Michalkova H, Strmiska V, Kudr J, et al. Int J Nanomedicine. 2019;14:7609–7624
The authors have advised due to an error at the time of figure assembly, on page 7614 and on page 7621 is incorrect. The correct figures are shown below.
Figure 1 Surface coating of IONs with biocompatible surfactant (POES) or polymers (PVP and Chit). (A) Schematic representation of surface coating of bare IONs with PVP, POES and Chit with a consequent tethering of cytotoxic substances Dox and Elli using incubation or ultrasonication, respectively. (B) SEM micrographs of morphology of bare IONs and their morphology after surface coatings. The scale bars, 400 nm (left) or 5 μm (right). (C) Content of organic matter in surface-coated formulations analyzed using CHNS/O analyzer. The values are expressed as the mean of three independent replicates (n=3). Vertical bars indicate standard error. (D) Photodocumentation of a colloidal stability of bare and surface-coated IONs. Time-evolution of (E) dhy and (F) PDI, both analyzed in Ringer’s solution. The values are expressed as the mean of six independent replicates (n=6). The vertical bars + and − errors.
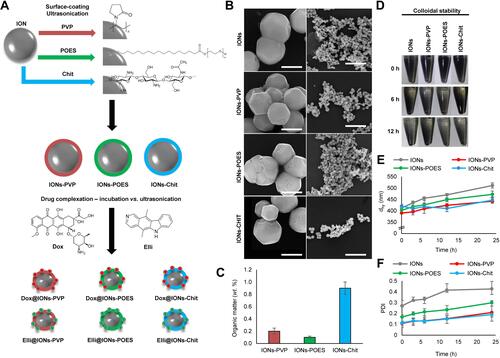
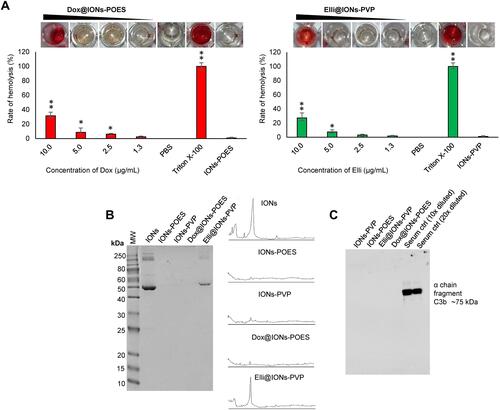
Figure 6 Examination of in vitro biocompatibility of Dox@IONs-POES and Elli@IONs-PVP. (A) Hemolysis of Dox@IONs-POES and Elli@IONs-PVP assayed on human RBCs. PBS (pH 7.4) and 0.1% Triton X-100 were utilized as negative and positive controls, respectively. Amount of tested IONs-POES and IONs-PVP without tethered Dox and Elli is adequate to the highest amount of IONs in Dox@IONs-POES and Elli@IONs-PVP treatments. Upper images depict representative photographs of tested samples. The values are expressed as the mean of three independent replicates (n=3). Vertical bars indicate + and −errors. *P<0.05, **P<0.01 related to the IONs-POES and IONs-PVP without tethered topo II poisons. (B) Protein corona patterns obtained after 30 mins incubation of annotated formulations with human plasma followed by extensive washing, elution, and loading onto 12% SDS-PAGE. As a control, human plasma (1,000× diluted) was loaded to the first lane. Figures on the right side show protein coronas quantified by densitometric analysis. (C) Immunoblot of C3b binding from human serum from male AB clotted whole blood.
The authors apologize for these errors and advise they do not affect the results of the paper.
