GiacconeGLinnSCWelinkJA dose-finding and pharmacokinetic study of reversal of multidrug resistance with SDZ PSC 833 in combination with doxorubicin in patients with solid tumorsClin Cancer Res1997311200520159815591
- TiwariAKSodaniKChenZSCurrent advances in modulation of ABC transporter-mediated multidrug resistance in cancerInt J Toxicol Pharmcol Res2009116
- ShiZLiangYJChenZSReversal of MDR1/P- glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivoCancer Biol Ther200651394716319528
- LiuCQureshiIADingXModulation of multidrug resistance gene (MDR-1) with antisense oligodeoxynucleotidesClin Sci (Lond)199691193988774266
- AlahariSKDeLongRFisherMHDeanNMVilietPJulianoRLNovel chemically modified oligonucleotides provide potent inhibition of P-glycoprotein expressionJ Pharmacol Exp Ther199828614194289655887
- StuartDDKaoGYAllenTMA novel, long-circulating, and functional liposomal formulation of antisense oligodeoxynucleotides targeted against MDR1Cancer Gene Ther20007346647510766353
- KobayashiHDoraiTHollandJFOhnumaTReversal of drug sensitivity in multidrug-resistant tumor cells by an MDR1(PGY1) ribozymeCancer Res1994545127112758118816
- HolmPSScanlonKJDietelMReversion of multidrug resistance in the P-glycoprotein-positive human pancreatic cell line (EPP85-181RDB) by introduction of a hammerhead ribozymeBr J Cancer19947022392437914421
- GaoZFieldsJZBomanBMTumor-specific expression of anti- MDR1 ribozyme selectively restores chemosensitivity in multidrug-resistant colon-adenocarcinoma cellsInt J Cancer199982334635210399951
- ChowdhuryEHStrategies for tumor-directed delivery of siRNAExpert Opin Drug Deliv20118338940121314230
- WuHHaitWNYangJMSmall interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cellsCancer Res20036371515151912670898
- EePLHeXRossDDBeckWTModulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interferenceMol Cancer Ther20043121577158315634651
- StanislausAHossainSChuaMJFabrication and intracellular delivery of siRNA/carbonate apatite nano-composites for effective knockdown of cyclin B1 geneDrugs and Therapy Studies20111e810.4081/dts.2011.e8.
- HossainSStanislausAChuaMJCarbonate apatite-facilitated intracellularly delivered siRNA for efficient knockdown of functional genesJ Control Release2010147110110820620182
- ElbashirSMHarborthJLendeckelWYalcinAWeberKTuschlTDuplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cellsNature2011411683649449811373684
- HarborthJElbashirSMBechertKTuschlTWeberKIdentification of essential genes in cultured mammalian cells using small interfering RNAsJ Cell Sci2001114Pt 244557456511792820
- CarmichaelJDeGraffWGGazdarAFMinnaJDMitchellJBEvaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testingCancer Res19874749369423802100
- SmythMJKrasovskisESuttonVRJohnstoneRWThe drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosisProc Natl Acad Sci U S A19989512702470299618532
- JohnstoneRWCretneyESmythMJP-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell deathBlood1999933107510859920858
- PallisMRussellNP-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathwayBlood20009592897290410779437
- XuDKangHFisherMJulianoRLStrategies for inhibition of MDR1 gene expressionMol Pharmacol200466226827515266017
- CohenDYangCPHorwitzSBThe products of the mdr1a and mdr1b genes from multidrug resistant murine cells have similar degradation ratesLife Sci19904674894952304382
- RichertNDAldwinLNiteckiDGottesmanMMPastanIStability and covalent modification of P-glycoprotein in multidrug-resistant KB cellsBiochemistry19882720760776132905169
- AkhtarSBenterIFNonviral delivery of synthetic siRNAs in vivoJ Clin Invest2007117123623363218060020